Use of PET Imaging in Neuro-Oncological Surgery
Abstract
Simple Summary
Abstract
1. Introduction
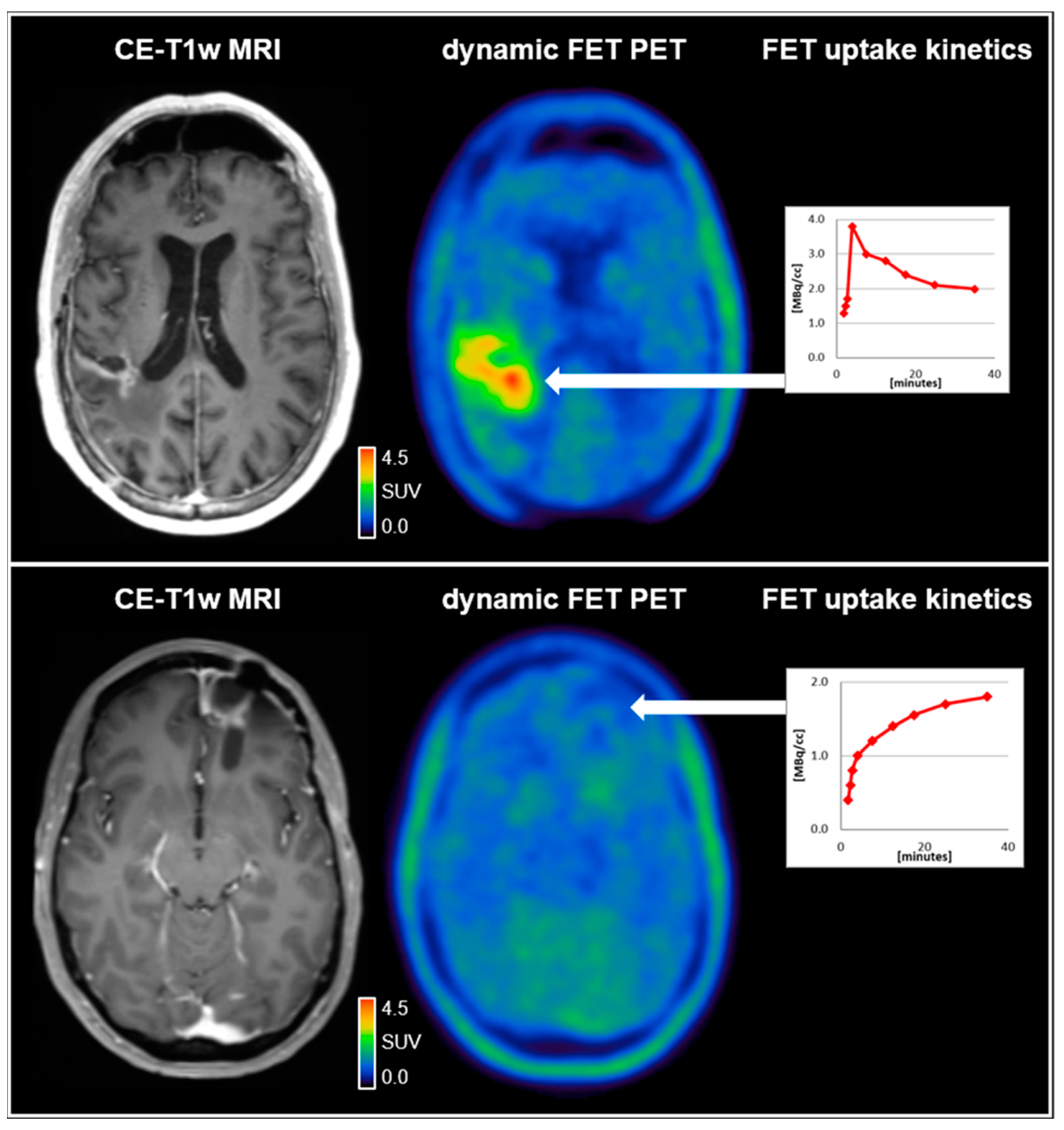
2. PET Imaging in the Management of Patients with Glioma: Past and Future
3. Clinical Value of PET in Patients with Brain Metastases
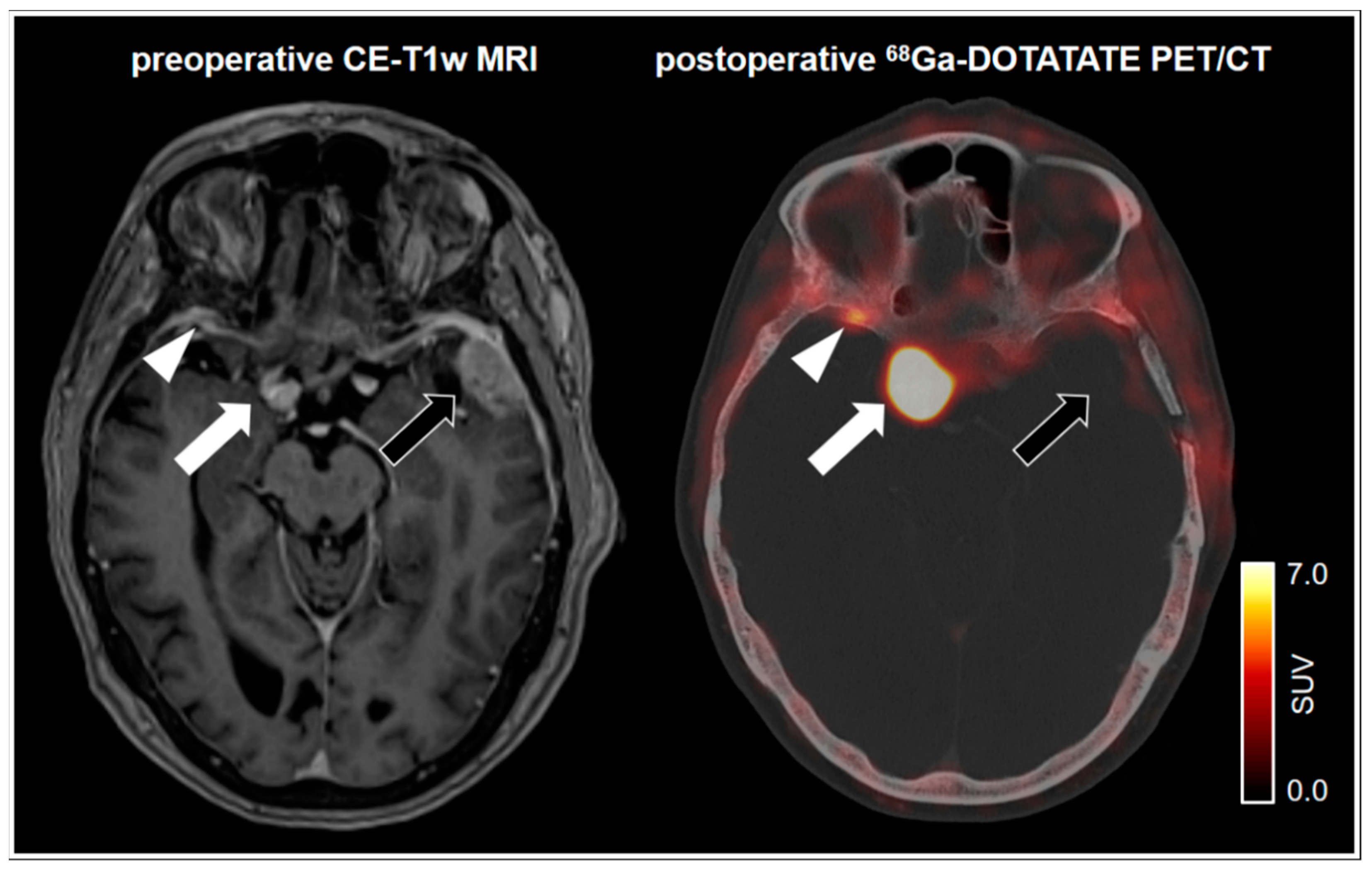
4. Specific Somatostatin Receptor Ligand PET in Meningioma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gambhir, S.S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2002, 2, 683–693. [Google Scholar] [CrossRef] [PubMed]
- La Fougère, C.; Suchorska, B.; Bartenstein, P.; Kreth, F.-W.; Tonn, J.-C. Molecular imaging of gliomas with PET: Opportunities and limitations. Neuro-Oncology 2011, 13, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Bergström, M.; Collins, V.P.; Ehrin, E.; Ericson, K.; Eriksson, L.; Greitz, T.; Halldin, C.; Von Hoist, H.; Långström, B.; Lilja, A.; et al. Discrepancies in Brain Tumor Extent as Shown by Computed Tomography and Positron Emission Tomography Using [68Ga]EDTA, [11C]Glucose, and [11C]Methionine. J. Comput. Assist. Tomogr. 1983, 7, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.; Berntsen, E.M.; Johansen, H.; Skjulsvik, A.J.; Reinertsen, I.; Dai, H.Y.; Xiao, Y.; Rivaz, H.; Borghammer, P.; Solheim, O.; et al. 18F-FACBC PET/MRI in Diagnostic Assessment and Neurosurgery of Gliomas. Clin. Nucl. Med. 2019, 44, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; La Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; La Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology 2016, 18, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Albert, N.L.; Sommerauer, M.; Grosu, A.L.; Ganswindt, U.; Law, I.; Preusser, M.; Le Rhun, E.; Vogelbaum, M.A.; Zadeh, G.; et al. PET imaging in patients with meningioma—Report of the RANO/PET Group. Neuro-Oncology 2017, 19, 1576–1587. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis—Report of the RANO/PET group. Neuro-Oncology 2019, 21, 585–595. [Google Scholar] [CrossRef]
- Galldiks, N.; Niyazi, M.; Grosu, A.L.; Kocher, M.; Langen, K.-J.; Law, I.; Minniti, G.; Kim, M.M.; Tsien, C.; Dhermain, F.; et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients—A report of the PET/RANO group. Neuro-Oncology 2021. [Google Scholar] [CrossRef]
- Wiriyasermkul, P.; Nagamori, S.; Tominaga, H.; Oriuchi, N.; Kaira, K.; Nakao, H.; Kitashoji, T.; Ohgaki, R.; Tanaka, H.; Endou, H.; et al. Transport of 3-Fluoro-L-Methyl-Tyrosine by Tumor-Upregulated L-Type Amino Acid Transporter 1: A Cause of the Tumor Uptake in PET. J. Nucl. Med. 2012, 53, 1253–1261. [Google Scholar] [CrossRef]
