Histological Features of Sporadic and Familial Testicular Germ Cell Tumors Compared and Analysis of Age-Related Changes of Histology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Familial and Sporadic Testicular Germ Cell Tumors
2.2. Central Pathology Review
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurney, J.K.; Florio, A.A.; Znaor, A.; Ferlay, J.; Laversanne, M.; Sarfati, D.; Bray, F.; McGlynn, K.A. International Trends in the Incidence of Testicular Cancer: Lessons from 35 Years and 41 Countries. Eur. Urol. 2019, 76, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Devesa, S.S.; Sigurdson, A.J.; McGlynn, K.A. International patterns and trends in testis cancer incidence. Int. J. Cancer 2005, 115, 822–827. [Google Scholar] [CrossRef]
- Sampson, J.N.; Wheeler, W.A.; Yeager, M.; Panagiotou, O.; Wang, Z.; Berndt, S.I.; Lan, Q.; Abnet, C.C.; Amundadottir, L.T.; Figueroa, J.D.; et al. Analysis of Heritability and Shared Heritability Based on Genome-Wide Association Studies for Thirteen Cancer Types. J. Natl. Cancer Inst. 2015, 107, djv279. [Google Scholar] [CrossRef]
- Gundy, S.; Babosa, M.; Baki, M.; Bodrogi, I. Increased predisposition to cancer in brothers and offspring of testicular tumor patients. Pathol. Oncol. Res. 2004, 10, 197–203. [Google Scholar] [CrossRef]
- Hanson, H.A.; Anderson, R.E.; Aston, K.I.; Carrell, D.T.; Smith, K.R.; Hotaling, J.M. Subfertility increases risk of testicular cancer: Evidence from population-based semen samples. Fertil. Steril. 2016, 105, 322–328.e321. [Google Scholar] [CrossRef] [PubMed]
- Fosså, S.D.; Chen, J.; Schonfeld, S.J.; McGlynn, K.A.; McMaster, M.L.; Gail, M.H.; Travis, L.B. Risk of contralateral testicular cancer: A population-based study of 29,515 U.S. men. J. Natl. Cancer Inst. 2005, 97, 1056–1066. [Google Scholar] [CrossRef]
