Evaluation of SSTR2 Expression in SI-NETs and Relation to Overall Survival after PRRT
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
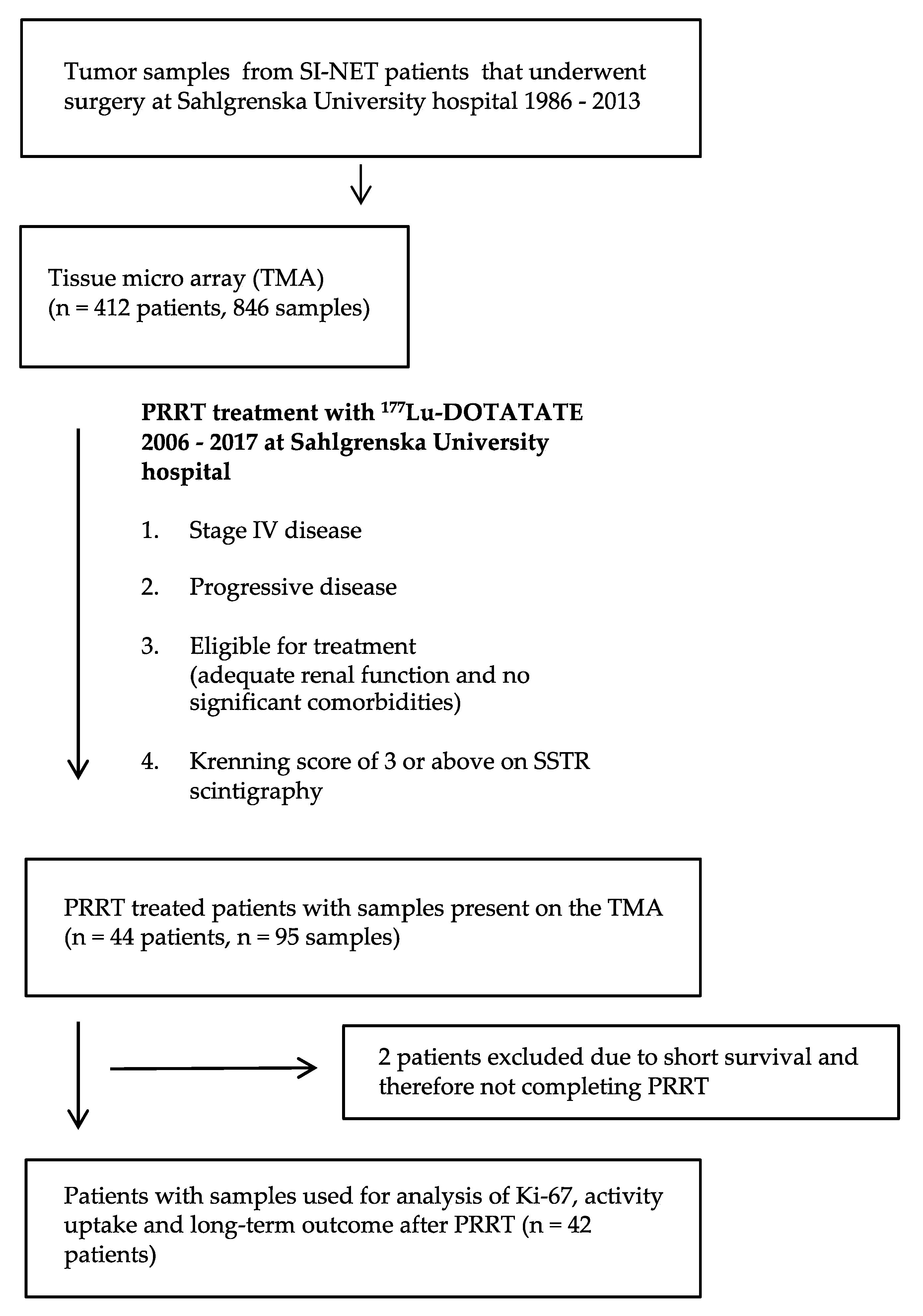
2.1. Tumour Tissue Samples
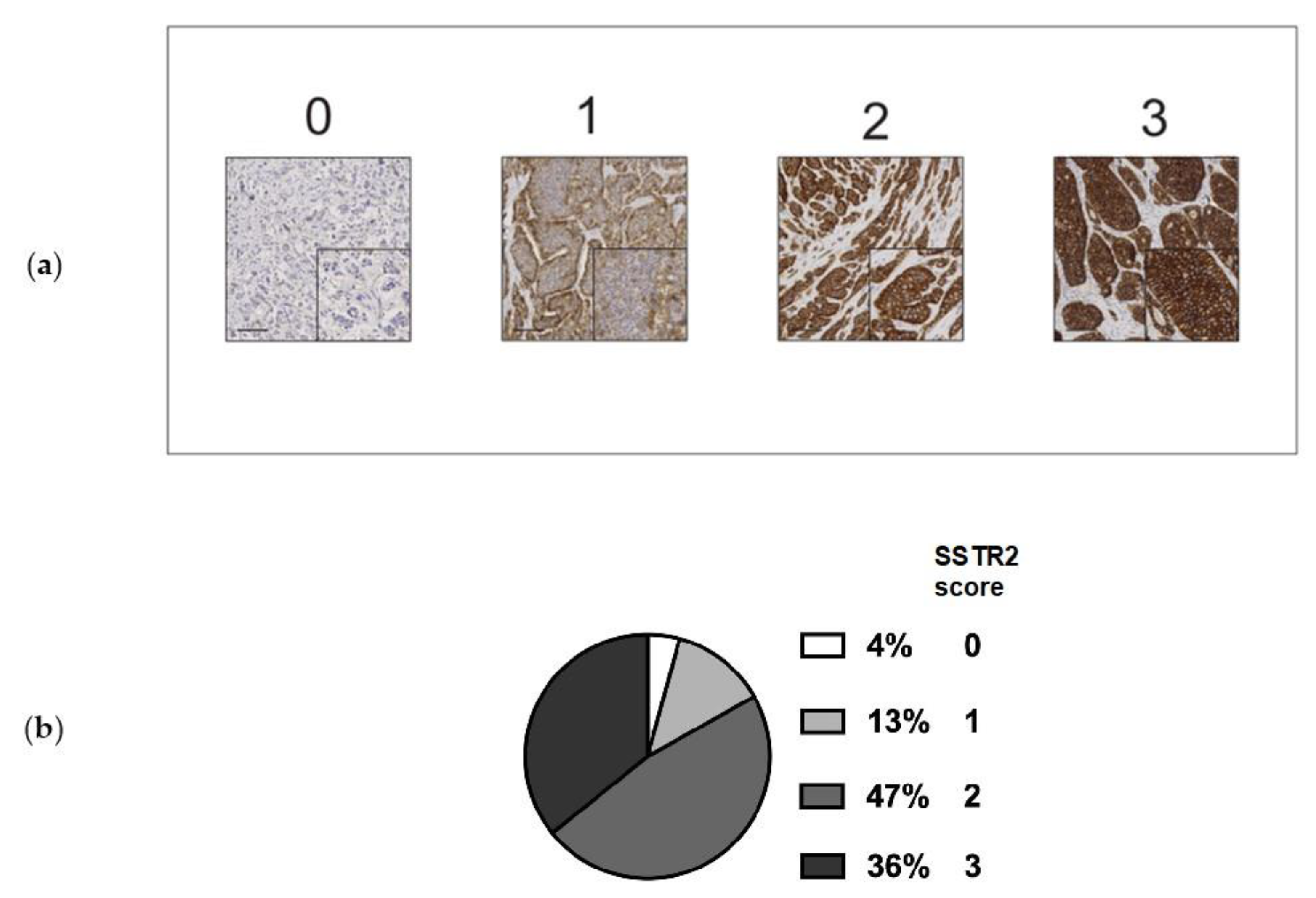
2.2. Immunohistochemistry and Scoring
2.3. Patients and Clinical Characteristics
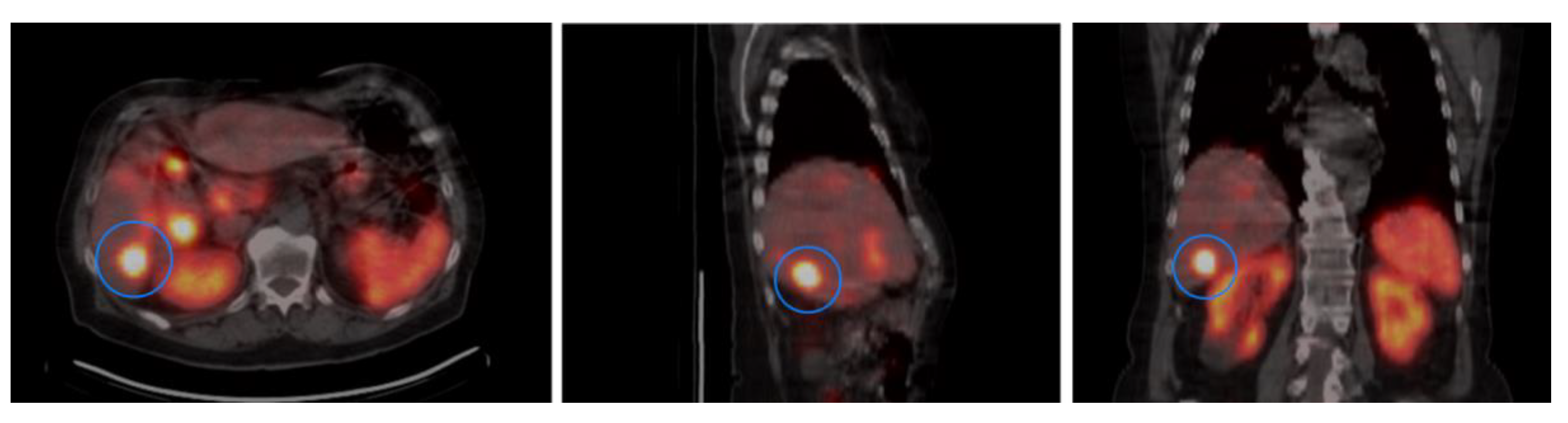
2.4. Activity Concentration in Tumors
2.5. Statistical Analysis
3. Results
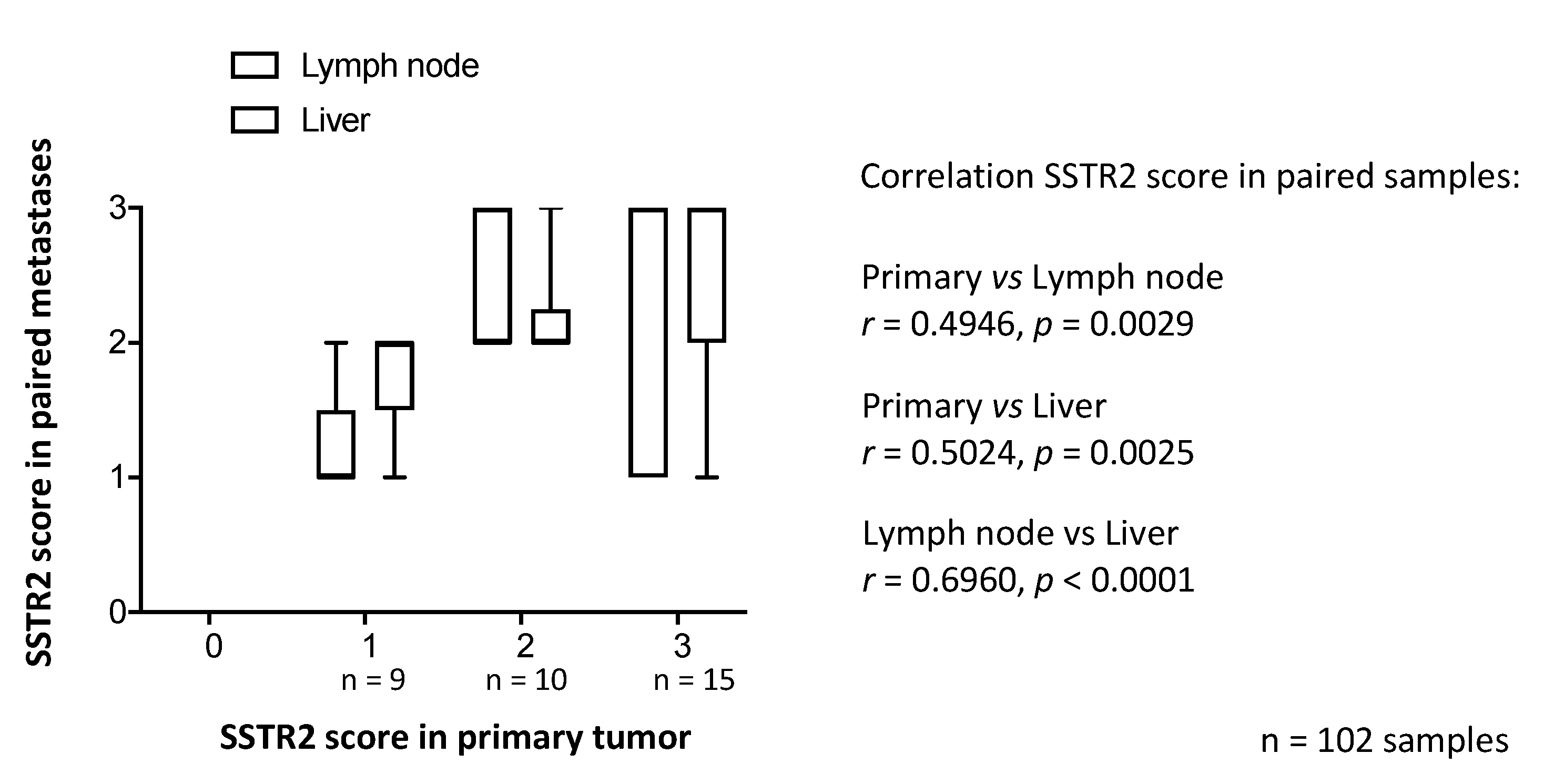
3.1. SSTR2 Scoring and Distribution of SSTR2 Expression Among Samples
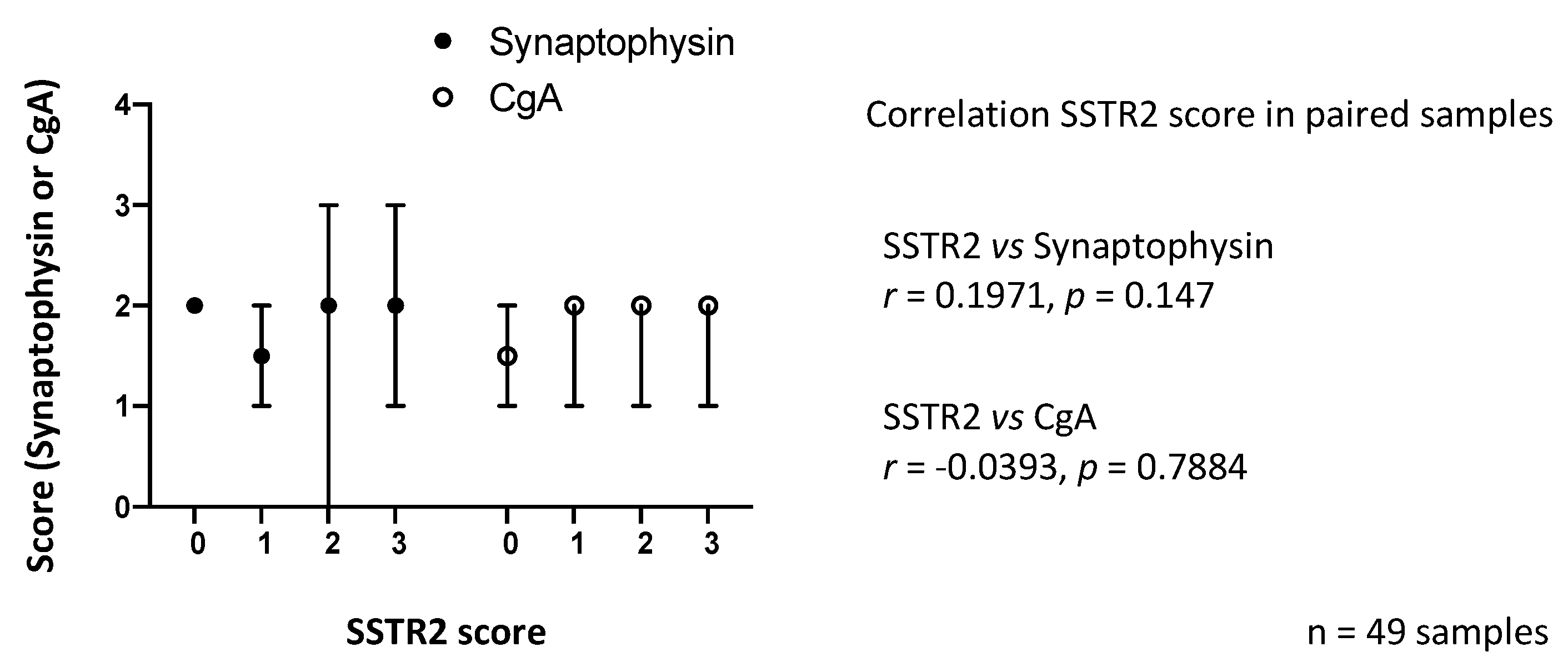
3.2. SSTR2 Expression Does Not Correlate with Synaptophysin or CgA Expression
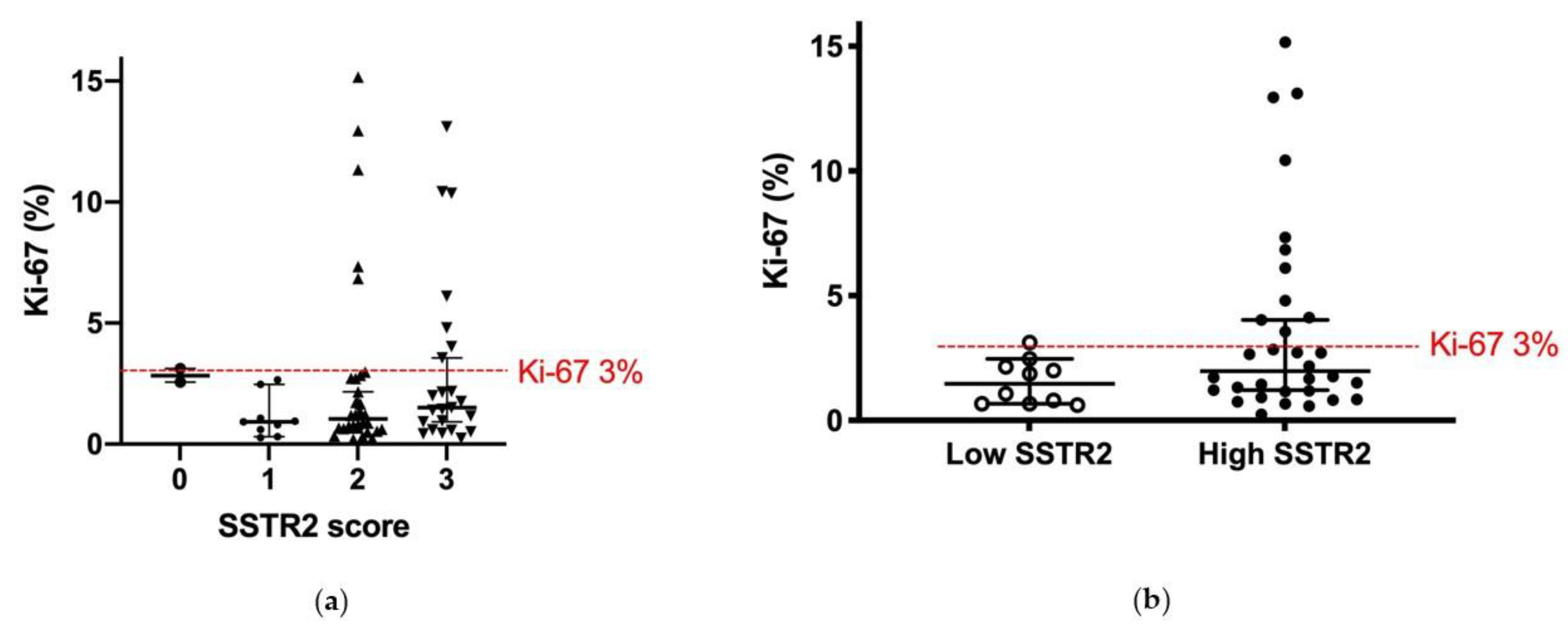
3.3. SSTR2 Expression and Proliferation Rate
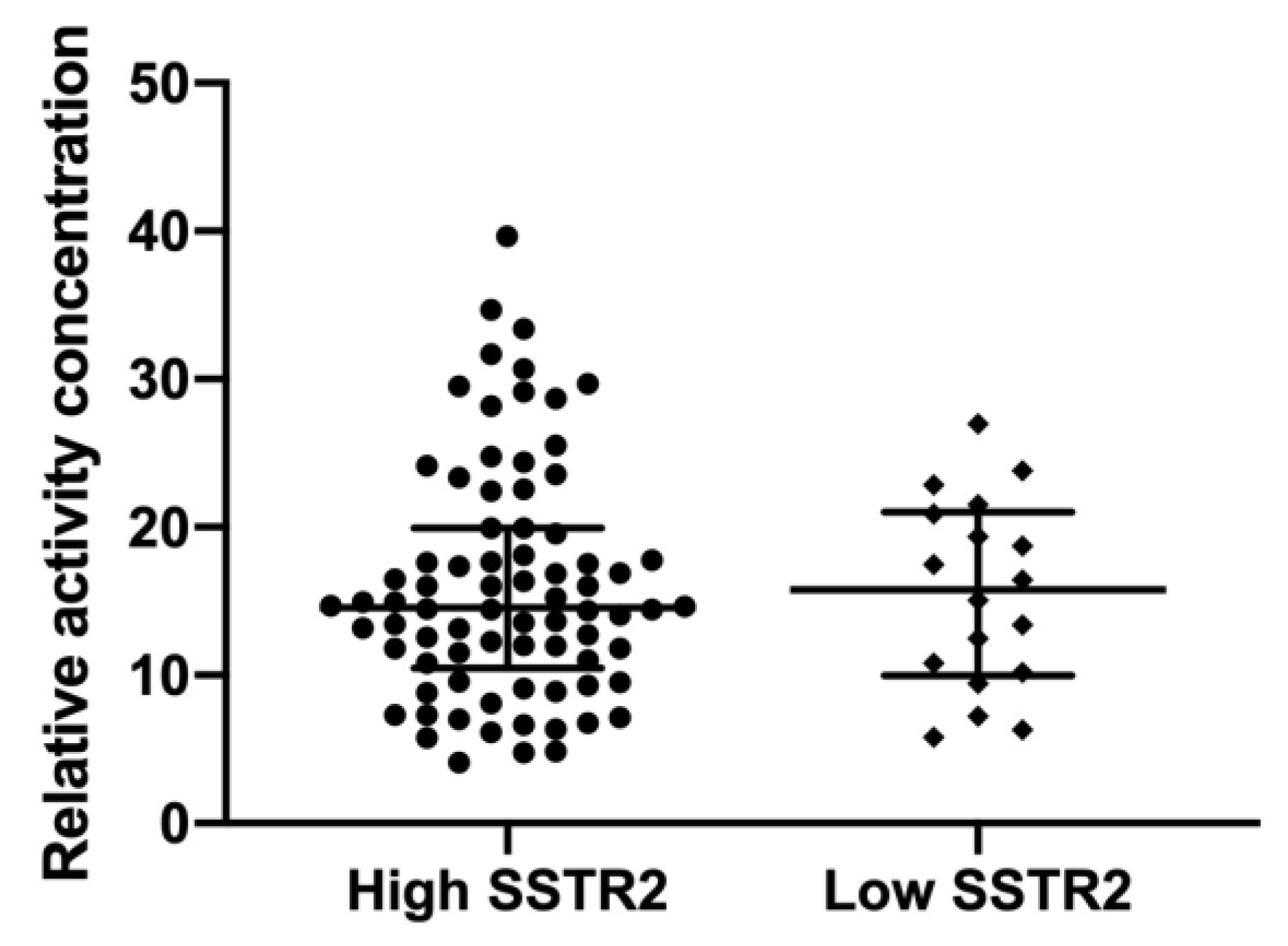
3.4. SSTR2 Expression and Activity Concentration
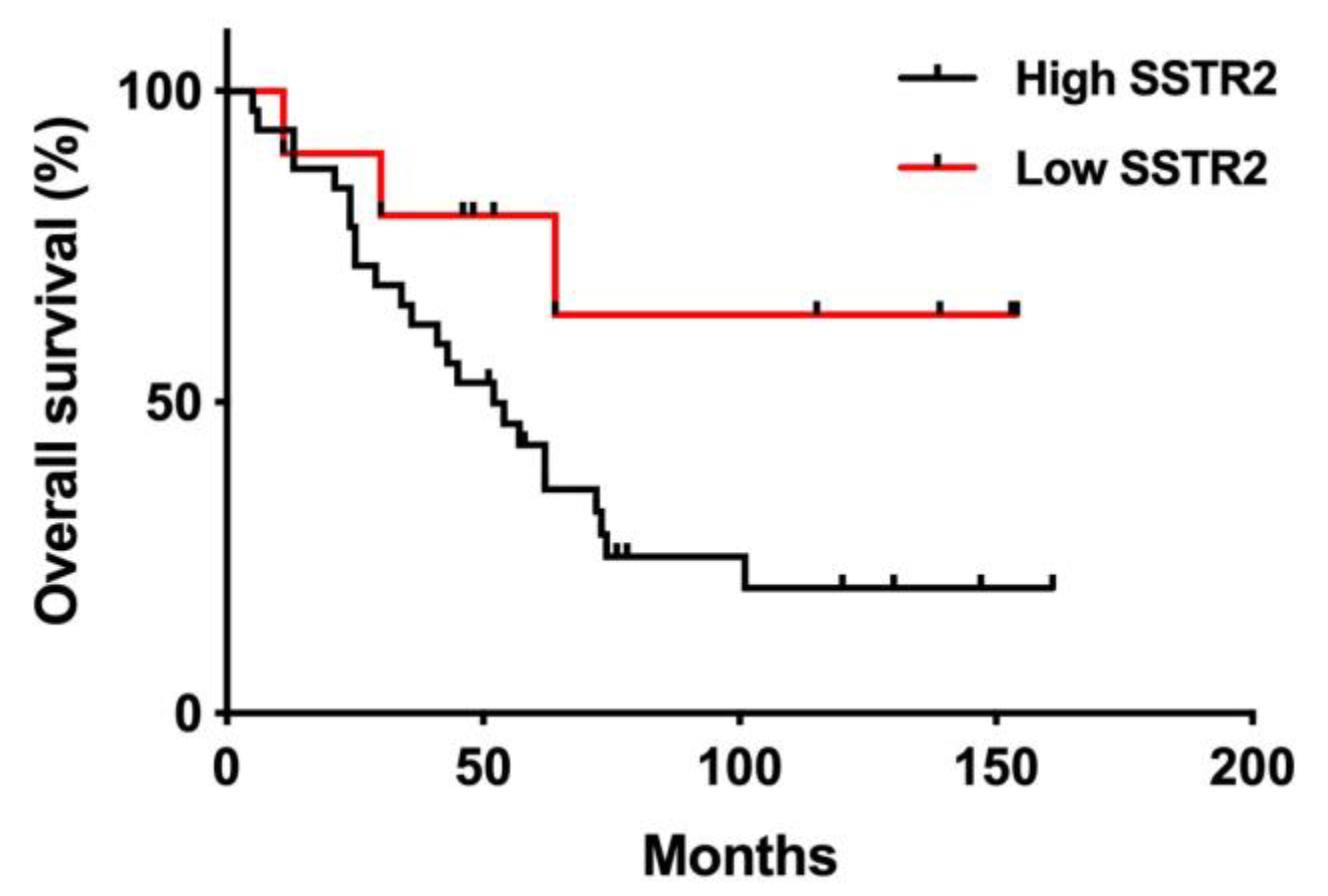
3.5. SSTR2 Expression and Long-Term Outcome
3.6. SSTR2 Expression and Treatment Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after "carcinoid": Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Berge, T.; Linell, F. Carcinoid tumours. Frequency in a defined population during a 12-year period. Acta Pathol. Microbiol. Scand. Sect. A Pathol. 