- Suchorska, B.; Albert, N.L.; Tonn, J.-C. Usefulness of PET Imaging to Guide Treatment Options in Gliomas. Curr. Treat. Options Neurol. 2016, 18, 4. [Google Scholar] [CrossRef]
- Ginet, M.; Zaragori, T.; Marie, P.-Y.; Roch, V.; Gauchotte, G.; Rech, F.; Blonski, M.; Lamiral, Z.; Taillandier, L.; Imbert, L.; et al. Integration of dynamic parameters in the analysis of 18F-FDopa PET imaging improves the prediction of molecular features of gliomas. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1381–1390. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Ceccon, G.; Lohmann, P.; Stoffels, G.; Judov, N.; Filss, C.P.; Rapp, M.; Bauer, E.; Hamisch, C.; Ruge, M.I.; Kocher, M.; et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro-Oncology 2016, 19, 281–288. [Google Scholar] [CrossRef]
- Zaragori, T.; Ginet, M.; Marie, P.-Y.; Roch, V.; Grignon, R.; Gauchotte, G.; Rech, F.; Blonski, M.; Lamiral, Z.; Taillandier, L.; et al. Use of static and dynamic [18F]-F-DOPA PET parameters for detecting patients with glioma recurrence or progression. EJNMMI Res. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schucht, P.; Knittel, S.; Slotboom, J.; Seidel, K.; Murek, M.; Jilch, A.; Raabe, A.; Beck, J. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir. 2013, 156, 305–312. [Google Scholar] [CrossRef]
- Floeth, F.W.; Sabel, M.; Ewelt, C.; Stummer, W.; Felsberg, J.; Reifenberger, G.; Steiger, H.J.; Stoffels, G.; Coenen, H.H.; Langen, K.-J. Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur. J. Nucl. Med. Mol. Imaging 2010, 38, 731–741. [Google Scholar] [CrossRef]
- Müther, M.; Koch, R.; Weckesser, M.; Sporns, P.; Schwindt, W.; Stummer, W. 5-Aminolevulinic Acid Fluorescence-Guided Resection of 18F-FET-PET Positive Tumor Beyond Gadolinium Enhancing Tumor Improves Survival in Glioblastoma. Neurosurgery 2019, 85, E1020–E1029. [Google Scholar] [CrossRef]
- Kunz, M.; Thon, N.; Eigenbrod, S.; Hartmann, C.; Egensperger, R.; Herms, J.; Geisler, J.; La Fougere, C.; Lutz, J.; Linn, J.; et al. Hot spots in dynamic 18FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro-Oncology 2011, 13, 307–316. [Google Scholar] [CrossRef]
- Bauer, E.K.; Stoffels, G.; Blau, T.; Reifenberger, G.; Felsberg, J.; Werner, J.M.; Lohmann, P.; Rosen, J.; Ceccon, G.; Tscherpel, C.; et al. Prediction of survival in patients with IDH-wildtype astrocytic gliomas using dynamic O-(2-[18F]-fluoroethyl)-l-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1486–1495. [Google Scholar] [CrossRef]
