Ductal Dilatation of ≥5 mm in Intraductal Papillary Mucinous Neoplasm Should Trigger the Consideration for Pancreatectomy: A Meta-Analysis and Systematic Review of Resected Cases
Abstract
Simple Summary
Abstract
1. Introduction
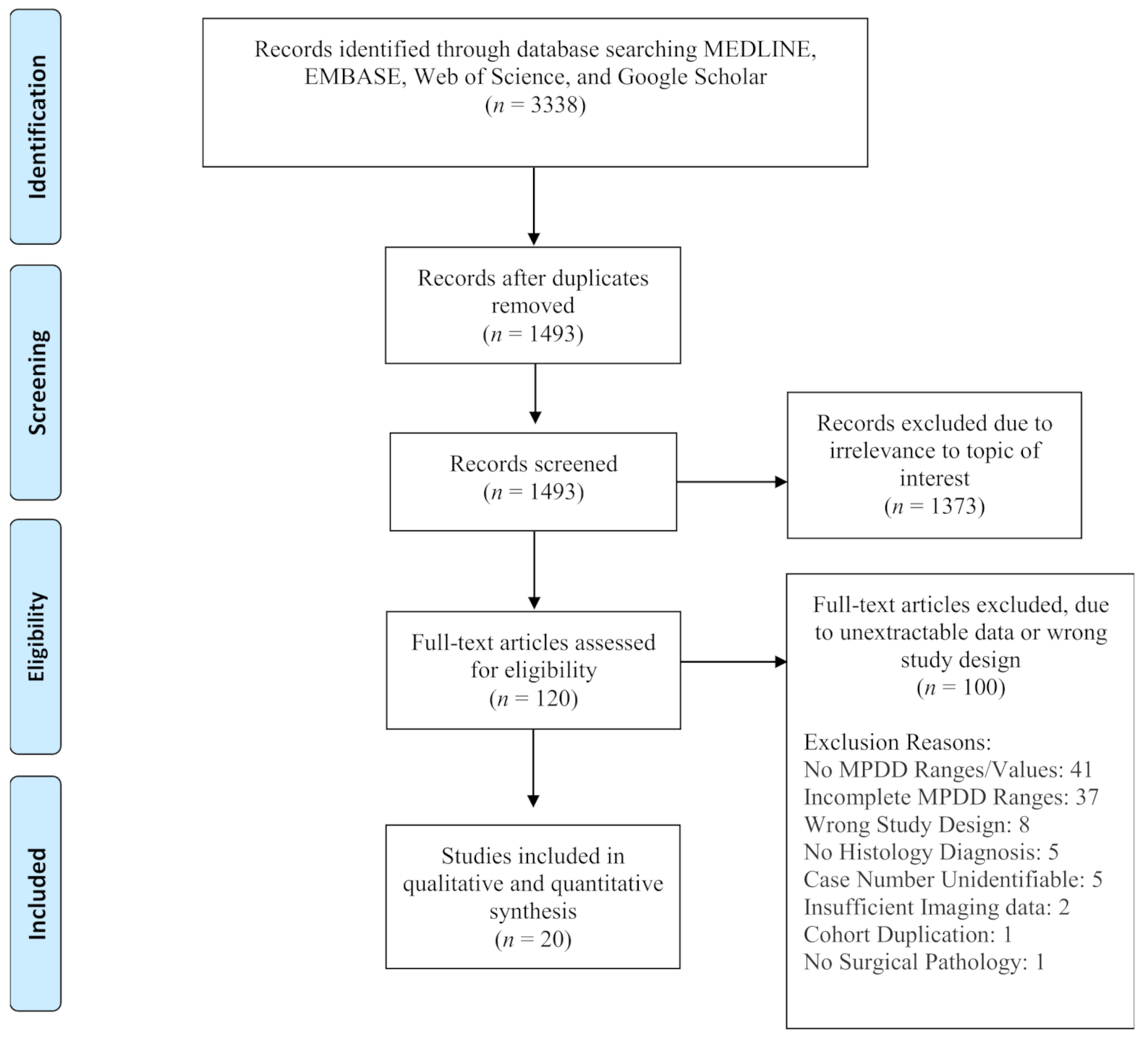
2. Materials and Methods
2.1. Article Search/Selection and Outcome Assessment
2.2. Data Extraction
2.3. Risk of Bias Analysis
2.4. Data Synthesis and Statistical Analysis
3. Results
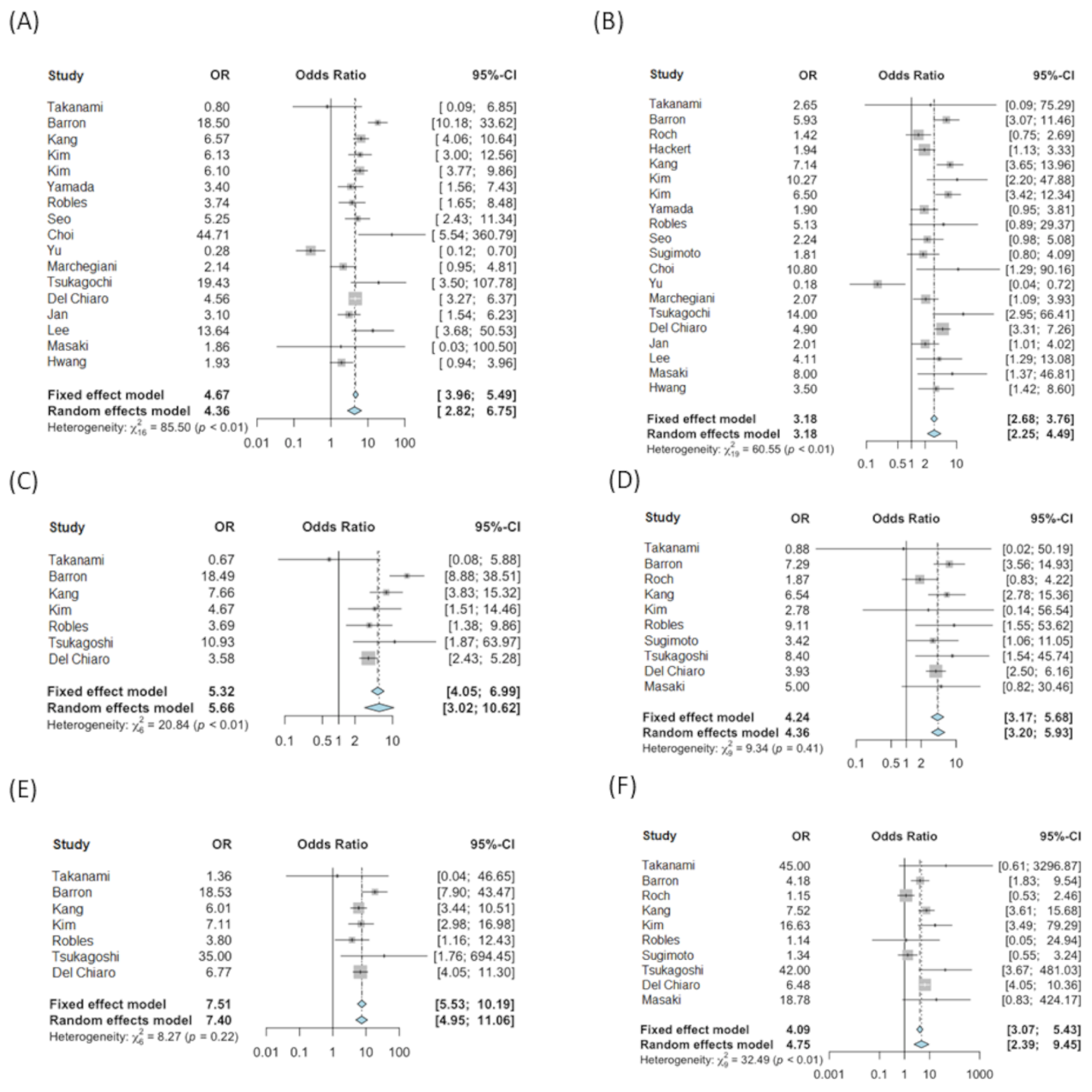
3.1. Tests of Association
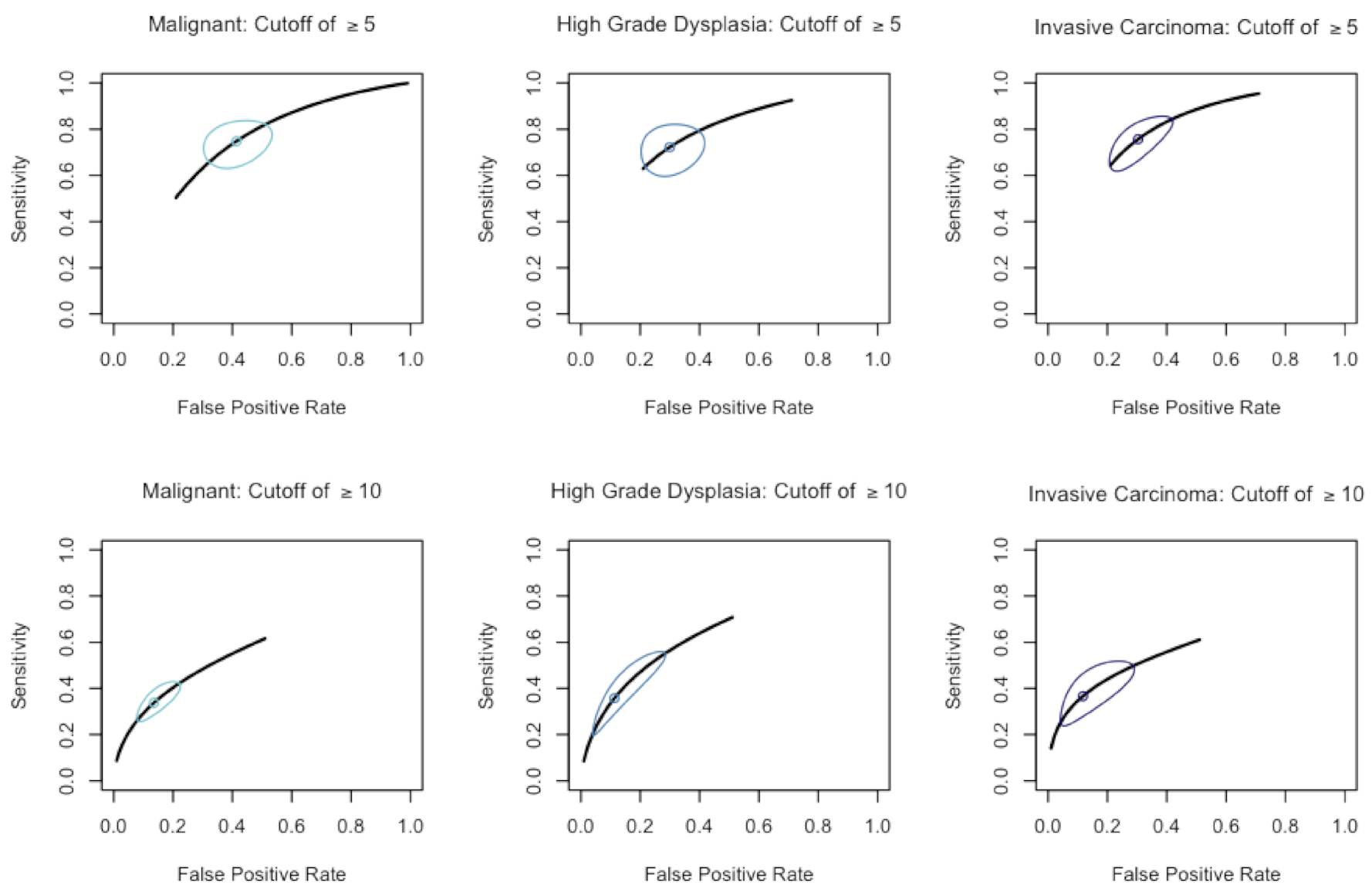
3.2. Diagnostic Tests
3.3. Subset Analysis Excluding Del Chiaro et al.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kromrey, M.-L.; Bülow, R.; Hübner, J.; Paperlein, C.; Lerch, M.M.; Ittermann, T.; Völzke, H.; Mayerle, J.; Kühn, J.-P. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018, 67, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Verbeke, C.; Salvia, R.; Klöppel, G.; Werner, J.; McKay, C.; Friess, H.; Manfredi, R.; Van Cutsem, E.; Löhr, M.; et al. European experts consensus statement on cystic tumours of the pancreas. Dig. Liver Dis. 2013, 45, 703–711. [Google Scholar] [CrossRef]
- Nilsson, L.N.; Keane, M.G.; Shamali, A.; Bocos, J.M.; van Zanten, M.M.; Antila, A.; Gil, C.V.; Del Chiaro, M.; Laukkarinen, J. Nature and management of pancreatic mucinous cystic neoplasm (MCN): A systematic review of the literature. Pancreatology 2016, 16, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.G.; Shamali, A.; Nilsson, L.N.; Antila, A.; Bocos, J.M.; Van Zanten, M.M.; Gil, C.V.; Maisonneuve, P.; Vaalavuo, Y.; Hoskins, T.; et al. Risk of malignancy in resected pancreatic mucinous cystic neoplasms. Br. J. Surg. 2018, 105, 439–446. [Google Scholar] [CrossRef]
- Jais, B.; Rebours, V.; Malleo, G.; Salvia, R.; Fontana, M.; Maggino, L.; Bassi, C.; Manfredi, R.; Moran, R.; Lennon, A.M.; et al. Serous cystic neoplasm of the pancreas: A multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2015, 65, 305–312. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Verbeke, C. Intraductal papillary mucinous neoplasms of the pancreas: Reporting clinically relevant features. Histopathology 2017, 70, 850–860. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Ateeb, Z.; Hansson, M.R.; Rangelova, E.; Segersvärd, R.; Kartalis, N.; Ansorge, C.; Löhr, M.J.; Arnelo, U.; Verbeke, C. Survival analysis and risk for progression of intraductal papillary mucinous neoplasia of the pancreas (IPMN) under surveillance: A single-institution experience. Ann. Surg. Oncol. 