Distinct Cerebrovascular Reactivity Patterns for Brain Radiation Necrosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. BOLD-CVR Acquisition and Calculation
2.3. Masking of the Contrast-Enhancing Lesion (Volume-Of-Interest, VOI)
2.4. Data Analysis
3. Results
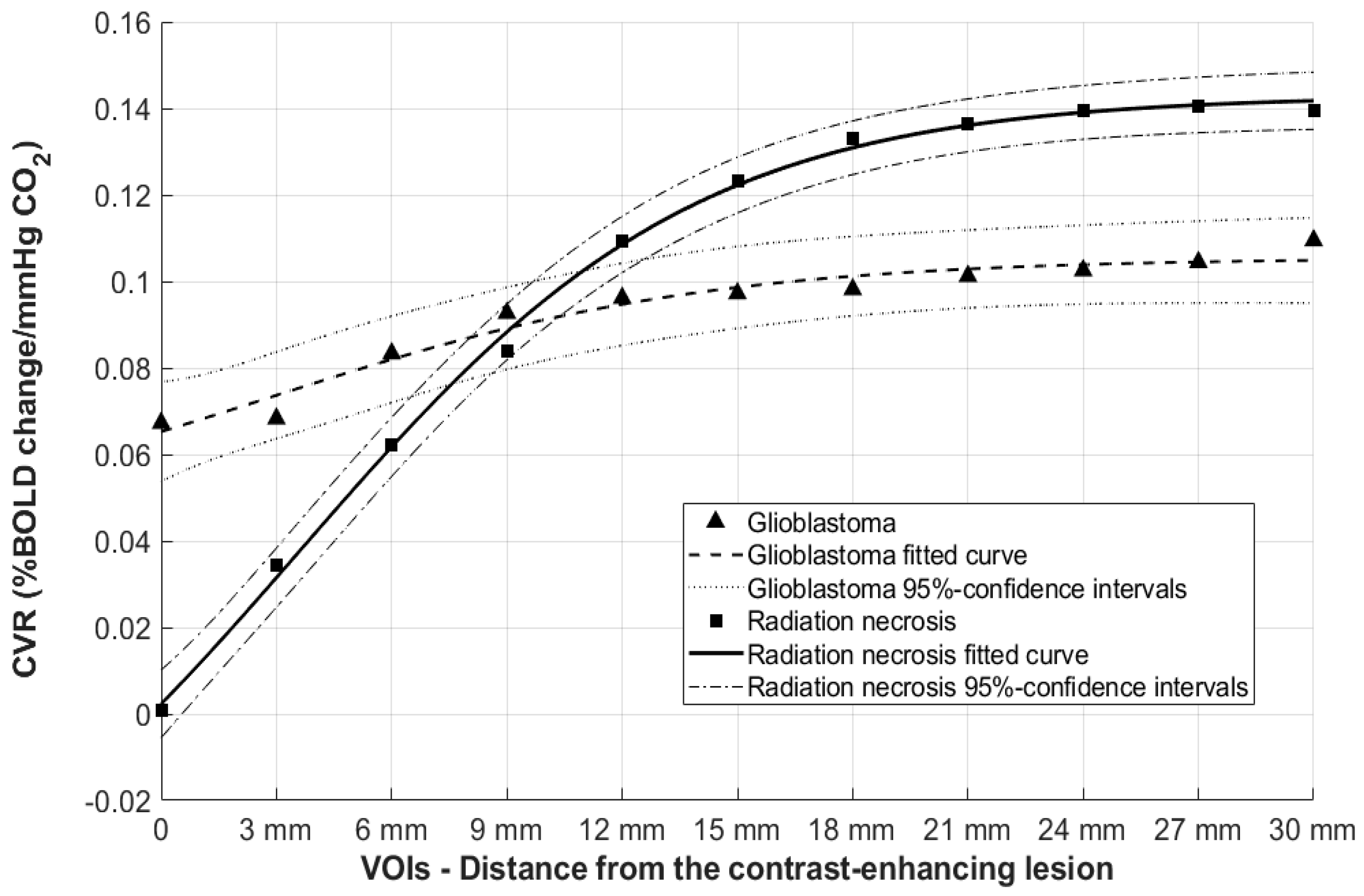
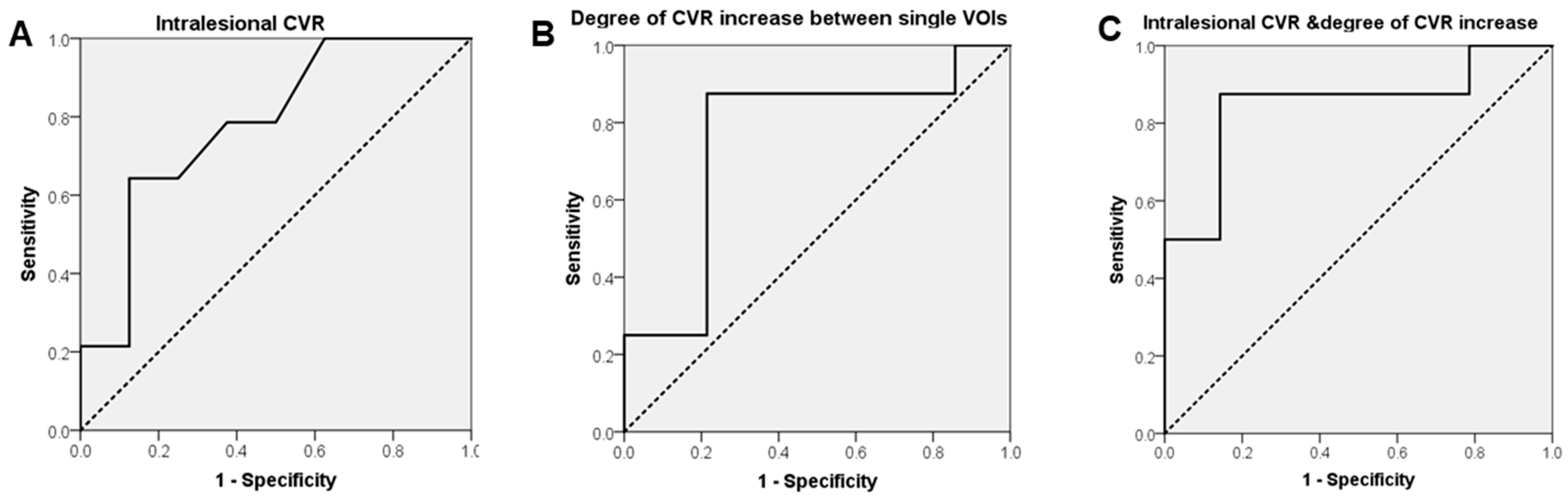
BOLD-CVR Findings for Patients with Radiation Necrosis and Newly Diagnosed Glioblastoma
4. Discussion
4.1. BOLD-CVR and Vascular Pathophysiology in Glioblastoma and Radiation Necrosis
4.2. Current Advances in Follow-Up Management of Glioblastoma
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado-Lopez, P.D.; Rinones-Mena, E.; Corrales-Garcia, E.M. Treatment-related changes in glioblastoma: A review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin. Transl. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Raimbault, A.; Cazals, X.; Lauvin, M.A.; Destrieux, C.; Chapet, S.; Cottier, J.P. Radionecrosis of malignant glioma and cerebral metastasis: A diagnostic challenge in MRI. Diagn. Interv. Imaging 2014, 95, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Langen, K.J.; Galldiks, N.; Hattingen, E.; Shah, N.J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 2017, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Siu, A.; Wind, J.J.; Iorgulescu, J.B.; Chan, T.A.; Yamada, Y.; Sherman, J.H. Radiation necrosis following treatment of high grade glioma-a review of the literature and current understanding. Acta Neurochir. (Wien) 2012, 154, 191–201; discussion 201. [Google Scholar] [CrossRef]
- Walker, A.J.; Ruzevick, J.; Malayeri, A.A.; Rigamonti, D.; Lim, M.; Redmond, K.J.; Kleinberg, L. Postradiation imaging changes in the CNS: How can we differentiate between treatment effect and disease progression? Future Oncol. 2014, 10, 1277–1297. [Google Scholar] [CrossRef]
- Rani, N.; Singh, B.; Kumar, N.; Singh, P.; Hazari, P.P.; Singh, H.; Kumar, G.; Radotra, B.; Kumar, M.; Bhattacharya, A.; et al. Differentiation of recurrent/residual glioma from radiation necrosis using semi quantitative 99mTc MDM (bis-methionine-DTPA) drain SPECT/CT and dynamic susceptibility contrast-enhanced MR perfusion: A comparative study. Clin. Nucl. Med. 2018, 43, e74–e81. [Google Scholar] [CrossRef] [PubMed]