- Thon, N.; Kunz, M.; Lemke, L.; Jansen, N.L.; Eigenbrod, S.; Kreth, S.; Lutz, J.; Egensperger, R.; Giese, A.; Herms, J.; et al. Dynamic18F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int. J. Cancer 2014, 136, 2132–2145. [Google Scholar] [CrossRef]
- Tommasini-Ghelfi, S.; Murnan, K.; Kouri, F.M.; Mahajan, A.S.; May, J.L.; Stegh, A.H. Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease. Sci. Adv. 2019, 5, eaaw4543. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; Westphal, M.; Schackert, G.; Meyermann, R.; Pietsch, T.; et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathol. 2010, 120, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Thon, N.; Eigenbrod, S.; Grasbon-Frodl, E.M.; Ruiter, M.; Mehrkens, J.H.; Kreth, S.; Tonn, J.C.; Kretzschmar, H.A.; Kreth, F.W. Novel Molecular Stereotactic Biopsy Procedures Reveal Intratumoral Homogeneity of Loss of Heterozygosity of 1p/19q and TP53 Mutations in World Health Organization Grade II Gliomas. J. Neuropathol. Exp. Neurol. 2009, 68, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Wijnenga, M.M.J.; French, P.J.; Dubbink, H.J.; Dinjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Smits, M.; Gahrmann, R.; Rutten, G.-J.; Verheul, J.B.; et al. The impact of surgery in molecularly defined low-grade glioma: An integrated clinical, radiological, and molecular analysis. Neuro-Oncology 2017, 20, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Vettermann, F.; Suchorska, B.; Unterrainer, M.; Nelwan, D.; Forbrig, R.; Ruf, V.; Wenter, V.; Kreth, F.-W.; Herms, J.; Bartenstein, P.; et al. Non-invasive prediction of IDH-wildtype genotype in gliomas using dynamic 18F-FET PET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Lerche, C.; Bauer, E.K.; Steger, J.; Stoffels, G.; Blau, T.; Dunkl, V.; Kocher, M.; Viswanathan, S.; Filss, C.P.; et al. Predicting IDH genotype in gliomas using FET PET radiomics. Sci. Rep. 2018, 8, 13328. [Google Scholar] [CrossRef]
- Suchorska, B.; Giese, A.; Biczok, A.; Unterrainer, M.; Weller, M.; Drexler, M.; Bartenstein, P.; Schüller, U.; Tonn, J.-C.; Albert, N.L. Identification of time-to-peak on dynamic 18F-FET-PET as a prognostic marker specifically in IDH1/2 mutant diffuse astrocytoma. Neuro-Oncology 2018, 20, 279–288. [Google Scholar] [CrossRef]
