Overview of Prognostic Systems for Hepatocellular Carcinoma and ITA.LI.CA External Validation of MESH and CNLC Classifications
Abstract
Simple Summary
Abstract
1. Introduction
- (1)
- Prognostic scores, derived from real cohort populations.
- (2)
- Staging systems, derived from the literature review
- (3)
- Combined prognostic systems, based on the literature evidences but weighted in a real population, and with the possibility to be used both as scores and as staging systems.
2. Overview of Available Prognostic Systems for HCC
2.1. Prognostic Scores
2.2. Staging Systems
2.3. Combined Staging Systems
2.4. Summary of the Pros and Cons of Prognostic Systems
3. Comparison of Available Prognostic Systems
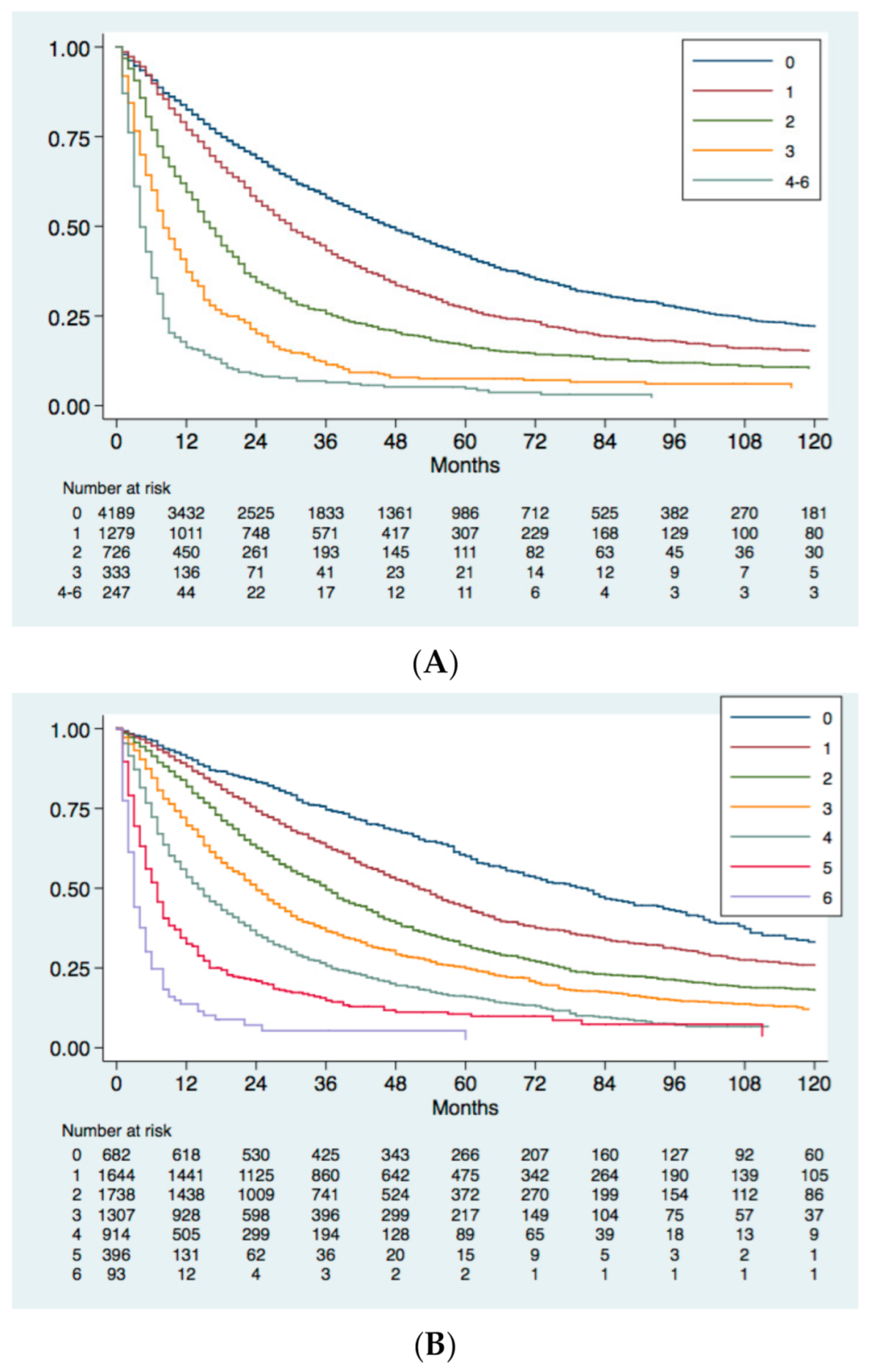
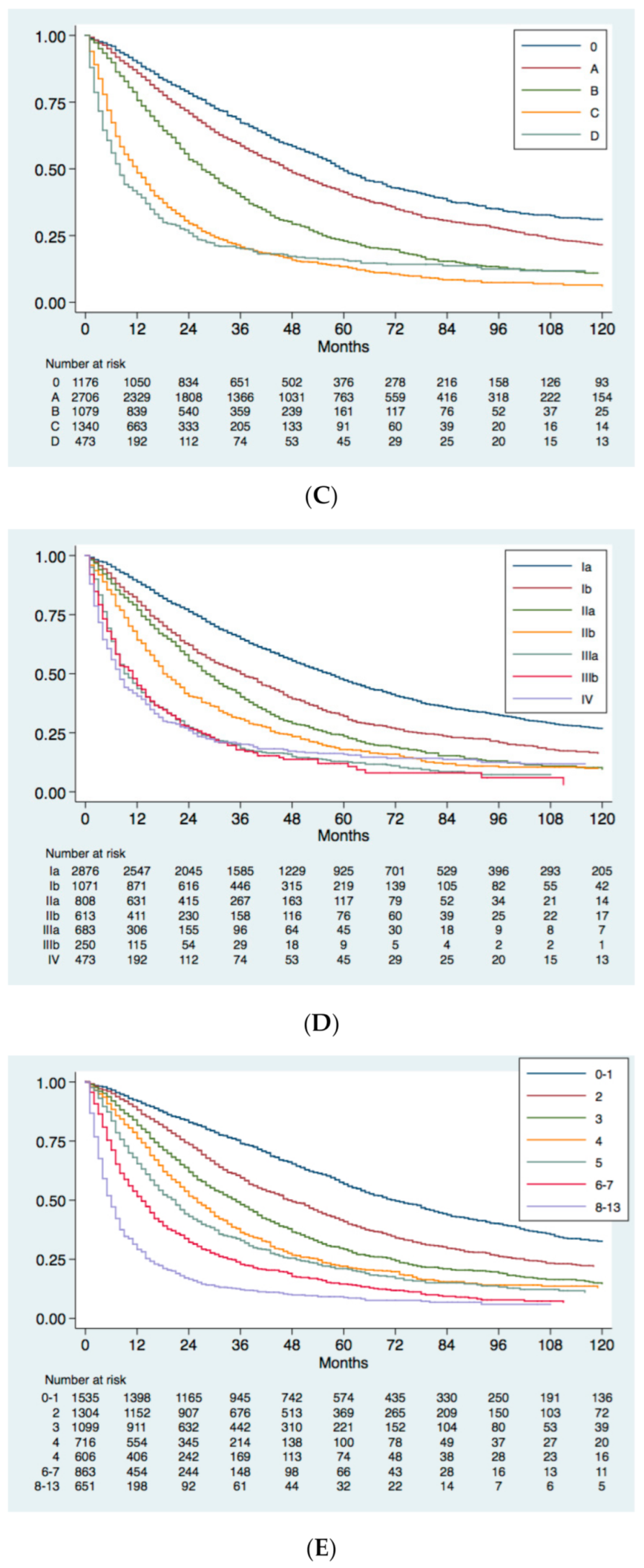
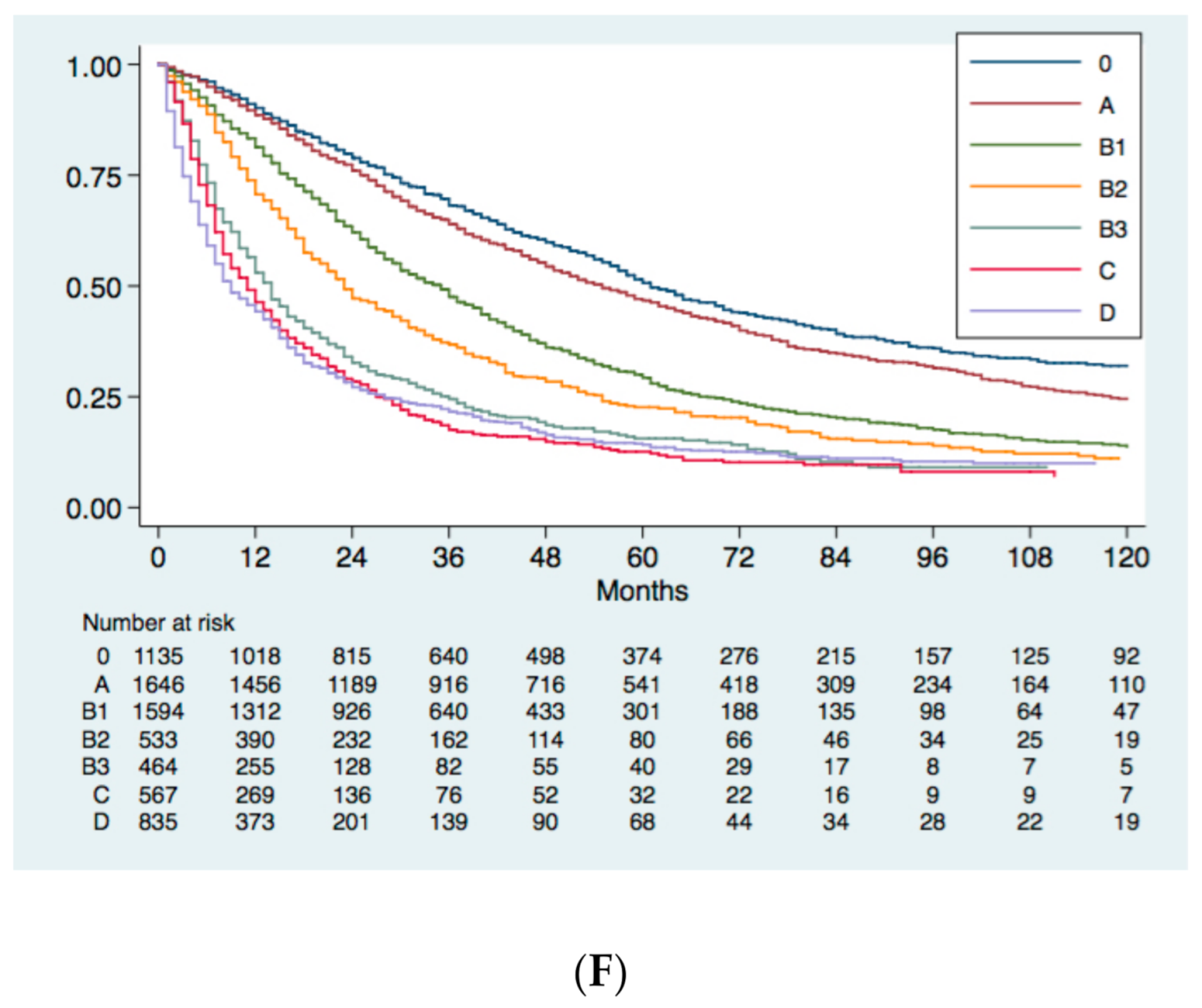
4. External Validation of MESH and CNLC Prognostic Systems in the ITA.LI.CA Database
4.1. Study Population
4.2. Statistical Analysis
4.3. Survival Analysis and Comparison between HCC Prognostic Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Levi, F.; Boffetta, P.; Lucchini, F.; Negri, E.; La Vecchia, C. Trends in Mortality from Hepatocellular Carcinoma in Europe, 1980–2004. Hepatology 2008, 48, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Kim, W.R. The Model for End-Stage Liver Disease (MELD) & Dagger. Hepatology 2007, 45, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the Oesophagus for Bleeding Oesophageal Varices. BJS 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients with Hepatocellular Carci-Noma: A New Evidence-Based Approach—The ALBI Grade. J. Clin. Oncol 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Ohya, T.; Kikuchi, S. Clinical Evaluation of Chemotherapeutic Agents in the Treatment of Primary Liver Cancer. Gan Kagaku Ryoho. Cancer Chemother. 1982, 9, 1623–1627. [Google Scholar]
- Liu, P.-H.; Hsu, C.-Y.; Hsia, C.-Y.; Lee, Y.-H.; Huang, Y.-H.; Su, C.-W.; Lee, F.-Y.; Lin, H.-C.; Huo, T.-I. Proposal and Validation of a New Model to Estimate Survival for Hepatocellular Carcinoma Patients. Eur. J. Cancer 2016, 63, 25–33. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.-C.; Wang, Z.; Cong, W.-M.; Wang, J.-H.; Zeng, M.-S.; Yang, J.-M.; Bie, P.; Liu, L.-X.; Wen, T.-F.; et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 edition). Liver Cancer 2018, 7, 235–260. [Google Scholar] [CrossRef]
- Xie, D.Y.; Ren, Z.G.; Zhou, J.; Fan, J.; Gao, Q. Chinese Clinical Guidelines for the Management of Hepatocellular Carcinoma: Updates and Insights. Hepatobiliary Surg. Nutr. 2020, 9, 452–463. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Wang, J.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020, 9, 682–720. [Google Scholar] [CrossRef]
- Farinati, F.; Vitale, A.; Spolverato, G.; Pawlik, T.M.; Huo, T.-L.; Lee, Y.-H.; Frigo, A.C.; Giacomin, A.; Giannini, E.G.; Ciccarese, F.; et al. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med. 2016, 13, e1002006. [Google Scholar] [CrossRef]
- Buell, J.F.; Lee, L.; Martin, J.E.; Dake, N.A.; Cavanaugh, T.M.; Hanaway, M.J.; Weiskittel, P.; Munda, R.; Alexander, J.W.; Cardi, M.; et al. Laparoscopic Donor Nephrectomy vs. Open Live Donor Nephrectomy: A Quality of Life and Functional Study. Clin. Transplant. 2005, 19, 102–109. [Google Scholar] [CrossRef]
- Cancer of the Liver Italian Program (CLIP) Investigators. A New Prognostic System for Hepatocellular Carcinoma: A Retrospective Study of 435 Patients: The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology 1998, 28, 751–755. [Google Scholar] [CrossRef]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Okuda, K.; Ohtsuki, T.; Obata, H.; Tomimatsu, M.; Okazaki, N.; Hasegawa, H.; Nakajima, Y.; Ohnishi, K. Natural History of Hepatocellular Carcinoma and Prognosis in Relation to Treatment. Study of 850 Patients. Cancer 1985, 56, 918–928. [Google Scholar] [CrossRef]
- Tateishi, R.; Yoshida, H.; Shiina, S.; Imamura, H.; Hasegawa, K.; Teratani, T.; Obi, S.; Sato, S.; Koike, Y.; Fujishima, T.; et al. Proposal of a New Prognostic Model for Hepatocellular Carcinoma: An Analysis of 403 Patients. Gut 2005, 54, 419–425. [Google Scholar] [CrossRef]
- Kudo, M.; Chung, H.; Osaki, Y. Prognostic Staging System for Hepatocellular Carcinoma (CLIP Score): Its Value and Limitations, and a Proposal for a New Staging System, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 2003, 38, 207–215. [Google Scholar] [CrossRef]
- Heinrich, S.; Sprinzl, M.; Schmidtmann, I.; Heil, E.; Koch, S.; Czauderna, C.; Heinrich, B.; Diggs, L.P.P.; Wörns, M.; Kloeckner, R.; et al. Validation of Prognostic Accuracy of MESH, HKLC, and BCLC Classifications in a Large German Cohort of Hepatocellular Carcinoma Patients. United Eur. Gastroenterol. J. 2020, 8, 444–452. [Google Scholar] [CrossRef]
