Efficacy and Safety of Checkpoint Inhibitor Treatment in Patients with Advanced Renal or Urothelial Cell Carcinoma and Concomitant Chronic Kidney Disease: A Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Assessments
2.3. Statistics
3. Results
3.1. Patient Collective
3.2. Exposure and Safety
| Characteristics of CPI Therapy | CKD n = 49 | Non-CKD n = 77 | p |
|---|---|---|---|
| Type of CPI—n (%) | |||
| Pembrolizumab | 7 (14.3) | 3 (3.9) | * (0.0460) |
| Pembrolizumab + axitinib | 0 (0.00) | 1 (1.3) | ns (>0.9999) |
| Nivolumab | 32 (65.3) | 52 (67.5) | ns (0.84759) |
| Atezolizumab | 4 (8.2) | 4 (5.2) | ns (0.2067) |
| Nivolumab + ipilimumab | 6 (12.2) | 16 (20.8) | ns (0.1459) |
| Tremelimumab + durvalumab | 0 (0.00) | 1 (1.3) | ns (>0.9999) |
| Number of doses—median ± SD | 15.00 ± 20.49 | 12.95 ± 12.43 | ns (0.7089) |
| Line of treatment (RCC) | N = 32 (100.0) | N = 53 (100.0) | |
| 1st | 7 (21.9) | 14 (26.4) | ns (0.7963) |
| 2nd | 19 (59.4) | 27 (50.9) | ns (0.5053) |
| 3rd | 3 (9.4) | 7 (13.2) | ns (0.7362) |
| 4th | 2 (6.3) | 3 (5.7) | ns (>0.9999) |
| 5th | 1 (3.1) | 2 (3.8) | ns (>0.9999) |
| Prior treatment (RCC) (1st–4th line) | N = 35 (100.0) | N = 58 (100.0) | |
| Sunitinib | 13 (37.1) | 34 (58.6) | ns (0.0555) |
| Pazopanib | 14 (40.0) | 8 (13.8) | ** (0.0056) |
| Cabozantinib | 4 (11.4) | 9 (15.5) | ns (0.7603) |
| Interferon | 1 (2.9) | 0 (0.0) | ns (0.3763) |
| Axitinib | 1 (2.9) | 2 (3.4) | ns (>0.9999) |
| Temsirolimus | 1 (2.9) | 0 (0.0) | ns (0.3763) |
| Everolimus | 1 (2.9) | 3 (5.2) | ns (>0.9999) |
| Sorafenib | 0 (0.0) | 1 (1.7) | ns (>0.9999) |
| Bevacizumab + interferon | 0 (0.0) | 1 (1.7) | ns (>0.9999) |
| Line of treatment (UC) (1st–4th line) | N = 17 (100.0) | N = 24 (100.0) | |
| 1st | 6 (35.3) | 2 (8.3) | * (0.0486) |
| 2nd | 10 (58.8) | 14 (58.3) | ns (>0.9999) |
| 3rd | 1 (5.9) | 7 (29.2) | ns (0.1102) |
| 4th | 0 (0.0) | 1 (4.2) | ns (>0.9999) |
| Prior treatment (UC) | N = 11 (100.0) | N = 22 (100.0) | |
| Cisplatin-backbone | 6 (54.5) | 18 (81.8) | ns (0.1210) |
| Carboplatin-backbone | 3 (27.3) | 3 (13.6) | ns (0.3752) |
| Gemcitabine mono | 2 (18.2) | 1 (4.5) | ns (>0.9999) |
| Adverse Events—n (%) | CKD n = 49 | Non-CKD n = 77 | p |
|---|---|---|---|
| Patients with adverse event | 24 (49.0) | 37 (48.1) | ns (>0.99999) |
| All adverse events | 35 | 81 | |
| Type of adverse event | |||
