Prognostic Role of Plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in Patients Affected by Metastatic Gastrointestinal Stromal Tumors: Can Immune Checkpoints Act as a Sentinel for Short-Term Survival?
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Clinico-Pathological Features of Metastatic GIST Patients
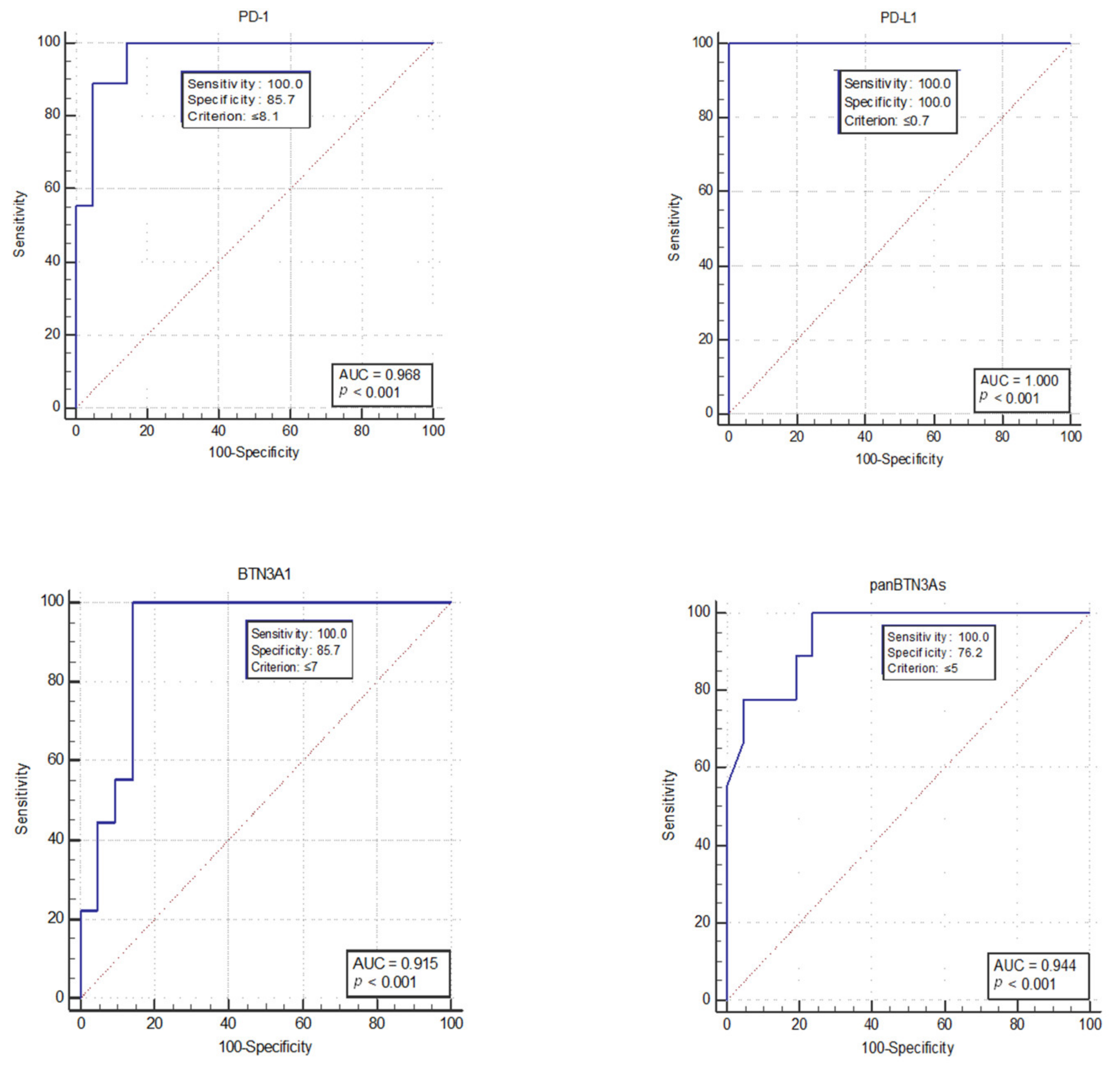
2.2. Discrimination between Short-Term versus Long-Term mGIST Survivors
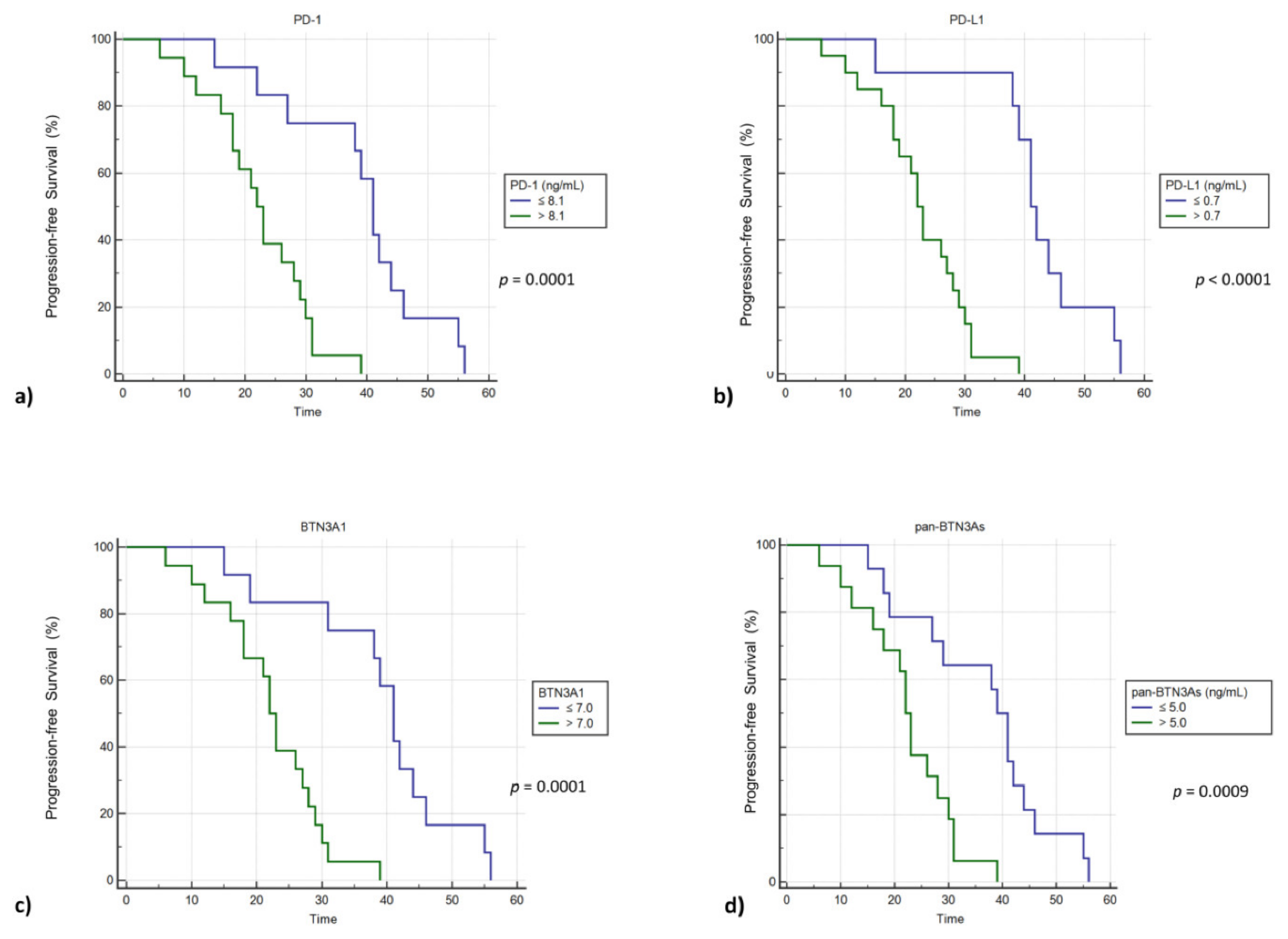
2.3. High Plasma Concentrations of sPD-1, sPD-L1, sBTN3A1 and pan-sBTN3As Are Negatively Correlated with Progression-Free Survival in mGIST Patients
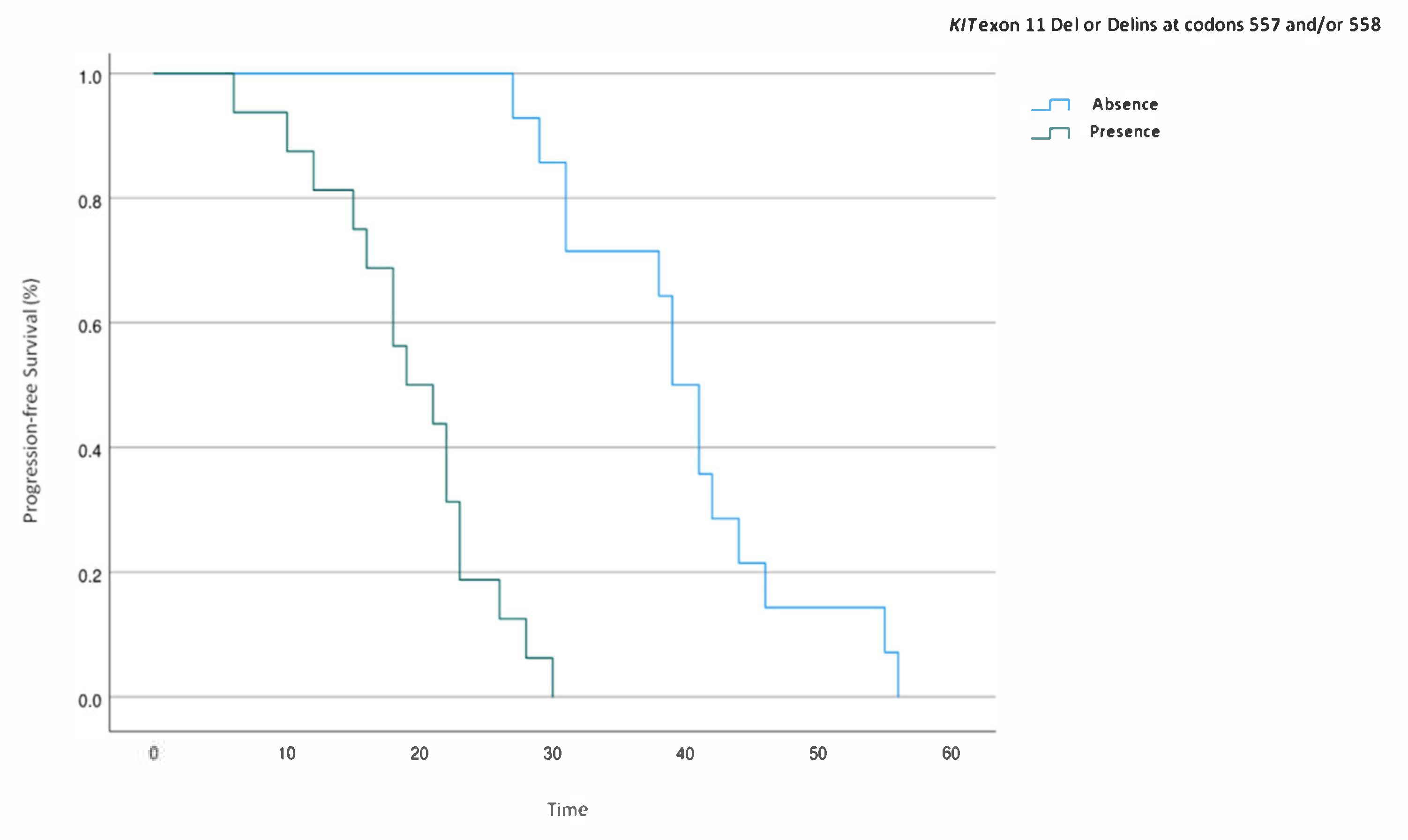
2.4. Multivariate Analysis of Prognostic Factors for PFS in KIT Exon 11-Mutated mGIST Patients
3. Discussion
4. Patients and Methods
4.1. Study Population
4.2. Measurement of Plasma PD-1, PD-L1, pan-BTN3As, and BTN3A1 Levels
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors—Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archiv 2001, 438, 1–12. [Google Scholar] [CrossRef]
- Fanale, D.; Incorvaia, L.; Castiglia, M.; Barraco, N.; Badalamenti, G.; Le Cesne, A.; Russo, A. Liquid Biopsy in Gastrointestinal Stromal Tumor. J. Path. 2017, 202. [Google Scholar] [CrossRef]
- Ma, G.L.; Murphy, J.D.; Martinez, M.E.; Sicklick, J.K. Epidemiology of Gastrointestinal Stromal Tumors in the Era of Histology Codes: Results of a Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2015, 24, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hidalgo, J.M.; Duran-Martinez, M.; Molero-Payan, R.; Rufian-Peña, S.; Arjona-Sanchez, A.; Casado-Adam, A.; Cosano-Alvarez, A.; Briceño-Delgado, J. Gastrointestinal stromal tumors: A multidisciplinary challenge. World J. Gastroenterol. 2018, 24, 1925–1941. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yue, C. Gastrointestinal stromal tumor. J. Gastrointest. Oncol. 2012, 3, 189–208. [Google Scholar] [PubMed]
