Substance P Antagonism Prevents Chemotherapy-Induced Cardiotoxicity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Murine Model of Doxorubicin Induced Cardiotoxicity
2.2. Echo Procedure
2.3. Measurement of Heart-to-Body Weight Ratio
2.4. Measurement of Cardiomyocyte Hypertrophy
2.5. Measurement of Substance P and NK1R Levels within the Heart
2.6. Measurement of Annexin V Levels, An Indicator of Apoptosis within the Heart
2.7. Measurement of GSH to GSSG Ratio, an Indicator of Oxidative Stress within the Heart
2.8. Statistics
3. Results
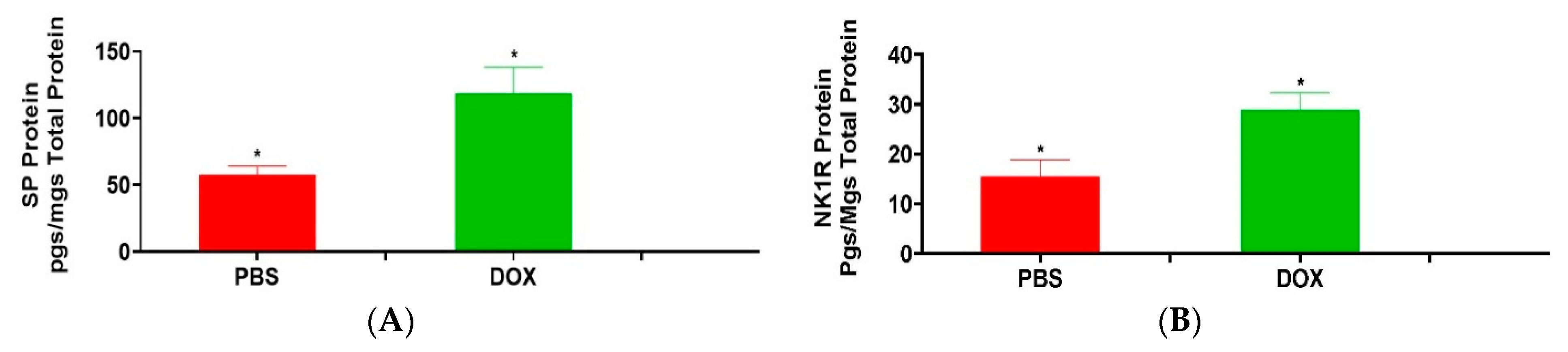
3.1. Effect of Doxorubicin on Heart SP and NK1R Protein Levels
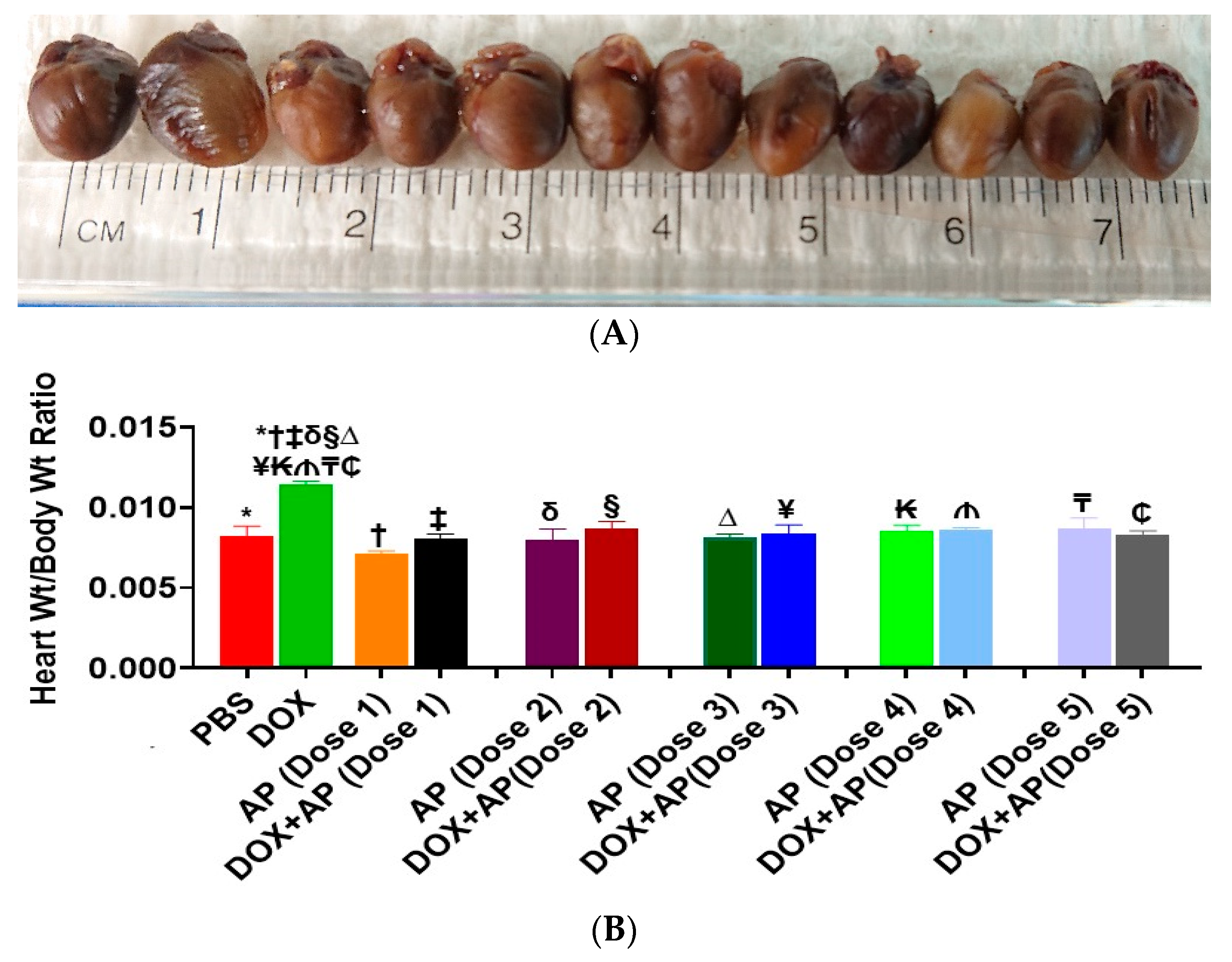
3.2. Effect of Aprepitant on Doxorubicin Induced Changes in Heart Weight to Body Weight Ratios
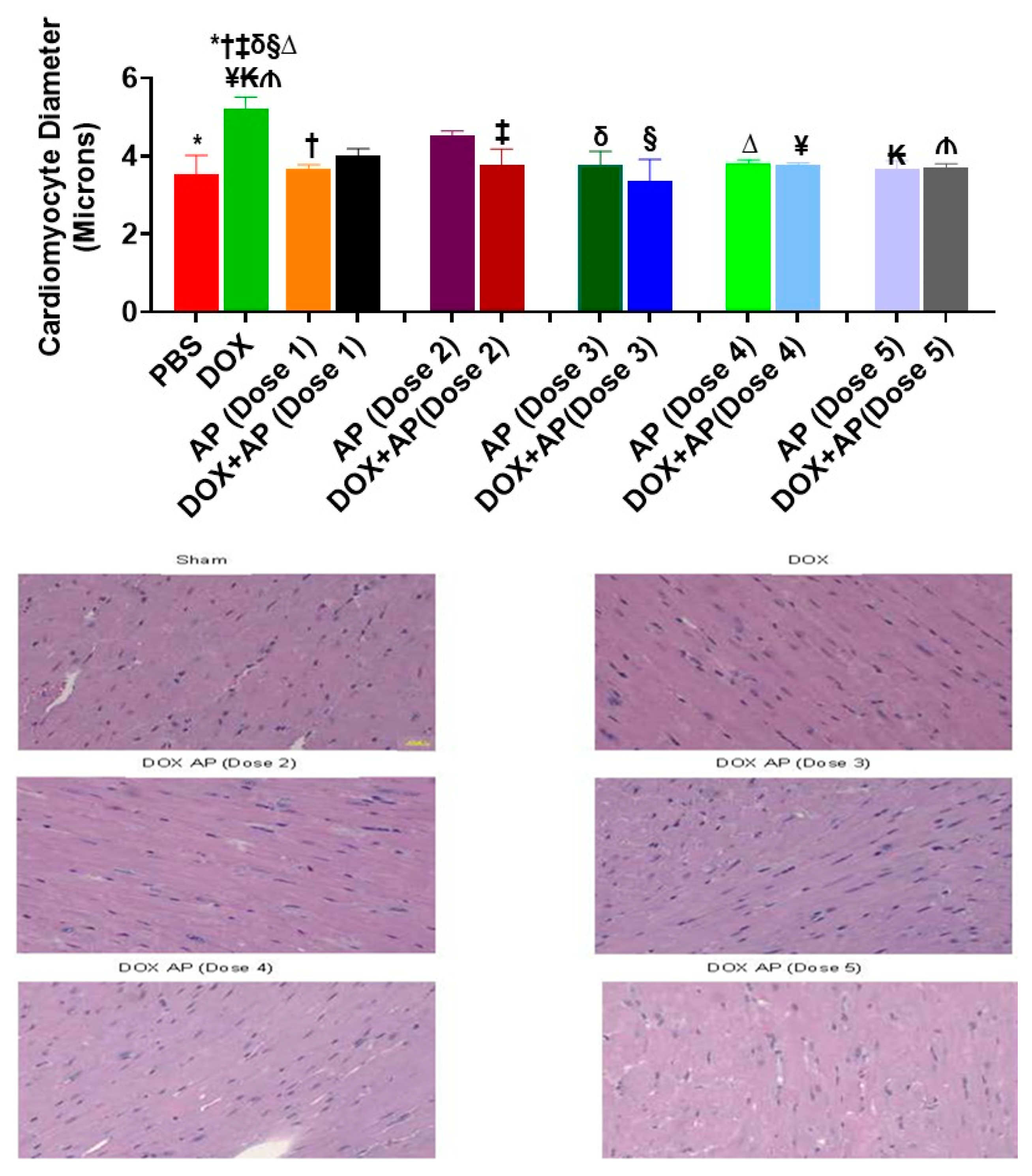
3.3. Effect of Aprepitant on Doxorubicin Induced Cardiomyocyte Hypertrophy
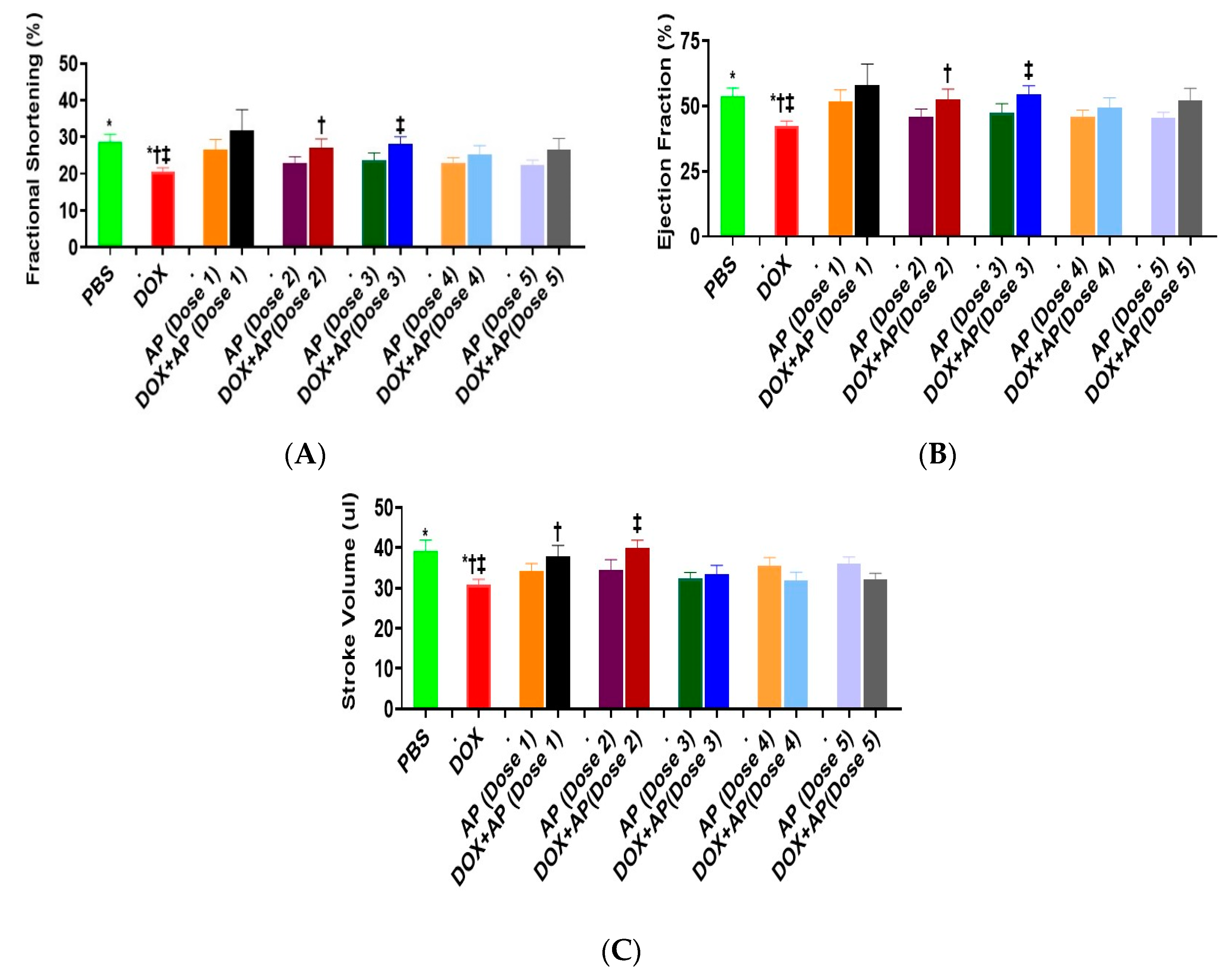
3.4. Effect of Aprepitant on Doxorubicin Induced Changes in Heart Functions
3.4.1. Fractional Shortening (FS)
3.4.2. Ejection Fraction (EF)
3.4.3. Stroke Volume
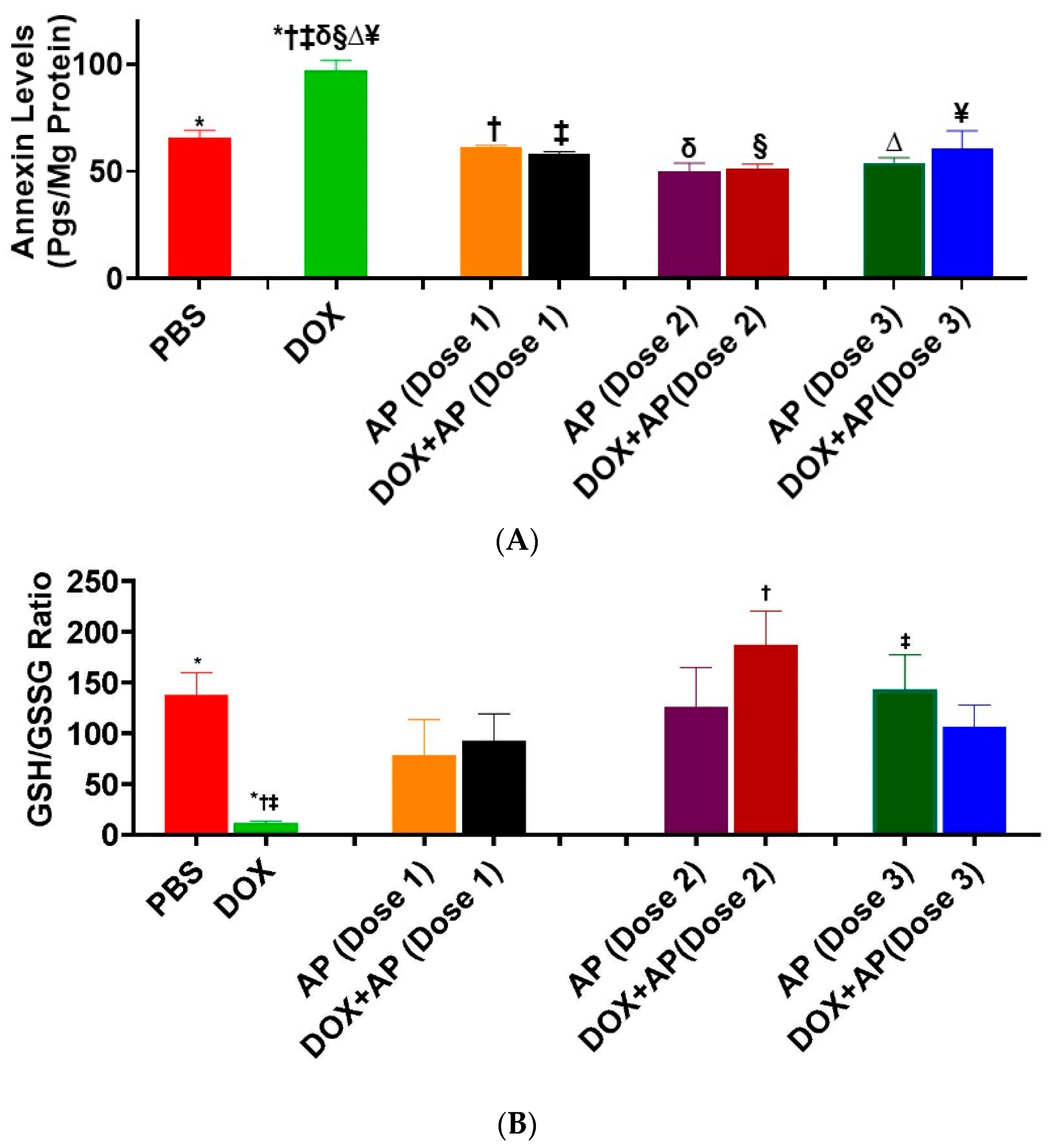
3.5. Effect of Aprepitant on Doxorubicin Induced Cardiomyocyte Apoptosis