2017, 24, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.A.; Attiyeh, M.A.; Seier, K.; Gönen, M.; Schattner, M.; Haviland, D.L.; Balachandran, V.P.; Kingham, T.P.; D’Angelica, M.I.; DeMatteo, R.P.; et al. should patients with cystic lesions of the pancreas undergo long-term radiographic surveillance? Results of 3024 patients evaluated at a single institution. Ann. Surg. 2017, 266, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Pezzilli, R.; Bissolati, M.; Capurso, G.; Romano, L.; Brunori, M.P.; Calculli, L.; Tamburrino, D.; Piccioli, A.; Ruffo, G.; et al. Active surveillance beyond 5 years is required for presumed branch-duct intraductal papillary mucinous neoplasms undergoing non-operative management. Am. J. Gastroenterol. 2017, 112, 1153–1161. [Google Scholar] [CrossRef]
- Tanaka, M.; Castillo, C.F.-D.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Fritz, S.; Klauss, M.; Bergmann, F.; Hinz, U.; Strobel, O.; Schneider, L.; Büchler, M.W. Main-duct intraductal papillary mucinous neoplasm: High cancer risk in duct diameter of 5 to 9 mm. Ann. Surg. 2015, 262, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ateeb, Z.; Valente, R.; Pozzi-Mucelli, R.M.; Malgerud, L.; Schlieper, Y.; Rangelova, E.; Fernandez-Moro, C.; Löhr, J.M.; Arnelo, U.; Del Chiaro, M. Main pancreatic duct dilation greater than 6 mm is associated with an increased risk of high-grade dysplasia and cancer in IPMN patients. Langenbecks Arch. Chir. 2019, 404, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Elliott, I.A.; Nguyen, A.H.; Kim, S.; Muthusamy, V.R.; Watson, R.; Hines, O.J.; Dawson, D.W.; Reber, H.A.; Donahue, T.R. Assessment of a revised management strategy for patients with intraductal papillary mucinous neoplasms involving the main pancreatic duct. JAMA Surg. 2017, 152, e163349. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Beckman, R.; Ateeb, Z.; Orsini, N.; Rezaee, N.; Manos, L.; Valente, R.; Yuan, C.; Ding, D.; Margonis, G.A.; et al. Main duct dilatation is the best predictor of high-grade dysplasia or invasion in intraductal papillary mucinous neoplasms of the pancreas. Ann. Surg. 2019, 272, 1118–1124. [Google Scholar] [CrossRef]
- Crippa, S.; Bassi, C.; Salvia, R.; Malleo, G.; Marchegiani, G.; Rebours, V.; Levy, P.; Partelli, S.; Suleiman, S.L.; Banks, P.A.; et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: A mid-term follow-up analysis. Gut 2017, 66, 495–506. [Google Scholar] [CrossRef]
- Marchegiani, G.; Andrianello, S.; Morbin, G.; Secchettin, E.; D’Onofrio, M.; De Robertis, R.; Malleo, G.; Bassi, C.; Salvia, R. Importance of main pancreatic duct dilatation in IPMN undergoing surveillance. Br. J. Surg. 2018, 105, 1825–1834. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Segersvärd, R.; Mucelli, R.P.; Rangelova, E.; Kartalis, N.; Ansorge, C.; Arnelo, U.; Blomberg, J.; Löhr, M.; Verbeke, C. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann. Surg. Oncol. 2014, 21, 1539–1544. [Google Scholar] [CrossRef]