- Lohmann, P.; Stavrinou, P.; Lipke, K.; Bauer, E.K.; Ceccon, G.; Werner, J.-M.; Neumaier, B.; Fink, G.R.; Shah, N.J.; Langen, K.-J.; et al. FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 591–602. [Google Scholar] [CrossRef]
- Suchorska, B.; Jansen, N.L.; Linn, J.; Kretzschmar, H.; Janssen, H.; Eigenbrod, S.; Simon, M.; Pöpperl, G.; Kreth, F.W.; La Fougere, C.; et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 2015, 84, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Piroth, M.D.; Pinkawa, M.; Holy, R.; Klotz, J.; Nussen, S.; Stoffels, G.; Coenen, H.H.; Kaiser, H.J.; Langen, K.J.; Eble, M.J. Prognostic Value of Early [18F]Fluoroethyltyrosine Positron Emission Tomography After Radiochemotherapy in Glioblastoma Multiforme. Int. J. Radiat. Oncol. 2011, 80, 176–184. [Google Scholar] [CrossRef]
- Fleischmann, D.F.; Unterrainer, M.; Bartenstein, P.; Belka, C.; Albert, N.L.; Niyazi, M. 18F-FET PET prior to recurrent high-grade glioma re-irradiation—Additional prognostic value of dynamic time-to-peak analysis and early static summation images? J. Neurooncol. 2017, 132, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Dunkl, V.; Kracht, L.W.; Vollmar, S.; Jacobs, A.H.; Fink, G.R.; Schroeter, M. Volumetry of [11C]-Methionine Positron Emission Tomographic Uptake as a Prognostic Marker before Treatment of Patients with Malignant Glioma. Mol. Imaging 2012, 11, 516–527. [Google Scholar] [CrossRef]
- Oehlke, O.; Mix, M.; Graf, E.; Schimek-Jasch, T.; Nestle, U.; Götz, I.; Schneider-Fuchs, S.; Weyerbrock, A.; Mader, I.; Baumert, B.G.; et al. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA)—Protocol of a randomized phase II trial (NOA 10/ARO 2013-1). BMC Cancer 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ceccon, G.; Lohmann, P.; Werner, J.-M.; Tscherpel, C.; Dunkl, V.; Stoffels, G.; Rosen, J.; Rapp, M.; Sabel, M.; Herrlinger, U.; et al. Early treatment response assessment using 18F-FET PET compared to contrast-enhanced MRI in glioma patients following adjuvant temozolomide chemotherapy. J. Nucl. Med. 2020. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Holy, R.; Pinkawa, M.; Stoffels, G.; Nolte, K.W.; Kaiser, H.J.; Filss, C.P.; Fink, G.R.; Coenen, H.H.; et al. Assessment of Treatment Response in Patients with Glioblastoma Using O-(2-18F-Fluoroethyl)-L-Tyrosine PET in Comparison to MRI. J. Nucl. Med. 2012, 53, 1048–1057. [Google Scholar] [CrossRef]