- Rubin, B.P.; Fletcher, J.A.; Fletcher, C.D.M. Molecular Insights into the Histogenesis and Pathogenesis of Gastrointestinal Stromal Tumors. Int. J. Surg. Pathol. 2000, 8, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.A.; Kamalidehghan, B.; Saleem, M.; Huri, H.Z.; Ahmadipour, F. Receptor tyrosine kinase (c-Kit) inhibitors: A potential therapeutic target in cancer cells. Drug Des. Dev. Ther. 2016, 10, 2443–2459. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Valli, R.; Bertolini, F.; Marchioni, A.; Cavazza, A.; Mucciarini, C.; Migaldi, M.; Federico, M.; Trentini, G.P.; Sgambato, A. PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft-tissue tumours of the gastrointestinal tract. Histopathology 2005, 46, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Sjögren, H.; Meis-Kindblom, J.M.; Stenman, G.; Åman, P.; Kindblom, L.-G. The Complexity of KIT Gene Mutations and Chromosome Rearrangements and Their Clinical Correlation in Gastrointestinal Stromal (Pacemaker Cell) Tumors. Am. J. Pathol. 2002, 160, 15–22. [Google Scholar] [CrossRef]
- Mussi, C.; Schildhaus, H.-U.; Gronchi, A.; Wardelmann, E.; Hohenberger, P. Therapeutic Consequences from Molecular Biology for Gastrointestinal Stromal Tumor Patients Affected by Neurofibromatosis Type 1. Clin. Cancer Res. 2008, 14, 4550–4555. [Google Scholar] [CrossRef]
- Boikos, S.A.; Pappo, A.S.; Killian, J.K.; Laquaglia, M.P.; Weldon, C.B.; George, S.; Trent, J.C.; Von Mehren, M.; Wright, J.A.; Schiffman, J.D.; et al. Molecular Subtypes ofKIT/PDGFRAWild-Type Gastrointestinal Stromal Tumors. JAMA Oncol. 2016, 2, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Plaat, B.E.C.; Hollema, H.; Molenaar, W.M.; Broers, G.H.T.; Pijpe, J.; Mastik, M.F.; Hoekstra, H.J.; Berg, E.V.D.; Scheper, R.J.; Van Der Graaf, W.T.A. Soft Tissue Leiomyosarcomas and Malignant Gastrointestinal Stromal Tumors: Differences in Clinical Outcome and Expression of Multidrug Resistance Proteins. J. Clin. Oncol. 2000, 18, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Roberts, P.J.; Sarlomo-Rikala, M.; Andersson, L.C.; Tervahartiala, P.; Tuveson, D.; Silberman, S.L.; Capdeville, R.; Dimitrijevic, S.; Druker, B.; et al. Effect of the Tyrosine Kinase Inhibitor STI571 in a Patient with a Metastatic Gastrointestinal Stromal Tumor. N. Engl. J. Med. 2001, 344, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Blanke, C.D.; Demetri, G.D.; Von Mehren, M.; Heinrich, M.C.; Eisenberg, B.; Fletcher, J.A.; Corless, C.L.; Fletcher, C.D.M.; Roberts, P.J.; Heinz, D.; et al. Long-Term Results From a Randomized Phase II Trial of Standard- Versus Higher-Dose Imatinib Mesylate for Patients With Unresectable or Metastatic Gastrointestinal Stromal Tumors Expressing KIT. J. Clin. Oncol. 2008, 26, 620–625. [Google Scholar] [CrossRef]