- Crockford, G.P.; Linger, R.; Hockley, S.; Dudakia, D.; Johnson, L.; Huddart, R.; Tucker, K.; Friedlander, M.; Phillips, K.A.; Hogg, D.; et al. Genome-wide linkage screen for testicular germ cell tumour susceptibility loci. Hum. Mol. Genet. 2006, 15, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Loveday, C.; Levy, M.; Dudakia, D.; Rapley, E.; Nsengimana, J.; Bishop, D.T.; Reid, A.; Huddart, R.; Broderick, P.; et al. Large-scale Sequencing of Testicular Germ Cell Tumour (TGCT) Cases Excludes Major TGCT Predisposition Gene. Eur. Urol. 2018, 73, 828–831. [Google Scholar] [CrossRef]
- Greene, M.H.; Pfeiffer, R.M. Familial TGCT: Polygenic aetiology advanced. Nat. Rev. Urol. 2018, 15, 665–666. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Looijenga, L.H.J. Human germ cell tumours from a developmental perspective. Nat. Rev. Cancer 2019, 19, 522–537. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Kersemaekers, A.M.F.; Jacobsen, G.K.; Timmer, A.; Steyerberg, E.W.; Molier, M.; Van Weeren, P.C.; Stoop, H.; Looijenga, L.H.J. Morphology of testicular parenchyma adjacent to germ cell tumours. An interim report. Apmis 2003, 111, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Premkumar, A.; Mueller, C.; Rosenberg, P.; Soho, C.; Bratslavsky, G.; Greene, M.H. Increased prevalence of testicular microlithiasis in men with familial testicular cancer and their relatives. Br. J. Cancer 2008, 99, 1748–1753. [Google Scholar] [CrossRef]
- Linehan, W.M.; Pinto, P.A.; Bratslavsky, G.; Pfaffenroth, E.; Merino, M.; Vocke, C.D.; Toro, J.R.; Bottaro, D.; Neckers, L.; Schmidt, L.S.; et al. Hereditary Kidney Cancer: Unique Opportunity for Disease-based Therapy. Cancer 2009, 115, 2252–2261. [Google Scholar] [CrossRef]
- Dudani, S.; de Velasco, G.; Wells, J.C.; Gan, C.L.; Donskov, F.; Porta, C.; Fraccon, A.; Pasini, F.; Lee, J.L.; Hansen, A.; et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association With Survival. JAMA Netw. Open 2021, 4, e2021869. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.L.; Friedlander, M.; Tucker, K.; Phillips, K.A.; Hogg, D.; Jewett, M.A.S.; Lohynska, R.; Daugaard, G.; Richard, S.; Bonaiti-Pellie, C.; et al. The International Testicular Cancer Linkage Consortium: A clinicopathologic descriptive analysis of 461 familial malignant testicular germ cell tumor kindred. Urol. Oncol.