Adjuvant Systemic Therapy after Chemoradiation and Brachytherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Article Characteristics
2.2. Study Population Characteristics
2.3. Treatment Characteristics
2.4. Outcomes Measures
2.5. Literature Searches
2.6. Selection of Studies
2.7. Risk of Bias Assessment
2.8. Data Extraction
2.9. Statistical Methods
3. Results
3.1. Systematic Searches
3.2. Characteristics Included Studies
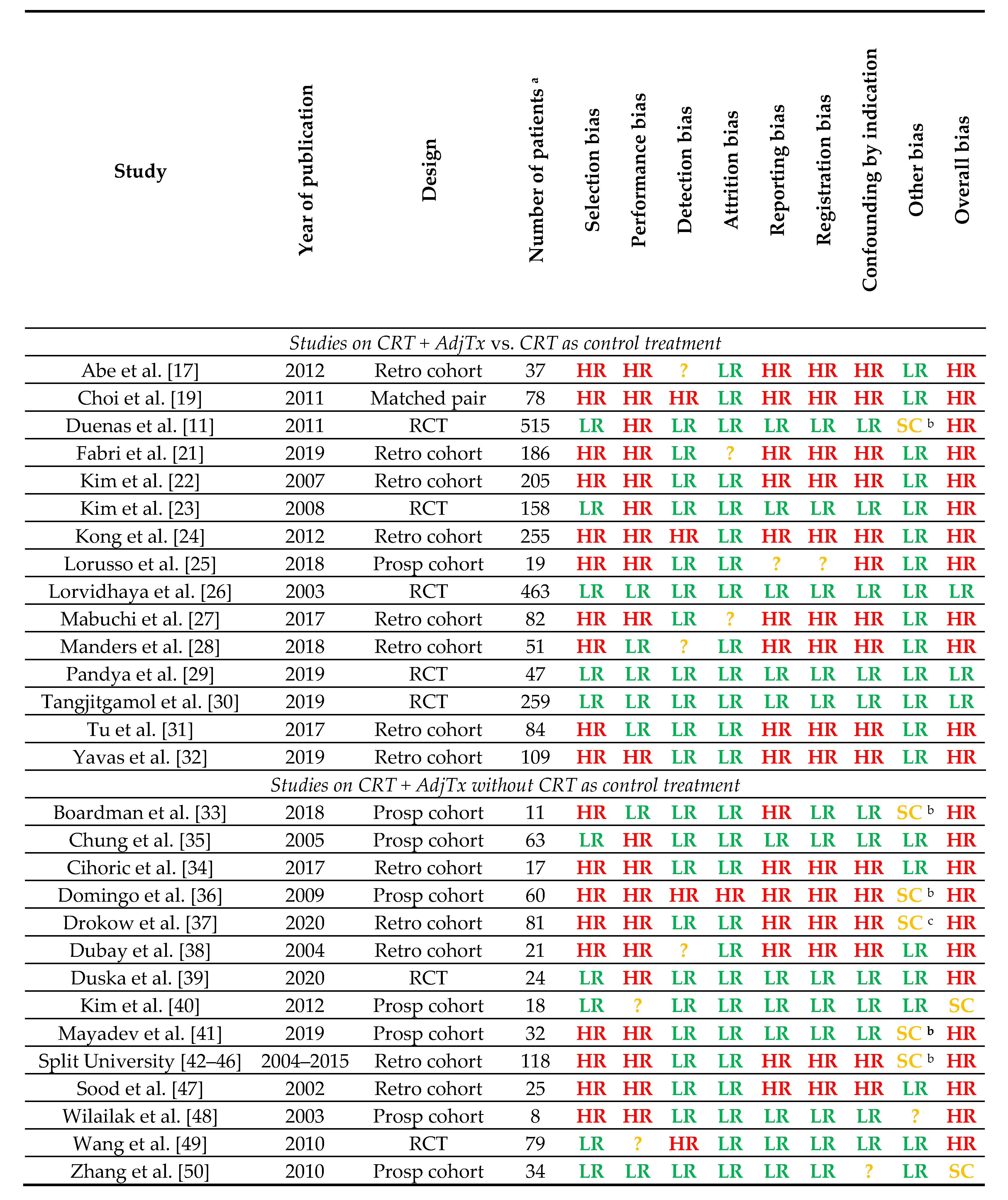