- Salvia, R.; Malleo, G.; Marchegiani, G.; Pennacchio, S.; Paiella, S.; Paini, M.; Pea, A.; Butturini, G.; Pederzoli, P.; Bassi, C. Pancreatic resections for cystic neoplasms: From the surgeon’s presumption to the pathologist’s reality. Surgery 2012, 152, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Correa-Gallego, C.; Ferrone, C.R.; Thayer, S.P.; Wargo, J.A.; Warshaw, A.L.; Castillo, C.F.-D. Incidental pancreatic cysts: Do we really know what we are watching? Pancreatology 2010, 10, 144–150. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Valente, R.; Wolfgang, C. Response to comment on “Main duct dilatation is the best predictor of high-grade dysplasia or invasion in intraductal papillary mucinous neoplasms of the pancreas”. Ann. Surg. 2019, 270, e109–e110. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Basturk, O.; Hong, S.M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Adsay, N.V.; Furukawa, T.; Hruban, R.H.; Klimstra, D.S.; Klöppel, G.; Offerhaus, G.J.A.; Pitman, M.B.; Shimizu, M.; Zamboni, G. Intraductal neoplasms of the pancreas. In WHO Classification of Tumours of the Digestive System; IARC: Lyon, France, 2010; pp. 304–313. [Google Scholar]
- Takanami, K.; Hiraide, T.; Tsuda, M.; Nakamura, Y.; Kaneta, T.; Takase, K.; Fukuda, H.; Takahashi, S. Additional value of FDG PET/CT to contrast-enhanced CT in the differentiation between benign and malignant intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Ann. Nucl. Med. 2011, 25, 501–510. [Google Scholar] [CrossRef]
- Barron, M.R.; Roch, A.M.; Waters, J.A.; Parikh, J.A.; DeWitt, J.M.; Al-Haddad, M.A.; Ceppa, E.P.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; et al. Does preoperative cross-sectional imaging accurately predict main duct involvement in intraductal papillary mucinous neoplasm? J. Gastrointest. Surg. 2014, 18, 447–456. [Google Scholar] [CrossRef]
- Roch, A.M.; DeWitt, J.M.; Al-Haddad, M.A.; Schmidt, I.I.C.M.; Ceppa, E.P.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Schmidt, C.M. Nonoperative management of main pancreatic duct-involved intraductal papillary mucinous neoplasm might be indicated in select patients. J. Am. Coll. Surg. 2014, 219, 122–129. [Google Scholar] [CrossRef]
- Kang, M.J.; Jang, J.Y.; Lee, S.; Park, T.; Lee, S.Y.; Kim, S.W. Clinicopathological meaning of size of main-duct dilatation in intraductal papillary mucinous neoplasm of pancreas: Proposal of a simplified morphological classification based on the investigation on the size of main pancreatic duct. World J. Surg. 2015, 39, 2006–2013. [Google Scholar] [CrossRef]
- Kim, Y.I.; Shin, S.H.; Song, K.B.; Hwang, D.W.; Lee, J.H.; Park, K.-M.; Lee, Y.-J.; Kim, S.C. Branch duct intraductal papillary mucinous neoplasm of the pancreas: Single-center experience with 324 patients who underwent surgical resection. Korean J. Hepato Biliary Pancreat. Surg. 2015, 19, 113–120. [Google Scholar] [CrossRef]