-Semin. Orig. Invest. 2010, 28, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. (Eds.) International Classification of Diseases for Oncology (ICD-O), 3rd ed.; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Skakkebaek, N.E. Possible Carcinoma-in-Situ of Testis. Lancet 1972, 2, 516–517. [Google Scholar] [CrossRef]
- Dorssers, L.C.J.; Gillis, A.J.M.; Stoop, H.; van Marion, R.; Nieboer, M.M.; van Riet, J.; van de Werken, H.J.G.; Oosterhuis, J.W.; de Ridder, J.; Looijenga, L.H.J. Molecular heterogeneity and early metastatic clone selection in testicular germ cell cancer development. Br. J. Cancer 2019, 120, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W. Clinical Prediction Models. A Pratical Approach to Development, Validation, and Updating, 2nd ed.; Springer International Publishing: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Sterne, J.A.; Davey Smith, G. Sifting the evidence-what’s wrong with significance tests? Br. Med. J. 2001, 322, 226–231. [Google Scholar] [CrossRef]
- Lash, T.L. Heuristic thinking and inference from observational epidemiology. Epidemiology 2007, 18, 67–72. [Google Scholar] [CrossRef]
- Dagash, H.; MacKinnon, E.A. Testicular microlithiasis: What does it mean clinically? BJU Int. 2007, 99, 157–160. [Google Scholar] [CrossRef]
- Winter, T.C.; Kim, B.; Lowrance, W.T.; Middleton, W.D. Testicular Microlithiasis: What Should You Recommend? Am. J. Roentgenol. 2016, 206, 1164–1169. [Google Scholar] [CrossRef]
- Pedersen, M.R.; Rafaelsen, S.R.; Moller, H.; Vedsted, P.; Osther, P.J. Testicular microlithiasis and testicular cancer: Review of the literature. Int. Urol. Nephrol. 2016, 48, 1079–1086. [Google Scholar] [CrossRef]
- Richenberg, J.; Belfield, J.; Ramchandani, P.; Rocher, L.; Freeman, S.; Tsili, A.C.; Cuthbert, F.; Studniarek, M.; Bertolotto, M.; Turgut, A.T.; et al. Testicular microlithiasis imaging and follow-up: Guidelines of the ESUR scrotal imaging subcommittee. Eur. Radiol. 2015, 25, 323–330. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.H.; Luo, E.T.; Liu, T.S.; Wei, A.Y. A Meta-Analysis of the Relationship between Testicular Microlithiasis and Incidence of Testicular Cancer. Urol. J. 2015, 12, 2057–2064. [Google Scholar] [PubMed]