- Rutkowski, P.; Nowecki, Z.I.; Dębiec-Rychter, M.; Grzesiakowska, U.; Michej, W.; Woźniak, A.; Siedlecki, J.A.; Limon, J.; Dobosz, A.J.V.; Kąkol, M.; et al. Predictive factors for long-term effects of imatinib therapy in patients with inoperable/metastatic CD117(+) gastrointestinal stromal tumors (GISTs). J. Cancer Res. Clin. Oncol. 2007, 133, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Badalamenti, G.; Rinaldi, G.; Iovanna, J.L.; Olive, D.; Swayden, M.; Terruso, L.; Vincenzi, B.; Fulfaro, F.; Bazan, V.; et al. Can the plasma PD-1 levels predict the presence and efficiency of tumor-infiltrating lymphocytes in patients with metastatic melanoma? Ther. Adv. Med. Oncol. 2019, 11, 175883591984887. [Google Scholar] [CrossRef]
- Bian, B.; Fanale, D.; Dusetti, N.; Roque, J.; Pastor, S.; Chretien, A.-S.; Incorvaia, L.; Russo, A.; Olive, D.; Iovanna, J. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. OncoImmunology 2019, 8, e1561120. [Google Scholar] [CrossRef]
- Badalamenti, G.; Fanale, D.; Incorvaia, L.; Barraco, N.; Listì, A.; Maragliano, R.; Vincenzi, B.; Calò, V.; Iovanna, J.L.; Bazan, V.; et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell. Immunol. 2019, 343, 103753. [Google Scholar] [CrossRef]
- Lamberti, G.; Sisi, M.; Andrini, E.; Palladini, A.; Giunchi, F.; Lollini, P.-L.; Ardizzoni, A.; Gelsomino, F. The Mechanisms of PD-L1 Regulation in Non-Small-Cell Lung Cancer (NSCLC): Which Are the Involved Players? Cancers 2020, 12, 3129. [Google Scholar] [CrossRef]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Barraco, N.; Bono, M.; Corsini, L.R.; Galvano, A.; Gristina, V.; Listì, A.; Vieni, S.; et al. Programmed Death Ligand 1 (PD-L1) as a Predictive Biomarker for Pembrolizumab Therapy in Patients with Advanced Non-Small-Cell Lung Cancer (NSCLC). Adv. Ther. 2019, 36, 2600–2617. [Google Scholar] [CrossRef]
