Prognostic Factors and Current Treatment Strategies for Renal Cell Carcinoma Metastatic to the Brain: An Overview
Simple Summary
Abstract
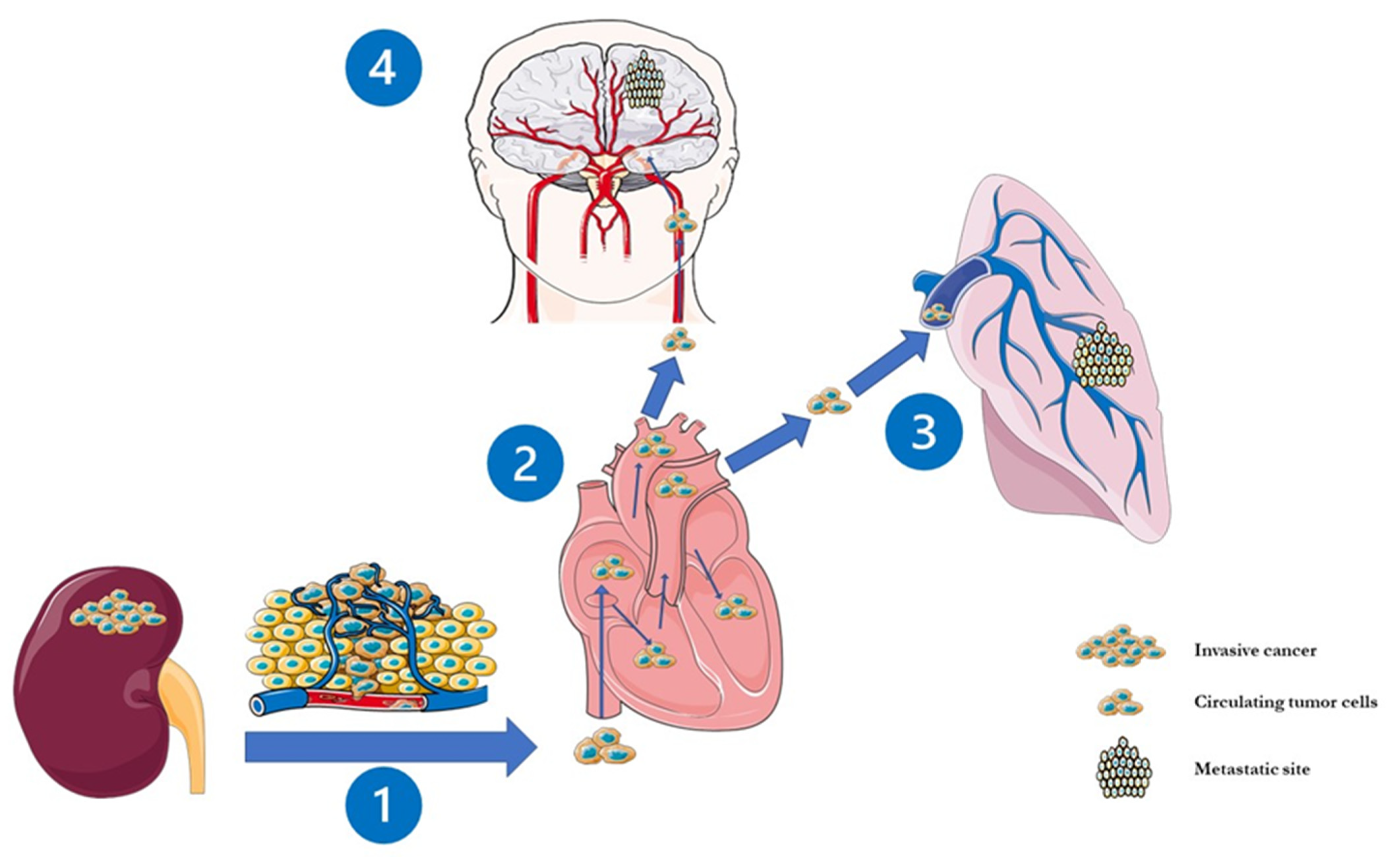
1. Introduction
2. Clinico-Radiological Features of BMRCC and Indications for Brain Surveillance in RCC Patients
3. BMRCC’s Prognostic Factors and Risk Scales
4. Role of Cytoreductive Nephrectomy
5. Current Treatment Strategies
5.1. Surgical Resection
5.2. The Role of Radiotherapy
5.3. TKIs
5.4. Immune Checkpoint Inhibitors (ICIs)
5.5. Ongoing Clinical Trials
6. General Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A. Renal Cell Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Sun, M.; Jeldres, C.; Shariat, S.F.; Trinh, Q.-D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; et al. Distribution of Metastatic Sites in Renal Cell Carcinoma: A Population-Based Analysis. Ann. Oncol. 2012, 23, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Sun, M.-H.; Kondziolka, D.; Flickinger, J.; Lunsford, L.D. Radiosurgery in Patients with Renal Cell Carcinoma Metastasis to the Brain: Long-Term Outcomes and Prognostic Factors Influencing Survival and Local Tumor Control. J. Neurosurg. 2003, 98, 342–349. [Google Scholar] [CrossRef]
- Sun, M.; De Velasco, G.; Brastianos, P.K.; Aizer, A.A.; Martin, A.; Moreira, R.; Nguyen, P.L.; Trinh, Q.-D.; Choueiri, T.K. The Development of Brain Metastases in Patients with Renal Cell Carcinoma: Epidemiologic Trends, Survival, and Clinical Risk Factors Using a Population-Based Cohort. Eur. Urol. Focus 2019, 5, 474–481. [Google Scholar] [CrossRef]
- Shuch, B.; La Rochelle, J.C.; Klatte, T.; Riggs, S.B.; Liu, W.; Kabbinavar, F.F.; Pantuck, A.J.; Belldegrun, A.S. Brain Metastasis from Renal Cell Carcinoma. Cancer 2008, 113, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.E.; Youn, P.; Peterson, C.R., 3rd; Usuki, K.Y.; Walter, K.A.; Okunieff, P.; Milano, M.T. Radiotherapy for Brain Metastases from Renal Cell Carcinoma in the Targeted Therapy Era: The University of Rochester Experience. Am. J. Clin. Oncol. 2017, 40, 439–443. [Google Scholar] [CrossRef]
- Verma, J.; Jonasch, E.; Allen, P.K.; Weinberg, J.S.; Tannir, N.; Chang, E.L.; Mahajan, A. The Impact of Tyrosine Kinase Inhibitors on the Multimodality Treatment of Brain Metastases from Renal Cell Carcinoma. Am. J. Clin. Oncol. 2013, 36, 620–624. [Google Scholar] [CrossRef]