- Sharmeen, F.; Rosenthal, M.H.; Wood, M.J.; Tirumani, S.H.; Sweeney, C.; Howard, S.A. Relationship Between the Pathologic Subtype/Initial Stage and Microliths in Testicular Germ Cell Tumors. J. Ultras Med. 2015, 34, 1977–1982. [Google Scholar] [CrossRef]
- Pedersen, M.R.; Horsfield, C.; Foot, O.; Lindebjerg, J.; Osther, P.J.S.; Vedsted, P.; Chandra, A.; Rafaelsen, S.R.; Moller, H. Testicular microlithiasis in patients with testicular cancer in the United Kingdom and in Denmark. Dan Med. J. 2018, 65, 1–5. [Google Scholar]
- Heller, H.T.; Oliff, M.C.; Doubilet, P.M.; O’Leary, M.P.; Benson, C.B. Testicular Microlithiasis: Prevalence and Association with Primary Testicular Neoplasm. J. Clin. Ultrasound 2014, 42, 423–426. [Google Scholar] [CrossRef] [PubMed]
- de Jong, B.W.D.; Brazao, C.A.D.; Stoop, H.; Wolffenbuttel, K.P.; Oosterhuis, J.W.; Puppels, G.J.; Weber, R.F.A.; Looijenga, L.H.J.; Kok, D.J. Raman spectroscopic analysis identifies testicular microlithiasis as intratubular hydroxyapatite. J. Urol. 2004, 171, 92–96. [Google Scholar] [CrossRef]
- Vigorita, V.J.; Ghelman, B.; Mintz, D. Orthopedic Pathology; Wolters Kluwer: Philadelphia, PA, USA, 2008. [Google Scholar]
- Holm, M.; Hoei-Hansen, C.E.; Rajpert-De Meyts, E.; Skakkebaek, N.E. Increased risk of carcinoma in situ in patients with testicular germ cell cancer with ultrasonic microlithiasis in the contralateral testicle. J. Urol. 2003, 170, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.; Huddart, R.A.; Elliott, F.; Sohaib, S.A.; Parker, E.; Dudakia, D.; Pugh, J.L.; Easton, D.F.; Bishop, D.T.; Stratton, M.R.; et al. Testicular microlithiasis as a familial risk factor for testicular germ cell tumour. Br. J. Cancer 2007, 97, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Milosevic, M.; Panzarella, T.; Banerjee, D.; Jewett, M.; Catton, C.; Tew-George, B.; Gospodarowicz, M.; Warde, P. The prognostic significance of the tumour infiltrating lymphocyte count in stage I testicular seminoma managed by surveillance. Eur. J. Cancer 2002, 38, 2014–2019. [Google Scholar] [CrossRef]
