Induction of Apoptosis, Inhibition of MCL-1, and VEGF-A Expression Are Associated with the Anti-Cancer Efficacy of Magnolol Combined with Regorafenib in Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Cultures, Viability Analysis, and Combination Index Analysis
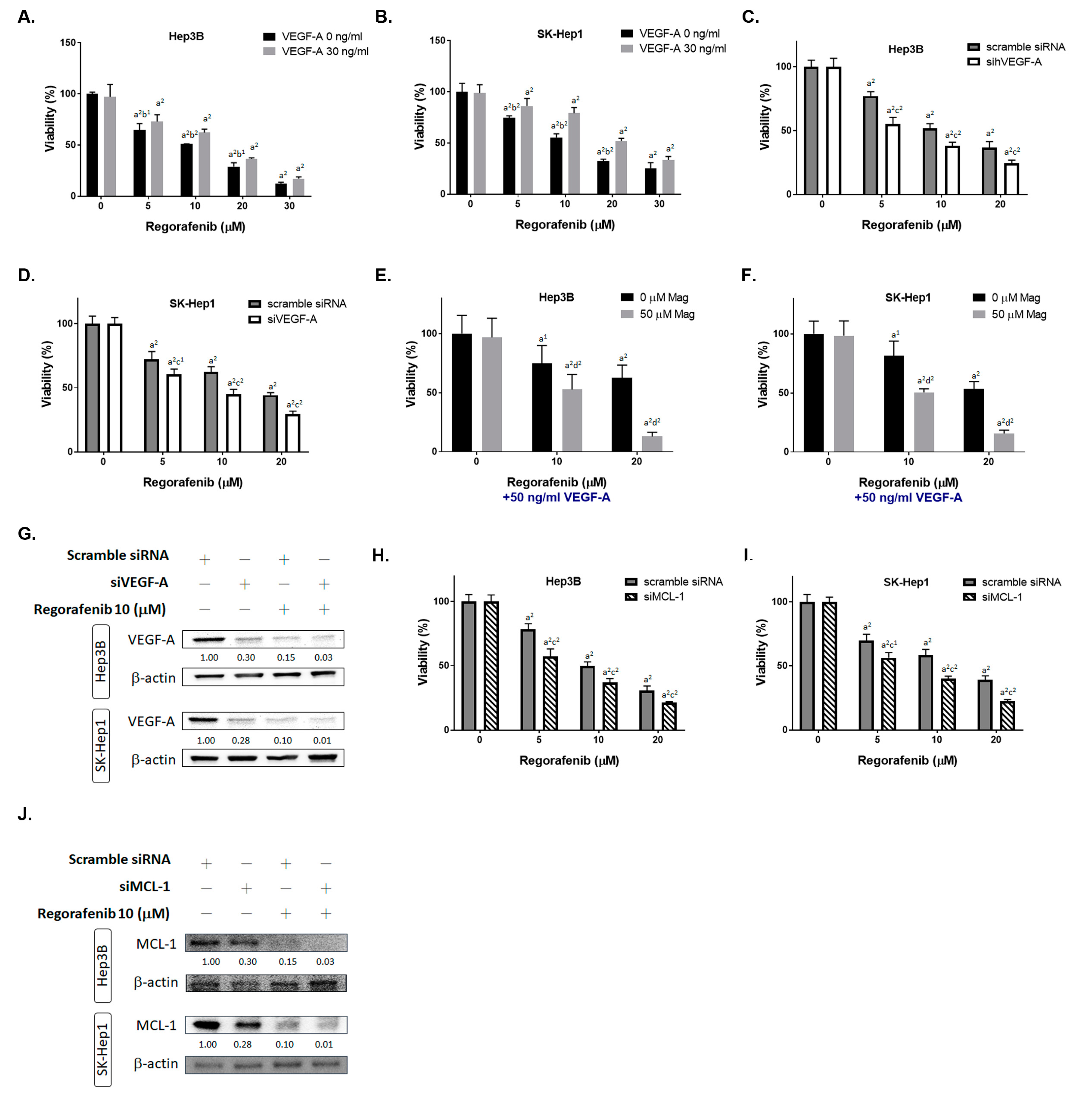
2.3. Transfection Procedure of siMCL-1 and siVEGF-A
2.4. Immunofluorescence Staining
2.5. Comet Assay
2.6. Western Blotting
2.7. Flow Cytometry Analysis
2.8. Wound Healing Assay
2.9. Invasion and Migration Assay
2.10. Animal Experiment
2.11. Hematoxylin and Eosin (H&E) and Immunohistochemistry (IHC)
2.12. Statistical Analysis
3. Results
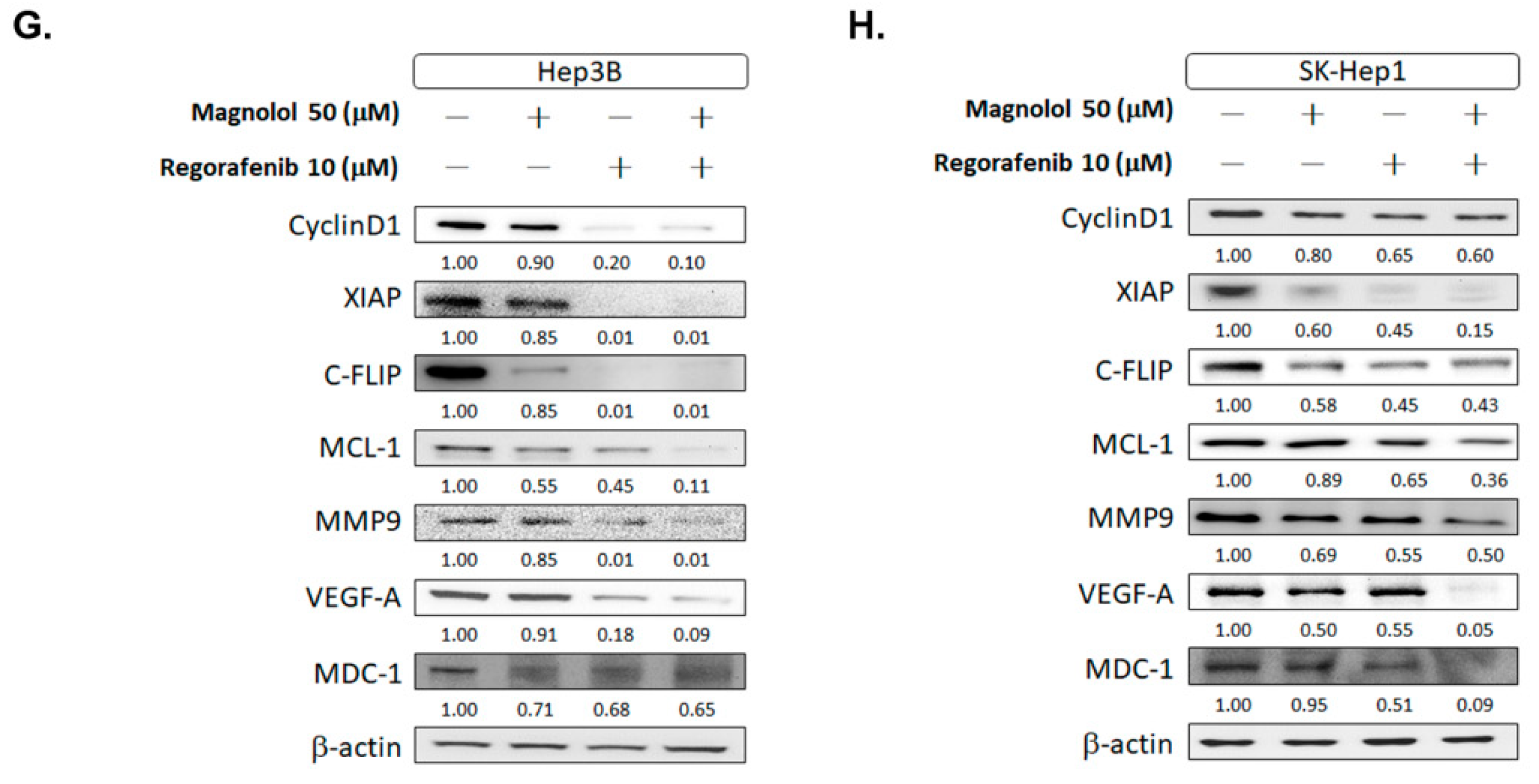
3.1. Magnolol Effectively Induced the Cytotoxicity of Regorafenib in HCC Cells
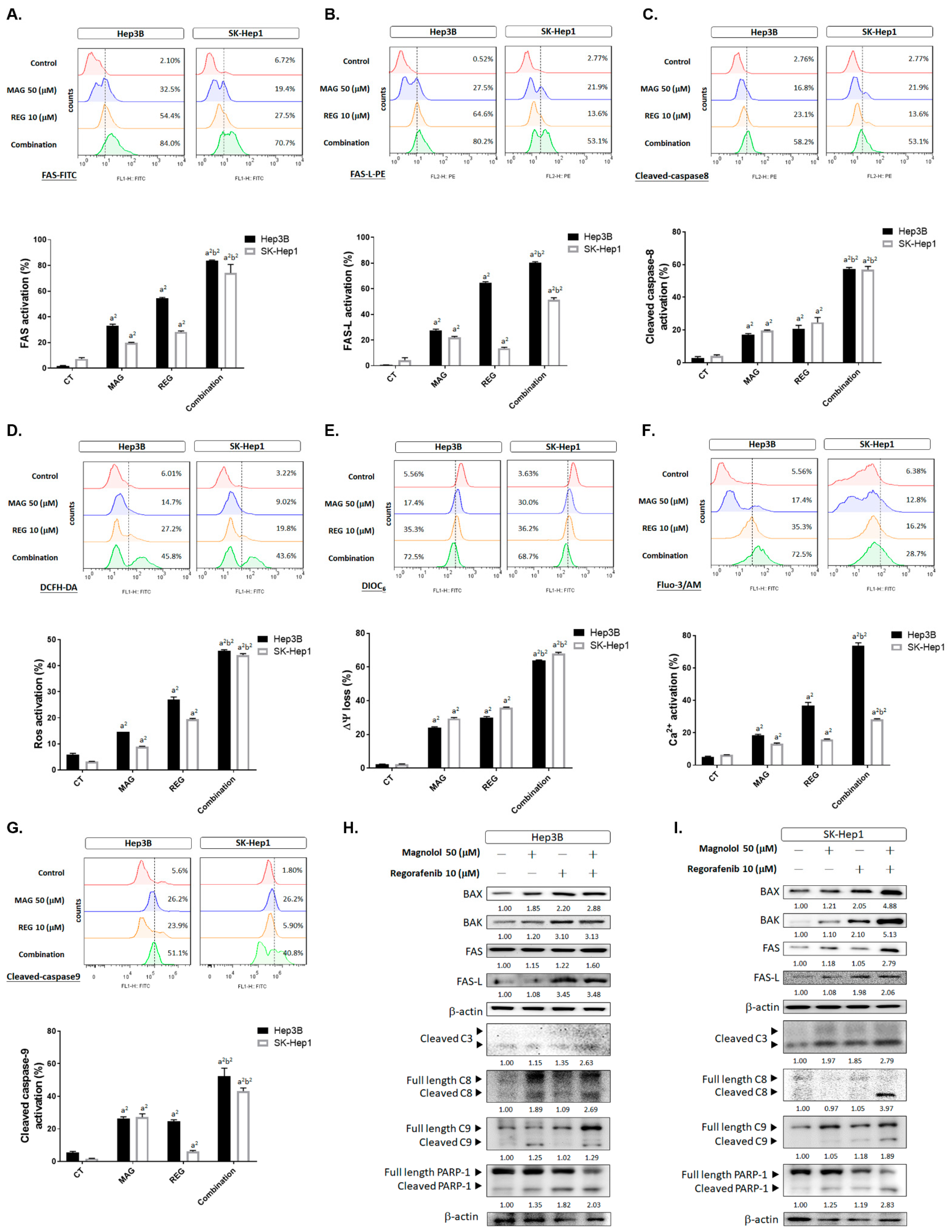
3.2. Inhibition of Both VEGF-A and MCL-1 Expression Sensitize HCC Cells to Regorafenib
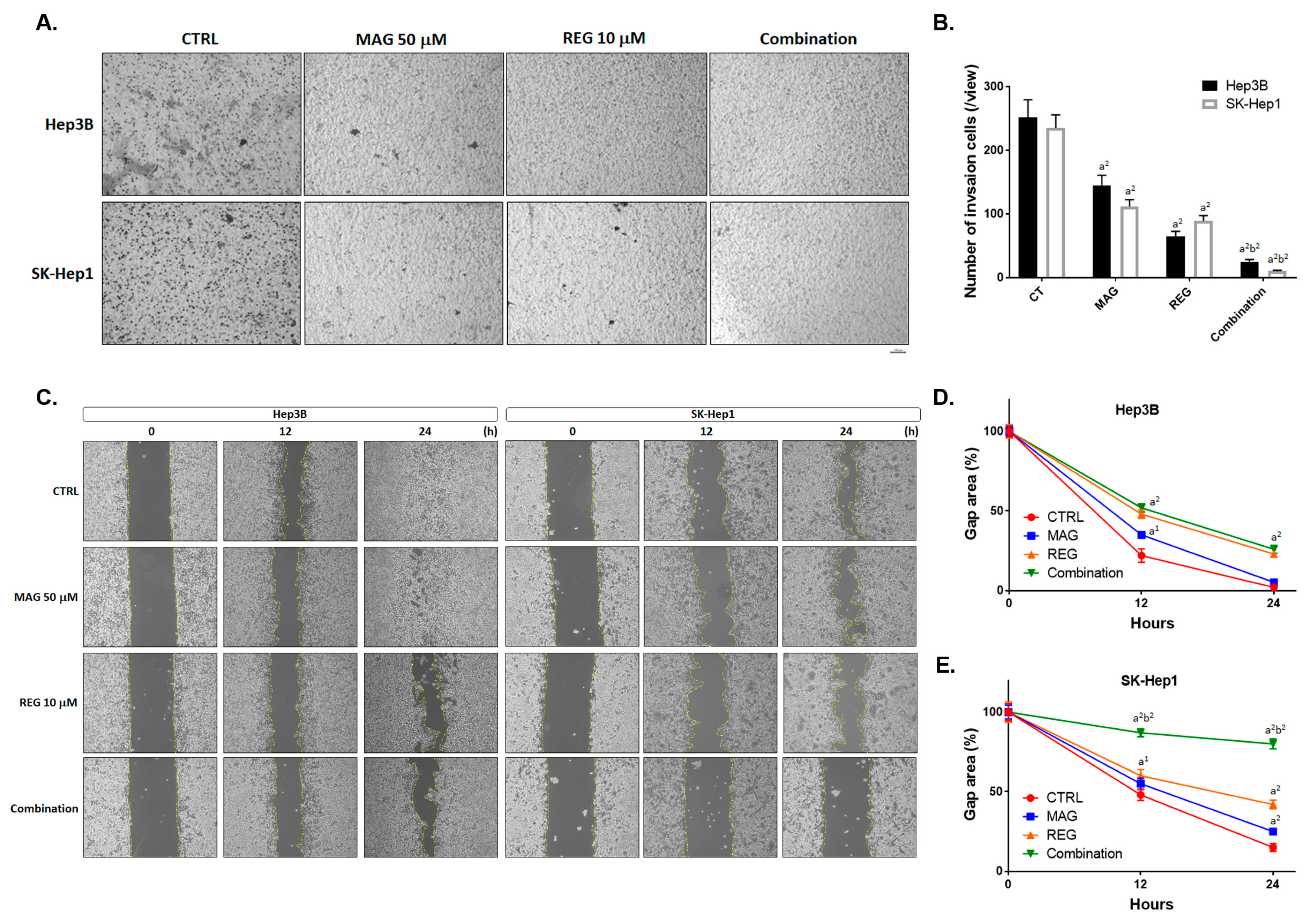
3.3. Magnolol Enhanced the Metastasis Inhibiton of Regorafenib in HCC Cells
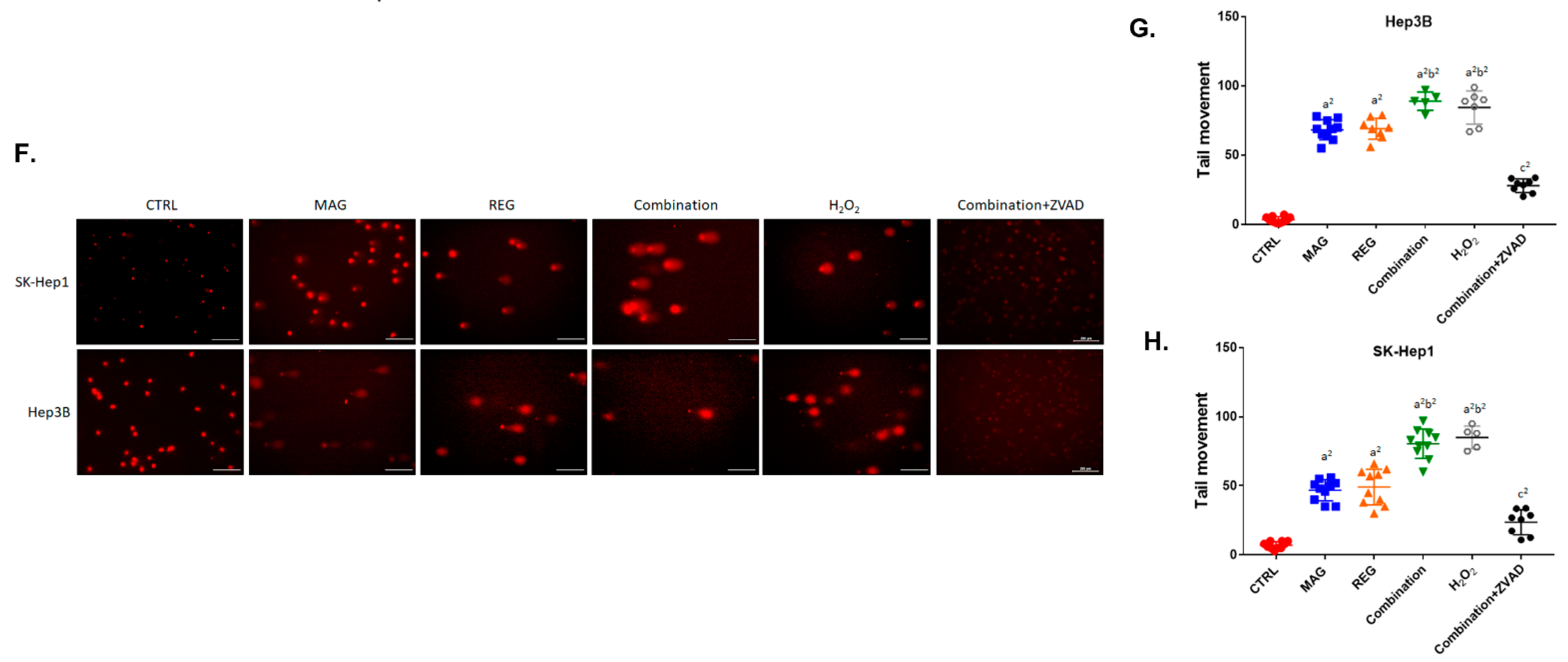
3.4. Magnolol Induced the Apoptosis and DNA Damage Effect of Regorafenib in HCC Cells
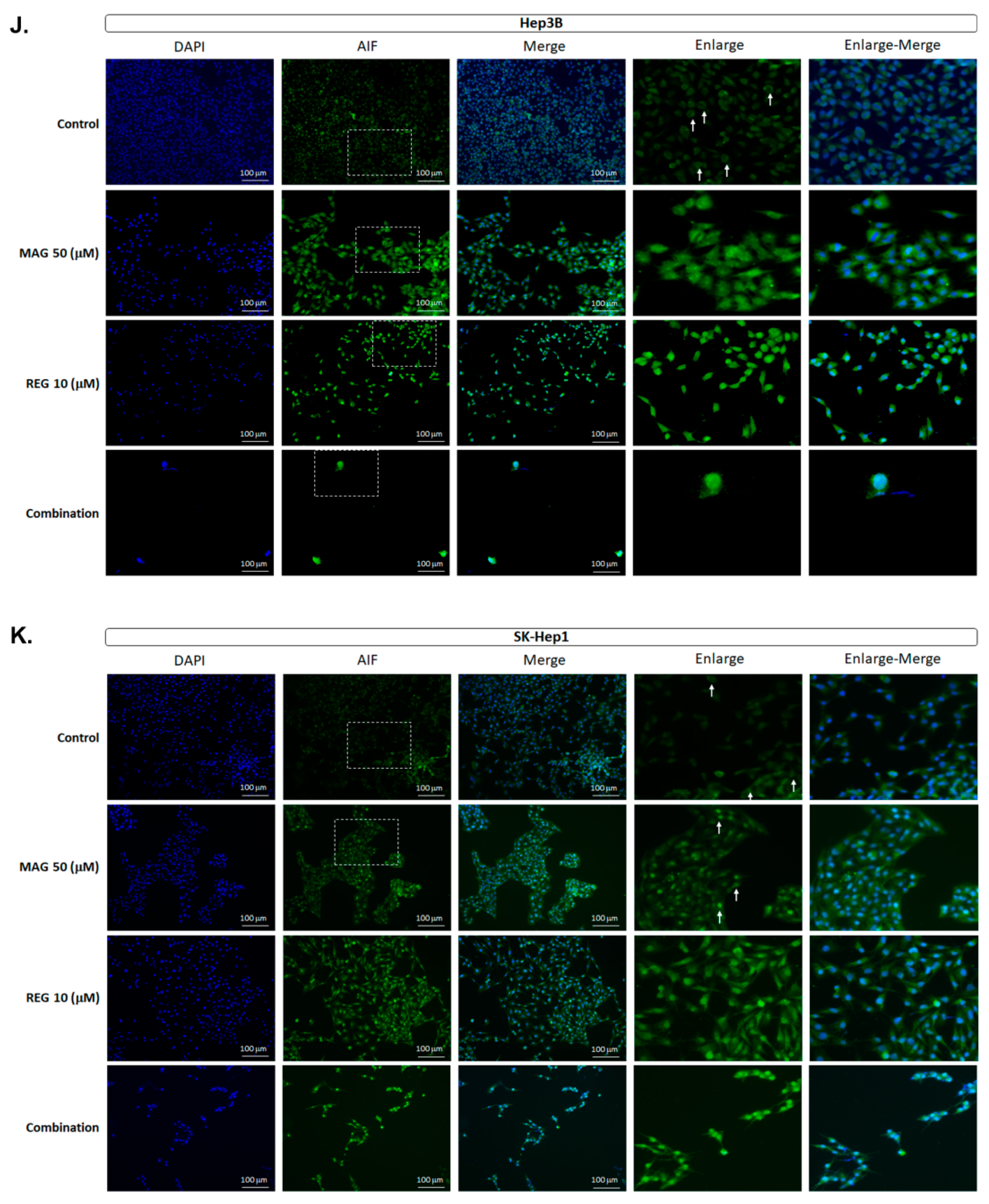
3.5. Magnolol Triggered the Caspase-Dependent and Caspase-Independent Apoptotic Effects of Regorafenib in HCC Cells
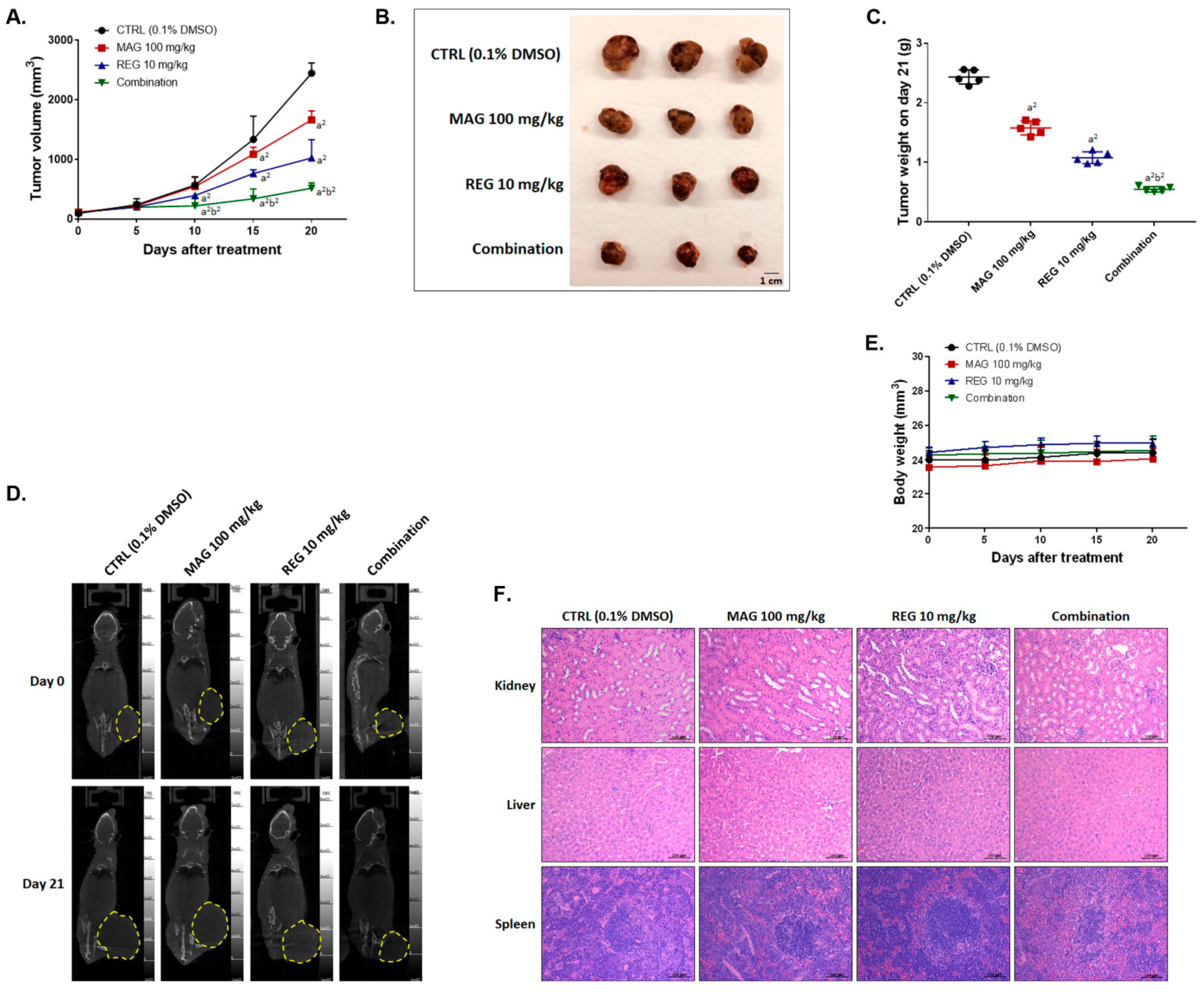
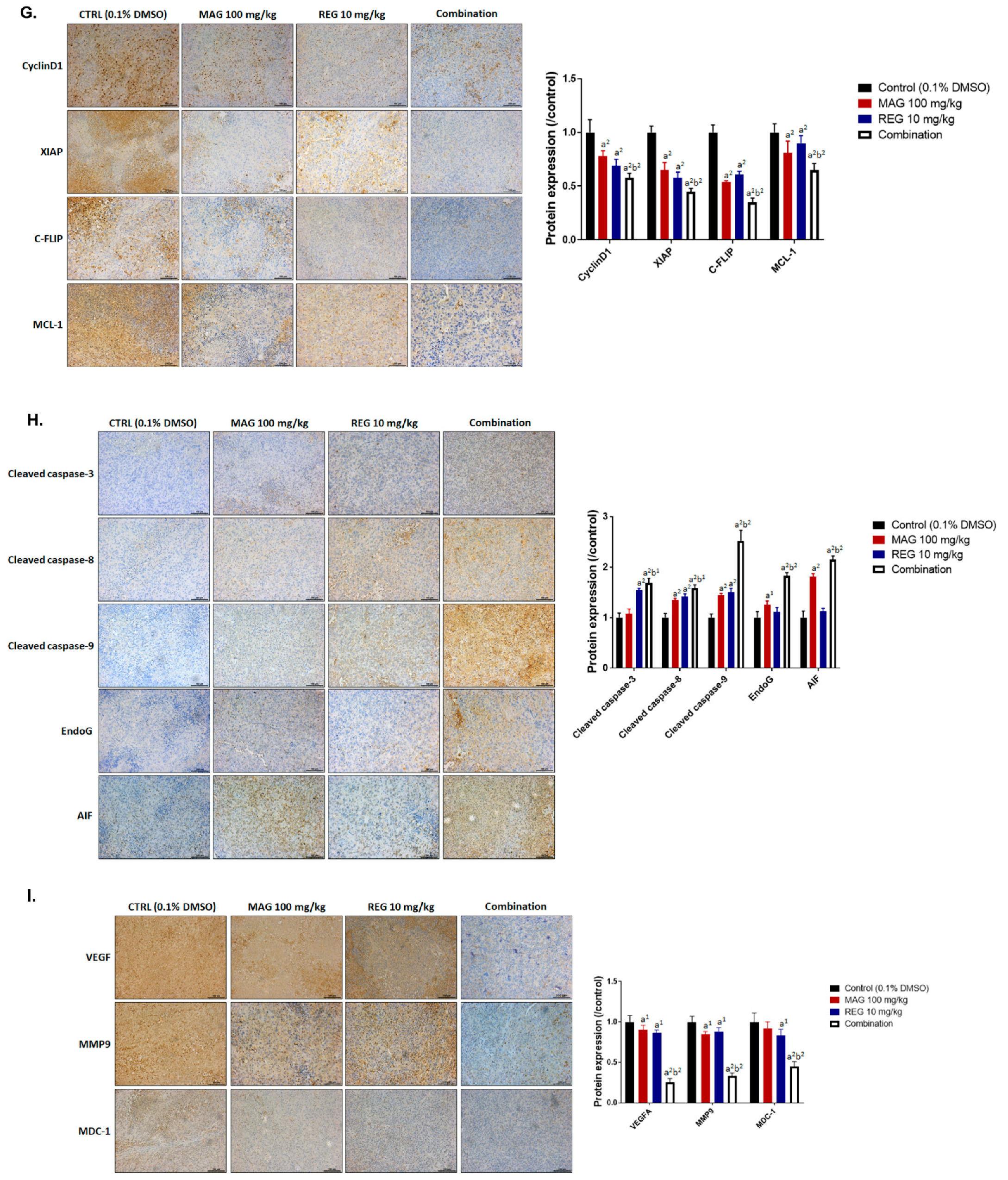
3.6. Magnolol Enhanced the Anti-HCC Efficacy of Regorafenib in an Hep3B-Bearing Animal Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grothey, A.; Blay, J.Y.; Pavlakis, N.; Yoshino, T.; Bruix, J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat. Rev. 2020, 86, 101993. [Google Scholar] [CrossRef]