- Chevreau, C.; Ravaud, A.; Escudier, B.; Amela, E.; Delva, R.; Rolland, F.; Tosi, D.; Oudard, S.; Blanc, E.; Ferlay, C.; et al. A Phase II Trial of Sunitinib in Patients with Renal Cell Cancer and Untreated Brain Metastases. Clin. Genitourin. Cancer 2014, 12, 50–54. [Google Scholar] [CrossRef]
- Pellerino, A.; Internò, V.; Muscolino, E.; Mo, F.; Bruno, F.; Pronello, E.; Franchino, F.; Soffietti, R.; Rudà, R. Leptomeningeal Metastases from Non-Small Cell Lung Cancer: State of the Art and Recent Advances. J. Cancer Metastasis Treat. 2020, 6, 41. [Google Scholar] [CrossRef]
- Pellerino, A.; Internò, V.; Mo, F.; Franchino, F.; Soffietti, R.; Rudà, R. Management of Brain and Leptomeningeal Metastases from Breast Cancer. Int. J. Mol. Sci. 2020, 21, 8534. [Google Scholar] [CrossRef]
- Wroński, M.; Arbit, E.; Russo, P.; Galicich, J.H. Surgical Resection of Brain Metastases from Renal Cell Carcinoma in 50 Patients. Urology 1996, 47, 187–193. [Google Scholar] [CrossRef]
- Hanzly, M.; Abbotoy, D.; Creighton, T.; Diorio, G.; Mehedint, D.; Murekeyisoni, C.; Attwood, K.; Kauffman, E.; Fabiano, A.; Schwaab, T. Early Identification of Asymptomatic Brain Metastases from Renal Cell Carcinoma. Clin. Exp. Metastasis 2015, 32, 783–788. [Google Scholar] [CrossRef]
- Bennani, O.; Derrey, S.; Langlois, O.; Castel, H.; Laquerriere, A.; Freger, P.; Proust, F. Brain Metastasis from Renal Cell Carcinoma. Neurochirurgie 2014, 60, 12–16. [Google Scholar] [CrossRef]
- Choi, S.Y.; Yoo, S.; You, D.; Jeong, I.G.; Song, C.; Hong, B.; Hong, J.H.; Ahn, H.; Kim, C.-S. Prognostic Factors for Survival of Patients with Synchronous or Metachronous Brain Metastasis of Renal Cell Carcinoma. Clin. Genitourin. Cancer 2017, 15, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; George, D.J.; Harrison, M.R. How We Treat Brain Metastases in Metastatic Renal Cell Carcinoma. Clin. Adv. Hematol. Oncol. 2018, 16, 110–114. [Google Scholar]
- Yekedüz, E.; Arzu Yaşar, H.; Utkan, G.; Ürün, Y. A Systematic Review: Role of Systemic Therapy on Treatment and Prevention of Brain Metastasis in Renal Cell Carcinoma. J. Oncol. Pharm. Pract. 2020, 26, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Alamdari, F.I.; Rasmuson, T.; Roos, G. Follow-up Guidelines for Nonmetastatic Renal Cell Carcinoma Based on the Occurrence of Metastases after Radical Nephrectomy. BJU Int. 1999, 84, 405–411. [Google Scholar] [CrossRef]