3.3. Risk of Bias Assessment
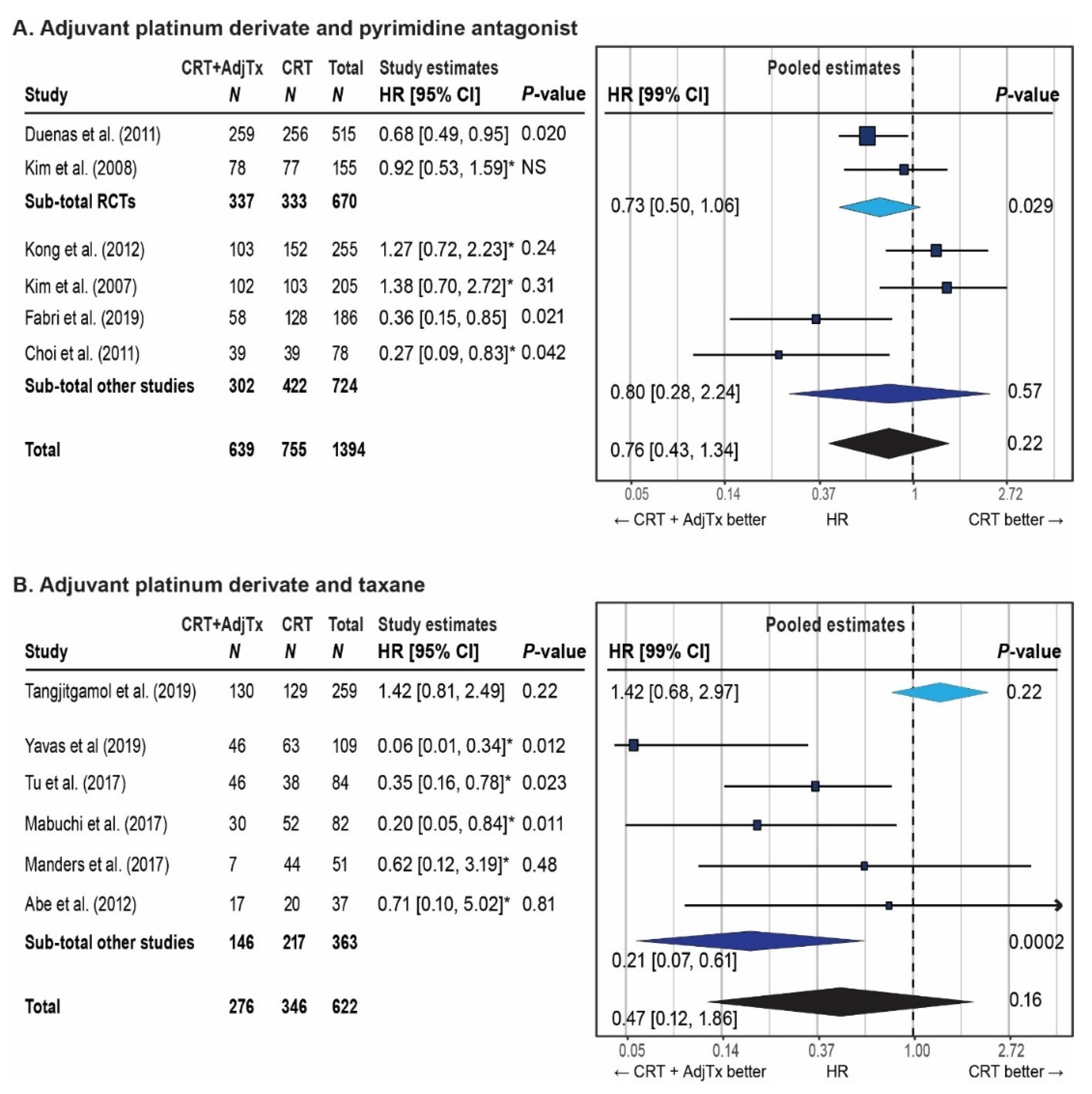
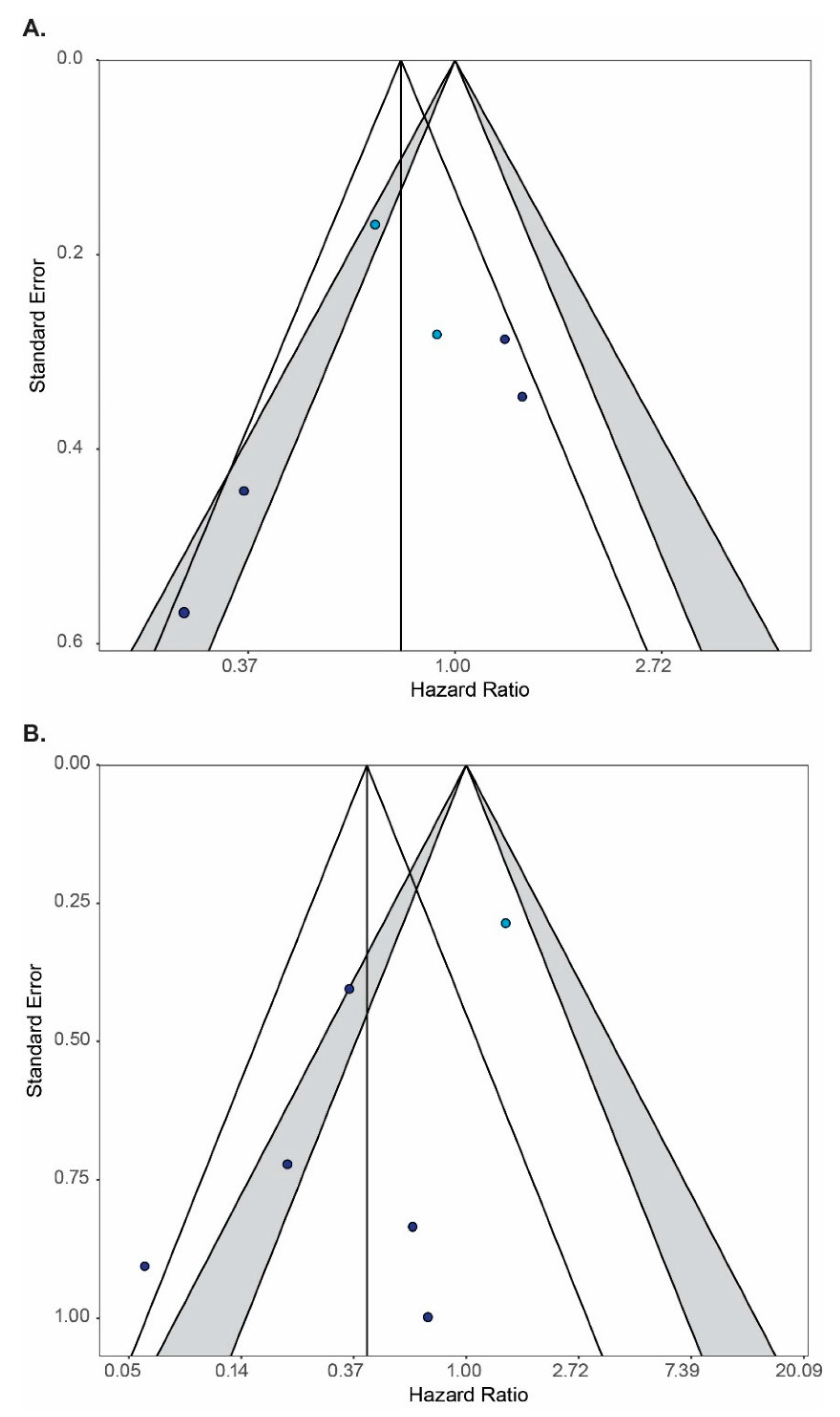
3.4. Meta-Analysis
3.5. Systematic Review of Survival Outcomes
3.6. Systematic Review of Feasibility and Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay:, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Obser-vatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2018. Available online: https://gco.iarc.fr/today (accessed on 4 September 2020).