- Chevret, S.; Trinchet, J.-C.; Mathieu, D.; Rached, A.A.; Beaugrand, M.; Chastang, C. A New Prognostic Classification for Predicting Survival in Patients with Hepatocellular Carcinoma. J. Hepatol. 1999, 31, 133–141. [Google Scholar] [CrossRef]
- Leung, W.T.W.; Tang, A.M.Y.; Zee, B.; Lau, W.Y.; Lai, P.B.S.; Leung, T.W.T.; Lau, J.T.F.; Yu, S.C.H.; Johnson, P.J. Construction of the Chinese University Prognostic Index for Hepatocellular Carcinoma and Comparison with the TNM Staging System, the Okuda Staging System, and the Cancer of the Liver Italian Program Staging System. Cancer 2002, 94, 1760–1769. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Huang, Y.-H.; Hsia, C.-Y.; Su, C.-W.; Lin, H.-C.; Loong, C.-C.; Chiou, Y.-Y.; Chiang, J.-H.; Lee, P.-C.; Huo, T.-I.; et al. A New Prognostic Model for Hepatocellular Carcinoma Based on Total Tumor Volume: The Taipei Integrated Scoring System. J. Hepatol. 2010, 53, 108–117. [Google Scholar] [CrossRef]
- Yang, J.D.; Kim, W.R.; Park, K.W.; Chaiteerakij, R.; Kim, B.; Sanderson, S.O.; Larson, J.J.; Pedersen, R.A.; Therneau, T.M.; Gores, G.J.; et al. Model to Estimate Survival in Ambulatory Patients with Hepatocellular Carcinoma. Hepatology 2012, 56, 614–621. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Diseases AAftSoL. Management of Hepatocellular Carcinoma: An Update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- European Association for The Study of The Liver. EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Otto, G.; Pitton, M.B.; Hoppe-Lotichius, M.; Weinmann, A. Liver Transplantation and BCLC Classification: Limitations Impede Optimum Treatment. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 6–12. [Google Scholar] [CrossRef]
- Cho, Y.K.; Chung, J.W.; Kim, J.K.; Ahn, Y.S.; Kim, M.Y.; Park, Y.O.; Kim, W.T.; Byun, J.H. Comparison of 7 Staging Systems for Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Cancer 2008, 112, 352–361. [Google Scholar] [CrossRef]
- Yau, T.; Tang, V.Y.; Yao, T.J.; Fan, S.T.; Lo, C.-M.; Poon, R.T.P. Development of Hong Kong Liver Cancer Staging System with Treatment Stratification for Patients with Hepatocellular Carcinoma. Gastroenterology 2014, 146, 1691–1700. [Google Scholar] [CrossRef]
- Kim, K.M.; Sinn, D.H.; Jung, S.H.; Gwak, G.Y.; Paik, J.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. The Recommended Treatment Algorithms of the BCLC and HKLC Staging Systems: Does Following These Always Improve Survival Rates for HCC Patients? Liver Int. 2016, 36, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Adhoute, X.; Penaranda, G.; Bronowicki, J.P. Usefulness of the HKLC vs. the BCLC staging system in a European HCC cohort. J. Hepatol. 2015, 62, 492–493. [Google Scholar] [CrossRef] [PubMed]
- Borzio, M.; Dionigi, E.; Rossini, A.; Marignani, M.; Sacco, R.; De Sio, I.; Bertolini, E.; Francica, G.; Giacomin, A.; Parisi, G.; et al. External Validation of the ITA.LI.CA Prognostic System for Patients with Hepatocellular Carcinoma: A Multicenter Cohort Study. Hepatology 2018, 67, 2215–2225. [Google Scholar] [CrossRef]
- Vitale, A.; Farinati, F.; Noaro, G.; Burra, P.; Pawlik, T.M.; Bucci, L.; Giannini, E.G.; Faggiano, C.; Ciccarese, F.; Rapaccini, G.L.; et al. Restaging Patients With Hepatocellular Carcinoma Before Additional Treatment Decisions: A Multicenter Cohort Study. Hepatology 2018, 68, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Farinati, F.; Pawlik, T.M.; Frigo, A.C.; Giannini, E.G.; Napoli, L.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. The Concept of Therapeutic Hierarchy for Patients with Hepatocellular Carcinoma: A Multicenter Cohort Study. Liver Int. 2019, 39, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Zustovich, F.; Farinati, F.; Cillo, U.; Vitale, A.; Zanus, G.; Donach, M.; Farina, M.; Zovato, S.; Pastoreli, D. Pegylated Liposomal Doxorubicin and Gemcitabine in Patients with Advanced Hepatocellular Carcinoma: Results of a Phase 2 Study. Cancer 2011, 117, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Vitale, A.; Volk, M.L.; Frigo, A.C.; Grigoletto, F.; Brolese, A.; Zanus, A.; D’Amico, F.; Farinati, F.; Burra, P.; et al. The Survival Benefit of Liver Transplantation in Hepatocellular Carcinoma Patients. Dig Liver Dis. 2010, 42, 642–649. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Tabrizian, P.; Park, J.-W.; Yang, J.; Yan, L.; Schwartz, M.; Han, G.; Izzo, F.; Chen, M.; et al. The Role of Hepatic Resection in the Treatment of Hepatocellular Cancer. Hepatology 2015, 62, 440–451. [Google Scholar] [CrossRef]
- Pecorelli, A.; Lenzi, B.; Gramenzi, A. Curative Therapies Are Superior to Standard of Care (Transarterial Chemoembolization) for Intermediate Stage Hepatocellular Carcinoma. Liver Int. 2017, 37, 423–433. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, E.W.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Maida, M.; Orlando, E.; Camma, C.; Cabibbo, G. Staging Systems of Hepatocellular Carcinoma: A Review of Literature. World J. Gastroenterol. 2014, 20, 4141–4150. [Google Scholar] [CrossRef]