- Levy, D.A.; Slaton, J.W.; Swanson, D.A.; Dinney, C.P. Stage Specific Guidelines for Surveillance after Radical Nephrectomy for Local Renal Cell Carcinoma. J. Urol. 1998, 159, 1163–1167. [Google Scholar] [CrossRef]
- Sandock, D.S.; Seftel, A.D.; Resnick, M.I. A New Protocol for the Followup of Renal Cell Carcinoma Based on Pathological Stage. J. Urol. 1995, 154, 28–31. [Google Scholar] [CrossRef]
- Wyler, L.; Napoli, C.U.; Ingold, B.; Sulser, T.; Heikenwälder, M.; Schraml, P.; Moch, H. Brain Metastasis in Renal Cancer Patients: Metastatic Pattern, Tumour-Associated Macrophages and Chemokine/Chemoreceptor Expression. Br. J. Cancer 2014, 110, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Li, Y.; Chen, P.; Wang, J.; Liu, W.; Chen, J. Do Renal Cell Carcinoma Patients with Brain Metastases Still Need Nephrectomy? Int. Urol. Nephrol. 2019, 51, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.-B.; Chen, S.-H.; Chen, Y.-H.; Wu, Y.-P.; Lin, F.; Xue, X.-Y.; Zheng, Q.-S.; Xu, N.; Wei, Y. Risk Factors for Brain Metastases in Patients with Renal Cell Carcinoma. Biomed Res. Int. 2020, 2020, 6836234. [Google Scholar] [CrossRef] [PubMed]
- Bitoh, S.; Hasegawa, H.; Ohtsuki, H.; Obashi, J.; Fujiwara, M.; Sakurai, M. Cerebral Neoplasms Initially Presenting with Massive Intracerebral Hemorrhage. Surg. Neurol. 1984, 22, 57–62. [Google Scholar] [CrossRef]
- Mori, Y.; Kondziolka, D.; Flickinger, J.C.; Logan, T.; Lunsford, L.D. Stereotactic Radiosurgery for Brain Metastasis from Renal Cell Carcinoma. Cancer 1998, 83, 344–353. [Google Scholar] [CrossRef]
- Muacevic, A.; Kreth, F.W.; Mack, A.; Tonn, J.-C.; Wowra, B. Stereotactic Radiosurgery without Radiation Therapy Providing High Local Tumor Control of Multiple Brain Metastases from Renal Cell Carcinoma. Minim. Invasive Neurosurg. 2004, 47, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, S.; Jokura, H.; Nakamura, H.; Shintaku, I.; Ohyama, C.; Satoh, M.; Saito, S.; Fukuzaki, A.; Orikasa, S.; Yoshimoto, T. Gamma-Knife Radiosurgery for Brain Metastasis of Renal Cell Carcinoma: Results in 42 Patients. Int. J. Urol. 2002, 9, 618–625; discussion 626; author reply 627. [Google Scholar] [CrossRef]
- Seute, T.; Leffers, P.; Wilmink, J.T.; ten Velde, G.P.M.; Twijnstra, A. Response of Asymptomatic Brain Metastases from Small-Cell Lung Cancer to Systemic First-Line Chemotherapy. J. Clin. Oncol. 2006, 24, 2079–2083. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Kidney Cancer (Version 4.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (accessed on 19 April 2021).