| Diarrhea | 1 (2.0) | 12 (15.6) | * (0.0157) |
| Vomiting | 5 (10.2) | 7 (9.1) | ns (>0.99999) |
| Constipation | 5 (10.2) | 5 (6.5) | ns (0.5093) |
| Nausea | 5 (10.2) | 9 (11.7) | ns (>0.99999) |
| Loss of appetite | 2 (4.1) | 8 (10.4) | ns (0.3137) |
| Fatigue | 4 (8.2) | 13 (16.9) | ns (0.1909) |
| Rash | 5 (10.2) | 14 (18.2) | ns (0.3086) |
| Pruritus | 2 (4.1) | 6 (7.8) | ns (0.4819) |
| Infusion reaction | 0 (0.0) | 2 (2.6) | ns (0.5209) |
| Mucositis | 1 (2.0) | 1 (1.3) | ns (>0.99999) |
| Exacerbation RA | 1 (2.0) | 0 (0.0) | ns (>0.99999) |
| Xerostomia | 0 (0.0) | 3 (3.9) | ns (0.2813) |
| Acute kidney injury | 4 (8.2) | 1 (1.3) | ns (0.0746) |
| irAE—no. (%) | CKD n = 49 | Non-CKD n = 77 | p |
|---|---|---|---|
| Patients with irAE | 14 (28.6) | 19 (24.7) | ns (>0.9999) |
| All irAE | 15 | 23 | |
| Type of irAE | |||
| Colitis | 1 (2.0) | 6 (7.8) | ns (0.2459) |
| Nephritis | 4 (8.2) | 1 (1.3) | ns (0.0746) |
| Elev. of liver enzymes | 1 (2.0) | 3 (3.9) | ns (>0.99999) |
| Dermatitis | 2 (4.1) | 2 (2.6) | ns (0.6419) |
| Elev. of pancr. enzymes | 0 (0.0) | 3 (3.9) | ns (0.2813) |
| Gastritis | 0 (0.0) | 2 (2.6) | ns (0.5209) |
| Pneumonitis | 4 (8.2) | 1 (1.3) | ns (0.0746) |
| Myocarditis/myositis | 0 (0.0) | 1 (1.3) | ns (>0.99999) |
| Arthritis/myalgia | 0 (0.0) | 1 (1.3) | ns (>0.99999) |
| Thyroid dysfunction | 2 (4.1) | 2 (2.6) | ns (0.6419) |
| Adrenocortical insuff. | 0 (0.0) | 1 (1.3) | ns (>0.99999) |
| Encephalitis | 1 (2.0) | 0 (0.0) | ns (>0.99999) |
| CTCAE-stage of irAE | |||
| 1 | 3 (6.1) | 2 (2.6) | ns (0.3762) |
| 2 | 9 (18.4) | 10 (13.0) | ns (0.4503) |
| 3 | 1 (2.0) | 9 (11.7) | ns (0.0871) |
| 4 | 1 (2.0) | 2 (2.6) | ns (>0.99999) |
| 5 | 1 (2.0) | 0 (0.0) | ns (>0.99999) |
| Therapy of irAE | |||
| Glucocorticoids | 11 (22.4) | 21 (27.3) | ns (0.6753) |
| Therapy interruption | 7 (14.3) | 8 (10.4) | ns (0.5775) |
| Therapy discontinuation | 4 (8.2) | 11 (14.3) | ns (0.4018) |
| Therapy continued | 4 (8.2) | 4 (5.2) | ns (0.7102) |
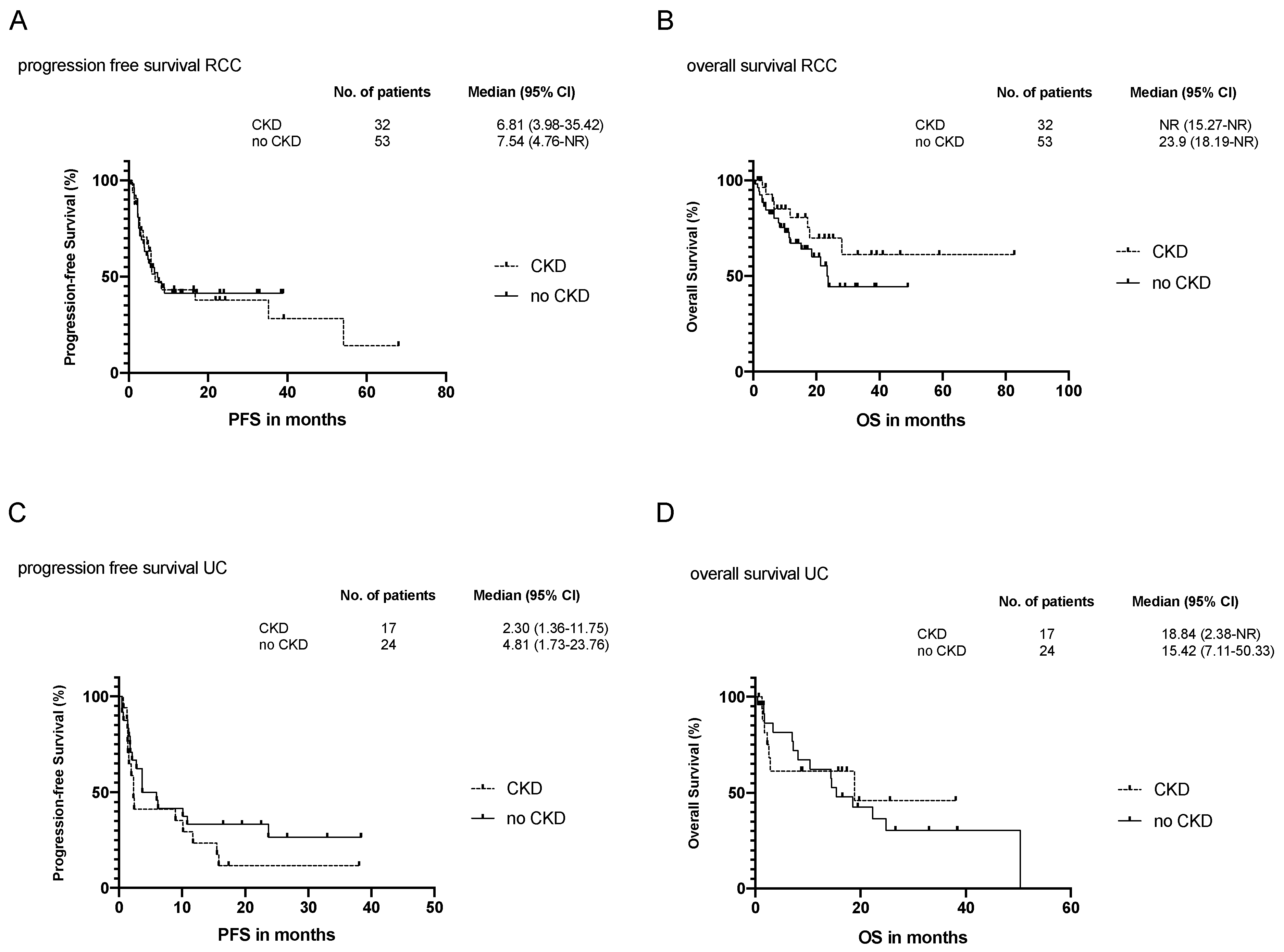