- D’Alessandro, R.; Refolo, M.G.; Iacovazzi, P.A.; Pesole, P.L.; Messa, C.; Carr, B.I. Ramucirumab and GSK1838705A Enhance the Inhibitory Effects of Low Concentration Sorafenib and Regorafenib Combination on HCC Cell Growth and Motility. Cancers 2019, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Voutsadakis, I.A. A Systematic Review and Meta-analysis of Retrospective Series of Regorafenib for Treatment of Metastatic Colorectal Cancer. Anticancer Res. 2017, 37, 5925–5934. [Google Scholar] [CrossRef][Green Version]
- Zeiner, P.S.; Kinzig, M.; Divé, I.; Maurer, G.D.; Filipski, K.; Harter, P.N.; Senft, C.; Bähr, O.; Hattingen, E.; Steinbach, J.P.; et al. Regorafenib CSF Penetration, Efficacy, and MRI Patterns in Recurrent Malignant Glioma Patients. J. Clin Med. 2019, 8, 2031. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; Lippolis, C.; Carella, N.; Cavallini, A.; Messa, C.; D’Alessandro, R. Chlorogenic Acid Improves the Regorafenib Effects in Human Hepatocellular Carcinoma Cells. Int. J. Mol Sci 2018, 19, 1518. [Google Scholar] [CrossRef]
- Belli, V.; Sforza, V.; Cardone, C.; Martinelli, E.; Barra, G.; Matrone, N.; Napolitano, S.; Morgillo, F.; Tuccillo, C.; Federico, A.; et al. Regorafenib in combination with silybin as a novel potential strategy for the treatment of metastatic colorectal cancer. Oncotarget 2017, 8, 68305–68316. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef]
- Tang, C.Y.; Lai, C.C.; Huang, P.H.; Yang, A.H.; Chiang, S.C.; Huang, P.C.; Tseng, K.W.; Huang, C.H. Magnolol Reduces Renal Ischemia and Reperfusion Injury via Inhibition of Apoptosis. Am. J. Chin. Med. 2017, 45, 1421–1439. [Google Scholar] [CrossRef]