- Suarez-Sarmiento, A.J.; Nguyen, K.A.; Syed, J.S.; Nolte, A.; Ghabili, K.; Cheng, M.; Liu, S.; Chiang, V.; Kluger, H.; Hurwitz, M.; et al. Brain Metastasis from Renal-Cell Carcinoma: An Institutional Study. Clin. Genitourin. Cancer 2019, 17, e1163–e1170. [Google Scholar] [CrossRef]
- Remon, J.; Lianes, P.; Martínez, S. Brain Metastases from Renal Cell Carcinoma. Should We Change the Current Standard? Cancer Treat. Rev. 2012, 38, 249–257. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Chao, S.T.; Sneed, P.K.; Luo, X.; Suh, J.; Roberge, D.; Bhatt, A.; Jensen, A.W.; Brown, P.D.; Shih, H.; et al. Diagnosis-Specific Prognostic Factors, Indexes, and Treatment Outcomes for Patients with Newly Diagnosed Brain Metastases: A Multi-Institutional Analysis of 4,259 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 655–661. [Google Scholar] [CrossRef]
- Takeshita, N.; Otsuka, M.; Kamasako, T.; Somoto, T.; Uemura, T.; Shinozaki, T.; Kobayashi, M.; Kawana, H.; Itami, M.; Iuchi, T.; et al. Prognostic Factors and Survival in Japanese Patients with Brain Metastasis from Renal Cell Cancer. Int. J. Clin. Oncol. 2019, 24, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- El Ali, Z.; Rottey, S.; Barthelemy, P.; Kotecki, N.; VAN Paemel, R.; Devrient, D.; Awada, A.; Gil, T.; Pannier, D.; Ryckewaert, T.; et al. Brain Metastasis and Renal Cell Carcinoma: Prognostic Scores Assessment in the Era of Targeted Therapies. Anticancer Res. 2019, 39, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.; Daugherty, E.; Jacob, J.; Shapiro, O.; Mollapour, M.; Bratslavsky, G. Renal Cell Carcinoma and Brain Metastasis: Questioning the Dogma of Role for Cytoreductive Nephrectomy. Urol. Oncol. 2019, 37, 182.e9–182.e15. [Google Scholar] [CrossRef]
- Heng, D.Y.; Signorovitch, J.; Swallow, E.; Li, N.; Zhong, Y.; Qin, P.; Zhuo, D.Y.; Wang, X.; Park, J.; Stergiopoulos, S.; et al. Comparative Effectiveness of Second-Line Targeted Therapies for Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis of Real-World Observational Studies. PLoS ONE 2014, 9, e114264. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, M.; Coleman, N.; Welsh, L.; Saran, F.; Lopez, J. CNS Cancer Immunity Cycle and Strategies to Target This for Glioblastoma. Oncotarget 2018, 9, 22802–22816. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.W.; Chung, H.-T.; Paek, S.H.; Kim, D.G.; Jung, H.-W. Brain Metastasis from Renal Cell Carcinoma. Prog. Neurol. Surg. 2012, 25, 163–175. [Google Scholar] [CrossRef]
- Decker, D.A.; Decker, V.L.; Herskovic, A.; Cummings, G.D. Brain Metastases in Patients with Renal Cell Carcinoma: Prognosis and Treatment. J. Clin. Oncol. 1984, 2, 169–173. [Google Scholar] [CrossRef]
- Hernandez, L.; Zamorano, L.; Sloan, A.; Fontanesi, J.; Lo, S.; Levin, K.; Li, Q.; Diaz, F. Gamma Knife Radiosurgery for Renal Cell Carcinoma Brain Metastases. J. Neurosurg. 2002, 97, 489–493. [Google Scholar] [CrossRef]
- Maor, M.H.; Frias, A.E.; Oswald, M.J. Palliative Radiotherapy for Brain Metastases in Renal Carcinoma. Cancer 1988, 62, 1912–1917. [Google Scholar] [CrossRef]
- Shuto, T.; Inomori, S.; Fujino, H.; Nagano, H. Gamma Knife Surgery for Metastatic Brain Tumors from Renal Cell Carcinoma. J. Neurosurg. 2006, 105, 555–560. [Google Scholar] [CrossRef]