- Marrero, J.A.; Fontana, R.J.; Barrat, A.; Askari, F.; Conjeevaram, H.S.; Su, G.L.; Lok, A.S. Prognosis of Hepatocellular Carcinoma: Comparison of 7 Staging Systems in an American Cohort. Hepatology 2005, 41, 707–716. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Park, M.S.; Kim, E.H.; Seong, J.; Lee, D.J.; Kan, K.H. Applicability of BCLC Stage for Prognostic Stratification in Comparison with Other Staging Systems: Single Centre Experience from Long-Term Clinical Outcomes of 1717 Treatment-Naive Patients with Hepatocellular Carcinoma. Liver Int. 2012, 32, 1120–1127. [Google Scholar] [CrossRef]
- Cillo, U.; Bassanello, M.; Vitale, A.; Grigoletto, F.A.; Burra, P.; Fagiuoli, S.; D’Amico, F.; Ciarleglio, F.A.; Boccagni, P.; Brolese, A.; et al. The Critical Issue of Hepatocellular Carcinoma Prognostic Classification: Which Is the Best Tool Available? J. Hepatol. 2004, 40, 124–131. [Google Scholar] [CrossRef]
- Liu, P.-H.; Hsu, C.-Y.; Hsia, C.-Y.; Lee, Y.-H.; Su, C.-W.; Huang, Y.-H.; Lee, F.-Y.; Lin, H.-C.; Huo, T.-I. Prognosis of Hepatocellular Carcinoma: Assessment of Eleven Staging Systems. J. Hepatol. 2016, 64, 601–608. [Google Scholar] [CrossRef]
- Farinati, F.; Sergio, A.; Baldan, A.; Giacomin, A.; Di Nolfo, M.A.; Del Poggio, P.; Benvegnù, L.; Rapaccini, G.L.; Zoli, M.; Borzio, F.; et al. Early and Very Early Hepatocellular Carcinoma: When and How Much Do Staging and Choice of Treatment Really Matter? A Multi-Center Study. BMC Cancer 2009, 9, 33. [Google Scholar] [CrossRef]
- Sirivatanauksorn, Y.; Tovikkai, C. Comparison of Staging Systems of Hepatocellular Carcinoma. HPB Surg. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Choo, S.P.; Tan, W.L.; Goh, B.K.P. Comparison of Hepatocellular Carcinoma in Eastern versus Western Populations. Cancer 2016, 122, 3430–3446. [Google Scholar] [CrossRef]
- Finotti, M.; Vitale, A.; Volk, M.; Cillo, U. A 2020 update on liver transplant for hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Tanabe, G.; Sako, K.; Hiwaki, T.; Hokotate, H.; Fukukura, Y.; Baba, Y.; Imamura, Y.; Aikou, T. Discrimination Value of the New Western Prognostic System (CLIP Score) for Hepatocellular Carcinoma in 662 Japanese Patients. Hepatology 2001, 34, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Tanabe, G.; Nuruki, K.; Hamanoue, M.; Komorizono, Y.; Oketani, M.; Hokotate, H.; Inoue, H.; Baba, Y.; Imamura, Y.; et al. Prognostic Performance of the New Classification of Primary Liver Cancer of Japan (4th Edition) for Patients with Hepatocellular Carcinoma: A Validation Analysis. Hepatol. Res. 2002, 24, 395–403. [Google Scholar] [CrossRef]
- Adam, R.; Karam, V.; Cailliez, V.; O Grady, J.G.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-Year Evolution of Liver Transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Kudo, M.; Chung, H.; Haji, S.; Osaki, Y.; Oka, H.; Seki, T.; Kasugai, H.; Sasaki, Y.; Matsunaga, T. Validation of a New Prognostic Staging System for Hepatocellular Carcinoma: The JIS Score Compared with the CLIP Score. Hepatology 2004, 40, 1396–1405. [Google Scholar] [CrossRef]
- Grieco, A.; Pompili, M.; Caminiti, G.; Miele, L.; Covino, M.; Alfei, B.; Rapaccini, G.L.; Gasbarrini, G. Prognostic Factors for Survival in Patients with Early-Intermediate Hepatocellular Carcinoma Undergoing Non-surgical Therapy: Comparison of Okuda, CLIP, and BCLC Staging Systems in a Single Italian Centre. Gut 2005, 54, 411–418. [Google Scholar] [CrossRef]
- Toyoda, H.; Kumada, T.; Kiriyama, S. Comparison of the Usefulness of Three Staging Systems for Hepatocellular Carcinoma (CLIP, BCLC, and JIS) in Japan. Am. J. Gastroenterol. 2005, 100, 1764–1771. [Google Scholar] [CrossRef]
- Cillo, U.; Vitale, A.; Grigoletto, F.; Farinati, F.; Brolese, A.; Zanus, G.; Neri, D.; Boccagni, P.; Srsen, N.; D’Amico, F.; et al. Prospective Validation of the Barcelona Clinic Liver Cancer Staging System. J. Hepatol. 2006, 44, 723–731. [Google Scholar] [CrossRef]
- Nanashima, A.; Sumida, Y.; Abo, T.; Shindou, H.; Fukuoka, H.; Takeshita, H.; Hidaka, S.; Tanaka, K.; Sawai, T.; Yasutake, T.; et al. Modified Japan Integrated Staging Is Currently the Best Available Staging System for Hepatocellular Carcinoma Patients Who Have Undergone Hepatectomy. J. Gastroenterol. 2006, 41, 250–256. [Google Scholar] [CrossRef]
- Chung, H.; Kudo, M.; Takahashi, S.; Hagiwara, S.; Sakaguchi, Y.; Inoue, T.; Minami, Y.; Ueshima, K.; Fukunaga, T.; Matsunaga, T. Comparison of Three Current Staging Systems for Hepatocellular Carcinoma: Japan Integrated Staging Score, New Barcelona Clinic Liver Cancer Staging Classification, and Tokyo Score. J. Gastroenterol. Hepatol. 2008, 23, 445–452. [Google Scholar] [CrossRef]