- Zhang, F.H.; Ren, H.Y.; Shen, J.X.; Zhang, X.Y.; Ye, H.M.; Shen, D.Y. Magnolol suppresses the proliferation and invasion of cholangiocarcinoma cells via inhibiting the NF-κB signaling pathway. Biomed. Pharmacother. 2017, 94, 474–480. [Google Scholar] [CrossRef]
- Chen, J.H.; Chiang, I.T.; Hsu, F.T. Protein Kinase B Inactivation Is Associated with Magnolol-Enhanced Therapeutic Efficacy of Sorafenib in Hepatocellular Carcinoma In Vitro and In Vivo. Cancers 2019, 12, 87. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.H.; Yang, J.S.; Ma, C.Y.; Yang, M.D.; Huang, H.Y.; Hsia, T.C.; Kuo, H.M.; Wu, P.P.; Lee, T.H.; Chung, J.G. Danthron, an anthraquinone derivative, induces DNA damage and caspase cascades-mediated apoptosis in SNU-1 human gastric cancer cells through mitochondrial permeability transition pores and Bax-triggered pathways. Chem. Res. Toxicol. 2011, 24, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Chen, S.C.; Kao, C.M.; Huang, M.H.; Shih, M.K.; Chen, Y.L.; Huang, S.P.; Liu, T.-Z. Assessment of Genotoxicity of Benzidine and Its Structural Analogues to Human Lymphocytes Using Comet Assay. Toxicol. Sci. 2003, 72, 283–288. [Google Scholar] [CrossRef][Green Version]
- Hsu, F.T.; Chiang, I.T.; Wang, W.S. Induction of apoptosis through extrinsic/intrinsic pathways and suppression of ERK/NF-κB signalling participate in anti-glioblastoma of imipramine. J. Cell Mol. Med. 2020, 24, 3982–4000. [Google Scholar] [CrossRef]
- Pan, P.J.; Liu, Y.C.; Hsu, F.T. Protein Kinase B and Extracellular Signal-Regulated Kinase Inactivation is Associated with Regorafenib-Induced Inhibition of Osteosarcoma Progression In Vitro and In Vivo. J. Clin. Med. 2019, 8, 900. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chung, J.G.; Tan, Z.L.; Hsu, F.T.; Liu, Y.C.; Lin, S.S. ERK/AKT Inactivation and Apoptosis Induction Associate With Quetiapine-inhibited Cell Survival and Invasion in Hepatocellular Carcinoma Cells. In Vivo 2020, 34, 2407–2417. [Google Scholar] [CrossRef]