- Rades, D.; Dziggel, I.; Blanck, O.; Gebauer, N.; Bartscht, T.; Schild, S.E. A Score to Identify Patients with Brain Metastases from Colorectal Cancer Who May Benefit from Whole-Brain Radiotherapy in Addition to Stereotactic Radiosurgery/Radiotherapy. Anticancer Res. 2018, 38, 3111–3114. [Google Scholar] [PubMed]
- Klausner, G.; Troussier, I.; Biau, J.; Jacob, J.; Schernberg, A.; Canova, C.-H.; Simon, J.-M.; Borius, P.-Y.; Malouf, G.; Spano, J.-P.; et al. Stereotactic Radiation Therapy for Renal Cell Carcinoma Brain Metastases in the Tyrosine Kinase Inhibitors Era: Outcomes of 120 Patients. Clin. Genitourin. Cancer 2019, 17, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and Gene Delivery to the Brain: The Vascular Route. Neuron 2002, 36, 555–558. [Google Scholar] [CrossRef]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of Brain Metastases According to Molecular Subtypes. Nature reviews. Neurology 2020, 16, 557–574. [Google Scholar] [CrossRef]
- Beck, J.; Procopio, G.; Bajetta, E.; Keilholz, U.; Negrier, S.; Szczylik, C.; Bokemeyer, C.; Bracarda, S.; Richel, D.J.; Staehler, M.; et al. Final Results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) Expanded-Access Study: A Large Open-Label Study in Diverse Community Settings. Ann. Oncol. 2011, 22, 1812–1823. [Google Scholar] [CrossRef]
- Bukowski, R.M.; Stadler, W.M.; McDermott, D.F.; Dutcher, J.P.; Knox, J.J.; Miller, W.H., Jr.; Hainsworth, J.D.; Henderson, C.A.; Hajdenberg, J.; Kindwall-Keller, T.L.; et al. Safety and Efficacy of Sorafenib in Elderly Patients Treated in the North American Advanced Renal Cell Carcinoma Sorafenib Expanded Access Program. Oncology 2010, 78, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Imarisio, I.; Paglino, C. High Frequency of Intracerebral Hemorrhage in Metastatic Renal Carcinoma Patients with Brain Metastases Treated with Tyrosine Kinase Inhibitors Targeting the Vascular Endothelial Growth Factor Receptor. Eur. Urol. 2008, 53, 1092–1093. [Google Scholar] [CrossRef]
- Jäger, D.; Ma, J.H.; Mardiak, J.; Ye, D.W.; Korbenfeld, E.; Zemanova, M.; Ahn, H.; Guo, J.; Leonhartsberger, N.; Stauch, K.; et al. Sorafenib Treatment of Advanced Renal Cell Carcinoma Patients in Daily Practice: The Large International PREDICT Study. Clin. Genitourin. Cancer 2015, 13, 156–164.e1. [Google Scholar] [CrossRef]
- Massard, C.; Zonierek, J.; Gross-Goupil, M.; Fizazi, K.; Szczylik, C.; Escudier, B. Incidence of Brain Metastases in Renal Cell Carcinoma Treated with Sorafenib. Ann. Oncol. 2010, 21, 1027–1031. [Google Scholar] [CrossRef]
- Verma, J.; Jonasch, E.; Allen, P.; Tannir, N.; Mahajan, A. Impact of Tyrosine Kinase Inhibitors on the Incidence of Brain Metastasis in Metastatic Renal Cell Carcinoma. Cancer 2011, 117, 4958–4965. [Google Scholar] [CrossRef]
- Gore, M.E.; Szczylik, C.; Porta, C.; Bracarda, S.; Bjarnason, G.A.; Oudard, S.; Lee, S.-H.; Haanen, J.; Castellano, D.; Vrdoljak, E.; et al. Final Results from the Large Sunitinib Global Expanded-Access Trial in Metastatic Renal Cell Carcinoma. Br. J. Cancer 2015, 113, 12–19. [Google Scholar] [CrossRef]