- Trimble, E.L.; Gius, D.; Harlan, L.C. Impact of NCI Clinical Announcement upon use of chemoradiation for women with cervical cancer. J. Clin. Oncol. 2007, 25, 5537. [Google Scholar] [CrossRef]
- Vale, C.; Jakobsen, A. Reducing Uncertainties About the Effects of Chemoradiotherapy for Cervical Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data From 18 Randomized Trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar] [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Mahantshetty, U.; Krishnatry, R.; Hande, V.; Jamema, S.; Ghadi, Y.; Engineer, R.; Chopra, S.; Gurram, L.; Deshpande, D.; Shrviastava, S. Magnetic Resonance Image Guided Adaptive Brachytherapy in Locally Advanced Cervical Cancer: An Experience From a Tertiary Cancer Center in a Low and Middle Income Countries Setting. Int. J. Radiat. Oncol. 2017, 99, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Dimopoulos, J.; Georg, P.; Lang, S.; Waldhäusl, C.; Wachter-Gerstner, N.; Weitmann, H.; Reinthaller, A.; Knocke, T.H.; Wachter, S.; et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother. Oncol. 2007, 83, 148–155. [Google Scholar] [CrossRef]
- Lindegaard, J.C.; Fokdal, L.U.; Nielsen, S.K.; Juul-Christensen, J.; Tanderup, K. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol. 2013, 52, 1510–1519. [Google Scholar] [CrossRef]
- Rijkmans, E.; Nout, R.; Rutten, I.; Ketelaars, M.; Neelis, K.; Laman, M.; Coen, V.; Gaarenstroom, K.; Kroep, J.; Creutzberg, C. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol. Oncol. 2014, 135, 231–238. [Google Scholar] [CrossRef]
- Tan, L.-T.; Pötter, R.; Sturdza, A.; Fokdal, L.; Haie-Meder, C.; Schmid, M.; Gregory, D.; Petric, P.; Jürgenliemk-Schulz, I.; Gillham, C.; et al. Change in Patterns of Failure After Image-Guided Brachytherapy for Cervical Cancer: Analysis From the RetroEMBRACE Study. Int. J. Radiat. Oncol. 2019, 104, 895–902. [Google Scholar] [CrossRef]
- Horeweg, N.; Creutzberg, C.L.; Rijkmans, E.C.; Laman, M.S.; A Velema, L.; A Coen, V.L.M.; Stam, T.C.; Kerkhof, E.M.; Kroep, J.R.; De Kroon, C.D.; et al. Efficacy and toxicity of chemoradiation with image-guided adaptive brachytherapy for locally advanced cervical cancer. Int. J. Gynecol. Cancer 2019, 29, 257–265. [Google Scholar] [CrossRef]
- Dueñas-González, A.; Zarbá, J.J.; Patel, F.; Alcedo, J.C.; Beslija, S.; Casanova, L.; Pattaranutaporn, P.; Hameed, S.; Blair, J.M.; Barraclough, H.; et al. Phase III, Open-Label, Randomized Study Comparing Concurrent Gemcitabine Plus Cisplatin and Radiation Followed by Adjuvant Gemcitabine and Cisplatin Versus Concurrent Cisplatin and Radiation in Patients with Stage IIB to IVA Carcinoma of the Cervix. J. Clin. Oncol. 2011, 29, 1678–1685. [Google Scholar] [CrossRef]
- Tangjitgamol, S.; Katanyoo, K.; Laopaiboon, M.; Lumbiganon, P.; Manusirivithaya, S.; Supawattanabodee, B. Adjuvant chemotherapy after concurrent chemoradiation for locally advanced cervical cancer. Cochrane Database Syst. Rev. 2014, 2014, 010401. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; A Stewart, L.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. For the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Furumoto, H.; Nishimura, M.; Irahara, M.; Ikushima, H. Adjuvant chemotherapy following concurrent chemoradiotherapy for uterine cervical cancer with lymphadenopathy. Oncol. Lett. 2011, 3, 571–576. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, J.-W.; Kim, T.-J.; Kim, W.Y.; Nam, H.R.; Kim, B.-G.; Huh, S.J.; Lee, J.-H.; Bae, D.-S. Phase II Study of Consolidation Chemotherapy After Concurrent Chemoradiation in Cervical Cancer: Preliminary Results. Int. J. Radiat. Oncol. 2007, 68, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Lee, Y.-Y.; Kim, M.K.; Kim, T.-J.; Lee, J.-W.; Nam, H.R.; Huh, S.J.; Lee, J.-H.; Bae, D.-S.; Kim, B.-G. A Matched-Case Comparison to Explore the Role of Consolidation Chemotherapy After Concurrent Chemoradiation in Cervical Cancer. Int. J. Radiat. Oncol. 2011, 81, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-González, A.; Orlando, M.; Zhou, Y.; Quinlivan, M.; Barraclough, H. Efficacy in high burden locally advanced cervical cancer with concurrent gemcitabine and cisplatin chemoradiotherapy plus adjuvant gemcitabine and cisplatin: Prognostic and predictive factors and the impact of disease stage on outcomes from a prospective randomized phase III trial. Gynecol. Oncol. 2012, 126, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Fabri, V.A.; Queiroz, A.C.M.; Mantoan, H.; Sanches, S.M.; Guimarães, A.P.G.; Ribeiro, A.R.G.; Souza, R.P.; Maya, J.M.L.; Santos, E.S.; Castro, F.S.; et al. The Impact of Addition of Consolidation Chemotherapy to Standard Cisplatin-Based Chemoradiotherapy in Uterine Cervical Cancer: Matter of Distant Relapse. J. Oncol. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Kim, Y.B.; Cho, J.H.; Keum, K.C.; Lee, C.G.; Seong, J.; Suh, C.O.; Kim, G.E. Concurrent chemoradiotherapy followed by adjuvant chemotherapy in uterine cervical cancer patients with high-risk factors. Gynecol. Oncol. 2007, 104, 58–63. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, S.S.; Nam, J.-H.; Kim, Y.-M.; Kim, J.H.; Choi, E.K. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high-dose rate brachytherapy for locally advanced cervical cancer. Gynecol. Oncol. 2008, 108, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.W.; Chang, S.-J.; Paek, J.; Yoo, S.-C.; Yoon, J.-H.; Chang, K.-H.; Chun, M.; Ryu, H.-S. Comparison of concurrent chemoradiation therapy with weekly cisplatin versus monthly fluorouracil plus cisplatin in FIGO stage IIB-IVA cervical cancer. J. Gynecol. Oncol. 2012, 23, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Martinelli, F.; Maltese, G.; Fontanella, C.; Sabatucci, I.; Ditto, A.; Signorelli, M.; Bogani, G.; Lepori, S.; Tripodi, E.; et al. Locally Advanced Cervical Cancer: Is a Trimodality Treatment a Safe and Effective Approach? Oncology 2018, 95, 239–245. [Google Scholar] [CrossRef]
- Lorvidhaya, V.; Chitapanarux, I.; Sangruchi, S.; Lertsanguansinchai, P.; Kongthanarat, Y.; Tangkaratt, S.; Visetsiri, E. Concurrent mitomycin C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix: A randomized trial. Int. J. Radiat. Oncol. 2003, 55, 1226–1232. [Google Scholar] [CrossRef]
- Mabuchi, S.; Isohashi, F.; Okazawa, M.; Kitada, F.; Maruoka, S.; Ogawa, K.; Kimura, T. Chemoradiotherapy followed by consolidation chemotherapy involving paclitaxel and carboplatin and in FIGO stage IIIB/IVA cervical cancer patients. J. Gynecol. Oncol. 2017, 28, e15. [Google Scholar] [CrossRef]
- Manders, D.B.; Sims, T.T.; Bailey, A.; Hwang, L.; Richardson, D.L.; Miller, D.S.; Kehoe, S.M.; Albuquerque, K.V.; Lea, J.S. The Significance of Para-Aortic Nodal Size and the Role of Adjuvant Systemic Chemotherapy in Cervical Cancer. Am. J. Clin. Oncol. 2018, 41, 1225–1230. [Google Scholar] [CrossRef]
- Sunita, B.; Pandya, T.; Suhag, V.; Ranjan, S.; Pandya, S. Toxicity profile of double-agent adjuvant chemotherapy after concurrent chemoradiation and brachytherapy in locally advanced cervical cancer: Comparison with standard chemoradiation protocol. Indian J. Med. Paediatr. Oncol. 2019, 40, 6. [Google Scholar] [CrossRef]
- Tangjitgamol, S.; Tharavichitkul, E.; Tovanabutra, C.; Rongsriyam, K.; Asakij, T.; Paengchit, K.; Sukhaboon, J.; Penpattanagul, S.; Kridakara, A.; Hanprasertpong, J.; et al. A randomized controlled trial comparing concurrent chemoradiation versus concurrent chemoradiation followed by adjuvant chemotherapy in locally advanced cervical cancer patients: ACTLACC trial. J. Gynecol. Oncol. 2019, 30, e82. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.J.; Chen, C.; Cheng, X.X.; Li, L.Y. Comparison of the curative effect and safety of consolidation chemotherapy after concurrent chemoradiotherapy with concurrent chemoradiotherapy alone for locally advanced cervical cancer. Eur. J. Gynaecol. Oncol. 2018, 39, 558–563. [Google Scholar] [CrossRef]
- Yavas, G.; Yavas, C.; Sen, E.; Oner, I.; Celik, C.; Ata, O. Adjuvant carboplatin and paclitaxel after concurrent cisplatin and radiotherapy in patients with locally advanced cervical cancer. Int. J. Gynecol. Cancer 2019, 29, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Boardman, C.H.; Brady, W.E.; Dizon, D.S.; Kunos, C.A.; Moore, K.N.; Zanotti, K.M.; Matthews, C.; Cosin, J.A.; Aghajanian, C.; Fracasso, P.M. A Phase I Evaluation of Extended Field Radiation Therapy with Concomitant Cisplatin Chemotherapy Followed by Paclitaxel and Carboplatin Chemotherapy in Women With Cervical Carcinoma Metastatic to the Para-aortic Lymph Nodes: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2018, 151, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Cihoric, N.; Tsikkinis, A.; Badra, E.V.; Glatzer, M.; Novak, U.; Scherz, A.; Shelan, M.; Soldatovic, I.; Yojena, C.K.K.; Aebersold, D.M.; et al. Highly conformal combined radiotherapy with cisplatin and gemcitabine for treatment of loco-regionally advanced cervical cancer—A retrospective study. Radiat. Oncol. 2017, 12, 202. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Jian, J.J.-M.; Cheng, S.H.; Hsieh, C.-I.; Tan, T.-D.; Chang, H.-J.; Horng, C.-F.; Soong, T.; Tsou, M.-H. Extended-field radiotherapy and high-dose-rate brachytherapy with concurrent and adjuvant cisplatin-based chemotherapy for locally advanced cervical cancer: A phase I/II study. Gynecol. Oncol. 2005, 97, 126–135. [Google Scholar] [CrossRef]