- Suchorska, B.; Unterrainer, M.; Biczok, A.; Sosnova, M.; Forbrig, R.; Bartenstein, P.; Tonn, J.-C.; Albert, N.L.; Kreth, F.-W. 18F-FET-PET as a biomarker for therapy response in non-contrast enhancing glioma following chemotherapy. J. Neurooncol. 2018, 139, 721–730. [Google Scholar] [CrossRef]
- Roelcke, U.; Wyss, M.T.; Nowosielski, M.; Rudà, R.; Roth, P.; Hofer, S.; Galldiks, N.; Crippa, F.; Weller, M.; Soffietti, R. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro-Oncology 2015, 18, 744–751. [Google Scholar] [CrossRef][Green Version]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.P.; Hamid, O.; Hodi, F.S.; Moschos, S.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Mottaghy, M.F.; Buck, K.A.; Maschke, S.; Kley, H.; Frechen, D.; Wibmer, T.; Reske, N.S.; Pauls, S.; Krüger, S.; Mottaghy, F.M.; et al. Brain metastasis in lung cancer. Nuklearmedizin 2011, 50, 101–106. [Google Scholar] [CrossRef]
- Unterrainer, M.; Galldiks, N.; Suchorska, B.; Kowalew, L.-C.; Wenter, V.; Schmid-Tannwald, C.; Niyazi, M.; Bartenstein, P.; Langen, K.-J.; Albert, N.L. 18F-FET PET Uptake Characteristics in Patients with Newly Diagnosed and Untreated Brain Metastasis. J. Nucl. Med. 2016, 58, 584–589. [Google Scholar] [CrossRef]
- Kumar, A.J.; Leeds, N.E.; Fuller, G.N.; Van Tassel, P.; Maor, M.H.; Sawaya, R.E.; Levin, V.A. Malignant Gliomas: MR Imaging Spectrum of Radiation Therapy- and Chemotherapy-induced Necrosis of the Brain after Treatment. Radiology. 2000, 217, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Romagna, A.; Unterrainer, M.; Schmid-Tannwald, C.; Brendel, M.; Tonn, J.-C.; Nachbichler, S.B.; Muacevic, A.; Bartenstein, P.; Kreth, F.-W.; Albert, N.L. Suspected recurrence of brain metastases after focused high dose radiotherapy: Can [18F]FET- PET overcome diagnostic uncertainties? Radiat. Oncol. 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuguchi, N.; Sunada, I.; Iwai, Y.; Yamanaka, K.; Tanaka, K.; Takami, T.; Otsuka, Y.; Sakamoto, S.; Ohata, K.; Goto, T.; et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: Is a differential diagnosis possible? J. Neurosurg. 2003, 98, 1056–1064. [Google Scholar] [CrossRef]
- Yomo, S.; Oguchi, K. Prospective study of 11C–methionine PET for distinguishing between recurrent brain metastases and radiation necrosis: Limitations of diagnostic accuracy and long-term results of salvage treatment. BMC Cancer 2017, 17, 713. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.P.; Piroth, M.D.; Sabel, M.; Ruge, M.I.; Herzog, H.; Shah, N.J.; Fink, G.R.; Coenen, H.H.; et al. Role of O-(2-18F-Fluoroethyl)-L-Tyrosine PET for Differentiation of Local Recurrent Brain Metastasis from Radiation Necrosis. J. Nucl. Med. 2012, 53, 1367–1374. [Google Scholar] [CrossRef]