- Gore, M.E.; Hariharan, S.; Porta, C.; Bracarda, S.; Hawkins, R.; Bjarnason, G.A.; Oudard, S.; Lee, S.-H.; Carteni, G.; Nieto, A.; et al. Sunitinib in Metastatic Renal Cell Carcinoma Patients with Brain Metastases. Cancer 2011, 117, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.E.; Szczylik, C.; Porta, C.; Bracarda, S.; Bjarnason, G.A.; Oudard, S.; Hariharan, S.; Lee, S.-H.; Haanen, J.; Castellano, D.; et al. Safety and Efficacy of Sunitinib for Metastatic Renal-Cell Carcinoma: An Expanded-Access Trial. Lancet Oncol. 2009, 10, 757–763. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Calabrò, F.; Bracarda, S.; Cartenì, G.; Lo Re, G.; Ruggeri, E.M.; Basso, U.; Gasparini, G.; Ciuffreda, L.; Ferrari, V.; et al. Safety and Efficacy of Sunitinib in Patients from Italy with Metastatic Renal Cell Carcinoma: Final Results from an Expanded-Access Trial. Oncology 2015, 88, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Kim, D.W.N.; Straka, C.; Timmerman, R.D.; Brugarolas, J. Prolonged Survival of a Patient with Papillary Renal Cell Carcinoma and Brain Metastases Using Pazopanib. J. Clin. Oncol. 2013, 31, e114–e117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberto, M.; Bassanelli, M.; Iannicelli, E.; Giacinti, S.; D’Antonio, C.; Aschelter, A.M.; Marchetti, P. Clinical Outcome of Third-Line Pazopanib in a Patient with Metastatic Renal Cell Carcinoma. Case Rep. Oncol. Med. 2015, 2015, 629046. [Google Scholar] [CrossRef]
- Santoni, M.; Conti, A.; Massari, F.; Arnaldi, G.; Iacovelli, R.; Rizzo, M.; De Giorgi, U.; Trementino, L.; Procopio, G.; Tortora, G.; et al. Treatment-Related Fatigue with Sorafenib, Sunitinib and Pazopanib in Patients with Advanced Solid Tumors: An up-to-Date Review and Meta-Analysis of Clinical Trials. Int. J. Cancer 2015, 136, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Matrana, M.R.; Duran, C.; Shetty, A.; Xiao, L.; Atkinson, B.J.; Corn, P.; Pagliaro, L.C.; Millikan, R.E.; Charnsangave, C.; Jonasch, E.; et al. Outcomes of Patients with Metastatic Clear-Cell Renal Cell Carcinoma Treated with Pazopanib after Disease Progression with Other Targeted Therapies. Eur. J. Cancer 2013, 49, 3169–3175. [Google Scholar] [CrossRef][Green Version]
- Négrier, S.; Moriceau, G.; Attignon, V.; Haddad, V.; Pissaloux, D.; Guerin, N.; Carrie, C. Activity of Cabozantinib in Radioresistant Brain Metastases from Renal Cell Carcinoma: Two Case Reports. J. Med. Case Rep. 2018, 12, 351. [Google Scholar] [CrossRef]
- Hirsch, L.; Martinez Chanza, N.; Farah, S.; Flippot, R.; Rathi, N.; Collier, K.; de Velasco, G.; Seront, E.; Beuselinck, B.; Xu, W.; et al. Activity and Safety of Cabozantinib (Cabo) in Brain Metastases (BM) from Metastatic Renal Cell Carcinoma (MRCC): An International Multicenter Study. J. Clin. Oncol. 2021, 39, 310. [Google Scholar] [CrossRef]
- Porta, C.; Calvo, E.; Climent, M.A.; Vaishampayan, U.; Osanto, S.; Ravaud, A.; Bracarda, S.; Hutson, T.E.; Escudier, B.; Grünwald, V.; et al. Efficacy and Safety of Everolimus in Elderly Patients with Metastatic Renal Cell Carcinoma: An Exploratory Analysis of the Outcomes of Elderly Patients in the RECORD-1 Trial. Eur. Urol. 2012, 61, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grünwald, V.; Thompson, J.A.; Figlin, R.A.; Hollaender, N.; et al. Phase 3 Trial of Everolimus for Metastatic Renal Cell Carcinoma: Final Results and Analysis of Prognostic Factors. Cancer 2010, 116, 4256–4265. [Google Scholar] [CrossRef]