- Falcone, I.; Conciatori, F.; Bazzichetto, C.; Ferretti, G.; Cognetti, F.; Ciuffreda, L.; Milella, M. Tumor Microenvironment: Implications in Melanoma Resistance to Targeted Therapy and Immunotherapy. Cancers 2020, 12, 2870. [Google Scholar] [CrossRef]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Porta, C.; Olive, D.; De Luca, I.; Brando, C.; Rizzo, M.; Messina, C.; Rediti, M.; et al. Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: A step toward a biomarker for therapeutic decisions. OncoImmunology 2020, 9, 1832348. [Google Scholar] [CrossRef]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Brando, C.; Bono, M.; De Luca, I.; Algeri, L.; Bonasera, A.; Corsini, L.R.; Scurria, S.; et al. A “Lymphocyte MicroRNA Signature” as Predictive Biomarker of Immunotherapy Response and Plasma PD-1/PD-L1 Expression Levels in Patients with Metastatic Renal Cell Carcinoma: Pointing towards Epigenetic Reprogramming. Cancers 2020, 12, 3396. [Google Scholar] [CrossRef]
- Corsini, L.R.; Fanale, D.; Passiglia, F.; Incorvaia, L.; Gennusa, V.; Bazan, V.; Russo, A. Monoclonal antibodies for the treatment of non-hematological tumors: A safety review. Expert Opin. Drug Saf. 2018, 17, 1197–1209. [Google Scholar] [CrossRef]
- Melero, I.; Berman, D.M.; Aznar, M.A.; Korman, A.J.; Gracia, J.L.P.; Haanen, J.B.A.G. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 2015, 15, 457–472. [Google Scholar] [CrossRef]
- Benyamine, A.; Loncle, C.; Foucher, E.; Blazquez, J.-L.; Castanier, C.; Chrétien, A.-S.; Modesti, M.; Secq, V.; Chouaib, S.; Gironella, M.; et al. BTN3A is a prognosis marker and a promising target for Vγ9Vδ2 T cells based-immunotherapy in pancreatic ductal adenocarcinoma (PDAC). OncoImmunology 2018, 7, e1372080. [Google Scholar] [CrossRef] [PubMed]
- Arnett, H.A.; Viney, J.L. Immune modulation by butyrophilins. Nat. Rev. Immunol. 2014, 14, 559–569. [Google Scholar] [CrossRef]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Nakamura, T.; Hayashi, A.; Okamoto, T.; Iino, T.; Asanuma, Y.; Hagi, T.; Kita, K.; Nakamura, K.; Sudo, A. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci. Rep. 2020, 10, 9077. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Mamessier, E.; Pantaleo, M.A.; Astolfi, A.; Ostrowski, J.; Birnbaum, D. PDL1 expression is an independent prognostic factor in localized GIST. OncoImmunology 2015, 4, e1002729. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Fanale, D.; Vincenzi, B.; De Luca, I.; Bartolotta, T.; Cannella, R.; Pantuso, G.; Cabibi, D.; Russo, A.; Bazan, V.; et al. Type and Gene Location of KIT Mutations Predict Progression-Free Survival to First-Line Imatinib in Gastrointestinal Stromal Tumors: A Look into the Exon. Cancers 2021, 13, 993. [Google Scholar] [CrossRef]
- Tsai, K.K.; Daud, A.I. The Role of Anti-PD-1/PD-L1 Agents in Melanoma: Progress to Date. Drugs 2015, 75, 563–575. [Google Scholar] [CrossRef]
- Menon, S.; Shin, S.; Dy, G. Advances in Cancer Immunotherapy in Solid Tumors. Cancers 2016, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Boland, P.M.; Ma, W.W. Immunotherapy for Colorectal Cancer. Cancers 2017, 9, 50. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Rusakiewicz, S.; Semeraro, M.; Sarabi, M.; Desbois, M.; Locher, C.; Mendez, R.; Vimond, N.; Concha, A.; Garrido, F.; Isambert, N.; et al. Immune Infiltrates Are Prognostic Factors in Localized Gastrointestinal Stromal Tumors. Cancer Res. 2013, 73, 3499–3510. [Google Scholar] [CrossRef]
- Tan, Y.; Trent, J.C.; Wilky, A.B.; Kerr, A.D.; Rosenberg, A.E. Current status of immunotherapy for gastrointestinal stromal tumor. Cancer Gene Ther. 2017, 24, 130–133. [Google Scholar] [CrossRef]
- Seifert, A.M.; Zeng, S.; Zhang, J.Q.; Kim, T.S.; Cohen, N.A.; Beckman, M.J.; Medina, B.D.; Maltbaek, J.H.; Loo, J.K.; Crawley, M.H.; et al. PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2017, 23, 454–465. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Shoushtari, A.N.; Keohan, M.L.; Dickson, M.A.; Gounder, M.M.; Chi, P.; Loo, J.K.; Gaffney, L.; Schneider, L.; Patel, Z.; et al. Combined KIT and CTLA-4 Blockade in Patients with Refractory GIST and Other Advanced Sarcomas: A Phase Ib Study of Dasatinib plus Ipilimumab. Clin. Cancer Res. 2017, 23, 2972–2980. [Google Scholar] [CrossRef]