3.6. Effect of Aprepitant on Doxorubicin Induced Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lipshultz, S.E.; Colan, S.D.; Gelber, R.D.; Perez-Atayde, A.R.; Sallan, S.E.; Sanders, S.P. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 1991, 324, 808–815. [Google Scholar] [CrossRef]
- Sinha, B.K.; Mimnaugh, E.G.; Rajagopalan, S.; Myers, C.E. Adriamycin activation and oxygen free radical formation in human breast tumor cells: Protective role of glutathione peroxidase in adriamycin resistance. Cancer Res. 1989, 49, 3844–3848. [Google Scholar]
- Young, R.J.; Natukunda, A.; Litiere, S.; Woll, P.J.; Wardelmann, E.; van der Graaf, W.T. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: Pooled analysis of eleven European organisation for research and treatment of cancer soft tissue and bone sarcoma group trials. Eur. J. Cancer 2014, 50, 3178–3186. [Google Scholar] [CrossRef]
- Novakova, Z.; Stastna, J.; Honzikova, K.; Hrstkova, H.; Honzikova, N.; Zavodna, E.; Fišer, B.; Honzík, P. Anthracycline therapy and 24-hour blood-pressure profile in long-term survivors of childhood cancer. Physiol. Res. 2010, 59 (Suppl. 1), S97–S102. [Google Scholar] [CrossRef]
- Tassone, P.; Tagliaferri, P.; Perricelli, A.; Blotta, S.; Quaresima, B.; Martelli, M.L.; Goel, A.; Barbieri, V.; Costanzo, F.; Boland, C.R.; et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br. J. Cancer 2003, 88, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann. Oncol. 2012, 23 (Suppl. 7), vii155–vii66. [Google Scholar] [CrossRef]