- Hudes, G.; Carducci, M.; Tomczak, P.; Dutcher, J.; Figlin, R.; Kapoor, A.; Staroslawska, E.; Sosman, J.; McDermott, D.; Bodrogi, I.; et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Kattan, J.; El Rassy, E.; Assi, T.; Bakouny, Z.; Pavlidis, N. A Comprehensive Review of the Role of Immune Checkpoint Inhibitors in Brain Metastasis of Renal Cell Carcinoma Origin. Crit. Rev. Oncol./Hematol. 2018, 130, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Venur, V.A.; Preusser, M.; Ahluwalia, M.S. Immune Checkpoint Inhibitors in Brain Metastases: From Biology to Treatment. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annu. Meet. 2016, 35, e116–e122. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, X.; Zhang, H.; Sun, G.; Yang, Y.; Chen, J.; Zhu, X.; Zhao, P.; Zhao, J.; Liu, J.; et al. Differential Expressions of PD-1, PD-L1 and PD-L2 between Primary and Metastatic Sites in Renal Cell Carcinoma. BMC Cancer 2019, 19, 360. [Google Scholar] [CrossRef]
- Derosa, L.; Le Teuff, G.; Khordahi, M.; Chanez, B.; Zalcman, E.; Gravis, G.; Negrier, S.; Radulescu, C.; Albiges, L.; Merabet, Z.; et al. Inter and Intra-Tumor Heterogeneity of PD-L1 and MET Expression in Metastatic Renal Cell Carcinoma (MRCC). J. Clin. Oncol. 2017, 35, 4569. [Google Scholar] [CrossRef]
- Escudier, B.; Sharma, P.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. CheckMate 025 Randomized Phase 3 Study: Outcomes by Key Baseline Factors and Prior Therapy for Nivolumab Versus Everolimus in Advanced Renal Cell Carcinoma [Figure Presented]. Eur. Urol. 2017, 72, 962–971. [Google Scholar] [CrossRef]
- Bracarda, S.; Galli, L.; Maruzzo, M.; Lo Re, G.; Buti, S.; Favaretto, A.; Di Costanzo, F.; Sacco, C.; Merlano, M.; Mucciarini, C.; et al. Negative Prognostic Factors and Resulting Clinical Outcome in Patients with Metastatic Renal Cell Carcinoma Included in the Italian Nivolumab-Expanded Access Program. Future Oncol. 2018, 14, 1347–1354. [Google Scholar] [CrossRef]
- De Giorgi, U.; Cartenì, G.; Giannarelli, D.; Basso, U.; Galli, L.; Cortesi, E.; Caserta, C.; Pignata, S.; Sabbatini, R.; Bearz, A.; et al. Safety and Efficacy of Nivolumab for Metastatic Renal Cell Carcinoma: Real-World Results from an Expanded Access Programme. BJU Int. 2019, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.S.; Barata, P.C.; Zhang, T.; George, D.J.; Atkins, M.B.; Kelly, W.J.; Vogelzang, N.J.; Pal, S.K.; Hsu, J.; Appleman, L.J.; et al. Clinical Activity of Nivolumab in Patients with Non-Clear Cell Renal Cell Carcinoma. J. Immunother. Cancer 2018, 6, 9. [Google Scholar] [CrossRef]
- Min, L.; Hodi, F.S.; Kaiser, U.B. Corticosteroids and Immune Checkpoint Blockade. Aging 2015, 7, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Garant, A.; Guilbault, C.; Ekmekjian, T.; Greenwald, Z.; Murgoi, P.; Vuong, T. Concomitant Use of Corticosteroids and Immune Checkpoint Inhibitors in Patients with Hematologic or Solid Neoplasms: A Systematic Review. Critical Rev. Oncol./Hematol. 2017, 120, 86–92. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03967522 (accessed on 26 April 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04187872 (accessed on 26 April 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04434560 (accessed on 26 April 2021).