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.-Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Ryckewaert, T.; et al. Combination of pembrolizumab and metronomic cyclophosphamide in patients with advanced sarcomas and GIST: A French Sarcoma Group phase II trial. J. Clin. Oncol. 2017, 35, 11053. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Shoushtari, A.N.; Agaram, N.P.; Kuk, D.; Qin, L.-X.; Carvajal, R.D.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2015, 46, 357–365. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Zhang, J.; Teng, F.; Xing, L.; Yu, J. PD-1/PD-L1 checkpoint blockades in non-small cell lung cancer: New development and challenges. Cancer Lett. 2017, 405, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Lin, H.-H.; Lin, C.-W.; Li, C.-C.; Yao, M.; Tang, J.-L.; Hou, H.-A.; Tsay, W.; Chou, S.-J.; Cheng, C.-L.; et al. Soluble PD-L1: A biomarker to predict progression of autologous transplantation in patients with multiple myeloma. Oncotarget 2016, 7, 62490–62502. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Liu, L. Prognostic significance of PD-L1 in solid tumor. Medicine 2017, 96, e6369. [Google Scholar] [CrossRef]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.-H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Zhao, R.; Song, Y.; Wang, Y.; Huang, Y.; Li, Z.; Cui, Y.; Yi, M.; Xia, L.; Zhuang, W.; Wu, X.; et al. PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif. 2019, 52, e12571. [Google Scholar] [CrossRef]
- Kruger, S.; Legenstein, M.-L.; Rösgen, V.; Haas, M.; Modest, D.P.; Westphalen, C.B.; Ormanns, S.; Kirchner, T.; Heinemann, V.; Holdenrieder, S.; et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. OncoImmunology 2017, 6, e1310358. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J.; Jin, J.; Chen, H.; Zhen, Z.; Jiang, W.; Lin, T.; Huang, H.; Xia, Z.; Sun, X. High Serum Level of Soluble Programmed Death Ligand 1 is Associated With a Poor Prognosis in Hodgkin Lymphoma. Transl. Oncol. 2018, 11, 779–785. [Google Scholar] [CrossRef]
- Takeuchi, M.; Doi, T.; Obayashi, K.; Hirai, A.; Yoneda, K.; Tanaka, F.; Iwai, Y. Soluble PD-L1 with PD-1-binding capacity exists in the plasma of patients with non-small cell lung cancer. Immunol. Lett. 2018, 196, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Kamarudin, A.N.; Cox, T.; Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: Current methods and applications. BMC Med. Res. Methodol. 2017, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | N of Patients (%) |