- Domingo, E.; Lorvidhaya, V.; Reyes, R.D.L.; SyOrtin, T.; Kamnerdsupaphon, P.; Lertbutsayanukul, C.; Vito-Cruz, E.; Tharavichitkul, E.; Jin, K.; Yoshihara, M.; et al. Capecitabine-Based Chemoradiotherapy with Adjuvant Capecitabine for Locally Advanced Squamous Carcinoma of the Uterine Cervix: Phase II Results. Oncologist 2009, 14, 828–834. [Google Scholar] [CrossRef]
- Drokow, E.K.; Zi, L.; Qian, H.; Xu, L.; Foli, F.; Ahmed, H.A.W.; Akpabla, G.S.; Wu, G.; Agyekum, E.B.; Gao, W.; et al. Tolerability, Efficacy and Feasibility of Concurrent Gemcitabine and Cisplatin (CGP) Combined with Intensity Modulated Radiotherapy for Loco-Regionally Advanced Carcinoma of the Cervix. J. Cancer 2020, 11, 2632–2638. [Google Scholar] [CrossRef]
- A Dubay, R.; Rose, P.G.; O’Malley, D.M.; Shalodi, A.D.; Ludin, A.; A Selim, M. Evaluation of concurrent and adjuvant carboplatin with radiation therapy for locally advanced cervical cancer. Gynecol. Oncol. 2004, 94, 121–124. [Google Scholar] [CrossRef]
- Duska, L.R.; Scalici, J.M.; Temkin, S.M.; Schwarz, J.K.; Crane, E.K.; Moxley, K.M.; Hamilton, C.A.; Wethington, S.L.; Petroni, G.R.; Ms, N.E.V.; et al. Results of an early safety analysis of a study of the combination of pembrolizumab and pelvic chemoradiation in locally advanced cervical cancer. Cancer 2020, 126, 4948–4956. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, M.-K.; Kim, H.J.; Han, S.-S.; Kim, J.-W. Phase II Study of Consolidation Chemotherapy after Adjuvant or Primary Concurrent Chemoradiation Using Paclitaxel and Carboplatin to Treat High-Risk Early-Stage or Locally Advanced Cervical Cancer. Cancer Res. Treat. 2012, 44, 97–103. [Google Scholar] [CrossRef]
- Mayadev, J.S.; Enserro, D.; Lin, Y.G.; Da Silva, D.M.; Lankes, H.A.; Aghajanian, C.; Ghamande, S.; Moore, K.N.; Kennedy, V.A.; Fracasso, P.M.; et al. Sequential Ipilimumab After Chemoradiotherapy in Curative-Intent Treatment of Patients With Node-Positive Cervical Cancer. JAMA Oncol. 2020, 6, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Jelavic, T.B.; Mise, B.P.; Strikic, A.; Ban, M.; Vrdoljak, E. Adjuvant Chemotherapy in Locally Advanced Cervical Cancer After Treatment with Concomitant Chemoradiotherapy—Room for Improvement? Anticancer Res. 2015, 35, 4161–4165. [Google Scholar] [PubMed]
- Miše, B.P.; Jelavic, T.B.; Strikic, A.; Hrepic, D.; Tomic, K.; Hamm, W.; Tomic, S.; Prskalo, T.; Vrdoljak, E. Long Follow-up of Patients With Locally Advanced Cervical Cancer Treated With Concomitant Chemobrachyradiotherapy With Cisplatin and Ifosfamide Followed by Consolidation Chemotherapy. Int. J. Gynecol. Cancer 2015, 25, 315–319. [Google Scholar] [CrossRef]
- Vrdoljak, E.; Jelavic, T.B.; Saratlija-Novakovic, Z.; Hamm, W. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy in the treatment of locally advanced adenocarcinoma or adenosquamous carcinoma of the cervix uteri. Eur. J. Gynaecol. Oncol. 2005, 26, 602–604. [Google Scholar] [PubMed]
- Vrdoljak, E.; Omrčen, T.; Novaković, Ž.S.; Jelavić, T.B.; Prskalo, T.; Hrepić, D.; Hamm, W. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy for women with locally advanced carcinoma of the uterine cervix—Final results of a prospective phase II-study. Gynecol. Oncol. 2006, 103, 494–499. [Google Scholar] [CrossRef]
- Vrdoljak, E.; Prskalo, T.; Omrčen, T.; Situm, K.; Boraska, T.; Ilić, N.F.; Janković, S.; Hamm, W. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: Results of a phase II study. Int. J. Radiat. Oncol. 2005, 61, 824–829. [Google Scholar] [CrossRef]
- Sood, B.M.; Timmins, P.F.; Gorla, G.R.; Garg, M.; Anderson, P.S.; Vikram, B.; Goldberg, G.L. Concomitant cisplatin and ex-tended field radiation therapy in patients with cervical and endometrial cancer. Int. J. Gynecol. Cancer 2002, 12, 459–464. [Google Scholar] [CrossRef]
- Wilailak, S.; Dangprasert, S.; Srisupundit, S. Phase I clinical trial of chemoimmunotherapy in combination with radiotherapy in stage IIIB cervical cancer patients. Int. J. Gynecol. Cancer 2003, 13, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, D.-S.; Pan, T.; Liu, S.; Wang, M.-K. Efficacy of concurrent chemoradiotherapy plus adjuvant chemotherapy on advanced cervical cancer. Chin. J. Cancer 2010, 29, 959–963. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, M.-Q.; Liu, S.-P.; Wang, X.-E. Concurrent Chemoradiotherapy with Paclitaxel and Nedaplatin Followed by Consolidation Chemotherapy in Locally Advanced Squamous Cell Carcinoma of the Uterine Cervix: Preliminary Results of a Phase II Study. Int. J. Radiat. Oncol. 2010, 78, 821–827. [Google Scholar] [CrossRef] [PubMed]
- A Phase III Trial of Adjuvant Chemotherapy Following Chemoradiation as Primary Treatment for Locally Advanced Cervical Cancer Compared to Chemoradiation Alone: The OUTBACK Trial. Available online: https://clinicaltrials.gov/show/NCT01414608 (accessed on 22 December 2017).