- Lohmann, P.; Kocher, M.; Ceccon, G.; Bauer, E.K.; Stoffels, G.; Viswanathan, S.; Ruge, M.I.; Neumaier, B.; Shah, N.J.; Fink, G.R.; et al. Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. NeuroImage Clin. 2018, 20, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef]
- Kebir, S.; Rauschenbach, L.; Galldiks, N.; Schlaak, M.; Hattingen, E.; Landsberg, J.; Bundschuh, R.A.; Langen, K.-J.; Scheffler, B.; Herrlinger, U.; et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro-Oncology 2016, 18, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, D.S.; Scheffler, M.; Brandes, V.; Ruge, M.; Kunze, S.; Merkelbach-Bruse, S.; Nogova, L.; Michels, S.; Fischer, R.; Riedel, R.; et al. Monitoring Treatment Response to Erlotinib in EGFR-mutated Non–small-cell Lung Cancer Brain Metastases Using Serial O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin. Lung Cancer 2019, 20, e148–e151. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Abdulla, D.S.; Scheffler, M.; Wolpert, F.; Werner, J.-M.; Huellner, M.W.; Stoffels, G.; Schweinsberg, V.; Schlaak, M.; Kreuzberg, N.; et al. Treatment Monitoring of Immunotherapy and Targeted Therapy using 18F-FET PET in Patients with Melanoma and Lung Cancer Brain Metastases: Initial Experiences. J. Nucl. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.F.; Grierson, J.R.; Dohmen, B.M.; Machulla, H.-J.; Stayanoff, J.C.; Lawhorn-Crews, J.M.; Obradovich, J.E.; Muzik, O.; Mangner, T.J. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998, 4, 1334–1336. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Yee, M.K.; Tuchayi, A.M.; Kirkwood, J.M.; Tawbi, H.; Mountz, J.M. Targeted Therapy and Immunotherapy Response Assessment with F-18 Fluorothymidine Positron-Emission Tomography/Magnetic Resonance Imaging in Melanoma Brain Metastasis: A Pilot Study. Front. Oncol. 2018, 8, 18. [Google Scholar] [CrossRef]
- Whittle, I.R.; Smith, C.; Navoo, P.; Collie, D. Meningiomas. Lancet 2004, 363, 1535–1543. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef]
- Reubi, J.C.; Maurer, R.; Klijn, J.; Stefanko, S.; Foekens, J.; Blaauw, G.; Blankenstein, M.; Lamberts, S. High Incidence of Somatostatin Receptors in Human Meningiomas: Biochemical Characterization. J. Clin. Endocrinol. Metab. 1986, 63, 433–438. [Google Scholar] [CrossRef]