- Kim, J.R.; Jang, J.Y.; Kang, M.J.; Park, T.; Lee, S.Y.; Jung, W.; Chang, J.; Shin, Y.; Han, Y.; Kim, S.W. Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19-9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas. J. Hepatobiliary Pancreat. Sci. 2015, 22, 699–707. [Google Scholar] [CrossRef]
- Yamada, S.; Fujii, T.; Murotani, K.; Kanda, M.; Sugimoto, H.; Nakayama, G.; Koike, M.; Fujiwara, M.; Nakao, A.; Kodera, Y. Comparison of the international consensus guidelines for predicting malignancy in intraductal papillary mucinous neoplasms. Surgery 2016, 159, 878–884. [Google Scholar] [CrossRef]
- Robles, E.P.-C.; Maire, F.; Cros, J.; Vullierme, M.-P.; Rebours, V.; Sauvanet, A.; Aubert, A.; Dokmak, S.; Lévy, P.; Ruszniewski, P. Accuracy of 2012 International Consensus Guidelines for the prediction of malignancy of branch-duct intraductal papillary mucinous neoplasms of the pancreas. United Eur. Gastroenterol. J. 2016, 4, 580–586. [Google Scholar] [CrossRef]
- Seo, N.; Byun, J.H.; Kim, J.H.; Kim, H.J.; Lee, S.S.; Song, K.B.; Kim, S.C.; Han, D.J.; Hong, S.M.; Lee, M.G. Validation of the 2012 International Consensus Guidelines using computed tomography and magnetic resonance imaging: Branch duct and main duct intraductal papillary mucinous neoplasms of the pancreas. Ann. Surg. 2016, 263, 557–564. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Kim, J.H.; Yu, M.H.; Eun, H.W.; Lee, H.K.; Han, J.K. Diagnostic performance and imaging features for predicting the malignant potential of intraductal papillary mucinous neoplasm of the pancreas: A comparison of EUS, contrast-enhanced CT and MRI. Abdom. Radiol. 2017, 42, 1449–1458. [Google Scholar] [CrossRef]
- Yu, S.; Takasu, N.; Watanabe, T.; Fukumoto, T.; Okazaki, S.; Tezuka, K.; Sugawara, S.; Hirai, I.; Kimura, W. Validation of the 2012 Fukuoka Consensus Guideline for intraductal papillary mucinous neoplasm of the pancreas from a single institution experience. Pancreas 2017, 46, 936–942. [Google Scholar] [CrossRef]
- Tsukagoshi, M.; Araki, K.; Saito, F.; Kubo, N.; Watanabe, A.; Igarashi, T.; Ishii, N.; Yamanaka, T.; Shirabe, K.; Kuwano, H. Evaluation of the international consensus guidelines for the surgical resection of intraductal papillary mucinous neoplasms. Dig. Dis. Sci. 2017, 63, 860–867. [Google Scholar] [CrossRef]
- Jan, I.-S.; Chang, M.-C.; Yang, C.-Y.; Tien, Y.-W.; Jeng, Y.-M.; Wu, C.-H.; Chen, B.-B.; Chang, Y.-T. Validation of indications for surgery of european evidence-based guidelines for patients with pancreatic intraductal papillary mucinous neoplasms. J. Gastrointest. Surg. 2020, 24, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Choi, S.