- Huo, T.-I.; Lin, H.-C.; Hsia, C.-Y.; Wu, J.-C.; Lee, P.-C.; Chi, C.-W.; Lee, S.-D. The Model for End-Stage Liver Disease Based Cancer Staging Systems Are Better Prognostic Models for Hepatocellular Carcinoma: A Prospective Sequential Survey. Am. J. Gastroenterol. 2007, 102, 1920–1930. [Google Scholar] [CrossRef]
- Guglielmi, A.; Ruzzenente, A.; Pachera, S.; Valdegamberi, A.; Sandri, M.; D’Onofrio, M.; Iacono, C. Comparison of Seven Staging Systems in Cirrhotic Patients with Hepatocellular Carcinoma in a Cohort of Patients Who Underwent Radiofrequency Ablation with Complete Response. Am. J. Gastroenterol. 2008, 103, 597–604. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hu, F.-C.; Huang, G.-T.; Lee, P.-H.; Tsang, Y.-M.; Cheng, A.-L.; Chen, D.-S.; Wang, J.-D.; Sheu, J.-C. Applicability of Staging Systems for Patients with Hepatocellular Carcinoma Is Dependent on Treatment Method—Analysis of 2010 Taiwanese Patients. Eur. J. Cancer 2009, 45, 1630–1639. [Google Scholar] [CrossRef]
- Chen, T.; Chu, C.; Yu, J.; Chen, C.; Chan, D.; Liu, Y.; Hsieh, C. Comparison of Clinical Staging Systems in Predicting Survival of Hepatocellular Carcinoma Patients Receiving Major or Minor Hepatectomy. Eur. J. Surg. Oncol. (EJSO) 2007, 33, 480–487. [Google Scholar] [CrossRef]
- Nanashima, A.; Omagari, K.; Tobinaga, S.; Shibata, K.; Sumida, Y.; Mine, M.; Morino, S.; Shibasaki, S.; Ide, N.; Shindou, H.; et al. Comparative Study of Survival of Patients with Hepatocellular Carcinoma Predicted by Different Staging Systems Using Multivariate Analysis. Eur. J. Surg. Oncol. 2005, 31, 882–890. [Google Scholar] [CrossRef]
- Yen, Y.-H.; Changchien, C.-S.; Wang, J.-H.; Kee, K.-M.; Hung, C.-H.; Hu, T.-H.; Lee, C.-M.; Lin, C.-Y.; Wang, C.-C.; Chen, T.-Y.; et al. A Modified TNM-Based Japan Integrated Score Combined with AFP Level May Serve as a Better Staging System for Early-Stage Predominant Hepatocellular Carcinoma Patients. Dig. Liver Dis. 2009, 41, 431–441. [Google Scholar] [CrossRef]
- Chan, S.L.; Mo, F.K.F.; Johnson, P.J.; Liem, G.S.; Chan, T.C.; Poon, M.C.; Ma, B.B.Y.; Leung, T.W.T.; Lai, P.B.S.; Chan, A.T.C.; et al. Prospective Validation of the Chinese University Prognostic Index and Comparison with Other Staging Systems for Hepatocellular Carcinoma in an Asian Population. J. Gastroenterol. Hepatol. 2011, 26, 340–347. [Google Scholar] [CrossRef]
- Tournoux-Facon, C.; Paoletti, X.; Barbare, J.-C.; Bouché, O.; Rougier, P.; Dahan, L.; Lombard-Bohas, C.; Faroux, R.; Raoul, J.L.; Bedenne, L.; et al. Development and Validation of a New Prognostic Score of Death for Patients with Hepatocellular Carcinoma in Palliative Setting. J. Hepatol. 2011, 54, 108–114. [Google Scholar] [CrossRef]
- Op den Winkel, M.; Nagel, D.; Sappl, J.; Op den Winkel, P.; Lamerz, R.; Zech, C.J.; Straub, G.; Nickel, T.; Rentsch, M.; Stieber, P.; et al. Prognosis of Patients with Hepatocellular Carcinoma. Validation and Ranking of Established Staging-Systems in a Large Western HCC-Cohort. PLoS ONE 2012, 7, e45066. [Google Scholar] [CrossRef]
- Gomaa, A.I.; Hashim, M.S.; Waked, I. Comparing Staging Systems for Predicting Prognosis and Survival in Patients with Hepatocellular Carcinoma in Egypt. PLoS ONE 2014, 9, e90929. [Google Scholar] [CrossRef]
- Memon, K.; Kulik, L.M.; Lewandowski, R.J. Comparative Study of Staging Systems for Hepatocellular Carci-Noma in 428 Patients Treated with Radioembolization. J. Vasc. Interv. Radiol. 2014, 25, 1056–1066. [Google Scholar] [CrossRef][Green Version]
- Yan, X.; Fu, X.; Cai, C.; Zi, X.; Yao, H.; Qiu, J. Validation of Models in Patients with Hepatocellular Carcinoma: Comparison of Hong Kong Liver Cancer with Barcelona Clinic Liver Cancer Staging System in a Chinese Cohort. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1180–1186. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Hong, Y.-F.; Lin, J.; Li, X.; Wu, D.-H.; Wen, J.-Y.; Chen, J.; Ruan, D.-Y.; Lin, Q.; Dong, M.; et al. Validation and Ranking of Seven Staging Systems of Hepatocellular Carcinoma. Oncol. Lett. 2017, 14, 705–714. [Google Scholar] [CrossRef]
- Sohn, J.H.; Duran, R.; Zhao, Y.; Fleckenstein, F.; Chapiro, J.; Sahu, S.; Schernthaner, R.E.; Qian, T.; Lee, H.; Zhao, L.; et al. Validation of the Hong Kong Liver Cancer Staging System in Determining Prognosis of the North American Patients Following Intra-arterial Therapy. Clin. Gastroenterol. Hepatol. 2017, 15, 746–755. [Google Scholar] [CrossRef]
- Selby, L.K.E.; Tay, R.X.Y.; Woon, W.W.L.; Low, J.K.; Bei, W.; Shelat, V.G.; Pang, T.C.Y.; Junnarkar, S.P. Validity of the Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer Staging Systems for Hepatocellular Carcinoma in Singapore. J. Hepato-Biliary-Pancreat. Sci. 2017, 24, 143–152. [Google Scholar] [CrossRef]
- Samawi, H.H.; Sim, H.W.; Chan, K.K. Prognosis of Patients with Hepatocellular Carcinoma Treated with Soraf-Enib: A Comparison of Five Models in a Large Canadian Database. Cancer Med. 2018. [Google Scholar] [CrossRef]