| DS-GPA * | |||
|---|---|---|---|
| Points | 0 | 1 | 2 |
| KPS | <70 | 70–80 | 90–100 |
| No of BMs | >3 | 2–3 | 1 |
| RTOG RPA | |||
| Class | I | II | III |
| KPS | ≥70 | ≥70 | <70 |
| and | and | and | |
| Age | <65 | all | all |
| and | and | and | |
| Extracranial metastases | No | No/Yes | No/Yes |
| BS-BM * | |||
| Points | 0 | 1 | |
| KPS | ≤70 | ≥80 | |
| Systemic disease | PD | SD-PR-CR-NED | |
| Extracranial metastases | Yes | No | |
| CERENAL * | |||
| Points | 0 | 1 | |
| KPS | >70 | ≤70 | |
| Age | ≤50 | >50 | |
| PD of systemic disease | No | Yes | |
| Extracranial metastases | No | Yes | |
| No of BM | 1 | ≥2 | |
| SRS | Yes | No |
| Authors | Drug | Previous Treatment | Main Results |
|---|---|---|---|
| TKIs | |||
| Jäger et al., 2015 (PREDICT trial) | Sorafenib | 37% of patients received sorafenib in first-line, while 63% were pre-treated with cytokines or TT (sunitinib, temsirolimus, pazopanib, or bevacizumab plus interferon). | Safety and efficacy of Sorafenib in BMRCC was confirmed. The median duration of sorafenib in the BM group was 7.3 months. |
| Gore et al., 2015 (Sunitinib global EAP) | Sunitinib | 12% treatment-naïve; 10% previously treated with VEGFR-TKIs; 68% previously treated with cytokines | 9% of BMRCC patients achieved an objective response rate, 33% had stable disease (SD) for more than 3 months, leading to an overall clinical benefit of 42% |
| Jacobs et al. 2013; Matrana et al., 2013; Roberto et al. 2015; Santoni et al. 2015 | Pazopanib | At list one previous treatment (range, 1–5) including all prior TT, cytokines, cytotoxics, and other experimental agents. | SD observed in 60% of patients, with a percentage of regression of brain lesions in 13% of cases |
| Negrier et al., 2018 | Cabozantinib | Anti VEGFr-TKI, anti-PD1 treatment and SRS | Cabozantinib proved able to reach the brain and to induce regressions of BMRCC that were resistant to radiation and previous angiogenic VEGFR-TKIs (warning: just case reports) |
| ICIs | |||
| Escudier et al., 2017 (NIVOREN trial); De Giorgi et al., 2019;Nivolumab Italian EAP) | Nivolumab Nivolumab | Previous anti-VEGFR-TKIs and/or cytokines; 67% of pts had not received local therapy for BM Anti-VEGFR-TKIs and/or cytokines | 60% of pts achieved a PFS of at least 3 months; among 44 patients assessed for BM response, ORR was 23%, while local progressive disease was 48%. Neurologic deterioration requiring steroids was observed in 32% of patients OS and ORR in 32 BMRCC patients were not different from those of the overall study population |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Internò, V.; De Santis, P.; Stefania Stucci, L.; Rudà, R.; Tucci, M.; Soffietti, R.; Porta, C. Prognostic Factors and Current Treatment Strategies for Renal Cell Carcinoma Metastatic to the Brain: An Overview. Cancers 2021, 13, 2114. https://doi.org/10.3390/cancers13092114
Internò V, De Santis P, Stefania Stucci L, Rudà R, Tucci M, Soffietti R, Porta C. Prognostic Factors and Current Treatment Strategies for Renal Cell Carcinoma Metastatic to the Brain: An Overview. Cancers. 2021; 13(9):2114. https://doi.org/10.3390/cancers13092114
Chicago/Turabian StyleInternò, Valeria, Pierluigi De Santis, Luigia Stefania Stucci, Roberta Rudà, Marco Tucci, Riccardo Soffietti, and Camillo Porta. 2021. "Prognostic Factors and Current Treatment Strategies for Renal Cell Carcinoma Metastatic to the Brain: An Overview" Cancers 13, no. 9: 2114. https://doi.org/10.3390/cancers13092114
APA StyleInternò, V., De Santis, P., Stefania Stucci, L., Rudà, R., Tucci, M., Soffietti, R., & Porta, C. (2021). Prognostic Factors and Current Treatment Strategies for Renal Cell Carcinoma Metastatic to the Brain: An Overview. Cancers, 13(9), 2114. https://doi.org/10.3390/cancers13092114