-Y.; Min, J.H.; Yi, B.H.; Lee, M.H.; Kim, S.S.; Hwang, J.A.; Kim, J.H. Determining malignant potential of intraductal papillary mucinous neoplasm of the pancreas: CT versus MRI by using revised 2017 international consensus guidelines. Radiology 2019, 293, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Masaki, Y.; Koshita, S.; Noda, Y.; Kanno, Y.; Ogawa, T.; Masu, K.; Sawai, T.; Ito, K. Should we regard all main duct type intraductal papillary mucinous neoplasms of the pancreas (MD-IPMN) as an indication of surgery? A retrospective study in 29 patients with MD-IPMN showing mural nodules. Pancreatology 2019, 19, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.A.; Choi, S.-Y.; Lee, J.E.; Kim, S.S.; Lee, S.; Moon, J.Y.; Heo, N.H. Pre-operative nomogram predicting malignant potential in the patients with intraductal papillary mucinous neoplasm of the pancreas: Focused on imaging features based on revised international guideline. Eur. Radiol. 2020, 30, 3711–3722. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid.-Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Yasuda, K.; Cho, E.; Uno, K.; Tanaka, K.; Nakajima, M. Differential diagnosis of intraductal papillary-mucinous tumor of the pancreas by endoscopic ultrasonography and intraductal ultrasonography. Dig. Endosc. 2004, 16, 101–106. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Baba, T.; Ishihara, T.; Kobayashi, A.; Nakamura, K.; Tadenuma, H.; Ito, H.; Miyazaki, M.; Saisho, H. Long-term follow-up of intraductal papillary mucinous neoplasm of the pancreas with ultrasonography. Clin. Gastroenterol. Hepatol. 2005, 3, 1136–1143. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Segersvard, R.; Lohr, M.; Verbeke, C. Early detection and prevention of pancreatic cancer: Is it really possible today? World J. Gastroenterol. 2014, 20, 12118–12131. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, R.A.; Pessia, A.; Ateeb, Z.; Davanian, H.; Moro, C.F.; Alkharaan, H.; Healy, K.; Ghazi, S.; Arnelo, U.; Valente, R.; et al. Integrated targeted metabolomic and lipidomic analysis: A novel approach to classifying early cystic precursors to invasive pancreatic cancer. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Gaiser, R.A.; Halimi, A.; Alkharaan, H.; Lu, L.; Davanian, H.; Healy, K.; Hugerth, L.W.; Ateeb, Z.; Valente, R.; Moro, C.F.; et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 2019, 68, 2186–2194. [Google Scholar] [CrossRef]
- Finks, J.F.; Osborne, N.H.; Birkmeyer, J.D. Trends in hospital volume and operative mortality for high-risk surgery. N. Engl. J. Med. 2011, 364, 2128–2137. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, P.; Muller, X.; Malleo, G.; Park, J.; Hwang, H.; Napoli, N.; Javed, A.; Inoue, Y.; Beghdadi, N.; Kalisvaart, M.; et al. Benchmarks in pancreatic surgery. A novel tool for unbiased outcome comparisons. Ann Surg. 2019, 270, 211–218. [Google Scholar] [CrossRef]
- Woo, S.M.; Ryu, J.K.; Lee, S.H.; Yoo, J.W.; Park, J.K.; Kim, Y.T.; Yoon, Y.B. Survival and prognosis of invasive intraductal papillary mucinous neo-plasms of the pancreas: Comparison with pancreatic ductal adenocarcinoma. Pancreas 2008, 36, 50–55. [Google Scholar] [CrossRef]