- Fierstra, J.; van Niftrik, C.; Warnock, G.; Wegener, S.; Piccirelli, M.; Pangalu, A.; Esposito, G.; Valavanis, A.; Buck, A.; Luft, A.; et al. Staging hemodynamic failure with blood oxygen-level-dependent functional magnetic resonance imaging cerebrovascular reactivity: A comparison versus gold standard ((15)O-)H2O-positron emission tomography. Stroke 2018. [Google Scholar] [CrossRef]
- Deuschl, C.; Kirchner, J.; Poeppel, T.D.; Schaarschmidt, B.; Kebir, S.; El Hindy, N.; Hense, J.; Quick, H.H.; Glas, M.; Herrmann, K.; et al. (11)C-MET PET/MRI for detection of recurrent glioma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 593–601. [Google Scholar] [CrossRef]
- Verger, A.; Langen, K.J. PET imaging in glioblastoma: Use in clinical practice. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications Copyright: The Authors; Codon Publications: Brisbane, Australia, 2017; Chapter 9. [Google Scholar] [CrossRef] [PubMed]
- Zaca, D.; Hua, J.; Pillai, J.J. Cerebrovascular reactivity mapping for brain tumor presurgical planning. World J. Clin. Oncol. 2011, 2, 289–298. [Google Scholar] [CrossRef]
- Pillai, J.J.; Mikulis, D.J. Cerebrovascular reactivity mapping: An evolving standard for clinical functional imaging. AJNR Am. J. Neuroradiol. 2015, 36, 7–13. [Google Scholar] [CrossRef]
- Fierstra, J.; van Niftrik, C.; Piccirelli, M.; Bozinov, O.; Pangalu, A.; Krayenbuhl, N.; Valavanis, A.; Weller, M.; Regli, L. Diffuse gliomas exhibit whole brain impaired cerebrovascular reactivity. Magn. Reson. Imaging 2018, 45, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Muscas, G.; van Niftrik, C.H.B.; Sebök, M.; Seystahl, K.; Piccirelli, M.; Stippich, C.; Weller, M.; Regli, L.; Fierstra, J. Hemodynamic investigation of peritumoral impaired blood oxygenation-level dependent cerebrovascular reactivity in patients with diffuse glioma. Magn. Reson. Imaging 2020, 70, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.J.; Zaca, D. Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technol. Cancer Res. Treat 2012, 11, 361–374. [Google Scholar] [CrossRef]
- Holodny, A.I.; Schulder, M.; Liu, W.C.; Wolko, J.; Maldjian, J.A.; Kalnin, A.J. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: Implications for image-guided neurosurgery. AJNR Am. J. Neuroradiol. 2000, 21, 1415–1422. [Google Scholar] [PubMed]