- Chen, Y.S.; Sun, R.; Chen, W.L.; Yau, Y.C.; Hsu, F.T.; Chung, J.G.; Tsai, C.J.; Hsieh, C.L.; Chiu, Y.M.; Chen, J.H. The In Vivo Radiosensitizing Effect of Magnolol on Tumor Growth of Hepatocellular Carcinoma. In Vivo 2020, 34, 1789–1796. [Google Scholar] [CrossRef]
- Tsai, J.J.; Pan, P.J.; Hsu, F.T.; Chung, J.G.; Chiang, I.T. Glycyrrhizic Acid Modulates Apoptosis through Extrinsic/Intrinsic Pathways and Inhibits Protein Kinase B- and Extracellular Signal-Regulated Kinase-Mediated Metastatic Potential in Hepatocellular Carcinoma In Vitro and In Vivo. Am. J. Chin. Med. 2020, 48, 223–244. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Park, S.J.; Kim, S.W.; Hariharasudhan, G.; Jeong, S.Y.; Chang, I.Y.; Lee, J.H. Karyopherin α-2 Mediates MDC1 Nuclear Import through a Functional Nuclear Localization Signal in the tBRCT Domain of MDC1. Int. J. Mol. Sci. 2020, 21, 2650. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Farshdousti Hagh, M.; Alivand, M.R.; Akbari, A.A.M.; Shams Asenjan, K.; Saraei, R.; Solali, S. Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL. J. Cell Physiol. 2018, 233, 6470–6485. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Sun, Y.; Zhang, M.; Fa, Z.; Wan, Y.; Min, Z.; Xu, H.; Xu, C.; Tang, J. GLS1 promotes proliferation in hepatocellular carcinoma cells via AKT/GSK3β/CyclinD1 pathway. Exp. Cell Res. 2019, 381, 1–9. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, W.; Niu, F. Adenoviral vector expressing IGF-1 protects murine chondrogenic ATDC5 cells against hydrogen peroxide-induced mitochondrial dysfunction and apoptosis. J. Toxicol. Sci. 2015, 40, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, M.; Zhu, J.; Qu, J.; Qin, K.; Zhao, D.; Wang, L.; Dong, L.; Zhang, X. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed. Pharmacother. 2020, 132, 110797. [Google Scholar] [CrossRef]
- Yu, C.C.; Huang, S.Y.; Chang, S.F.; Liao, K.F.; Chiu, S.C. The Synergistic Anti-Cancer Effects of NVP-BEZ235 and Regorafenib in Hepatocellular Carcinoma. Molecules 2020, 25, 2454. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Long, X.; Li, P.; Chen, S.; Kuang, W.; Guo, J. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27. [Google Scholar] [CrossRef]
- Peng, S.; Wang, Y.; Peng, H.; Chen, D.; Shen, S.; Peng, B.; Chen, M.; Lencioni, R.; Kuang, M. Autocrine vascular endothelial growth factor signaling promotes cell proliferation and modulates sorafenib treatment efficacy in hepatocellular carcinoma. Hepatology 2014, 60, 1264–1277. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Fan, F.; Wang, R.; Ye, X.; Xia, L.; Boulbes, D.; Ellis, L.M. Intracrine VEGF signalling mediates colorectal cancer cell migration and invasion. Br. J. Cancer 2017, 117, 848–855. [Google Scholar] [CrossRef]
- Suenaga, M.; Mashima, T.; Kawata, N.; Wakatsuki, T.; Horiike, Y.; Matsusaka, S.; Dan, S.; Shinozaki, E.; Seimiya, H.; Mizunuma, N.; et al. Serum VEGF-A and CCL5 levels as candidate biomarkers for efficacy and toxicity of regorafenib in patients with metastatic colorectal cancer. Oncotarget 2016, 7, 34811–34823. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Q.; Liu, Z. Ubiquitination and deubiquitination of MCL1 in cancer: Deciphering chemoresistance mechanisms and providing potential therapeutic options. Cell Death Dis. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wen, P.; Chen, B.; Hu, G.; Wu, L.; Xu, A.; Zhao, G. Downregulation of CDC20 Increases Radiosensitivity through Mcl-1/p-Chk1-Mediated DNA Damage and Apoptosis in Tumor Cells. Int. J. Mol. Sci. 2020, 21, 6692. [Google Scholar] [CrossRef]
- Song, X.; Shen, L.; Tong, J.; Kuang, C.; Zeng, S.; Schoen, R.E.; Yu, J.; Pei, H.; Zhang, L. Mcl-1 inhibition overcomes intrinsic and acquired regorafenib resistance in colorectal cancer. Theranostics 2020, 10, 8098–8110. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 2016, 354. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Selimovic, D.; Hannig, M.; Taylor, E.B.; Abd Elmageed, Z.Y.; Hassan, S.Y.; Haikel, Y.; Kandil, E.; Leverkus, M.; Brodell, R.T.; et al. Imiquimod-induced apoptosis of melanoma cells is mediated by ER stress-dependent Noxa induction and enhanced by NF-κB inhibition. J. Cell Mol. Med. 2016, 20, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Hung, F.M.; Lee, C.H.; Yeh, M.Y.; Lee, M.H.; Lu, H.F.; Chen, Y.L.; Liu, J.Y.; Chung, J.G. Fisetin Induces Apoptosis of HSC3 Human Oral Cancer Cells Through Endoplasmic Reticulum Stress and Dysfunction of Mitochondria-mediated Signaling Pathways. In Vivo 2017, 31, 1103–1114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitazumi, I.; Tsukahara, M. Regulation of DNA fragmentation: The role of caspases and phosphorylation. FEBS J. 2011, 278, 427–441. [Google Scholar] [CrossRef]
- Wang, W.H.; Chiang, I.T.; Ding, K.; Chung, J.G.; Lin, W.J.; Lin, S.S.; Hwang, J.J. Curcumin-induced apoptosis in human hepatocellular carcinoma j5 cells: Critical role of ca(+2)-dependent pathway. Evid.-Based Complement. Altern. Med. 2012, 2012, 512907. [Google Scholar] [CrossRef]