- Greene, M.H.; Kratz, C.P.; Mai, P.L.; Mueller, C.; Peters, J.A.; Bratslavsky, G.; Ling, A.; Choyke, P.M.; Premkumar, A.; Bracci, J.; et al. Familial testicular germ cell tumors in adults: 2010 summary of genetic risk factors and clinical phenotype. Endocr.-Relat. Cancer 2010, 17, R109–R121. [Google Scholar] [CrossRef] [PubMed]
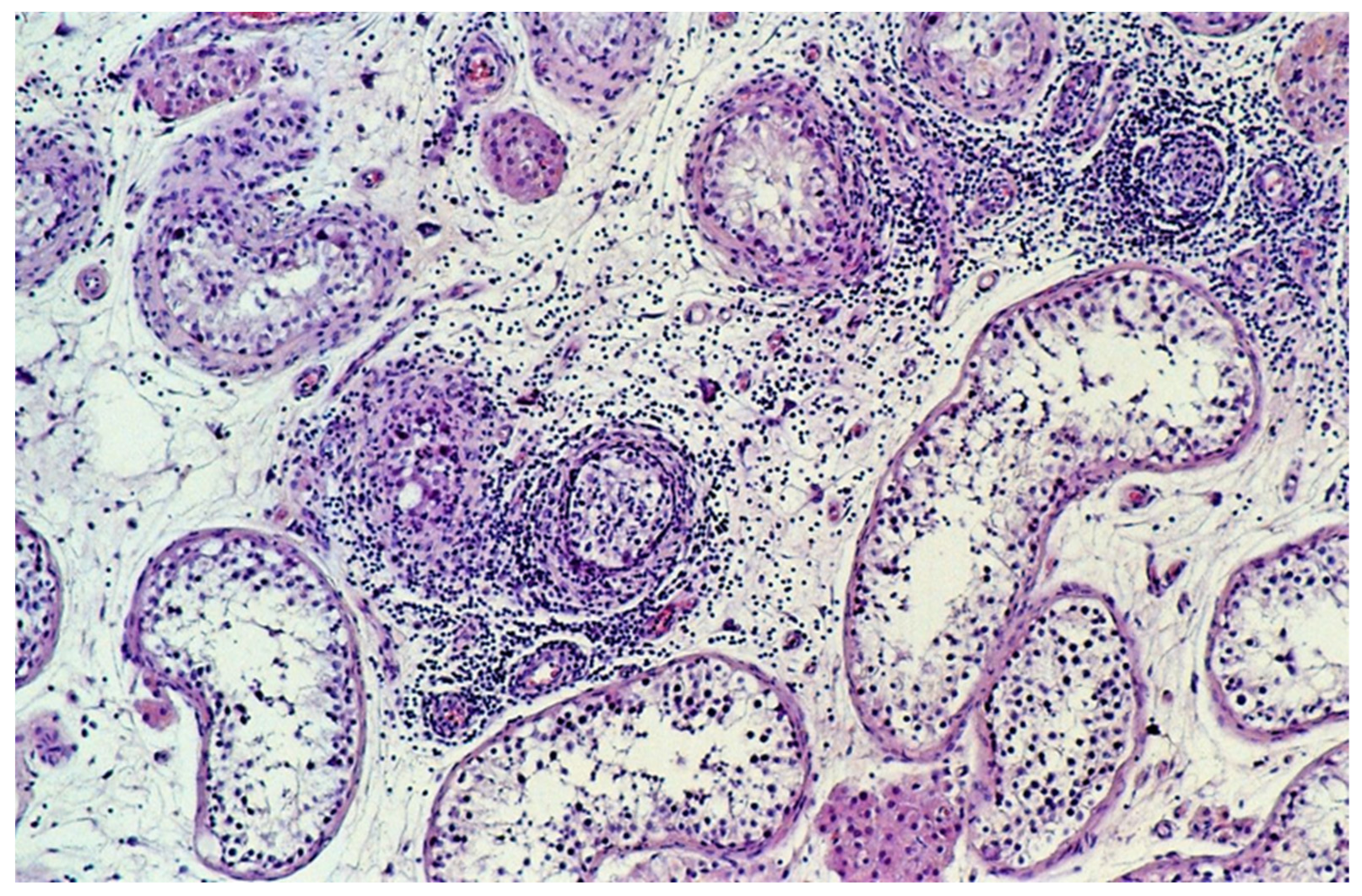

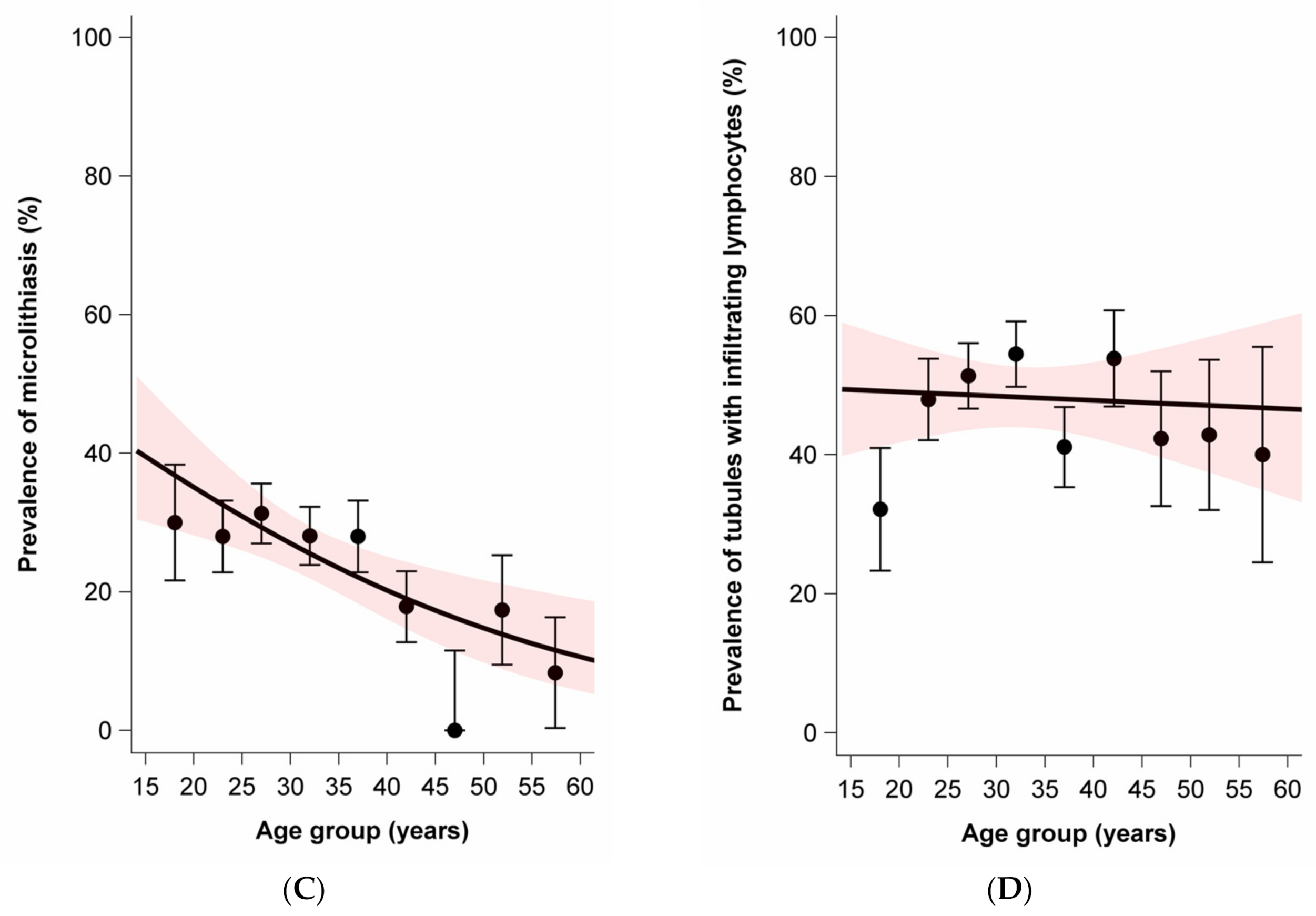

| Characteristic | Overall (n = 601) | Sporadic GCT (n = 296) | Familial GCT (n = 305) | |||
|---|---|---|---|---|---|---|
| Center, N, % | ||||||
| Norway | 131 | 21.8 | 58 | 20.0 | 73 | 23.9 |
| United Kingdom | 324 | 53.9 | 156 | 52.7 | 168 | 55.1 |
| United States | 146 | 24.3 | 82 | 27.7 | 64 | 21.0 |
| Age at diagnosis (years), N, % | ||||||
| <20 | 36 | 6.0 | 19 | 6.4 | 17 | 5.6 |
| 20–24 | 83 | 13.8 | 44 | 14.9 | 39 | 12.8 |
| 25–29 | 128 | 21.3 | 56 | 18.9 | 72 | 23.6 |
| 30–34 | 123 | 20.5 | 57 | 19.3 | 66 | 21.6 |
| 35–39 | 85 | 14.1 | 42 | 14.2 | 43 | 14.1 |
| 40–44 | 61 | 10.2 | 32 | 10.8 | 29 | 9.5 |
| 45–49 | 33 | 5.5 | 17 | 5.7 | 16 | 5.3 |
| 50–54 | 31 | 5.2 | 18 | 6.1 | 13 | 4.3 |
| 55+ | 14 | 2.3 | 7 | 2.4 | 7 | 2.3 |
| Missing | 7 | 1.2 | 4 | 1.4 | 3 | 1.0 |
| Mean age & SD | 33.2 | 9.8 | 33.4 | 10.2 | 33.0 | 9.5 |
| Age range | 14–73 | 15–73 | 14–66 | |||
| Histological type, N, % | ||||||
| Pure seminoma | 299 | 49.8 | 135 | 45.6 | 164 | 53.8 |
| Pure nonseminoma (≥1 N) | 221 | 36.8 | 118 | 39.9 | 103 | 33.8 |
| Seminoma + nonseminoma | 81 | 13.5 | 43 | 14.5 | 38 | 12.5 |
| Presence of histologic components % | ||||||
| Seminoma, classical | 62.2 | 58.8 | 65.6 | |||
| Seminoma, HMI | 0.5 | 0.7 | 0.3 | |||
| Spermatocytic tumor | 0.5 | 0.7 | 0.3 | |||
| Embryonal carcinoma | 40.6 | 42.6 | 38.7 | |||
| Yolk sac tumor | 33.0 | 34.1 | 31.8 | |||