|---|---|
| Total patients | 30 |
| Sex | |
| Male | 19 (63.3) |
| Female | 11 (36.7) |
| Age at diagnosis (y) | |
| Median | 58 |
| Mean | 57 |
| Range | 33–77 |
| Age groups (y) | |
| ≤40 | 2 (6.6) |
| 41–50 | 7 (23.4) |
| 51–60 | 9 (30) |
| >60 | 12 (40) |
| Type of KIT exon 11 PV | |
| Deletion/delins | 16 (53.3) |
| Duplication/insertion/SNV | 14 (46.7) |
| Site of origin | |
| Gastric | 14 (46.7) |
| Small bowel | 11 (36.6) |
| Other GI | 5 (16.7) |
| Primitive tumor diameter | |
| ≤5 cm | 12 (40) |
| >5 cm | 18 (60) |
| Mitosis | |
| ≤5/50 HPF | 13 (43.3) |
| >5/50 HPF | 17 (56.7) |
| Site of metastasis | |
| Liver | 11 (36.6) |
| Peritonuem | 13 (43.4) |
| Liver and peritoneum | 6 (20) |
| PFS | |
| ≤36 months | 20 (66.7) |
| >36 months | 10 (33.3) |
| Type of Mutations | Pathogenic Variant | No. Patients (%) |
|---|---|---|
| Deletions | p.W557_K558del | 6 (20%) |
| p.K550_K558del | 5 (16.8%) | |
| p.E554_K558del | 1 (3.3%) | |
| p.Q556_V560del | 2 (6.7%) | |
| Del/Ins | p.K558_V559delinsN | 1 (3.3%) |
| p.W557_V559delinsF | 1 (3.3%) | |
| SNV | p.W557R | 4 (13.3%) |
| p.V560D | 3 (10%) | |
| p.V559D | 3 (10%) | |
| Duplication | p.D572_D579dup | 3 (10%) |
| Insertion | p.K558_V559insS | 1 (3.3%) |
| PFS | Univariate Cox Regression | Multivariable Cox Regression | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Gender (M vs. F) | 0.90 (0.42–1.98) | NS | - | - |
| Age (≤55 vs. >55 years) | 2.25 (1.01–4.98) | 0.04 | 0.93 (0.33–2.61) | NS |
| Primitive tumor diameter (≤5 cm vs. >5 cm) | 0.67 (0.31–1.47) | NS | - | - |
| Mitosis (≤5/50 HPF vs. >5/50 HPF) | 0.74 (0.34–1.62) | NS | - | - |
| Gastric site of origin (No vs. yes) | 0.68 (0.31–1.45) | NS | - | - |
| sPD-L1 (≤0.7 vs. >0.7 ng/mL) | 0.05 (0.01–0.25) | <0.0001 | 0.01 (0.001–0.18) | 0.001 |
| sPD-1 (≤8.1 vs. >8.1 ng/mL) | 0.16 (0.05–0.45) | 0.0001 | 1.85 (0.47–7.27) | NS |
| sBTN3 A1 (≤0.7 vs. >0.7 ng/mL) | 0.14 (0.05–0.41) | 0.0001 | 0.84 (0.17–4.23) | NS |
| pan-sBTN3 s (≤5.0 vs. >5.0 ng/mL) | 0.23 (0.09–0.58) | <0.001 | 4.45 (0.96–20.5) | 0.05 |