3.3. Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Choueiri, T.; Powles, T.; Burotto, M.; Bourlon, M.; Zurawski, B.; Juárez, V.O.; Hsieh, J.; Basso, U.; Shah, A.; Suarez, C.; et al. 696O_PR Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann. Oncol. 2020, 31, S1159. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullén, A.; et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. J. Clin. Oncol. 2020, 38, LBA1. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Aguilera, J.V.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Park, H.; Lo-Ciganic, W. Immune Checkpoint Inhibitors and Immune-Related Adverse Events in Patients With Advanced Melanoma: A Systematic Review and Network Meta-analysis. JAMA Netw. Open. 2020, 3, e201611. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Levey, A.S.; Serio, A.M.; Snyder, M.; Vickers, A.J.; Raj, G.V.; Scardino, P.T.; Russo, P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 2006, 7, 735–740. [Google Scholar] [CrossRef]
- Stewart, J.H.; Vajdic, C.M.; Van Leeuwen, M.T.; Amin, J.; Webster, A.C.; Chapman, J.R.; McDonald, S.P.; Grulich, A.E.; McCredie, M.R.E. The pattern of excess cancer in dialysis and transplantation. Nephrol. Dial. Transplant. 2009, 24, 3225–3231. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.R.; Huisman, M.; Van den Dorpel, M.; Betjes, M.G.H. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J. Am. Soc. Nephrol. 2008, 19, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Basic Science and Dialysis: Disturbances of Acquired Immunity in Hemodialysis Patients. Semin. Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Loriot, Y.; James, N.; Choy, E.; Castellano, D.; Lopez-Rios, F.; Banna, G.L.; De Giorgi, U.; Masini, C.; Bamias, A.; et al. Primary Results from SAUL, a Multinational Single-arm Safety Study of Atezolizumab Therapy for Locally Advanced or Metastatic Urothelial or Nonurothelial Carcinoma of the Urinary Tract. Eur. Urol. 2019, 76, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Censits, J.; Pal, S.; Kaiser, C.; Ding, B.; Bellmunt, J. Atezolizumab in patients with renal insufficiency and mixed variant histology: Analyses from an expanded access program in platinum-treated locally advanced or metastatic urothelial carcinoma. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group, Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Gruenvald, V.; Horwich, A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v58–v68. [Google Scholar] [CrossRef]
- Bellmunt, J.A.; Orsola, J.J.; Leow, T.; Wiegel, M.; De Santis, M.; Horwich, A. Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii40–iii48. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 29 March 2021).