- Farinati, F.; Vanin, V.; Giacomin, A. BCLC Stage B Hepatocellular Carcinoma and Transcatheter Arterial Chemoembolization: A 20-Year Survey by the Italian Liver Cancer Group. Liver Int. 2015, 35, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Garuti, F.; Neri, A.; Avanzato, F.; Gramenzi, A.; Rampoldi, D.; Rucci, P.; Farinati, F.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.L.; et al. The Changing Scenario of Hepatocellular Carcinoma in Italy: An Update. Liver Int. 2021, 41, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, A.N.; Enders, C.K. An Introduction to Modern Missing Data Analyses. J. School Psychol. 2010, 48, 5–37. [Google Scholar] [CrossRef] [PubMed]
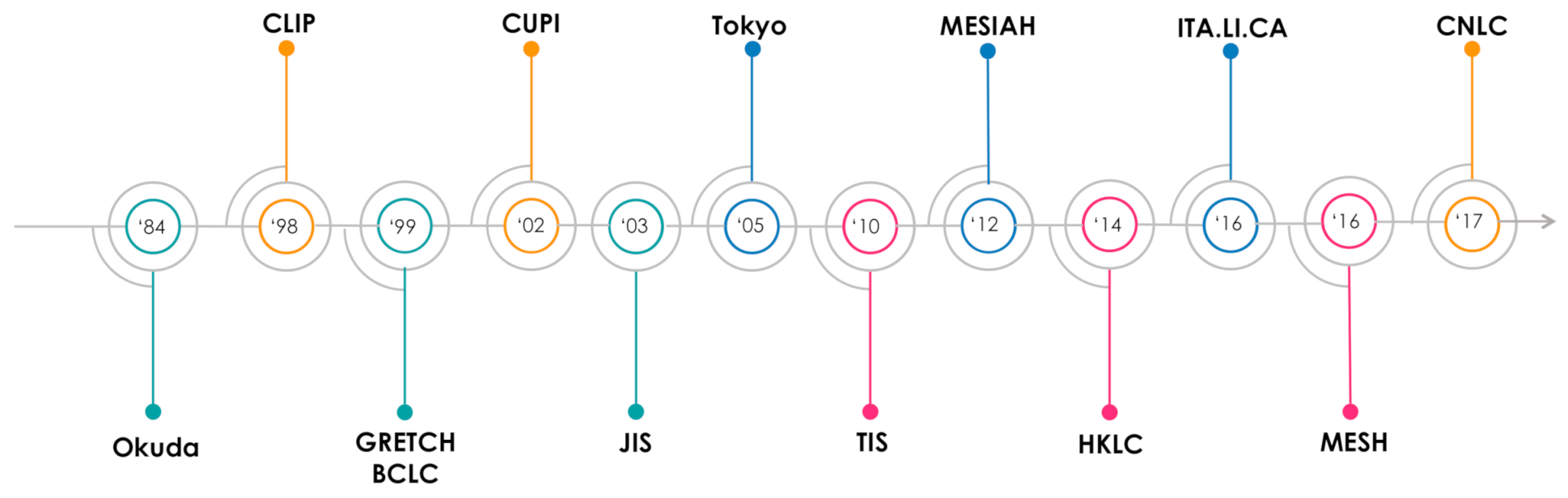
| Staging System | Year | Pts Number | Performance Status | Liver Function | HCC Number | Size | aFP | Vascular Invasion | Metastasis | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Okuda [16] | 1984 | 600 | No | Ascites Albumin Bilirubin | No | Yes | No | No | No | / |
| CLIP [14] | 1998 | 435 | No | CPS | Yes | Yes | Yes | Yes | No | / |
| French (GRETCH) [20] | 1999 | 761 | Karnofsky | Bilirubin | No | No | Yes | Yes | No | Alk-P |
| CUPI [21] | 2002 | 926 | Symptoms | Ascites Bilirubin | Yes | Yes | Yes | Yes | Yes | Alk-P |
| JIS [18] | 2003 | Review | No | CPS | Yes | Yes | No | Yes | Yes | / |
| Tokyo [17] | 2005 | 403 | No | Albumin Bilirubin | Yes | Yes | No | No | No | / |
| TIS [22] | 2010 | 2030 | No | CPS | TTV | TTV | Yes | No | No | / |
| MESIAH [23] | 2012 | 477 | No | MELD Albumin | Yes | Yes | Yes | Yes | Yes | Age |
| Taiwanese MESH [8] | 2016 | 3182 | ECOG | CPS | Yes | Yes | Yes | Yes | Yes | Alk-P |
| Prognostic Factors | Scores | |
|---|---|---|
| 0 | 1 | |
| Tumour burden | Within MC | Beyond MC |
| Vascular invasion or metastases | Absent | Present |
| CPS score | 5 | ≥6 |
| PS | 0–1 | ≥2 |
| Serum aFP | <20 ng/mL | ≥20 ng/mL |
| Serum Alk-P | <200 IU/L | ≥200 IU/L |
| Variables | CNCL Stage | ||||||
|---|---|---|---|---|---|---|---|
| Ia | Ib | IIa | IIb | IIIa | IIIb | IV | |
| PS | 0–2 | 0–2 | 0–2 | 0–2 | 0–2 | 0–2 | 3–4 |
| CPS | A-B | A-B | A-B | A-B | A-B | A-B | C |
| Number and size of HCC | Single ≤ 5 cm | Single HCC > 5 cm OR 2–3 HCC ≤ 3 cm | 2–3 HCC >3 cm | ≥4, no size limits | No number or size limits | No number or size limits | No number or size limits |
| Vascular invasion | No | No | No | No | Yes | Yes | Yes/No |
| Extrahepatic metastases | No | No | No | No | No | Yes | Yes/No |
| SCORE | 0 | 1 | 2 | 3 | 4 | 5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Stage | 0 | A | B1 | B2 | B3 | C | ||||
| Diameter (cm) | <2 | ≤3 | 2–5 | ≤5 | >5 | >5 | ≤5 | >5 | Any | Any |
| Number of Nodules | 1 | 2–3 | 1 | 2–3 | 1 | 2–3 | >3 | >3 | Any | Any |
| HVI and/or metastases | No | No | No | No | No | No | No | No | Intra HVI | Extra HVI or Metastases |
| Functional Score | ||||||||||
| CPS | 5 | 6 | 7 | 8 | 9 | 10–15 | ||||
| ECOG PS | 0 | 1 | 2 | 3–4 | ||||||
| aFP | ≤1000 ng/mL | >1000 ng/mL | ||||||||
| Tumor Stage | Diameter (cm) | <2 | ≤3 | ≤5 | 3–5 | >5 | ≤5 | >5 | >5 | Any | Any | Any |
| Number of Nodules | 1 | 2–3 | 1 | 2–3 | 1 | >3 | 2–3 | >3 | Any | Any | Any | |
| HVI and/or Metastases | No | No | No | No | No | No | No | No | Intra HVI | Extra HVI or Metastases | Any | |
| Functional Score | CPS ≤9 and PS 0 or CPS ≤7 and PS 1–2 | CPS 8–9 and PS 1–2, or CPS >9, or PS >2 | ||||||||||
| Staging | 0 | A | B1 | B2 | B3 | C | D | |||||
| Therapy | 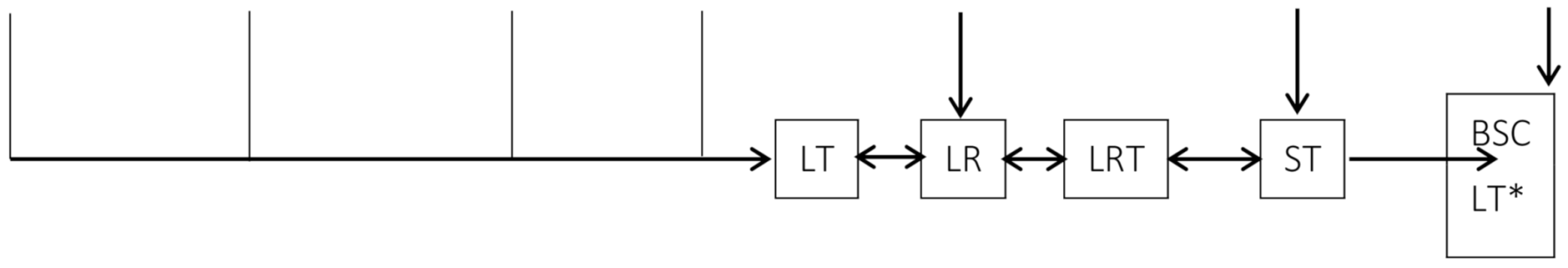 | |||||||||||
| Authors | Year | Country | Study | n. of Patients | Median F.U. Months | Modality Comparison | Compared Staging Systems | Most Representative Stages | Results of Comparison | Conclusions | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ueno et al. [52] | ‘00 | Japan | R | 662 | - | LR | CLIP | 1 (195); 2 (169) | 184.34 | - | The CLIP score has the highest stratification ability, especially in 3 subgroups of patients who received surgery, TACE, and PEI |
| AJCC | IV (253); II (193) | 102.24 | - | ||||||||
| Okuda | I (375); II (278) | 92.01 | - | ||||||||
| Ueno et al. [53] | ‘02 | Japan | R | 662 | - | LR | CLIP 4th edition | III (275); II 223 | 155.61 | - | CLIP score 4th edition has a higher stratification value than the 3rd edition. However, this benefit is due to the non-surgical patients, rather than to the surgical patients. |
| CLIP 3th edition | IVA (237); II (196) | 122.52 | - | ||||||||
| Cillo et al. [46] | ‘04 | Italy | R | 187 | 11 (0.3–120) | LR AIC | BCLC | B (43); C (25) | 70.67 | 953.02 | BCLC is superior in surgical and non surgical patients; |
| CLIP | 1 (61); 0 (55) | 41.29 | 984.40 | ||||||||
| Okuda | I (98); II (79) | 36.52 | 985.18 | ||||||||
| French | I.R. (92); L. R. (78) | 34.88 | 986.82 | ||||||||
| CUPI | L.R.(157); I.R. [54] | 27.49 | 994.21 | ||||||||