- Fierstra, J.; van Niftrik, B.; Piccirelli, M.; Burkhardt, J.K.; Pangalu, A.; Kocian, R.; Valavanis, A.; Weller, M.; Regli, L.; Bozinov, O. Altered intraoperative cerebrovascular reactivity in brain areas of high-grade glioma recurrence. Magn. Reson. Imaging 2016, 34, 803–808. [Google Scholar] [CrossRef]
- Holodny, A.I.; Schulder, M.; Liu, W.C.; Maldjian, J.A.; Kalnin, A.J. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: Implications for image-guided neurosurgery. AJNR Am. J. Neuroradiol. 1999, 20, 609–612. [Google Scholar]
- Iranmahboob, A.; Peck, K.K.; Brennan, N.P.; Karimi, S.; Fisicaro, R.; Hou, B.; Holodny, A.I. Vascular reactivity maps in patients with gliomas using breath-holding BOLD fMRI. J. Neuroimaging 2016, 26, 232–239. [Google Scholar] [CrossRef]
- Fisher, J.A.; Venkatraghavan, L.; Mikulis, D.J. Magnetic resonance imaging-based cerebrovascular reactivity and hemodynamic reserve: A review of method optimization and data interpretation. Stroke 2018. [Google Scholar] [CrossRef] [PubMed]
- van Niftrik, C.H.B.; Piccirelli, M.; Bozinov, O.; Pangalu, A.; Fisher, J.A.; Valavanis, A.; Luft, A.R.; Weller, M.; Regli, L.; Fierstra, J. Iterative analysis of cerebrovascular reactivity dynamic response by temporal decomposition. Brain Behav. 2017, 7, e00705. [Google Scholar] [CrossRef]
- Fierstra, J.; Sobczyk, O.; Battisti-Charbonney, A.; Mandell, D.M.; Poublanc, J.; Crawley, A.P.; Mikulis, D.J.; Duffin, J.; Fisher, J.A. Measuring cerebrovascular reactivity: What stimulus to use? J. Physiol. 2013, 591, 5809–5821. [Google Scholar] [CrossRef]
- van Niftrik, C.H.B.; Piccirelli, M.; Bozinov, O.; Maldaner, N.; Strittmatter, C.; Pangalu, A.; Valavanis, A.; Regli, L.; Fierstra, J. Impact of baseline CO2 on Blood-Oxygenation-Level-Dependent MRI measurements of cerebrovascular reactivity and task-evoked signal activation. Magn. Reson. Imaging 2018, 49, 123–130. [Google Scholar] [CrossRef]
- Pillai, J.J.; Zaca, D. Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World J. Clin. Oncol. 2011, 2, 397–403. [Google Scholar] [CrossRef]