- Yeh, E.T.; Tong, A.T.; Lenihan, D.J.; Yusuf, S.W.; Swafford, J.; Champion, C.; Durand, J.; Gibbs, H.; Zafarmand, A.A.; Ewer, M.S. Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation 2004, 109, 3122–3131. [Google Scholar] [CrossRef]
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010, 10, 337. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L.; Von Hoff, A.L., Jr.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Honore, P.; Rogers, S.D.; Schwei, M.J.; Salak-Johnson, J.L.; Luger, N.M.; Sabino, M.C.; Clohisy, D.; Mantyh, P. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000, 98, 585–598. [Google Scholar] [CrossRef]
- Weinstock, J.V.; Blum, A.; Walder, J.; Walder, R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J. Immunol. 1988, 141, 961–966. [Google Scholar] [PubMed]
- Maggi, C.A. The effects of tachykinins on inflammatory and immune cells. Regul. Pept. 1997, 70, 75–90. [Google Scholar] [CrossRef]
- Ho, W.Z.; Lai, J.P.; Zhu, X.H.; Uvaydova, M.; Douglas, S.D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997, 159, 5654–5660. [Google Scholar]
- Church, D.; Arkinstall, S.J.; Vallotton, M.B.; Chollet, A.; Kawashima, E.; Lang, U. Stimulation of atrial natriuretic peptide release by neurokinins in neonatal rat ventricular cardiomyocytes. Am. J. Physiol. 1996, 270 (Pt 2), H935–H944. [Google Scholar] [CrossRef]