- Poultsides, G.A.; Reddy, S.; Cameron, J.L.; Hruban, R.H.; Pawlik, T.M.; Ahuja, N.; Jain, A.; Edil, B.H.; Iacobuzio-Donahue, C.A.; Schulick, R.D.; et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann. Surg. 2010, 251, 470–476. [Google Scholar] [CrossRef]
- Chari, S.T.; Yadav, D.; Smyrk, T.C.; DiMagno, E.P.; Miller, L.J.; Raimondo, M.; Clain, J.E.; Norton, I.A.; Pearson, R.K.; Petersen, B.T.; et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 2002, 123, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.T.; Lewis, R.S.; Drebin, J.A.; Teitelbaum, U.R.; Lee, M.K.; Roses, R.E.; Fraker, D.L.; Vollmer, C.M. The efficacy of adjuvant therapy for pancreatic invasive intraductal papillary mucinous neoplasm (IPMN). Cancer 2015, 122, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Abdeljawad, K.; Vemulapalli, K.C.; Schmidt, C.M.; Dewitt, J.; Sherman, S.; Imperiale, T.F.; Al-Haddad, M. Prevalence of malignancy in patients with pure main duct intraductal papillary mucinous neoplasms. Gastrointest. Endosc. 2014, 79, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Itoh, S.; Ikeda, M.; Suzuki, K.; Naganawa, S. Intraductal papillary mucinous neoplasm of the pancreas: Assessment of the likelihood of invasiveness with multisection CT. Radiology 2008, 248, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Han, D.J.; Park, K.T.; Kim, Y.H.; Park, J.B.; Kim, S.C. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World J. Surg. 2010, 34, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Jun, D.Y.; Kwon, H.J.; Kim, S.G.; Kim, S.H.; Chun, J.M.; Kwon, Y.B.; Yoon, K.J.; Hwang, Y.J.; Yun, Y.K. Predictive factors for invasive intraductal papillary mucinous neo-plasm of the pancreas. Korean J. Hepatobiliary Pancreat. Surg. 2011, 15, 237–242. [Google Scholar] [CrossRef][Green Version]
- Kawakubo, K.; Tada, M.; Isayama, H.; Sasahira, N.; Nakai, Y.; Takahara, N.; Uchino, R.; Hamada, T.; Miyabayashi, K.; Yamamoto, K.; et al. Disease-specific mortality among patients with intra-ductal papillary mucinous neoplasm of the pancreas. Clin. Gastroenterol. Hepatol. 2014, 12, 486–491. [Google Scholar] [CrossRef]
- Brugge, W.R.; Lewandrowski, K.; Lee-Lewandrowski, E.; Centeno, B.A.; Szydlo, T.; Regan, S.; del Castillo, C.F.; Warshaw, A.L. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology 2004, 126, 1330–1336. [Google Scholar] [CrossRef]
- Arnelo, U.; Siiki, A.; Swahn, F.; Segersvärd, R.; Enochsson, L.; del Chiaro, M.; Lundell, L.; Verbeke, C.S.; Löhr, J.M. Single-operator pancreatoscopy is helpful in the evaluation of suspected intraductal papillary mucinous neoplasms (IPMN). Pancreatology 2014, 14, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Tyberg, A.; Raijman, I.; Siddiqui, A.; Arnelo, U.; Adler, D.G.; Xu, M.M.; Nassani, N.; Sejpal, D.V.; Kedia, P.; Nah Lee, Y.; et al. Digital pancreaticocholangioscopy for mapping of pancreaticobiliary neoplasia: Can we alter the surgical resection margin? J. Clin. Gastroenterol. 2019, 53, 71–75. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Country | Design | <5 mm (n) | 5–9 mm (n) | ≥10 mm (n) | |||