- Michot, J.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; Van Der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.-W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Baron, A.D.; Necchi, A.; Plimack, E.R.; Pal, S.K.; Bedke, J.; Zakharia, Y.; Grimm, M.O.; Bracarda, S.; Retz, M.; et al. Nivolumab monotherapy in patients with advanced platinum-resistant urothelial carcinoma: Efficacy and safety update from CheckMate 275. J. Clin. Oncol. 2019, 4524. [Google Scholar] [CrossRef]
- Elias, R.; Yan, F.; Singla, N.; Levonyack, N.; Formella, J.; Christie, A.; Kapur, P.; Bowman, A.I.; Hammers, H.J.; Hannan, R.; et al. Immune-related adverse events are associated with improved outcomes in ICI-treated renal cell carcinoma patients. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]
- Verzoni, E.; on behalf of the Italian Nivolumab Renal Cell Cancer Early Access Program group; Cartenì, G.; Cortesi, E.; Giannarelli, D.; De Giglio, A.; Sabbatini, R.; Buti, S.; Rossetti, S.; Cognetti, F.; et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: The Italian expanded access program. J. Immunother. Cancer 2019, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Morales-Barrera, R.; Rodriguez, C.S.; Gonzalez, M.; Ros, J.; Semidey, M.E.; Hernandez, E.S.; Mateo, J.; Sáez, C.F.; Lozano, F.; Mast, R.; et al. Impact of immune-related adverse events on survival in patients with metastastic urothelial carcinoma treated with immune-checkpoint inhibitors. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Kompotiatis, P.; Thongprayoon, C.; Cheungpasitporn, W.; Herrmann, J.; Herrmann, S.M. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta-analysis. Nephrol. Dial. Transplant. 2019, 34, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Motwani, S.; Riella, L.V. Renal complications of immune checkpoint blockade. Curr. Probl. Cancer 2017, 41, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, C.; Struck, J.P.; Ivanyi, P.; Kramer, M.W.; Hupe, M.C.; Hensen, B.; Fürschke, A.; Peters, I.; Merseburger, A.S.; Kuczyk, M.A.; et al. Checkpoint Inhibition for Metastatic Urothelial Carcinoma After Chemotherapy—Real-World Clinical Impressions and Comparative Review of the Literature. Front. Oncol. 2020, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Martin-Doyle, W.; Rajagopal, P.S.; Patel, C.G.; Anderson, E.M.; Rothman, A.T.; Cote, R.J.; Urun, Y.; Chang, S.L.; Choueiri, T.K.; et al. Adjuvant Chemotherapy for Invasive Bladder Cancer: A 2013 Updated Systematic Review and Meta-Analysis of Randomized Trials. Eur. Urol. 2014, 66, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Lin, J.F.; Lin, Y.C.; Chou, K.Y.; Chen, H.E.; Ho, C.Y.; Chen, P.C.; Hwang, T.I. Cisplatin contributes to programmed death-ligand 1 expression in bladder cancer through ERK1/2-AP-1 signaling pathway. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Rébé, C.; Demontoux, L.; Pilot, T.; Ghiringhelli, F. Platinum Derivatives Effects on Anticancer Immune Response. Biomolecules 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | CKD n = 49 | Non-CKD n = 77 | p |
|---|---|---|---|
| Median age—years ± SD | 68.52 ± 10.21 | 61.39 ± 11.36 | *** (0.0005) |
| <65 years—no. (%) | 18 (36.7) | 47 (61.0) | * (0.0104) |