- Conklin, J.; Fierstra, J.; Crawley, A.P.; Han, J.S.; Poublanc, J.; Mandell, D.M.; Silver, F.L.; Tymianski, M.; Fisher, J.A.; Mikulis, D.J. Impaired cerebrovascular reactivity with steal phenomenon is associated with increased diffusion in white matter of patients with Moyamoya disease. Stroke 2010, 41, 1610–1616. [Google Scholar] [CrossRef]
- Sam, K.; Poublanc, J.; Sobczyk, O.; Han, J.S.; Battisti-Charbonney, A.; Mandell, D.M.; Tymianski, M.; Crawley, A.P.; Fisher, J.A.; Mikulis, D.J. Assessing the effect of unilateral cerebral revascularisation on the vascular reactivity of the non-intervened hemisphere: A retrospective observational study. BMJ Open 2015, 5, e006014. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Reardon, D.A.; de Groot, J.F.; Wick, W.; Weller, M. Antiangiogenic therapy for glioblastoma: Current status and future prospects. Clin. Cancer Res. 2014, 20, 5612–5619. [Google Scholar] [CrossRef] [PubMed]
- Pak, R.W.; Hadjiabadi, D.H.; Senarathna, J.; Agarwal, S.; Thakor, N.V.; Pillai, J.J.; Pathak, A.P. Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. J. Cereb. Blood Flow. Metab. 2017, 37, 3475–3487. [Google Scholar] [CrossRef]
- Lombardi, G.; Pambuku, A.; Bellu, L.; Farina, M.; Della Puppa, A.; Denaro, L.; Zagonel, V. Effectiveness of antiangiogenic drugs in glioblastoma patients: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Oncol. Hematol. 2017, 111, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Drappatz, J.; Wen, P.Y. Antiangiogenic therapies for high-grade glioma. Nat. Rev. Neurol. 2009, 5, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Phillips, J.J.; Parvataneni, R.; Molinaro, A.; Essock-Burns, E.; Bourne, G.; Parsa, A.T.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol. 2012, 14, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sair, H.I.; Yahyavi-Firouz-Abadi, N.; Airan, R.; Pillai, J.J. Neurovascular uncoupling in resting state fMRI demonstrated in patients with primary brain gliomas. J. Magn. Reson. Imaging 2016, 43, 620–626. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.T.; Liu, Y.S.; Tsai, Y.S.; Chen, Y.C.; Wang, C.K. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: A meta-analysis. PLoS ONE 2016, 11, e0141438. [Google Scholar] [CrossRef]