1976, 84, 322–330. [Google Scholar]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Niederle, B.; Pape, U.F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Oberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Oberg, K.; Steinmuller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Weber, J.M.; Feldman, M.; Coppola, D.; Meredith, K.; Kvols, L.K. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J. Clin. Oncol 2013, 31, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793. [Google Scholar] [CrossRef]
- Rinke, A.; Muller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Blaker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Teunissen, J.J.; Bakker, W.H.; Kooij, P.P.; de Herder, W.W.; Feelders, R.A.; van Eijck, C.H.; Esser, J.P.; Kam, B.L.; Krenning, E.P. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 2754–2762. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Cremonesi, M.; Ferrari, M.; Bodei, L.; Tosi, G.; Paganelli, G. Dosimetry in Peptide radionuclide receptor therapy: A review. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2006, 47, 1467–1475. [Google Scholar]
- De Mestier, L.; Dromain, C.; d’Assignies, G.; Scoazec, J.Y.; Lassau, N.; Lebtahi, R.; Brixi, H.; Mitry, E.; Guimbaud, R.; Courbon, F.; et al. Evaluating digestive neuroendocrine tumor progression and therapeutic responses in the era of targeted therapies: State of the art. Endocr. Relat. Cancer 2014, 21, 105–120. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.S.; Singh, A.; van der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Malczewska, A.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: The NETest. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 895–906. [Google Scholar] [CrossRef]
- Arvidsson, Y.; Rehammar, A.; Bergstrom, A.; Andersson, E.; Altiparmak, G.; Sward, C.; Wangberg, B.; Kristiansson, E.; Nilsson, O. miRNA profiling of small intestinal neuroendocrine tumors defines novel molecular subtypes and identifies miR-375 as a biomarker of patient survival. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2018, 31, 1302–1317. [Google Scholar] [CrossRef]