|---|---|---|---|---|---|---|---|---|---|
| M | NM | M | NM | M | NM | ||||
| Takanami et al. [25] | 2011 | Japan | Retrospective | 3 | 2 | 5 | 5 | 1 | 0 |
| Barron et al. [26] | 2014 | U.S.A. | Retrospective | 17 | 149 | 74 | 40 | 40 | 14 |
| Roch et al. [27] | 2014 | U.S.A. | Retrospective | - | - | 50 | 64 | 30 | 27 |
| Hackert et al. [12] | 2015 | Germany | Retrospective | - | - | 93 | 64 | 76 | 27 |
| Kang et al. [28] | 2015 | S. Korea | Retrospective | 44 | 206 | 39 | 38 | 34 | 14 |
| Kim et al. [29] | 2015 | S. Korea | Retrospective | 15 | 212 | 19 | 50 | 4 | 3 |
| Kim et al. [30] | 2015 | S. Korea | Retrospective | 43 | 195 | 38 | 39 | 36 | 16 |
| Yamada et al. [31] | 2015 | Japan | Retrospective | 10 | 42 | 29 | 39 | 22 | 24 |
| Robles et al. [32] | 2016 | France | Retrospective | 13 | 57 | 19 | 25 | 4 | 2 |
| Seo et al. [33] | 2016 | S. Korea | Retrospective | 11 | 62 | 27 | 29 | 14 | 15 |
| Sugimoto et al. [14] | 2016 | U.S.A. | Retrospective | - | - | 22 | 19 | 42 | 20 |
| Choi et al. [34] | 2017 | S. Korea | Retrospective | 1 | 20 | 29 | 16 | 9 | 1 |
| Yu et al. [35] | 2017 | Japan | Retrospective | 39 | 13 | 14 | 12 | 3 | 8 |
| Marchegiani et al. [17] | 2018 | Italy | Retrospective | 8 | 43 | 43 | 126 | 20 | 32 |
| Tsukagoshi et al. [36] | 2018 | Japan | Retrospective | 2 | 17 | 4 | 4 | 12 | 3 |
| Del Chiaro et al. [15] | 2019 | U.S.A./Sweden | Retrospective | 65 | 240 | 134 | 152 | 107 | 43 |
| Jan et al. [37] | 2019 | Taiwan | Retrospective | 17 | 65 | 11 | 11 | 23 | 31 |
| Lee et al. [38] | 2019 | S. Korea | Retrospective | 3 | 36 | 16 | 16 | 9 | 6 |
| Masaki et al. [39] | 2019 | Japan | Retrospective | 0 | 0 | 3 | 6 | 16 | 4 |
| Hwang et al. [40] | 2020 | S. Korea | Retrospective | 25 | 45 | 11 | 18 | 18 | 9 |
| Total (% *) | 316 (18.4%) | 1404 (81.6%) | 680 (46.8%) | 773 (53.2%) | 520 (63.5%) | 289 (36.5%) | |||
| Author | Year | Design | <5 mm | 5–9 mm | ≥10 mm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HGD | IC | NM | HGD | IC | NM | HGD | IC | NM | |||
| Takanami et al. [25] | 2011 | Retrospective | 3 | 0 | 2 | 5 | 0 | 5 | 0 | 1 | 0 |
| Barron et al. [26] | 2014 | Retrospective | 10 | 7 | 149 | 40 | 34 | 40 | 27 | 13 | 14 |
| Roch et al. [27] | 2014 | Retrospective | - | - | - | 19 | 31 | 64 | 15 | 15 | 27 |
| Kang et al. [28] | 2015 | Retrospective | 15 | 29 | 206 | 17 | 22 | 38 | 12 | 22 | 14 |
| Kim et al. [29] | 2015 | Retrospective | 6 | 9 | 212 | 7 | 12 | 50 | 0 | 4 | 3 |