- Goode, T.; O'Connell, J.; Sternini, C.; Anton, P.; Wong, H.; O'Sullivan, G.C.; Collins, J.K.; Shanahan, F. Substance P (neurokinin-1) receptor is a marker of human mucosal but not peripheral mononuclear cells: Molecular quantitation and localization. J. Immunol. 1998, 161, 2232–2240. [Google Scholar] [PubMed]
- Cook, G.A.; Elliott, D.; Metwali, A.; Blum, A.M.; Sandor, M.; Lynch, R.; Weinstock, J.V. Molecular evidence that granuloma T lymphocytes in murine schistosomiasis mansoni express an authentic substance P (NK-1) receptor. J. Immunol. 1994, 152, 1830–1835. [Google Scholar] [PubMed]
- Tejero-Taldo, M.I.; Kramer, J.H.; Mak Iu, T.; Komarov, A.M.; Weglicki, W.B. The nerve-heart connection in the pro-oxidant response to Mg-deficiency. Heart Fail Rev. 2006, 11, 35–44. [Google Scholar] [CrossRef]
- Sterner-Kock, A.; Braun, R.K.; van der Vliet, A.; Schrenzel, M.D.; McDonald, R.J.; Kabbur, M.B.; Vulliet, P.R.; Hyde, D.M. Substance P primes the formation of hydrogen peroxide and nitric oxide in human neutrophils. J. Leukoc. Biol. 1999, 65, 834–840. [Google Scholar] [CrossRef]