- Tang, L.H.; Gonen, M.; Hedvat, C.; Modlin, I.M.; Klimstra, D.S. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: A comparison of digital image analysis with manual methods. Am. J. Surg. Pathol. 2012, 36, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Specht, E.; Kaemmerer, D.; Sanger, J.; Wirtz, R.M.; Schulz, S.; Lupp, A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology 2015, 67, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Ryden, T.; Heydorn Lagerlof, J.; Hemmingsson, J.; Marin, I.; Svensson, J.; Bath, M.; Gjertsson, P.; Bernhardt, P. Fast GPU-based Monte Carlo code for SPECT/CT reconstructions generates improved (177)Lu images. Ejnmmi Phys. 2018, 5, 1. [Google Scholar] [CrossRef]
- Brunner, P.; Jorg, A.C.; Glatz, K.; Bubendorf, L.; Radojewski, P.; Umlauft, M.; Marincek, N.; Spanjol, P.M.; Krause, T.; Dumont, R.A.; et al. The prognostic and predictive value of sstr2-immunohistochemistry and sstr2-targeted imaging in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Korner, M.; Waser, B.; Schonbrunn, A.; Perren, A.; Reubi, J.C. Somatostatin receptor subtype 2A immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting. Am. J. Surg. Pathol. 2012, 36, 242–252. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Peter, L.; Lupp, A.; Schulz, S.; Sanger, J.; Baum, R.P.; Prasad, V.; Hommann, M. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 2012, 5, 187–194. [Google Scholar] [PubMed]
- Qian, Z.R.; Li, T.; Ter-Minassian, M.; Yang, J.; Chan, J.A.; Brais, L.K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.; Nishihara, R.; et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Brizzi, M.P.; Faggiano, A.; La Rosa, S.; Rapa, I.; Ferrero, A.; Mansueto, G.; Righi, L.; Garancini, S.; Capella, C.; et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: A proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2007, 20, 1172–1182. [Google Scholar] [CrossRef]
- Ezziddin, S.; Lohmar, J.; Yong-Hing, C.J.; Sabet, A.; Ahmadzadehfar, H.; Kukuk, G.; Biersack, H.J.; Guhlke, S.; Reichmann, K. Does the pretherapeutic tumor SUV in 68Ga DOTATOC PET predict the absorbed dose of 177Lu octreotate? Clin. Nucl. Med. 2012, 37, 141–147. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Peter, L.; Lupp, A.; Schulz, S.; Sanger, J.; Prasad, V.; Kulkarni, H.; Haugvik, S.P.; Hommann, M.; Baum, R.P. Molecular imaging with (6)(8)Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1659–1668. [Google Scholar] [CrossRef]