- Lheureux, S.; Butler, M.O.; Clarke, B.; Cristea, M.C.; Martin, L.P.; Tonkin, K.; Fleming, G.F.; Tinker, A.V.; Hirte, H.W.; Tsoref, D.; et al. Association of Ipilimumab With Safety and Antitumor Activity in Women with Metastatic or Recurrent Human Papillomavirus–Related Cervical Carcinoma. JAMA Oncol. 2018, 4, e173776. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.-P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Study of Chemoradiotherapy with or without Pembrolizumab (MK-3475) for the Treatment of Locally Advanced Cervical Cancer (MK-3475-A18/KEYNOTE-A18/ENGOT-cx11). Available online: https://clinicaltrials.gov/ct2/show/NCT04221945 (accessed on 4 September 2020).
- Tomotherapy vs Conventional Radiation for Adjuvant Pelvic RT in Ca Cervix (PARCER). Available online: https://clinicaltrials.gov/ct2/show/NCT01279135 (accessed on 24 March 2021).
- Chinnachamy, A.N.; Chopra, S.; Krishnatry, R.; Kannan, S.; Thomas, B.; Mahantshetty, U.; Engineer, R.; Shrivastava, S.K. Evaluation of Interobserver and Interscale Agreement in Assessing Late Bowel Toxicity after Pelvic Radiation in Patients with Carcinoma of the Cervix. Jpn. J. Clin. Oncol. 2013, 43, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Kirisits, C.; De Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.; Potter, R.; Kossmeier, M.; Kirchheiner, K.; Mahantshetty, U.; Haie-Meder, C.; Lindegaard, J.; Jurgenliemk-Schulz, I.; Tan, L.; Hoskin, P.; et al. Nomograms Predicting Overall Survival in Locally Advanced Cervical Cancer treated by Image Guided Brachytherapy: A Retro-EMBRACE study. Int. J. Radiat. Oncol. 2019, 105, S50–S51. [Google Scholar] [CrossRef]
- Stanic, S.; Mayadev, J.S. Tolerance of the Small Bowel to Therapeutic Irradiation. Int. J. Gynecol. Cancer 2013, 23, 592–597. [Google Scholar] [CrossRef]
- Chopra, S.; Dora, T.; Chinnachamy, A.; Thomas, B.; Kannan, S.; Engineer, R.; Mahantshetty, U.; Phurailatpam, R.; Paul, S.N.; Shrivastava, S.K. Predictors of Grade 3 or Higher Late Bowel Toxicity in Patients Undergoing Pelvic Radiation for Cervical Cancer: Results from a Prospective Study. Int. J. Radiat. Oncol. 2014, 88, 630–635. [Google Scholar] [CrossRef] [PubMed]
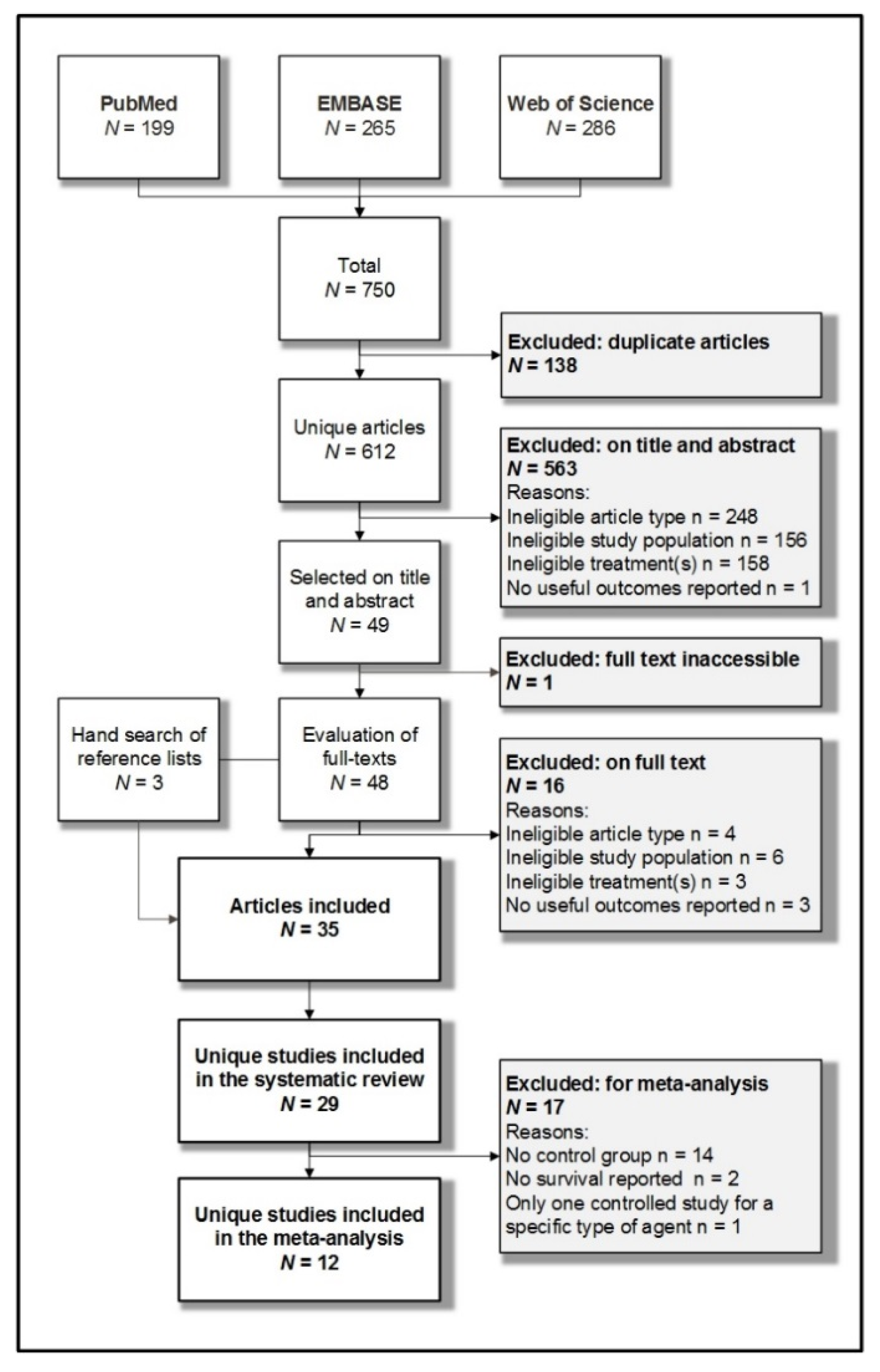
| Patient | Tumor characteristics: FIGO stage IB–IVA (including metastasis to the para-aortic lymph nodes) cervical cancer of squamous cell carcinoma, adenocarcinoma or adenosquamous carcinoma histotype Study characteristics: randomized controlled trials, non-randomized prospective and retrospective studies |
| Intervention | External beam radiotherapy to the whole pelvis (with or without integrated or sequential boosts or extended field) with concurrent chemotherapy and intracavitary or interstitial brachytherapy followed by adjuvant systemic therapy (e.g., chemotherapy or immuno therapy) |
| Control | External beam radiotherapy to the whole pelvis (with or without integrated or sequential boosts or extended field) with concurrent chemotherapy and intracavitary or interstitial brachytherapy |
| Outcomes | Overall survival, recurrence- or disease-free survival, metastasis-free survival, treatment completion, toxicity |
| Exclusion | Tumor characteristics: persistent or recurrent cervical cancer, distant metastasis Treatment characteristics: primary surgery, neo-adjuvant systemic therapy Publication types: conference abstracts, case-reports, review articles, meta-analyses, editorials, letters to the editor, guidelines, articles published before the year 2000 |
| Study | Country | Year | Design | Controlled | N a | Age b | Histology | Stage | Pelvic LN | PAO LN |
|---|---|---|---|---|---|---|---|---|---|---|
| Abe et al. [17] | Japan | 2012 | Retro | Yes | 37 | 55 (31–72) | SQ | IB–IVA | Yes | Yes |
| Choi et al. [18,19] | Korea | 2007, 2011 | m-pair | Yes | 78 | 53 (33–71) | SQ, AC, ASQ | IB–IVA | Yes | No |
| Duenas et al. [11,20] | Multiple c | 2011, 2012 | RCT | Yes | 515 | 46 (18–70) | SQ, AC, ASQ | IIB–IVA | NR | No |
| Fabri et al. [21] | Brazil | 2019 | Retro | Yes | 186 | 48 | SQ, AC | IB–IVA | Yes | Yes |
| Kim et al. [22] | Korea | 2007 | Retro | Yes | 205 | 51 (29–75) | SQ, SCC | IB, IIB | Yes | No |
| Kim et al. [23] | Korea | 2008 | RCT | Yes | 155 | 58 (34–75) | SQ, AC, ASQ | IIB–IVA | Yes | No |
| Kong et al. [24] | Korea | 2012 | Retro | Yes | 255 | 57 (25–87) | SQ, AC, ASQ | IIB–IVA | Yes | NR |
| Lorusso et al. [25] | Italy | 2018 | Pros | Yes | 19 | 48 (34–72) | SQ, AC, ASQ | II–IIIA | Yes | Yes |
| Lorvidhaya et al. [26] | Thailand | 2003 | RCT | Yes | 463 | 49 | SQ, AC, ASQ, SCC | IIB–IVA | Yes | Yes |
| Mabuchi et al. [27] | Japan | 2017 | Retro | Yes | 82 | 53 (30–68) | SQ | IIIB–IVA | Yes | NR |
| Manders et al. [28] | USA | 2018 | Retro | Yes | 51 | 48 (29–79) | SQ, AC, ASQ | IB–II, IIIB–IVA | Yes | Yes |
| Pandya et al. [29] | India | 2019 | RCT | Yes | 47 | 55 (33–70) | SQ, AC | IIB–IVA | Yes | Yes |