- Whinle, I.R.; Foo, M.S.; Besser, M.; Vanderfield, G.K. Progesterone and Oestrogen Receptors in Meningiomas: Biochemical and Clinicopathological Considerations. ANZ J. Surg. 2008, 54, 325–330. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Knigge, U.; Kjær, A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: Current status and review of the literature. Future Oncol. 2014, 10, 2259–2277. [Google Scholar] [CrossRef]
- Ilhan, H.; Lindner, S.; Todica, A.; Cyran, C.C.; Tiling, R.; Auernhammer, C.J.; Spitzweg, C.; Boeck, S.; Unterrainer, M.; Gildehaus, F.J.; et al. Biodistribution and first clinical results of 18F-SiFAlin-TATE PET: A novel 18F-labeled somatostatin analog for imaging of neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, J.F.; Stoffels, G.; Filß, C.; Galldiks, N.; Slotty, P.J.; Kamp, M.A.; El Khatib, M.; Hänggi, D.; Sabel, M.; Felsberg, J.; et al. Uptake and tracer kinetics of O-(2-18F-fluoroethyl)-l-tyrosine in meningiomas: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Nyuyki, F.; Plotkin, M.; Graf, R.; Michel, R.; Steffen, I.; Denecke, T.; Geworski, L.; Fahdt, D.; Brenner, W.; Wurm, R. Potential impact of 68Ga-DOTATOC PET/CT on stereotactic radiotherapy planning of meningiomas. Eur. J. Nucl. Med. Mol. Imaging 2009, 37, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Terpolilli, N.A.; Rachinger, W.; Kunz, M.; Thon, N.; Flatz, W.H.; Tonn, J.-C.; Schichor, C. Orbit-associated tumors: Navigation and control of resection using intraoperative computed tomography. J. Neurosurg. 2016, 124, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Klingenstein, A.; Haug, A.R.; Miller, C.; Hintschich, C. Ga-68-DOTA-TATE PET/CT for Discrimination of Tumors of the Optic Pathway. Orbit 2014, 34, 16–22. [Google Scholar] [CrossRef]
- Graf, R.; Nyuyki, F.; Steffen, I.G.; Michel, R.; Fahdt, D.; Wust, P.; Brenner, W.; Budach, V.; Wurm, R.; Plotkin, M. Contribution of 68Ga-DOTATOC PET/CT to Target Volume Delineation of Skull Base Meningiomas Treated with Stereotactic Radiation Therapy. Int. J. Radiat. Oncol. 2013, 85, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Milker-Zabel, S.; Bois, A.Z.-D.; Henze, M.; Huber, P.; Schulz-Ertner, D.; Hoess, A.; Haberkorn, U.; Debus, J. Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int. J. Radiat. Oncol. 2006, 65, 222–227. [Google Scholar] [CrossRef]
- Cornelius, J.F.; Langen, K.J.; Stoffels, G.; Hänggi, D.; Sabel, M.; Steiger, H.J. Positron Emission Tomography Imaging of Meningioma in Clinical Practice. Neurosurgery 2011, 70, 1033–1042. [Google Scholar] [CrossRef]