| Kudo et al. [55] | ‘04 | Japan | R | 4525 | 50 (14–156) | LR AIC | JIS | 1 (1399); 2 (1471) | 1238.05 | 33,642.3 | JIS score performed better than CLIP score |
| CLIP | 1 (1687); 0 (1181) | 1062.09 | 33,822.32 | ||||||||
| Grieco et al. [56] | ‘05 | Italy | R | 268 | 32 (3–130) | LR AIC | Okuda | I (190); II (78) | 50.4 | 0.669 | CLIP and BCLC more effective than in early-intermediate HCC in HCC underwent nonsurgical treatment or local treatments (PEI, RF, TACE). However, BCLC performed better in very early stage |
| CLIP | 0 (129); 1 (82) | 76.8 | 0.726 | ||||||||
| BCLC | A4 (93); A2 (68) | 89.9 | 0.731 | ||||||||
| Toyoda et al. [57] | ‘05 | Japan | R | 1508 | - | AIC | JIS | 1 (349); 2 (311) | - | 9987.96 | Comparison in two era: pre e post 1991. JIS system is the appropriate system in current era of early detection and treatment of HCC. |
| CLIP | 1 (396); 0 (332) | - | 10,031.8 | ||||||||
| BCLC | A (632); C (418); | - | 10,079.7 | ||||||||
| Marrero et al. [44] | ‘05 | USA | R | 244 | - | LR AIC | BCLC | C (31); A (28) | 76.8 | 943.7 | The BCLC staging system provided the best prognostic stratification. |
| GRETCH | B (42); C (39) | 59.2 | 970.4 | ||||||||
| TNM | III (36); II (31) | 54.3 | 978.5 | ||||||||
| Okuda | 2 (44); 3 (37) | 52.9 | 974.4 | ||||||||
| CUPI | I (44); H (37) | 52.3 | 990.8 | ||||||||
| CLIP | 1 (31); 0 (19) | 51.9 | 981.5 | ||||||||
| JIS | 1 (25); 2–4 (19) | 49.7 | 994.0 | ||||||||
| Cillo et al. [58] | ‘06 | Italy | P | 195 | 25 (5–54) | BCLC | A (89); B (58) | 43.01 | 885.98 | Including patient treated with LT, BCLC classification showed a better prognostic ability | |
| UNOS-TNM | II (75); IV (54) | 20.03 | 915.62 | ||||||||
| JIS | 2 (70); 1 (57) | 12.45 | 928.16 | ||||||||
| Okuda | I (117); II (71) | 3.98 | 933.06 | ||||||||
| CLIP | 1 (63); 2 (54) | 4.17 | 938.10 | ||||||||
| Nanashima et al. [59] | ‘06 | Japan | R | 230 | - | AIC | Modified JIS | - | - | 634.3 | For HCC after hepatic resection modified JIS score is the best predictor of prognosis |
| JIS | - | - | 635.8 | ||||||||
| Modified CLIP | - | - | 634.8 | ||||||||
| CLIP | - | - | 636.5 | ||||||||
| Japan TNM | - | - | 637.4 | ||||||||
| Chung et al. [60] | ‘07 | Japan | R | 290 | - | LR AIC | JIS | 2 (98); 1 (80) | 138.0 | 1635.6 | The JIS score provided the best prognostic stratification in a Japanese cohort of HCC patients who were mainly diagnosed at early stages and treated with radical therapies. |
| BCLC | A (131); B (63) | 111.0 | 1661.0 | ||||||||
| Tokyo | 2 (75); 3 (60) | 108.0 | 1671.4 | ||||||||
| Huo et al. [61] | ‘07 | Taiwan | R | - | - | LR AIC | CLIP + MELD | - | 192 | 1471.2 | The MELD-based CLIP and JIS staging systems have an improved predictive ability compared to the original system and are feasible models for HCC staging in the MELD era |
| CLIP | - | 173.5 | 1489.7 | ||||||||
| JIS + MELD | - | 140.7 | 1522.5 | ||||||||
| BCLC + MELD | - | 126.9 | 1536.3 | ||||||||
| JIS | - | 124.7 | 1538.5 | ||||||||
| BCLC | - | 122.9 | 1540.3 | ||||||||
| Cho et al. [29] | ‘07 | Korea | R | 131 | 24 (2–83) | LR AIC | CLIP | 1 (55); 0 (34) | 38.10 | 850.0 | The CLIP system provided the best prognostic stratification for a cohort the patients with HCC who underwent TACE. |
| JIS | 2 (50); 1 (44) | 33.6 | 854.5 | ||||||||
| Mod CLIP | 1 (54); 2–0 [54] | 26.9 | 863.2 | ||||||||
| Mod JIS | 2 (51); 1 (46) | 21.6 | 866.4 | ||||||||
| C-P score | 6 (47); 5 (45) | 18.8 | 869.2 | ||||||||
| Okuda | I (86); II (45) | 13.5 | 868.5 | ||||||||
| BCLC | - | 6.4 | 877.6 | ||||||||
| Guglielmi et al. [62] | ‘08 | Italy | R | 112 | 24 (3–92) | LR | BCLC | B (33); A4 (31) | 15.1 | - | BCLC performed better in HCC patients who underwent RF; moreover, it can give important prognostic information after complete response to treatment. |
| GRETCH | A (60); B (34) | 12.4 | - | ||||||||
| Okuda | I (63); II (28) | 10.5 | - | ||||||||
| CUPI | LR (88); I (8) | 8.0 | - | ||||||||
| JIS | 2 (53); 1 (28) | 3.9 | - | ||||||||
| CLIP | 1 (46); 0 [54] | 1.9 | - | ||||||||
| TNM | I (55); II (37) | 1.3 | - | ||||||||
| Chen et al. [63] | ‘08 | Taiwan | R | 2010 | - | LR AIC | Tokyo | - | 279.1 | 19,383.7 | The Tokyo score was the most informative one for predicting the survival of HCC patients as a whole, receiving LR, or TACE. CLIP score was the best fit system for HCC patients receiving CT or BSC. Each stagingsystem showed a significant difference in predicting the probability of survival across different stages. The applicability of staging systems for patients with HCC was dependent on treatment methods. |
| JIS | - | 213.3 | 19,492.3 | ||||||||
| CLIP | - | 205.3 | 19,508.3 | ||||||||
| BCLC | - | 191.2 | 19,540.1 | ||||||||
| Okuda | - | 184.7 | 19,551.7 | ||||||||
| TNM | - | 93.3 | 19,782.9 | ||||||||
| Chen et al. [64] | ‘07 | Taiwan | R | 382 | 21 (0.1–120) | LR | CLIP | - | 131.3 | - | While the CLIP system should be considered to stage major hepatectomy patients, the JIS system could be chosen to stage minor hepatectomy patients. |