- Chen, K.; Keaney, J.F., Jr. Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr. Atheroscler Rep. 2012, 14, 476–483. [Google Scholar] [CrossRef]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Kasembeli, M.; Bharadwaj, U.; Engineer, N.; Eckols, K.T.; Tweardy, D.J. Substance P receptor signaling mediates doxorubicin-induced cardiomyocyte apoptosis and triple-negative breast cancer chemoresistance. Biomed Res. Int. 2016, 2016, 1959270. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Garza, A.; Moore, J.; Eckols, T.K.; Parti, S.; Balaji, V.; Vallejo, J.; Tweardy, D.J. Substance P is required for the pathogenesis of EMCV infection in mice. Int. J. Clin. Exp. Med. 2009, 2, 76–86. [Google Scholar]
- Robinson, P.; Taffet, G.E.; Engineer, N.; Khumbatta, M.; Firozgary, B.; Reynolds, C.; Pham, T.; Bulsara, T.; Firozgary, G. Substance P receptor antagonism: A potential novel treatment option for viral-myocarditis. Biomed Res. Int. 2015, 2015, 645153. [Google Scholar] [CrossRef]
- Melendez, G.C.; Li, J.; Law, B.A.; Janicki, J.S.; Supowit, S.C.; Levick, S.P. Substance P induces adverse myocardial remodelling via a mechanism involving cardiac mast cells. Cardiovasc. Res. 2011, 92, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Weglicki, W.B.; Mak, I.T.; Phillips, T.M. Blockade of cardiac inflammation in Mg2+ deficiency by substance P receptor inhibition. Circ. Res. 1994, 74, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.T.; Chmielinska, J.J.; Kramer, J.H.; Spurney, C.F.; Weglicki, W.B. Loss of neutral endopeptidase activity contributes to neutrophil activation and cardiac dysfunction during chronic hypomagnesemia: Protection by substance P receptor blockade. Exp. Clin. Cardiol. 