- Miederer, M.; Seidl, S.; Buck, A.; Scheidhauer, K.; Wester, H.J.; Schwaiger, M.; Perren, A. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 48–52. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, H.S.; Kim, W.H. Clinical significance of protein expression of cyclooxygenase-2 and somatostatin receptors in gastroenteropancreatic neuroendocrine tumors. Cancer Res. Treat. 2011, 43, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Tan, T.M.; Toussi, S.T.; Ramachandran, R.; Martin, N.; Meeran, K.; Ngo, N.; Dina, R.; Sharma, R. An expression signature of the angiogenic response in gastrointestinal neuroendocrine tumours: Correlation with tumour phenotype and survival outcomes. Br. J. Cancer 2014, 110, 115–122. [Google Scholar] [CrossRef][Green Version]
- Ezziddin, S.; Attassi, M.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Willinek, W.; Grunwald, F.; Guhlke, S.; Biersack, H.J.; Sabet, A. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J. Nucl. Med. 2014, 55, 183–190. [Google Scholar] [CrossRef]
- Strosberg, J.; Nasir, A.; Coppola, D.; Wick, M.; Kvols, L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum. Pathol. 2009, 40, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Kampf, C.; Olsson, I.; Ryberg, U.; Sjöstedt, E.; Pontén, F. Production of tissue microarrays, immunohistochemistry staining and digitalization within the human protein atlas. J. Vis. Exp. 2012, 63, 3620. [Google Scholar] [CrossRef] [PubMed]
| All Patients | High SSTR2 a | Low SSTR2 b | ||
|---|---|---|---|---|
| Patients (n) | 42 | 32 | 10 | |
| Age at PRRT treatment, mean (range) | 66 (45–78) | 68 (48–77) | 65 (45–78) | p = ns |
| Male/female (n) | 19/23 | 15/17 | 4/6 | p = ns |
| Number of PRRT treatments, mean (range) | 3.8 (2–6) | 3.8 (2–6) | 3.7 (2–6) | p = ns |
| Ki-67%, median (range) | 1.82 | 1.98 (0.25–15.2) | 1.47 (0.62–3.1) | p = 0.10 |
| Months between surgery and PRRT, median | 60 | 62 | 53 | p = 0.94 |
| All Patients | High SSTR2 a | Low SSTR2 b | |
|---|---|---|---|
| Number of patients | 42 | 32 | 10 |
| Additional PRRT and/or HAE and/or external radiation | 8 | 8 | 0 |