| JIS | - | 122.8 | - | ||||||||
| BCLC | - | 94.7 | - | ||||||||
| Okuda | - | 81.3 | - | ||||||||
| CUPI | - | 55.8 | - | ||||||||
| AJCC | - | 50.5 | - | ||||||||
| Nanashima et al. [65] | ‘05 | Japan | R | 210 | - | AIC | Modified CLIP score | 1 (138); 2 (43) | - | 425.9 | The modified CLIP score showed the lowest AIC for DFS and OS in HCC underwent liver resection. |
| CLIP score | 1 (156); 2 (30) | - | 427.9 | ||||||||
| JIS score | 1 (114); 2 (66) | - | 436.4 | ||||||||
| AJCC TNM stage | I (125); II (61) | - | 441.3 | ||||||||
| Japan TNM stage | II (132); III (56) | - | 438.5 | ||||||||
| Yen et al. [66] | ‘09 | Taiwan | R | 2882 | - | AIC | CLIP | - | - | 35,21 | CLIP system provided the best prognostic stratification in late stages HCC. TNM-based JIS combined aFP may be the most applicable in early-stage HCC patients |
| TNM-based JIS | - | - | 35.42 | ||||||||
| TNM-based JIS + aFP | - | - | 35.27 | ||||||||
| BCLC | - | - | 35.58 | ||||||||
| JIS | - | - | 35.47 | ||||||||
| Chan et al. [67] | ‘10 | China | P | 595 | 41.4 (40–46.6) | LR AIC | CLIP | - | 213.05 | 5791.02 | CUPI is an appropriate staging system for HBV-related HCC. In patients with advanced HCC, both CUPI and CLIP offer good risk stratification |
| CUPI | - | 197.04 | 5807.03 | ||||||||
| Okuda | - | 154.57 | 5849.50 | ||||||||
| TNM | - | 90.17 | 5913.91 | ||||||||
| BCLC | - | 64.39 | 5939.68 | ||||||||
| Chen et al. [63] | ‘10 | Taiwan | R | 2010 | - | LR AIC | Tokyo | - | 552.2 | 19,383.7 | The Tokyo staging system was the best in predicting survival for patients receiving LR or TACE while CLIP scoring system was the most suitable in predicting survival in HCC patients receiving CT or BSC |
| JIS | - | 443.5 | 19,492.3 | ||||||||
| CLIP | - | 427.5 | 19,508.3 | ||||||||
| BCLC | - | 395.8 | 19,540.1 | ||||||||
| Okuda | - | 384.1 | 19,551.7 | ||||||||
| TNM | - | 153.0 | 19,782.9 | ||||||||
| Tournoux et al. [68] | ‘11 | France | P | 416 | 48 | AIC | Tournoux-Facon score | - | - | 3884 | The new prognostic score and CLIP + PS are recommended in palliative settings |
| CLIP + PS | - | - | 3894 | ||||||||
| CLIP | - | - | 3906 | ||||||||
| GRETCH | - | - | 3910 | ||||||||
| BCLC | - | - | 3928 | ||||||||
| Okuda | - | - | 3913 | ||||||||
| Op de Winkel et al. [69] | ‘12 | Germany | R | 405 | 14 (0.2–113) | LR AIC | CLIP | 1 (131); 2 (80) | - | 2286 | CLIP-score was identified as the most suitable staging system for predicting prognosis in a large German cohort of predominantly non-surgical HCC-patients |
| JIS | 2 (135); 3 (85) | - | 2293 | ||||||||
| Okuda | I (202); II (145) | - | 2337 | ||||||||
| GETCH | Intermediate 176; Low 103 | - | 2342 | ||||||||
| TNM | I (122); III (114) | - | 2342 | ||||||||
| BCLC | C (138); B (99) | - | 2343 | ||||||||
| Child | A (130); B (120) | - | 2369 | ||||||||
| Gomaa et al. [70] | ‘14 | Egypt | P | 2000 | 15 (13.6–16) | LR | BCLC | B (608); A (501) | 810 | - | BCLC staging system provided the best prognostic stratification for HCC patients. However, CLIP score has the highest stratification ability in patients with advanced HCC highlighting the importance of including aFP in best staging system. |
| JIS | 2 (625); 3 (579) | 694 | - | ||||||||
| CLIP | 2 (531); 1 (507) | 679 | - | ||||||||
| Okuda | II (917); I (696) | 363 | - | ||||||||
| Memon et al. [71] | ‘14 | USA | R | 428 | 23.2 | CLIP | 2 (115); 1 (113) | 127.22 | 2992.80 | CLIP was most accurate in predicting HCC survival in patients after Y-90 TARE treatment. | |
| JIS | 4 (140); 3 (139) | 103.98 | 3014.04 | ||||||||
| UNOS | T4b [54]; T2 (99) | 94.61 | 3023.41 | ||||||||
| BCLC | C (196); B (122) | 81.97 | 3032.05 | ||||||||
| GRETCH | B (239); A (95) | 73.40 | 3038.61 | ||||||||
| CUPI | LR (347); IR (72) | 64.45 | 3047.57 | ||||||||
| Okuda | 2 (266); 1 (151) | 53.13 | 3058.89 | ||||||||
| CTP | B 215; A 201 | 38.00 | 3074.02 | ||||||||
| Adhoute et al. [32] | ‘15 | Taiwan | R | 3182 | 17 | LR AIC | HKLC | I (1001); II (862) | 1370.15 | 5334.15 | Compared with the BCLC system, the HKLC system has better prognostic accuracy |
| BCLC | C (1282); A (736) | 920.99 | 5582.84 | ||||||||
| Yan et al. [72] | ‘15 | China | R | 668 | - | AIC | HKLC | I (267); IIb (201) | - | 4709.48 | Especially in HBV patient, the HKLC score is the best prognostic system in a Chinese cohort |
| BCLC | A1-A2 (264);C (117) | - | 4852.70 | ||||||||
| Liu et al. [47] | ‘16 | Taiwan | R | 3182 | 17 | LR AIC | CLIP | - | 1387.62 | 5666.83 | CLIP score is the most accurate prognostic model |
| TIS | - | 1204.24 | 5782.58 | ||||||||
| HKLC | - | 1078.52 | 5846.45 | ||||||||
| JIS | - | 1058.81 | 5850.54 | ||||||||
| Tokyo | - | 904.71 | 5966.41 | ||||||||
| BCLC | - | 854.90 | 5973.09 | ||||||||
| French | - | 874.57 | 5980.77 | ||||||||