- Sugahara, T.; Korogi, Y.; Tomiguchi, S.; Shigematsu, Y.; Ikushima, I.; Kira, T.; Liang, L.; Ushio, Y.; Takahashi, M. Posttherapeutic intraaxial brain tumor: The value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am. J. Neuroradiol. 2000, 21, 901–909. [Google Scholar] [PubMed]
- Anzalone, N.; Castellano, A.; Cadioli, M.; Conte, G.M.; Cuccarini, V.; Bizzi, A.; Grimaldi, M.; Costa, A.; Grillea, G.; Vitali, P.; et al. Brain gliomas: Multicenter standardized assessment of dynamic contrast-enhanced and dynamic susceptibility Ccntrast MR images. Radiology 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, L.; Wang, Q.; Zheng, X.; Wu, C.; Xu, B.N. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: A systematic review and meta-analysis. Eur. J. Radiol. 2014, 83, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Dowling, C.; Bollen, A.W.; Noworolski, S.M.; McDermott, M.W.; Barbaro, N.M.; Day, M.R.; Henry, R.G.; Chang, S.M.; Dillon, W.P.; Nelson, S.J.; et al. Preoperative proton MR spectroscopic imaging of brain tumors: Correlation with histopathologic analysis of resection specimens. AJNR Am. J. Neuroradiol. 2001, 22, 604–612. [Google Scholar]
- Plotkin, M.; Eisenacher, J.; Bruhn, H.; Wurm, R.; Michel, R.; Stockhammer, F.; Feussner, A.; Dudeck, O.; Wust, P.; Felix, R.; et al. 123I-IMT SPECT and 1H MR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: A comparative study. J. Neurooncol. 2004, 70, 49–58. [Google Scholar] [CrossRef]
- Rock, J.P.; Scarpace, L.; Hearshen, D.; Gutierrez, J.; Fisher, J.L.; Rosenblum, M.; Mikkelsen, T. Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 2004, 54, 1111–1119. [Google Scholar] [CrossRef]
- Shah, R.; Vattoth, S.; Jacob, R.; Manzil, F.F.; O’Malley, J.P.; Borghei, P.; Patel, B.N.; Cure, J.K. Radiation necrosis in the brain: Imaging features and differentiation from tumor recurrence. Radiographics 2012, 32, 1343–1359. [Google Scholar] [CrossRef]
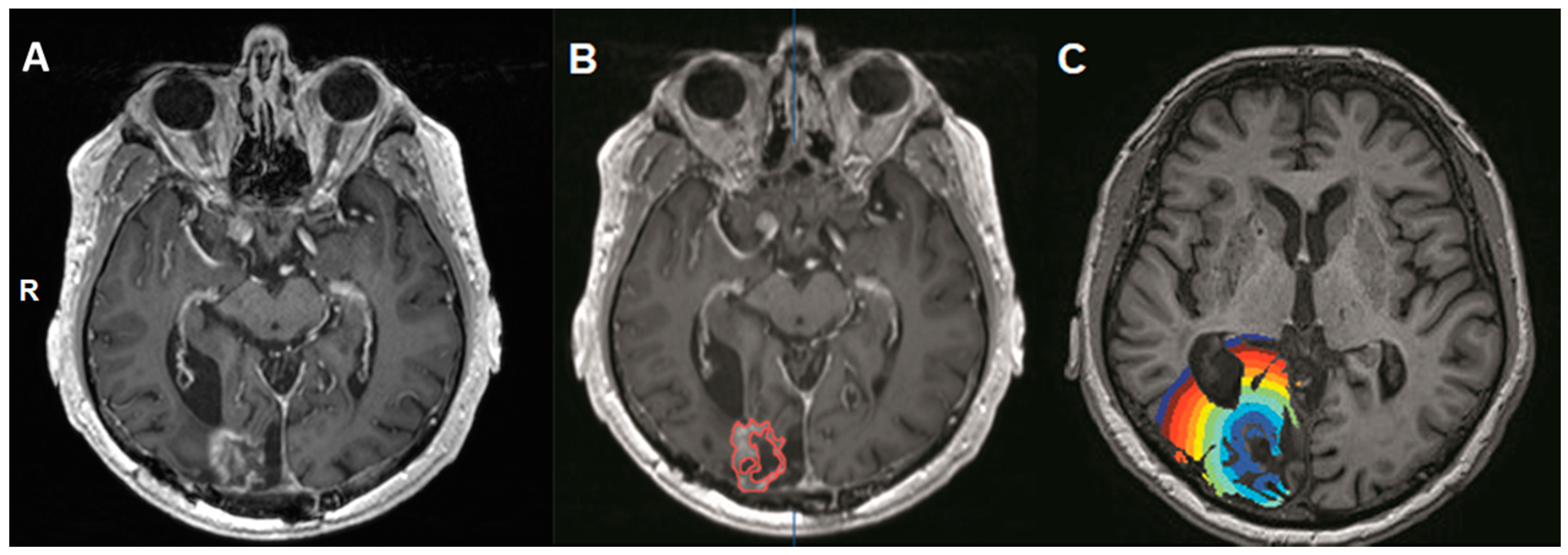
| Patient | Diagnosis | Sex/Age | Follow-Up (Months) | Radiotherapy Protocol |
|---|---|---|---|---|
| 1 | Atypical Meningioma (WHO grade II) | M/36 | 50 | 60 Gy—proton therapy |
| 2 | Anaplastic Oligodendroglioma (WHO grade III) | M/28 | 12 | 60 Gy |
| 3 | Meningotheliomatous meningioma (WHO grade I) | M/72 | 77 | 54 Gy |
| 4 | Anaplastic Oligodendroglioma (WHO grade III) | M/53 | 24 | 54 Gy |