| No additional treatment | 34 | 24 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elf, A.-K.; Johanson, V.; Marin, I.; Bergström, A.; Nilsson, O.; Svensson, J.; Wängberg, B.; Bernhardt, P.; Elias, E. Evaluation of SSTR2 Expression in SI-NETs and Relation to Overall Survival after PRRT. Cancers 2021, 13, 2035. https://doi.org/10.3390/cancers13092035
Elf A-K, Johanson V, Marin I, Bergström A, Nilsson O, Svensson J, Wängberg B, Bernhardt P, Elias E. Evaluation of SSTR2 Expression in SI-NETs and Relation to Overall Survival after PRRT. Cancers. 2021; 13(9):2035. https://doi.org/10.3390/cancers13092035
Chicago/Turabian StyleElf, Anna-Karin, Viktor Johanson, Ida Marin, Anders Bergström, Ola Nilsson, Johanna Svensson, Bo Wängberg, Peter Bernhardt, and Erik Elias. 2021. "Evaluation of SSTR2 Expression in SI-NETs and Relation to Overall Survival after PRRT" Cancers 13, no. 9: 2035. https://doi.org/10.3390/cancers13092035
APA StyleElf, A.-K., Johanson, V., Marin, I., Bergström, A., Nilsson, O., Svensson, J., Wängberg, B., Bernhardt, P., & Elias, E. (2021). Evaluation of SSTR2 Expression in SI-NETs and Relation to Overall Survival after PRRT. Cancers, 13(9), 2035. https://doi.org/10.3390/cancers13092035