| Okuda | - | 841.81 | 6029.90 | ||||||||
| AJCC TNM-7 | - | 820.24 | 6035.86 | ||||||||
| CUPI | - | 747.38 | 6100.00 | ||||||||
| TNM by LCSGJ | 586.74 | 6201.56 | |||||||||
| Farinati et al. [12] | ‘16 | Italy | R | 5183 | 58 (26–106) | LR AIC | ITA.LI.CA | - | - | 15,558 | Comparison between 2003–2012 in internal and external validation(n = 3281). The ITA.LI.CA score showed the best discriminatory ability and monotonicity of gradients among the most common HCC staging systems |
| CLIP | - | 215.38 | 15,721 | ||||||||
| HKLC | - | 179.83 | 15,728 | ||||||||
| MESIAH | - | 285.23 | 15,772 | ||||||||
| JIS | - | 356.03 | 15,898 | ||||||||
| Modified BCLC | - | 411.35 | 15,952 | ||||||||
| BCLC | - | 578.49 | 16,119 | ||||||||
| Chen et al. [73] | ‘17 | China | R | 220 | - | LR AIC | CLIP | 5 (57); 4 (50) | 70.6 | 1601.5 | CLIP performed better than others. CIS ranked second in predicting 3-month mortality |
| CIS | 2 (74); 1 (59) | 48.4 | 1632.3 | ||||||||
| CUPI | 1 (106); 2 (71) | 46.7 | 1629.9 | ||||||||
| Okuda | II (119); III (67) | 36.0 | 1641.1 | ||||||||
| TNM | III (127); IV (46) | 21.0 | 1654.8 | ||||||||
| JIS | 4 (83); 3 (56) | 46.8 | 1627.4 | ||||||||
| BCLC | C (138); D (82) | 7.24 | 1671.1 | ||||||||
| Sohn et al. [74] | ‘17 | USA | R | 1009 | - | LR AIC | HKLC-9 | - | 250 | 6200 | HKLC system determined prognosis in patients following TACE |
| HKLC-5 | - | 201 | 6241 | ||||||||
| BCLC | - | 119 | 6321 | ||||||||
| Selby et al. [75] | ‘17 | Singapore | R | 766 | - | AIC | HKLC | I (208); II (203) | - | 5711 | HKLC has better performance in guiding treatment. |
| BCLC | A (275); C (222) | - | 5764 | ||||||||
| Samawi et al. [76] | ‘18 | Canada | R | 681 | 37.6 (29.5–41) | LR AIC | CLIP | 2 (215); 1 (163) | 63.37 | 5725.76 | CLIP performed better while BCLC and TNM7 performed less favourably but the differences were small |
| Okuda | 1 (364); 2 (272) | 50.76 | 5730.38 | ||||||||
| ALBI | 2 (503); 1 (119) | 24.40 | 5756.73 | ||||||||
| BCLC | C (591); B (37) | 23.88 | 5759.25 | ||||||||
| TNM7 | IV (394); III (148) | 11.63 | 5771.51 | ||||||||
| Borzio et al. [33] | ‘18 | Italu | R | 1508 | 44 (23–63) | LR AIC | ITA.LI.CA | 2 (299); 1 (270) | 763 | 7087 | The ITA.LI.CA system performed better than other multidimensional prognostic systems, even after stratification by curative or palliative treatment. This new system appears to be particularly useful for predicting individual HCC prognosis in clinical practice. |
| CLIP | 0 (619); 1 (422) | 575 | 7233 | ||||||||
| HKLC | I (608); IIa (304) | 659 | 7194 | ||||||||
| MESIAH | Q3 (380); Q4 (377) | 642 | 7159 | ||||||||
| JIS | 1 (583); 2 (326) | 563 | 7206 | ||||||||
| ITA.LI.CA tumor stage | - | 482 | 7307 | ||||||||
| BCLC | A (687); B (310) | 514 | 7234 | ||||||||
| HCC Prognostic Systems | C index | Trend χ2 Test | AIC | LR Test, p Value |
|---|---|---|---|---|
| Study Group (n = 6882) | ||||
| ITA.LI.CA score [35] | 0.693 | 1749 | 74,138 | - |
| ITA.LI.CA staging [35] | 0.667 | 1211 | 74,520 | 472 < 0.0001 |
| MESH [8] | 0.662 | 1182 | 74,476 | 518 < 0.0001 |
| CNLC [9,10,11] | 0.661 | 1187 | 74,555 | 522 < 0.0001 |
| BCLC [15] | 0.659 | 1114 | 74,558 | 537 < 0.0001 |
| CLIP [14] | 0.620 | 1143 | 74,719 | 882 < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, A.; Farinati, F.; Finotti, M.; Di Renzo, C.; Brancaccio, G.; Piscaglia, F.; Cabibbo, G.; Caturelli, E.; Missale, G.; Marra, F.; et al. Overview of Prognostic Systems for Hepatocellular Carcinoma and ITA.LI.CA External Validation of MESH and CNLC Classifications. Cancers 2021, 13, 1673. https://doi.org/10.3390/cancers13071673
Vitale A, Farinati F, Finotti M, Di Renzo C, Brancaccio G, Piscaglia F, Cabibbo G, Caturelli E, Missale G, Marra F, et al. Overview of Prognostic Systems for Hepatocellular Carcinoma and ITA.LI.CA External Validation of MESH and CNLC Classifications. Cancers. 2021; 13(7):1673. https://doi.org/10.3390/cancers13071673
Chicago/Turabian StyleVitale, Alessandro, Fabio Farinati, Michele Finotti, Chiara Di Renzo, Giuseppina Brancaccio, Fabio Piscaglia, Giuseppe Cabibbo, Eugenio Caturelli, Gabriele Missale, Fabio Marra, and et al. 2021. "Overview of Prognostic Systems for Hepatocellular Carcinoma and ITA.LI.CA External Validation of MESH and CNLC Classifications" Cancers 13, no. 7: 1673. https://doi.org/10.3390/cancers13071673
APA StyleVitale, A., Farinati, F., Finotti, M., Di Renzo, C., Brancaccio, G., Piscaglia, F., Cabibbo, G., Caturelli, E., Missale, G., Marra, F., Sacco, R., Giannini, E. G., Trevisani, F., Cillo, U., Associazione Italiana per lo Studio del Fegato (AISF) HCC Special Interest Group, & Italian Liver Cancer (ITA.LI.CA) Study Group. (2021). Overview of Prognostic Systems for Hepatocellular Carcinoma and ITA.LI.CA External Validation of MESH and CNLC Classifications. Cancers, 13(7), 1673. https://doi.org/10.3390/cancers13071673