| Tangjitgamol et al. [30] | Thailand | 2019 | RCT | Yes | 259 | 50 (23–68) | SQ, AC, ASQ | IIB–IVA | Yes | No |
| Tu et al. [31] | China | 2017 | Retro | Yes | 84 | 46 (28–69) | SQ, AC, ASQ | IBM IIB–IIIB | No | No |
| Yavas et al. [32] | Turkey | 2019 | Retro | Yes | 109 | 53 (29–85) | SQ, AC, ASQ, SCC, LC | IB–IVA | Yes | Yes |
| Boardman et al. [33] | USA | 2018 | Pros | No | 10 | 42 (26–67) | SQ, AC | IB–IVA | Yes | Yes |
| Cihoric et al. [34] | Switzerland | 2017 | Retro | No | 17 | NR | SQ, AC | IB–IVA | Yes | Yes |
| Chung et al. [35] | Taiwan | 2005 | Pros | No | 63 | 52 (31–77) | SQ, AC, ASQ | IIB–IVA | Yes | Yes |
| Domingo et al. [36] | Multiple d | 2009 | Pros | No | 60 | 47 (28–72) | SQ | IIB–IIIB | NR | NR |
| Drokow et al. [37] | China | 2020 | Retro | No | 81 | 45 (25–60) | SQ, AC | IB2–IIIB | Yes | Yes |
| Dubay et al. [38] | USA | 2004 | Retro | No | 21 | 36 (25–72) | SQ | IIB–IVA | NR | NR |
| Duska et al. [39] | USA | 2020 | Pros | No | 24 | 49 (28–74) | SQ, AC | IB2–IVA | Yes | Yes |
| Kim et al. [40] | Korea | 2012 | Pros | No | 18 | 52 (37–74) | SQ, AC, ASQ | IIB–IVA | Yes | Yes |
| Mayadev et al. [41] | USA | 2019 | Pros | No | 32 | 50 (26–61) | SQ, AC, ASQ | IB2–IVA | Yes | Yes |
| Split University [42,43,44,45,46] | Croatia | 2004–2015 | Retro | No | 118 | 53 (27–77) | SQ, AC, ASQ | IB–IVA | Yes | No |
| Sood et al. [47] | USA | 2002 | Retro | No | 25 | 50 (36–73) | SQ | IB–IIIB | Yes | Yes |
| Wilailak et al. [48] | Thailand | 2003 | Pros | No | 8 | 45 (39–60) | SQ | IIIB | Yes | NR |
| Wang et al. [49] | China | 2010 | RCT | No | 79 | 52 (42–65) | SQ | IIA–IIIB | NR | NR |
| Zhang et al. [50] | China | 2010 | Pros | No | 34 | 47 (35–64) | SQ | IIB–IIIB | Yes | No |
| Study | Treatment Arm | N | Completion Rate | Severe Acute Toxicity | Severe Late Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | Leucopenia | Thrombopenia | GI | GU | Neuropathy | Liver | Renal | GI | GU | ||||
| Studies with CRT as control treatment | |||||||||||||
| Choi et al. [19] | CRT | 39 | 95% | 4% | 5% | 2% | 10% | . | 0% | 0% | 0% | 0% | 0% |
| CRT+AdjTx | 39 | 90% | 8% | 11% | 2% | 9% | . | 0% | 0% | 0% | 0% | 0% | |
| Duenas et al. [11] | CRT | 256 | . | 2% | 12% | 1% | 8% | . | . | 0% | 1% | 0% | 0% |
| CRT+AdjTx | 259 | 77% | 9% | 51% | 6% | 26% | . | . | 1% | 2% | 2% | 1% | |
| Fabri et al. [21] | CRT | 128 | . | . | . | . | . | . | . | . | . | . | . |
| CRT+AdjTx | 58 | 91% | . | . | . | . | . | . | . | . | . | . | |
| Kim et al. [22] | CRT | 103 | . | 3% | 42% | 11% | 12% | . | . | 2% | 4% | 0% | 1% |
| CRT+AdjTx | 102 | 63% | 12% | 77% | 13% | 23% | . | . | 7% | 8% | 8% | 3% | |
| Kim et al. [23] | CRT | 77 | 73% | Any hemat 25% | 0% | 0% | . | . | . | 4% | 3% | ||
| CRT+AdjTx | 78 | 65% | Any hemat 41% | 8% | 3% | . | . | . | 1% | 0% | |||
| Kong et al. [24] | CRT | 152 | 100% | 2% | 5% | 1% | 9% | . | . | . | . | . | . |
| CRT+AdjTx | 103 | 100% | 7% | 11% | 4% | 25% | . | . | . | . | . | . | |
| SUBTOTAL | CRT | 755 | 92% | 2% | 15% | 3% | 8% | 0% | 0% | 1% | 2% | 1% | 1% |
| 95%CI | 88–95% | 1–4% | 12–18% | 2–4% | 6–10% | 0–0% | 0–0% | 0–1% | 0–3% | 0–1% | 0–1% | ||
| CRT+AdjTx | 639 | 79% | 9% | 45% | 7% | 22% | 3% | 0% | 2% | 3% | 3% | 1% | |
| 95%CI | 76–82% | 7–12% | 41–49% | 5–9% | 18–25% | 0–7% | 0–0% | 1–4% | 2–5% | 1–4% | 0–2% | ||
| p-value | 1394 | <0.0001 | <0.0001 | <0.0001 | 0.004 | <0.0001 | 0.5 | NS | 0.037 | 0.26 | 0.012 | 0.51 | |
| Studies without CRT as control treatment | |||||||||||||
| Cihoric et al. [34] | CRT+AdjTx | 17 | 53% | . | . | . | 18% | 0% | . | . | . | 0% | 12% |
| Chung et al. [35] | CRT+AdjTx | 63 | 92% | 3% | 10% | 2% | 2% | . | . | . | . | 6% | . |
| Drokow et al. [37] | CRT+AdjTx | 81 | 100% | 0% | 0% | 0% | 3% | 0% | . | . | . | 0% | 0% |
| Wilailak et al. [48] | CRT+AdjTx | 8 | 75% | 0% | 38% | 0% | 26% | . | . | . | . | . | . |
| TOTAL | CRT | 755 | 92% | 2% | 15% | 3% | 8% | 0% | 0% | 1% | 2% | 1% | 1% |
| 95%CI | 88–95% | 1–4% | 12–18% | 2–4% | 6–10% | 0–0% | 0–0% | 0–1% | 0–3% | 0–1% | 0–1% | ||
| CRT+AdjTx | 808 | 82% | 7% | 36% | 5% | 18% | 1% | 0% | 2% | 3% | 3% | 1% | |
| 95%CI | 76–87% | 3–11% | 28–44% | 2–9% | 12–24% | 0–4% | 0–0% | 1–4% | 2–5% | 0–5% | 0–4% | ||
| p-value | 1563 | <0.0001 | <0.0001 | <0.0001 | 0.044 | <0.0001 | 1 | NS | 0.037 | 0.26 | 0.007 | 0.36 | |
| Study | Treatment Arm | N | Completion | Severe Acute Toxicity | Severe Late Toxicity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | Leucopenia | Thrombopenia | GI | GU | Neuropathy | GI | GU | ||||
| Studies with CRT as control treatment | |||||||||||
| Abe et al. [17] | CRT | 20 | . | . | . | . | . | . | . | 5% | . |
| CRT+AdjTx | 17 | 95% | 41% | 94% | 18% | . | . | 6% | . | ||
| Lorusso et al. [25] | CRT | 9 | . | . | . | . | . | . | . | . | . |