- Tsai, J.J.; Chen, J.H.; Chen, C.H.; Chung, J.G.; Hsu, F.T. Apoptosis induction and ERK/NF-κB inactivation are associated with magnolol-inhibited tumor progression in hepatocellular carcinoma in vivo. Environ. Toxicol. 2020, 35, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.J.; Pan, P.J.; Hsu, F.T. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-κB activation in hepatocellular carcinoma cells. Oncol. Rep. 2017, 37, 1036–1044. [Google Scholar] [CrossRef]
- Ting, C.Y.; Wang, H.E.; Yu, C.C.; Liu, H.C.; Liu, Y.C.; Chiang, I.T. Curcumin Triggers DNA Damage and Inhibits Expression of DNA Repair Proteins in Human Lung Cancer Cells. Anticancer Res. 2015, 35, 3867–3873. [Google Scholar] [PubMed]
- Wang, Z.; Zuo, W.; Zeng, Q.; Qian, Y.; Li, Y.; Liu, C.; Wang, J.; Zhong, S.; Bu, Y.; Hu, G. Loss of NFBD1/MDC1 disrupts homologous recombination repair and sensitizes nasopharyngeal carcinoma cells to PARP inhibitors. J. Biomed. Sci 2019, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zou, Z.; Luo, X.; Mi, Y.; Chang, H.; Xing, D. LRH1 enhances cell resistance to chemotherapy by transcriptionally activating MDC1 expression and attenuating DNA damage in human breast cancer. Oncogene 2018, 37, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
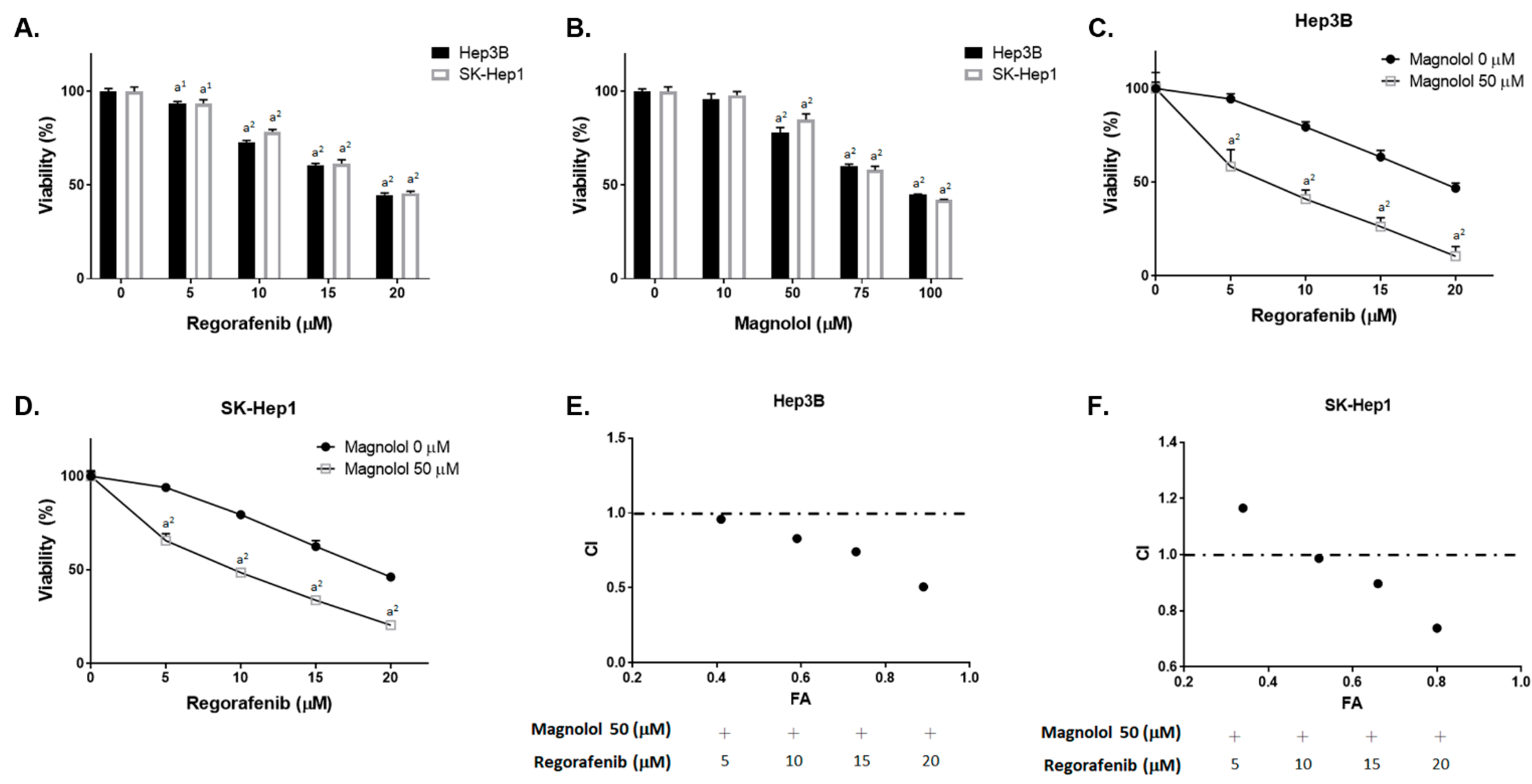

| Effect (FA) | Combination Index (CI) | ||||
|---|---|---|---|---|---|
| Regorafenib (μM) | Magnolol (μM) | Hep3B | SK-Hep1 | Hep3B | SK-Hep1 |
| 5 | 50 | 0.41 | 0.34 | 0.96 | 1.17 |
| 10 | 50 | 0.59 | 0.52 | 0.83 | 0.98 |
| 15 | 50 | 0.73 | 0.66 | 0.74 | 0.90 |
| 20 | 50 | 0.89 | 0.8 | 0.51 | 0.74 |
| Treatment | MTGT (Day) * | MTGDT (Day) # | MGIR $ | ER ★ |