| ≥65 years—no. (%) | 31 (63.3) | 30 (39.0) | * (0.0104) |
| Sex—n (%) | |||
| Male | 36 (73.5) | 63 (81.8) | ns (0.2752) |
| Female | 13 (26.5) | 14 (18.2) | ns (0.2752) |
| Tumor entity—n (%) | |||
| UC | 17 (34.7) | 24 (31.2) | ns (0.7004) |
| RCC | 32 (65.3) | 53 (68.8) | ns (0.7004) |
| IMDC-Score—n (%) (RCC only) | n = 32 | n = 53 | |
| Favorable | 5 (15.6) | 14 (26.4) | ns (0.2927) |
| Intermediate | 18 (56.3) | 29 (54.7) | ns (>0.9999) |
| Poor | 9 (28.1) | 10 (18.9) | ns (0.4213) |
| Grading RCC—n (%) | |||
| G1 | 0 (0.00) | 1 (1.89) | ns (>0.9999) |
| G2 | 16 (50.00) | 27 (50.94) | ns (>0.9999) |
| G3 | 10 (31.25) | 11 (20.75) | ns (0.3076) |
| G4 | 1 (3.13) | 2 (3.77) | ns (>0.9999) |
| Unknown | 5 (15.63) | 12 (22.64) | ns (0.5782) |
| Grading UC—n (%) | |||
| G1 | 0 (0.00) | 1 (4.17) | ns (>0.9999) |
| G2 | 5 (29.41) | 3 (12.5) | ns (0.2412) |
| G3 | 9 (52.94) | 12 (50.0) | ns (>0.9999) |
| G4 | 0 (0.00) | 0 (0.00) | ns (>0.9999) |
| Unknown | 3 (17.64) | 8 (33.33) | ns (0.3092) |
| Metastatic status—n (%) | |||
| Synchronous | 19 (38.8) | 25 (32.5) | ns (0.5658) |
| Metachronous | 30 (61.2) | 52 (67.5) | ns (0.5658) |
| Sites of distant metastasis—n (%) | |||
| Lung | 36 (73.5) | 52 (67.5) | ns (0.5528) |
| Lymph nodes | 25 (51.0) | 46 (59.8) | ns (0.3617) |
| Bone | 21 (42.9) | 35 (45.5) | ns (0.8548) |
| Liver | 19 (38.8) | 25 (32.5) | ns (0.5658) |
| Adrenal gland | 6 (12.2) | 11 (14.3) | ns (0.7960) |
| Brain | 3 (6.1) | 14 (18.2) | ns (0.0638) |
| Pleura | 6 (12.2) | 10 (13.0) | ns (>0.9999) |
| Peritoneum | 7 (14.3) | 7 (9.1) | ns (0.3946) |
| Skin | 1 (2.0) | 0 (0.00) | ns (0.3889) |
| Other | 17 (34.7) | 27 (35.1) | ns (>0.9999) |
| Causes of CKD—n (%) | CKD n = 49 |
|---|---|
| Nephrectomy | 37 (75.5) |
| Nephrotoxic chemotherapy | 11 (22.5) |
| Diabetes mellitus | 8 (16.3) |
| Hypertensive nephropathy | 1 (2.0) |
| Hydronephrosis | 1 (2.0) |
| Primary (atherosclerotic) cirrhotic kidney | 1 (2.0) |
| Efficacy of CPI Therapy | RCC CKD n = 32 | RCC Non-CKD n = 53 | p | UC CKD n = 17 | UC Non-CKD n = 24 | p |
|---|---|---|---|---|---|---|
| Best overall response—n (%) | ||||||
| Complete response | 0 (0.0) | 2 (3.8) | ns (0.5249) | 0 (0.0) | 0 (0.0) | ns (>0.99999) |
| Partial response | 7 (21.9) | 10 (18.9) | ns (0.7837) | 4 (23.5) | 4 (16.7) | ns (0.6975) |
| Stable disease | 9 (28.1) | 19 (35.8) | ns (0.4873) | 2 (11.8) | 7 (29.2) | ns (0.2623) |
| Progressive disease | 16 (50.0) | 22 (41.5) | ns (0.5035) | 11 (64.7) | 13 (54.2) | ns (0.5387) |
| Median time to response—months (95%CI) | 4.22 (3.00–51.73) | 3.10 (2.7–3.77) | ns (0.1003) | 3.33 (2.30–5.63) | 3.95 (1.83–7.30) | ns (0.6709) |
| Median duration of response—months (95%CI) | 13.37 (3.17–35.8) | 7.47 (3.03–12.27) | ns (0.0537) | 9.82 (6.23–14.10) | 18.97 (1.07–35.67) | ns (0.3717) |
| Patients with ongoing response—n (%) | 4 (12.50) | 9 (16.98) | ns (0.7584) | 1 (5.88) | 2 (8.33) | ns (>0.99999) |
| Median duration of therapy—months (95%CI) | 5.39 (2.8–11.60) | 5.20 (3.73–7.60) | ns (0.2347) | 2.33 (1.37–11.87) | 4.88 (1.80–19.44) | ns (0.3110) |
| Median follow-up—months (95%CI) | 15.49 (6.70–24.23) | 11.43 (6.63–16.57) | ns (0.0770) | 8.87 (2.30–17.63) | 14.64 (3.43–22.60) | ns (0.2416) |