| Choriocarcinoma | 2.8 | 3.0 | 2.6 | |||
| Teratoma | 29.6 | 32.1 | 27.2 | |||
| Teratoma WMA | 0.8 | 0.3 | 1.0 | |||
| Characteristic | Overall | Sporadic GCT (n = 296) | Familial GCT (n = 305) | Prev. Difference | 95%CI | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| GCNIS | ||||||||
| missing | 81 | 37 | 44 | |||||
| none | 89 | 17.1 | 45 | 17.4 | 44 | 16.9 | ||
| occasional | 69 | 13.3 | 38 | 14.7 | 31 | 11.9 | ||
| some | 86 | 16.5 | 38 | 14.7 | 48 | 18.4 | ||
| many | 276 | 53.1 | 138 | 53.3 | 138 | 52.9 | ||
| some-many | 362 | 69.6 | 176 | 68.0 | 186 | 71.3 | +3.3 | −4.6; +11.2 |
| TM | ||||||||
| missing | 68 | 33 | 35 | |||||
| no | 398 | 74.7 | 202 | 76.8 | 196 | 72.6 | ||
| yes | 135 | 25.3 | 61 | 23.2 | 74 | 27.4 | +4.2 | −3.2; +11.6 |
| Lymphocytic infiltration | ||||||||
| none | 21 | 3.5 | 9 | 3.0 | 12 | 3.9 | ||
| slight | 239 | 39.8 | 119 | 40.2 | 120 | 39.3 | ||
| moderate | 243 | 40.4 | 115 | 38.9 | 128 | 42.0 | ||
| extensive | 98 | 16.3 | 53 | 17.9 | 45 | 14.8 | ||
| mod. + extensive | 341 | 56.7 | 168 | 56.8 | 173 | 56.7 | 0.0 | −8.0; +8.0 |
| Tubules with infiltrating lymphocytes | ||||||||
| missing | 86 | 37 | 49 | |||||
| none | 128 | 24.6 | 66 | 25.5 | 62 | 23.7 | ||
| occasional | 138 | 26.5 | 72 | 27.8 | 66 | 25.2 | ||
| some | 100 | 19.2 | 50 | 19.3 | 50 | 19.1 | ||
| many | 149 | 28.6 | 71 | 27.4 | 78 | 29.8 | ||
| some-many | 249 | 48.4 | 121 | 46.7 | 128 | 50.0 | +3.3 | −5.4; +11.9 |
| Pure Nonseminoma (≥1 N) | Mixed (Seminoma + Nonseminoma) | Pure Seminoma | |
|---|---|---|---|
| Lymphocytic infiltration | n = 221 | n = 81 | n = 299 |
| none % | 5.9 | 0 | 2.7 |
| slight % | 55.2 | 29.6 | 31.1 |
| moderate % | 32.1 | 46.9 | 44.8 |
| extensive % | 6.8 | 23.5 | 21.4 |
| moderate-extensive % | 38.9 | 70.4 | 66.2 |
| Prevalence difference (95%CI) | Ref. | +31.5 (+19.6; +43.3) | +27.3 (+18.9; +35.7) |
| GCNIS | n = 189 | n = 74 | n = 257 |
| none % | 12.2 | 6.8 | 23.7 |
| occasional % | 12.7 | 4.1 | 16.3 |
| some % | 15.3 | 13.5 | 18.3 |
| many % | 59.8 | 75.7 | 41.6 |