|---|---|---|---|---|
| Control | 4.25 | NA | NA | NA |
| Magnolol (MAG) | 6.44 | 2.19 | 1.52 | 3.86 |
| Regorafenib (REG) | 10.93 | 6.68 | 2.57 | 2.28 |
| Combination | 24.89 | 20.64 | 2.28 | -- |
| Treatment | Mean Inhibitory * | Expected Inhibitory # | Combination Index $ |
|---|---|---|---|
| Control | -- | -- | -- |
| Magnolol (MAG) | 31.98 | -- | -- |
| Regorafenib (REG) | 58.11 | -- | -- |
| Combination | 78.87 | 50.37 | 0.43 (synergistic) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Hsu, F.-T.; Chen, W.-L.; Chen, J.-H. Induction of Apoptosis, Inhibition of MCL-1, and VEGF-A Expression Are Associated with the Anti-Cancer Efficacy of Magnolol Combined with Regorafenib in Hepatocellular Carcinoma. Cancers 2021, 13, 2066. https://doi.org/10.3390/cancers13092066
Chen C-H, Hsu F-T, Chen W-L, Chen J-H. Induction of Apoptosis, Inhibition of MCL-1, and VEGF-A Expression Are Associated with the Anti-Cancer Efficacy of Magnolol Combined with Regorafenib in Hepatocellular Carcinoma. Cancers. 2021; 13(9):2066. https://doi.org/10.3390/cancers13092066
Chicago/Turabian StyleChen, Cheng-Hsien, Fei-Ting Hsu, Wei-Lung Chen, and Jiann-Hwa Chen. 2021. "Induction of Apoptosis, Inhibition of MCL-1, and VEGF-A Expression Are Associated with the Anti-Cancer Efficacy of Magnolol Combined with Regorafenib in Hepatocellular Carcinoma" Cancers 13, no. 9: 2066. https://doi.org/10.3390/cancers13092066
APA StyleChen, C.-H., Hsu, F.-T., Chen, W.-L., & Chen, J.-H. (2021). Induction of Apoptosis, Inhibition of MCL-1, and VEGF-A Expression Are Associated with the Anti-Cancer Efficacy of Magnolol Combined with Regorafenib in Hepatocellular Carcinoma. Cancers, 13(9), 2066. https://doi.org/10.3390/cancers13092066