| CRT+AdjTx | 10 | 90% | 10% | . | . | . | . | . | . | ||
| Mabuchi et al. [27] | CRT | 52 | . | . | . | . | . | . | . | . | . |
| CRT+AdjTx | 30 | 63% | 3% | 57% | 3% | 10% | . | 0% | 13% | 10% | |
| Manders et al. [28] | CRT | 44 | 100% | Any acute hematological tox 11% | 5% | . | 0% | 3% | . | ||
| CRT+AdjTx | 7 | 86% | Any acute hematological tox 0% | 0% | . | 0% | 0% | . | |||
| Pandya et al. [29] | CRT | 23 | 70% | 17% | 17% | . | 4% | 8% | 0% | 4% | 13% |
| CRT+AdjTx | 24 | 79% | 13% | 33% | . | 17% | 0% | 8% | 0% | 4% | |
| Tangjitgamol et al. [30] | CRT | 129 | 95% | 3% | 0% | 6% | 2% | 2% | . | . | . |
| CRT+AdjTx | 130 | 65% | 5% | 13% | 4% | 5% | 3% | 3% | . | . | |
| Tu et al. [31] | CRT | 38 | 100% | Any acute hematological tox 24% | 13% | . | . | . | . | ||
| CRT+AdjTx | 46 | . | Any acute hematological tox 37% | 11% | . | . | . | . | |||
| Yavas et al. [32] | CRT | 63 | 100% | 0% | 0% | 0% | 0% | 0% | 0% | 3% | 0% |
| CRT+AdjTx | 46 | . | 0% | 13% | 4% | 4% | 0% | 4% | 2% | 0% | |
| CRT | 378 | 96% | 4% | 2% | 4% | 4% | 2% | 0% | 3% | 3% | |
| SUBTOTAL | 95%CI | 93–98% | 1–6% | 0–4% | 1–7% | 1–6% | 0–4% | 0–0% | 1–6% | 0–7% | |
| CRT+AdjTx | 310 | 70% | 19% | 26% | 5% | 9% | 2% | 3% | 5% | 4% | |
| 95%CI | 64–76% | 14–23% | 20–31% | 2–8% | 5–12% | 0–4% | 1–5% | 1–8% | 0–8% | ||
| p-value | 688 | <0.0001 | <0.0001 | <0.0001 | 0.82 | 0.022 | 1 | 0.055 | 0.55 | 1 | |
| Studies without CRT as control treatment | |||||||||||
| Boardman et al. [33] | CRT+AdjTx | 10 | 67% | 44% | 89% | 22% | . | . | 11% | Any late tox 0% | |
| Kim et al. [40] | CRT+AdjTx | 18 | 100% | . | 15% | . | 0% | . | . | 6% | 0% |
| Wang et al. [49] | CRT+AdjTx | 79 | . | 48% | 58% | 25% | 63% | . | . | . | . |
| Zhang et al. [50] | CRT+AdjTx | 34 | 82% | 0% | 82% | 0% | 3% | . | 0% | 6% | 3% |
| CRT | 378 | 96% | 4% | 2% | 4% | 4% | 2% | 0% | 3% | 3% | |
| TOTAL | 95%CI | 93–98% | 1–6% | 0–4% | 1–7% | 1–6% | 0–4% | 0–0% | 1–6% | 0–7% | |
| CRT+AdjTx | 451 | 74% | 24% | 38% | 19% | 19% | 2% | 4% | 5% | 3% | |
| 95%CI | 63–85% | 16–32% | 30–46% | 12–26% | 12–26% | 0–4% | 0–10% | 0–11% | 0–8% | ||
| p-value | 829 | <0.0001 | <0.0001 | <0.0001 | 0.027 | <0.0001 | 1 | 0.063 | 0.59 | 1 | |
| Study | Treatment Arm | N | Completion | Severe Acute Toxicity | Severe Late Toxicity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | Leucopenia | Thrombopenia | GI | GU | Renal | Other | GI | GU | ||||
| Adjuvant cisplatin + ifosfamide | ||||||||||||
| Split University [42,43,44,45,46] | CRT+AdjTx | 118 | 41% | 7% | 34% | 15% | 12% | . | 3% | . | Any late tox 19% | |
| Adjuvant cisplatin | ||||||||||||
| Dubay et al. [38] | CRT+AdjTx | 21 | 62% | 10% | 10% | 0% | 5% | . | 0% | . | . | . |
| Adjuvant carboplatin | ||||||||||||
| Sood et al. [47] | CRT+AdjTx | 25 | . | Any acute hematological tox 80% | Any acute non-hemat tox 28% | 4% | . | |||||
| Adjuvant 5-fluorouracil | ||||||||||||
| Lorvidhaya et al. [26] | CRT | 233 | 95% | 0% | 4% | 2% | . | . | . | . | Any late tox 3% | |
| CRT+AdjTx | 230 | 92% | 0% | 3% | 1% | . | . | . | . | Any late tox 6% | ||
| Adjuvant capecitabin | ||||||||||||
| Domingo et al. [36] | CRT+AdjTx | 60 | 90% | 5% | . | . | 3% | 2% | 3% | 3% a | . | . |
| Adjuvant cisplatin + 5-fluorouracil + interferon alpha + retinoic acid | ||||||||||||
| Wilailak et al. [48] | CRT+AdjTx | 8 | 75% | 0% | 38% | 0% | 25% | . | 38% | . | . | . |
| Adjuvant ipilimumab | ||||||||||||
| Mayadev et al. [41] | CRT+AdjTx | 21 b | 86% | 10% | 5% | 5% | 14% | 10% | . | 20% c | . | . |
| Adjuvant pembrolizumab | ||||||||||||
| Duska et al. [39] | CRT+AdjTx | 24 | 100% | 17% | 33% | 0% | 13% | . | . | 22% d | . | . |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horeweg, N.; Mittal, P.; Gradowska, P.L.; Boere, I.; Chopra, S.; Nout, R.A. Adjuvant Systemic Therapy after Chemoradiation and Brachytherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1880. https://doi.org/10.3390/cancers13081880
Horeweg N, Mittal P, Gradowska PL, Boere I, Chopra S, Nout RA. Adjuvant Systemic Therapy after Chemoradiation and Brachytherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(8):1880. https://doi.org/10.3390/cancers13081880
Chicago/Turabian StyleHoreweg, Nanda, Prachi Mittal, Patrycja L. Gradowska, Ingrid Boere, Supriya Chopra, and Remi A. Nout. 2021. "Adjuvant Systemic Therapy after Chemoradiation and Brachytherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis" Cancers 13, no. 8: 1880. https://doi.org/10.3390/cancers13081880
APA StyleHoreweg, N., Mittal, P., Gradowska, P. L., Boere, I., Chopra, S., & Nout, R. A. (2021). Adjuvant Systemic Therapy after Chemoradiation and Brachytherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis. Cancers, 13(8), 1880. https://doi.org/10.3390/cancers13081880