2011, 16, 121–124. [Google Scholar]
- Munoz, M.; Covenas, R. The Neurokinin-1 Receptor Antagonist Aprepitant: An intelligent bullet against cancer? Cancers 2020, 12, 2682. [Google Scholar] [CrossRef]
- Munoz, M.; Covenas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9. [Google Scholar] [CrossRef]
- Munoz, M.; Covenas, R.; Esteban, F.; Redondo, M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. J. Biosci. 2015, 40, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Covenas, R. Neurokinin-1 receptor: A new promising target in the treatment of cancer. Discov. Med. 2010, 10, 305–313. [Google Scholar] [PubMed]
- Munoz, M.; Gonzalez-Ortega, A.; Covenas, R. The NK-1 receptor is expressed in human leukemia and is involved in the antitumor action of aprepitant and other NK-1 receptor antagonists on acute lymphoblastic leukemia cell lines. Invest. N. Drugs 2012, 30, 529–540. [Google Scholar] [CrossRef]
- Munoz, M.; Berger, M.; Rosso, M.; Gonzalez-Ortega, A.; Carranza, A.; Covenas, R. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int. J. Oncol. 2014, 44, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Gonzalez-Ortega, A.; Salinas-Martin, M.V.; Carranza, A.; Garcia-Recio, S.; Almendro, V.; Coveñas, R. The neurokinin-1 receptor antagonist aprepitant is a promising candidate for the treatment of breast cancer. Int. J. Oncol. 2014, 45, 1658–1672. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Covenas, R. Targeting NK-1 receptors to prevent and treat pancreatic cancer: A new therapeutic approach. Cancers 2015, 7, 1215–1232. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 9–42. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legi, A.; Rodriguez, E.; Eckols, T.K.; Mistry, C.; Robinson, P. Substance P Antagonism Prevents Chemotherapy-Induced Cardiotoxicity. Cancers 2021, 13, 1732. https://doi.org/10.3390/cancers13071732
Legi A, Rodriguez E, Eckols TK, Mistry C, Robinson P. Substance P Antagonism Prevents Chemotherapy-Induced Cardiotoxicity. Cancers. 2021; 13(7):1732. https://doi.org/10.3390/cancers13071732
Chicago/Turabian StyleLegi, Ashiq, Emma Rodriguez, Thomas K. Eckols, Cyrus Mistry, and Prema Robinson. 2021. "Substance P Antagonism Prevents Chemotherapy-Induced Cardiotoxicity" Cancers 13, no. 7: 1732. https://doi.org/10.3390/cancers13071732
APA StyleLegi, A., Rodriguez, E., Eckols, T. K., Mistry, C., & Robinson, P. (2021). Substance P Antagonism Prevents Chemotherapy-Induced Cardiotoxicity. Cancers, 13(7), 1732. https://doi.org/10.3390/cancers13071732