| some-many % | 75.1 | 89.2 | 59.9 |
| Prevalence difference (95%CI) | Ref. | +14.1 (+4.7; +23.4) | −15.2 (−23.8; −6.6) |
| Microlithiasis | n = 193 | n = 74 | n = 266 |
| Present % | 25.4 | 37.8 | 21.8 |
| Prevalence difference (95%CI) | Ref. | +12.5 (−0.2; +25.1) | −3.6 (−11.5; +4.3) |
| Tubules with infiltrating lymphocytes | n = 189 | n = 74 | n = 258 |
| none | 30.2 | 10.8 | 24.4 |
| occasional | 32.8 | 14.9 | 25.2 |
| some | 17.5 | 18.9 | 20.5 |
| many | 19.6 | 55.4 | 27.5 |
| some-many | 37.0 | 74.3 | 49.2 |
| Prevalence difference (95%CI) | Ref. | +37.3 (+25.2; +49.4) | +12.2 (+2.9; +21.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stang, A.; McMaster, M.L.; Sesterhenn, I.A.; Rapley, E.; Huddart, R.; Heimdal, K.; McGlynn, K.A.; Oosterhuis, J.W.; Greene, M.H. Histological Features of Sporadic and Familial Testicular Germ Cell Tumors Compared and Analysis of Age-Related Changes of Histology. Cancers 2021, 13, 1652. https://doi.org/10.3390/cancers13071652
Stang A, McMaster ML, Sesterhenn IA, Rapley E, Huddart R, Heimdal K, McGlynn KA, Oosterhuis JW, Greene MH. Histological Features of Sporadic and Familial Testicular Germ Cell Tumors Compared and Analysis of Age-Related Changes of Histology. Cancers. 2021; 13(7):1652. https://doi.org/10.3390/cancers13071652
Chicago/Turabian StyleStang, Andreas, Mary L. McMaster, Isabell A. Sesterhenn, Elizabeth Rapley, Robert Huddart, Ketil Heimdal, Katherine A. McGlynn, Jan Wolter Oosterhuis, and Mark H. Greene. 2021. "Histological Features of Sporadic and Familial Testicular Germ Cell Tumors Compared and Analysis of Age-Related Changes of Histology" Cancers 13, no. 7: 1652. https://doi.org/10.3390/cancers13071652
APA StyleStang, A., McMaster, M. L., Sesterhenn, I. A., Rapley, E., Huddart, R., Heimdal, K., McGlynn, K. A., Oosterhuis, J. W., & Greene, M. H. (2021). Histological Features of Sporadic and Familial Testicular Germ Cell Tumors Compared and Analysis of Age-Related Changes of Histology. Cancers, 13(7), 1652. https://doi.org/10.3390/cancers13071652