| HR | CI 95% | p | |
|---|---|---|---|
| Progression-Free Survival RCC | |||
| CKD | 1.000 | 0.548–1.822 | 0.999 |
| Sex | 1.044 | 0.473–2.300 | 0.916 |
| Age | 1.010 | 0.982–1.040 | 0.481 |
| Distant metastasis bone and or brain and or liver | 1.409 | 0.641–2.676 | 0.334 |
| CPI treatment 2nd line * | 1.121 | 0.529–2.378 | 0.765 |
| CPI treatement 3–5th line * | 1.423 | 0.593–3.415 | 0.430 |
| Progression-Free Survival UC | |||
| CKD | 1.492 | 0.686–3.247 | 0.431 |
| Sex | 0.983 | 0.381–2.534 | 0.733 |
| Age | 1.021 | 0.981–1.063 | 0.311 |
| Distant metastasis bone and or brain and or liver | 1.141 | 0.553–2.353 | 0.623 |
| CPI treatment 2nd line * | 1.901 | 0.698–5.180 | 0.209 |
| CPI treatement 3–5th line * | 1.556 | 0.441–5.490 | 0.492 |
| Overall Survival RCC | |||
| CKD | 0.502 | 0.219–1.152 | 0.104 |
| Sex | 0.840 | 0.319–2.209 | 0.724 |
| Age | 1.039 | 1.002–1.077 | 0.039 |
| Distant metastasis bone and or brain and or liver | 1.229 | 0.463–3.265 | 0.679 |
| CPI treatment 2nd line * | 1.025 | 0.366–1.025 | 0.963 |
| CPI treatement 3–5th line * | 1.582 | 0.496–5.041 | 0.438 |
| Overall Survival UC | |||
| CKD | 0.656 | 0.296–1.454 | 0.299 |
| Sex | 1.737 | 0.774–3.899 | 0.181 |
| Age | 0.741 | 0.310–1.774 | 0.502 |
| Distant metastasis bone and or brain and or liver | 0.522 | 0.138–1.971 | 0.338 |
| CPI treatment 2nd line * | 2.447 | 1.020–5.874 | 0.045 |
| CPI treatement 3–5th line * | 0.997 | 0.985–1.009 | 0.599 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seydel, F.; Delecluse, S.; Zeier, M.; Holland-Letz, T.; Haag, G.M.; Berger, A.K.; Grün, B.C.; Bougatf, N.; Hohenfellner, M.; Duensing, S.; et al. Efficacy and Safety of Checkpoint Inhibitor Treatment in Patients with Advanced Renal or Urothelial Cell Carcinoma and Concomitant Chronic Kidney Disease: A Retrospective Cohort Study. Cancers 2021, 13, 1623. https://doi.org/10.3390/cancers13071623
Seydel F, Delecluse S, Zeier M, Holland-Letz T, Haag GM, Berger AK, Grün BC, Bougatf N, Hohenfellner M, Duensing S, et al. Efficacy and Safety of Checkpoint Inhibitor Treatment in Patients with Advanced Renal or Urothelial Cell Carcinoma and Concomitant Chronic Kidney Disease: A Retrospective Cohort Study. Cancers. 2021; 13(7):1623. https://doi.org/10.3390/cancers13071623
Chicago/Turabian StyleSeydel, Florian, Susanne Delecluse, Martin Zeier, Tim Holland-Letz, Georg Martin Haag, Anne Katrin Berger, Barbara Christine Grün, Nina Bougatf, Markus Hohenfellner, Stefan Duensing, and et al. 2021. "Efficacy and Safety of Checkpoint Inhibitor Treatment in Patients with Advanced Renal or Urothelial Cell Carcinoma and Concomitant Chronic Kidney Disease: A Retrospective Cohort Study" Cancers 13, no. 7: 1623. https://doi.org/10.3390/cancers13071623
APA StyleSeydel, F., Delecluse, S., Zeier, M., Holland-Letz, T., Haag, G. M., Berger, A. K., Grün, B. C., Bougatf, N., Hohenfellner, M., Duensing, S., Jäger, D., & Zschäbitz, S. (2021). Efficacy and Safety of Checkpoint Inhibitor Treatment in Patients with Advanced Renal or Urothelial Cell Carcinoma and Concomitant Chronic Kidney Disease: A Retrospective Cohort Study. Cancers, 13(7), 1623. https://doi.org/10.3390/cancers13071623