| 5 | Brain metastasis (breast cancer) | F/56 | 11 | radiosurgery (1 × 20 Gy) |
| 6 | Brain metastasis (breast cancer) | F/51 | 34 | 18 Gy interhemispheric + 20 Gy cerebellar |
| 7 | Glioblastoma (WHO grade IV) | F/77 | 10 | 40.5 Gy |
| 8 | Glioblastoma (WHO grade IV) | F/61 | 8 | 60 Gy |
| Concentric VOI from the Contrast-Enhancing Lesion | Glioblastoma Mean CVR Value | Glioblastoma (p-Value) | Radiation Necrosis Mean CVR Value | Radiation Necrosis (p-Value) |
|---|---|---|---|---|
| VOI 1 (3 mm) | 0.06 | 0.96 | 0.03 | 0.24 |
| VOI 2 (6 mm) | 0.07 | 0.50 | 0.06 | 0.04 |
| VOI 3 (9 mm) | 0.08 | 0.70 | 0.08 | 0.02 |
| VOI 4 (12 mm) | 0.09 | 0.89 | 0.11 | 0.01 |
| VOI 5 (15 mm) | 0.09 | 0.97 | 0.12 | 0.002 |
| VOI 6 (18 mm) | 0.10 | 0.97 | 0.13 | <0.001 |
| VOI 7 (21 mm) | 0.10 | 0.89 | 0.14 | <0.001 |
| VOI 8 (24 mm) | 0.10 | 0.95 | 0.14 | <0.001 |
| VOI 9 (27 mm) | 0.10 | 0.93 | 0.14 | <0.001 |
| VOI 10 (30 mm) | 0.11 | 0.83 | 0.14 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscas, G.; van Niftrik, C.H.B.; Sebök, M.; Della Puppa, A.; Seystahl, K.; Andratschke, N.; Brown, M.; Weller, M.; Regli, L.; Piccirelli, M.; et al. Distinct Cerebrovascular Reactivity Patterns for Brain Radiation Necrosis. Cancers 2021, 13, 1840. https://doi.org/10.3390/cancers13081840
Muscas G, van Niftrik CHB, Sebök M, Della Puppa A, Seystahl K, Andratschke N, Brown M, Weller M, Regli L, Piccirelli M, et al. Distinct Cerebrovascular Reactivity Patterns for Brain Radiation Necrosis. Cancers. 2021; 13(8):1840. https://doi.org/10.3390/cancers13081840
Chicago/Turabian StyleMuscas, Giovanni, Christiaan Hendrik Bas van Niftrik, Martina Sebök, Alessandro Della Puppa, Katharina Seystahl, Nicolaus Andratschke, Michelle Brown, Michael Weller, Luca Regli, Marco Piccirelli, and et al. 2021. "Distinct Cerebrovascular Reactivity Patterns for Brain Radiation Necrosis" Cancers 13, no. 8: 1840. https://doi.org/10.3390/cancers13081840
APA StyleMuscas, G., van Niftrik, C. H. B., Sebök, M., Della Puppa, A., Seystahl, K., Andratschke, N., Brown, M., Weller, M., Regli, L., Piccirelli, M., & Fierstra, J. (2021). Distinct Cerebrovascular Reactivity Patterns for Brain Radiation Necrosis. Cancers, 13(8), 1840. https://doi.org/10.3390/cancers13081840