| Robles et al. [32] | 2016 | Retrospective | 8 | 5 | 57 | 10 | 9 | 25 | 4 | 0 | 2 |
| Sugimoto et al. [14] | 2016 | Retrospective | - | - | - | 5 | 17 | 19 | 18 | 24 | 20 |
| Tsukagoshi et al. [36] | 2018 | Retrospective | 2 | 0 | 17 | 3 | 1 | 4 | 6 | 6 | 3 |
| Del Chiaro et al. [15] | 2019 | Retrospective | 45 | 20 | 240 | 78 | 56 | 152 | 53 | 54 | 43 |
| Masaki et al. [39] | 2019 | Retrospective | 0 | 0 | 0 | 3 | 0 | 6 | 10 | 6 | 4 |
| Total (% *) | 89 (8.5%) | 70 (6.7%) | 883 (84.7%) | 187 (24.2%) | 182 (23.6%) | 403 (52.2%) | 145 (34.5%) | 145 (34.5%) | 130 (31.0%) | ||
| Comparisons | Dilation | Sensitivity | 95% CI | Specificity | 95% CI | AUC | Studies Included |
|---|---|---|---|---|---|---|---|
| Malignancy to NM | ≥5 mm | 74.8% | (64.6–82.2%) | 58.6% | (49.0–67.6%) | 0.716 | 17 |
| ≥10 mm | 33.8% | (27.2–41.0%) | 86.4% | (79.6–91.2%) | 0.586 | 20 | |
| High-Grade Dysplasia to NM | ≥5 mm | 72.2% | (62.2–80.3%) | 70.1% | (60.7–78.0%) | 0.769 | 7 |
| ≥10 mm | 35.7% | (22.3–51.9%) | 88.7% | (75.8–95.1%) | 0.657 | 10 | |
| Invasive Carcinoma to NM | ≥5 mm | 75.6% | (64.8–83.9%) | 69.7% | (60.4–77.6%) | 0.786 | 7 |
| ≥10 mm | 36.6% | (26.0–48.7%) | 88.2% | (75.3–94.9%) | 0.587 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.H.A.; Oba, A.; Beaty, L.; Colborn, K.L.; Rodriguez Franco, S.; Harnke, B.; Meguid, C.; Negrini, D.; Valente, R.; Ahrendt, S.; et al. Ductal Dilatation of ≥5 mm in Intraductal Papillary Mucinous Neoplasm Should Trigger the Consideration for Pancreatectomy: A Meta-Analysis and Systematic Review of Resected Cases. Cancers 2021, 13, 2031. https://doi.org/10.3390/cancers13092031
Wu YHA, Oba A, Beaty L, Colborn KL, Rodriguez Franco S, Harnke B, Meguid C, Negrini D, Valente R, Ahrendt S, et al. Ductal Dilatation of ≥5 mm in Intraductal Papillary Mucinous Neoplasm Should Trigger the Consideration for Pancreatectomy: A Meta-Analysis and Systematic Review of Resected Cases. Cancers. 2021; 13(9):2031. https://doi.org/10.3390/cancers13092031
Chicago/Turabian StyleWu, Y.H. Andrew, Atsushi Oba, Laurel Beaty, Kathryn L. Colborn, Salvador Rodriguez Franco, Ben Harnke, Cheryl Meguid, Daniel Negrini, Roberto Valente, Steven Ahrendt, and et al. 2021. "Ductal Dilatation of ≥5 mm in Intraductal Papillary Mucinous Neoplasm Should Trigger the Consideration for Pancreatectomy: A Meta-Analysis and Systematic Review of Resected Cases" Cancers 13, no. 9: 2031. https://doi.org/10.3390/cancers13092031
APA StyleWu, Y. H. A., Oba, A., Beaty, L., Colborn, K. L., Rodriguez Franco, S., Harnke, B., Meguid, C., Negrini, D., Valente, R., Ahrendt, S., Schulick, R. D., & Del Chiaro, M. (2021). Ductal Dilatation of ≥5 mm in Intraductal Papillary Mucinous Neoplasm Should Trigger the Consideration for Pancreatectomy: A Meta-Analysis and Systematic Review of Resected Cases. Cancers, 13(9), 2031. https://doi.org/10.3390/cancers13092031