| KIT exon 11 Del or Delins at codons 557 and/or 558 (No vs. yes) | 0.04 (0.008–0.35) | <0.001 | 0.05 (0.007–0.31) | 0.003 |
| Antibodies | sPD-L1 | sPD-1 | pan-sBTN3As * | sBTN3A1 * |
|---|---|---|---|---|
| Coating Ab | α-sPD-L1 1.8 + α-sPD-L1 2.1 | α-sPD-1 6.4 | α-sBTN3A S148 | α-sBTN3A1 S240 |
| Detection Ab (biotinylated) | Α-sPD-L1 1.3.1 | α-sPD-1 3.1 | α-sBTN3A 103.2 | α-sBTN3A 103.2 |
| Detection limit (pg/mL) | 20 | 50 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanale, D.; Incorvaia, L.; Badalamenti, G.; De Luca, I.; Algeri, L.; Bonasera, A.; Corsini, L.R.; Brando, C.; Russo, A.; Iovanna, J.L.; et al. Prognostic Role of Plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in Patients Affected by Metastatic Gastrointestinal Stromal Tumors: Can Immune Checkpoints Act as a Sentinel for Short-Term Survival? Cancers 2021, 13, 2118. https://doi.org/10.3390/cancers13092118
Fanale D, Incorvaia L, Badalamenti G, De Luca I, Algeri L, Bonasera A, Corsini LR, Brando C, Russo A, Iovanna JL, et al. Prognostic Role of Plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in Patients Affected by Metastatic Gastrointestinal Stromal Tumors: Can Immune Checkpoints Act as a Sentinel for Short-Term Survival? Cancers. 2021; 13(9):2118. https://doi.org/10.3390/cancers13092118
Chicago/Turabian StyleFanale, Daniele, Lorena Incorvaia, Giuseppe Badalamenti, Ida De Luca, Laura Algeri, Annalisa Bonasera, Lidia Rita Corsini, Chiara Brando, Antonio Russo, Juan Lucio Iovanna, and et al. 2021. "Prognostic Role of Plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in Patients Affected by Metastatic Gastrointestinal Stromal Tumors: Can Immune Checkpoints Act as a Sentinel for Short-Term Survival?" Cancers 13, no. 9: 2118. https://doi.org/10.3390/cancers13092118
APA StyleFanale, D., Incorvaia, L., Badalamenti, G., De Luca, I., Algeri, L., Bonasera, A., Corsini, L. R., Brando, C., Russo, A., Iovanna, J. L., & Bazan, V. (2021). Prognostic Role of Plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in Patients Affected by Metastatic Gastrointestinal Stromal Tumors: Can Immune Checkpoints Act as a Sentinel for Short-Term Survival? Cancers, 13(9), 2118. https://doi.org/10.3390/cancers13092118