- Rachinger, W.; Stoecklein, V.M.; Terpolilli, N.A.; Haug, A.R.; Ertl, L.; Pöschl, J.; Schüller, U.; Schichor, C.; Thon, N.; Tonn, J.-C. Increased 68Ga-DOTATATE Uptake in PET Imaging Discriminates Meningioma and Tumor-Free Tissue. J. Nucl. Med. 2015, 56, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.G.; Jungblut, L.M.; Kazmierczak, P.M.; Vettermann, F.J.; Bollenbacher, A.; Tonn, J.C.; Schichor, C.; Rominger, A.; Albert, N.L.; Bartenstein, P.; et al. Improved Detection of Transosseous Meningiomas Using 68Ga-DOTATATE PET/CT Compared with Contrast-Enhanced MRI. J. Nucl. Med. 2017, 58, 1580–1587. [Google Scholar] [CrossRef]
- Unterrainer, M.; Ruf, V.; Ilhan, H.; Vettermann, F.J.; Cyran, C.C.; Niyazi, M.; Bartenstein, P.; Tonn, J.-C.; Albert, N.L. 68Ga-DOTATOC PET/CT Differentiates Meningioma from Dural Metastases. Clin. Nucl. Med. 2019, 44, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.; Kwekkeboom, D.J.; De Jong, M. Radiolabeled Peptides for Imaging and Therapy: A Radiant Future. J. Nucl. Med. 2015, 56, 1809–1812. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, M.; Bodei, L.; De Cicco, C.; Grana, C.M.; Cremonesi, M.; Botteri, E.; Baio, S.M.; Aricò, D.; Sansovini, M.; Paganelli, G. Peptide receptor radionuclide therapy with 90Y-DOTATOC in recurrent meningioma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Seystahl, K.; Stoecklein, V.; Schüller, U.; Rushing, E.; Nicolas, G.; Schäfer, N.; Ilhan, H.; Pangalu, A.; Weller, M.; Tonn, J.-C.; et al. Somatostatin-receptor-targeted radionuclide therapy for progressive meningioma: Benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro-Oncology 2016, 18, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzgreve, A.; Albert, N.L.; Galldiks, N.; Suchorska, B. Use of PET Imaging in Neuro-Oncological Surgery. Cancers 2021, 13, 2093. https://doi.org/10.3390/cancers13092093
Holzgreve A, Albert NL, Galldiks N, Suchorska B. Use of PET Imaging in Neuro-Oncological Surgery. Cancers. 2021; 13(9):2093. https://doi.org/10.3390/cancers13092093
Chicago/Turabian StyleHolzgreve, Adrien, Nathalie L. Albert, Norbert Galldiks, and Bogdana Suchorska. 2021. "Use of PET Imaging in Neuro-Oncological Surgery" Cancers 13, no. 9: 2093. https://doi.org/10.3390/cancers13092093
APA StyleHolzgreve, A., Albert, N. L., Galldiks, N., & Suchorska, B. (2021). Use of PET Imaging in Neuro-Oncological Surgery. Cancers, 13(9), 2093. https://doi.org/10.3390/cancers13092093







