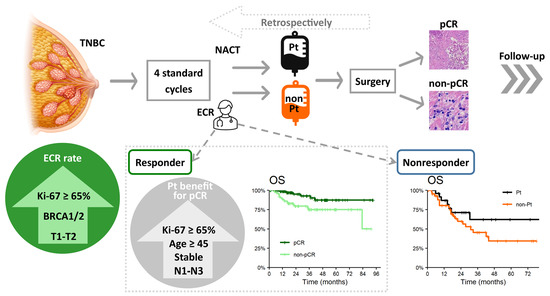
Neoadjuvant Chemotherapy of Triple-Negative Breast Cancer: Evaluation of Early Clinical Response, Pathological Complete Response Rates, and Addition of Platinum Salts Benefit Based on Real-World Evidence
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Follow-Up
2.2. Pathological Assessment, Breast Cancer Subtypes, and BRCA1/2 Germline Mutation Testing
2.3. Chemotherapy Regimens
2.4. Early Clinical Response Evaluation and Definition of pCR
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Early Clinical Response
3.2. Treatment Characteristics and Toxicity
3.3. Pathological Complete Response and Effect of Platinum Salts Adding
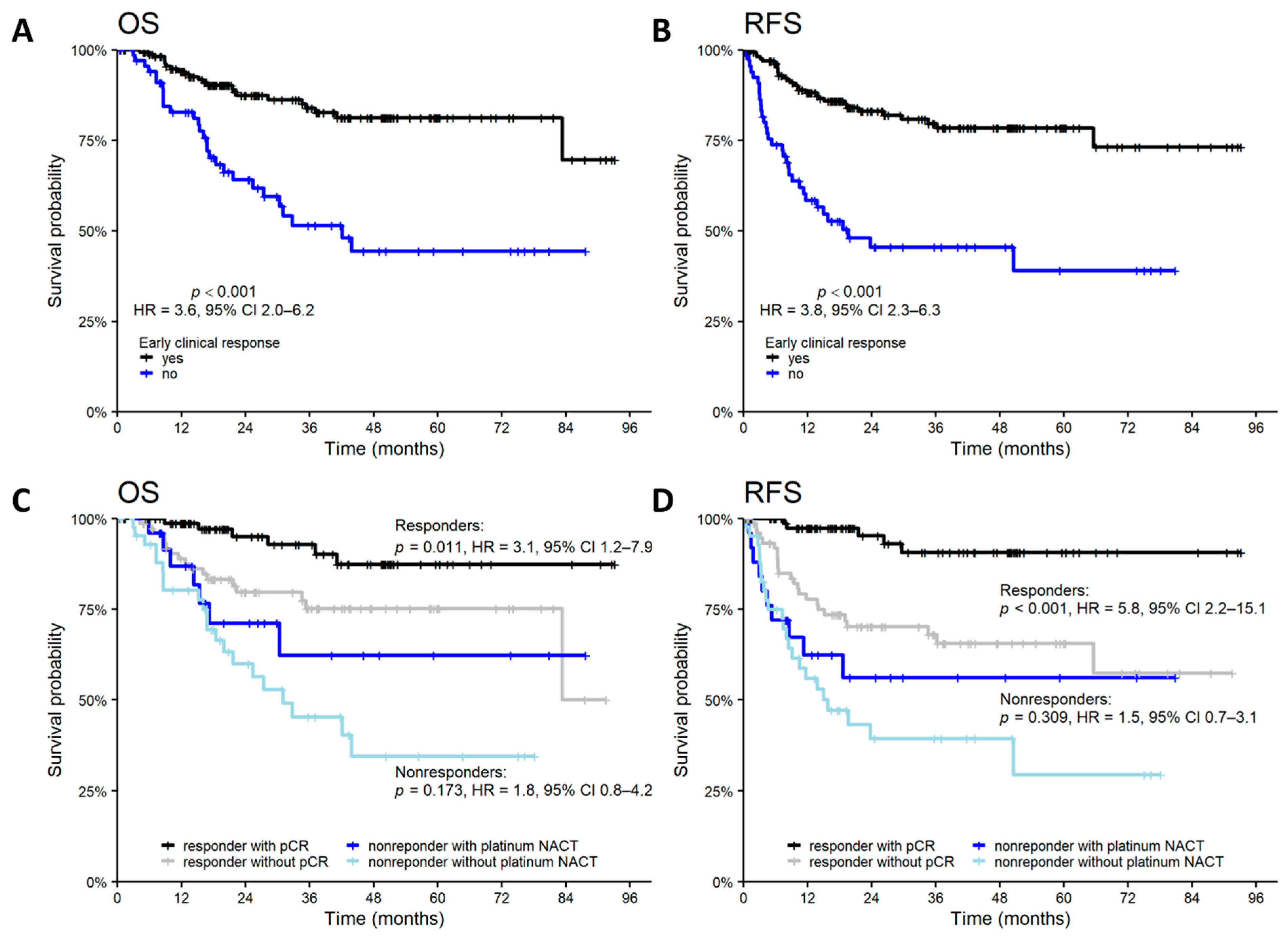
3.4. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2018, 27, 619–626. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef]
- Wolff, A.; Hammond, E.; Allison, K.; Harvey, B.; Mangu, P.; Bartlett, J.; Bilous, M.; Ellis, I.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.W.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive Genomic Analysis Identifies Novel Subtypes and Targets of Triple-Negative Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Jiang, Y.-Z.; Xu, X.-E.; Yu, K.-D.; Jin, X.; Hu, X.; Zuo, W.-J.; Hao, S.; Wu, J.; Liu, G.-Y.; et al. Comprehensive Transcriptome Analysis Identifies Novel Molecular Subtypes and Subtype-Specific RNAs of Triple-Negative Breast Cancer. Breast Cancer Res. BCR 2016, 18. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the Benefits of Therapy for Early-Stage Breast Cancer: The St. Gallen International Consensus Guidelines for the Primary Therapy of Early Breast Cancer 2019. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Foldi, J.; Dhawan, A.; DiGiovanna, M.P.; Mamounas, E.P. Changing Frameworks in Treatment Sequencing of Triple-Negative and HER2-Positive, Early-Stage Breast Cancers. Lancet Oncol. 2019, 20, e390–e396. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet Lond. Engl. 2014, 384, 164–172. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis after Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients with Triple-Negative Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Anderson, S.J.; Dignam, J.J.; Bear, H.D.; Julian, T.B.; Geyer, C.E.; Taghian, A.; Wickerham, D.L.; Wolmark, N. Predictors of Locoregional Recurrence after Neoadjuvant Chemotherapy: Results from Combined Analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3960–3966. [Google Scholar] [CrossRef]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative Chemotherapy in Patients with Operable Breast Cancer: Nine-Year Results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 2001, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Gass, P.; Lux, M.P.; Rauh, C.; Hein, A.; Bani, M.R.; Fiessler, C.; Hartmann, A.; Häberle, L.; Pretscher, J.; Erber, R.; et al. Prediction of Pathological Complete Response and Prognosis in Patients with Neoadjuvant Treatment for Triple-Negative Breast Cancer. BMC Cancer 2018, 18, 1051. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Wang, M.; Yu, X.; Guo, J.; Sun, T.; Yao, L.; Zhang, Q.; Xu, Y. Predictive and Prognostic Roles of Pathological Indicators for Patients with Breast Cancer on Neoadjuvant Chemotherapy. J. Breast Cancer 2019, 22, 497–521. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Blohmer, J.U.; Costa, S.D.; Denkert, C.; Eidtmann, H.; Eiermann, W.; Gerber, B.; Hanusch, C.; Hilfrich, J.; Huober, J.; et al. Response-Guided Neoadjuvant Chemotherapy for Breast Cancer. J. Clin. Oncol. 2013, 31, 3623–3630. [Google Scholar] [CrossRef]
- Cleator, S.J.; Makris, A.; Ashley, S.E.; Lal, R.; Powles, T.J. Good Clinical Response of Breast Cancers to Neoadjuvant Chemoendocrine Therapy Is Associated with Improved Overall Survival. Ann. Oncol. 2005, 16, 267–272. [Google Scholar] [CrossRef]
- Hong, J.; Wu, J.; Huang, O.; He, J.; Zhu, L.; Chen, W.; Li, Y.; Chen, X.; Shen, K. Early Response and Pathological Complete Remission in Breast Cancer with Different Molecular Subtypes: A Retrospective Single Center Analysis. J. Cancer 2020, 11, 6916–6924. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Marczyk, E.; Jasiowka, M.; Gronwald, J.; Jakubowicz, J.; Cybulski, C.; Wisniowski, R.; Godlewski, D.; et al. Pathologic Complete Response to Neoadjuvant Cisplatin in BRCA1-Positive Breast Cancer Patients. Breast Cancer Res. Treat. 2014, 147, 401–405. [Google Scholar] [CrossRef]
- Telli, M.L.; Jensen, K.C.; Vinayak, S.; Kurian, A.W.; Lipson, J.A.; Flaherty, P.J.; Timms, K.; Abkevich, V.; Schackmann, E.A.; Wapnir, I.L.; et al. Phase II Study of Gemcitabine, Carboplatin, and Iniparib as Neoadjuvant Therapy for Triple-Negative and BRCA1/2 Mutation-Associated Breast Cancer with Assessment of a Tumor-Based Measure of Genomic Instability: PrECOG 0105. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant Carboplatin in Patients with Triple-Negative and HER2-Positive Early Breast Cancer (GeparSixto; GBG 66): A Randomised Phase 2 Trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Alba, E.; Chacon, J.I.; Lluch, A.; Anton, A.; Estevez, L.; Cirauqui, B.; Carrasco, E.; Calvo, L.; Segui, M.A.; Ribelles, N.; et al. A Randomized Phase II Trial of Platinum Salts in Basal-like Breast Cancer Patients in the Neoadjuvant Setting. Results from the GEICAM/2006-03, Multicenter Study. Breast Cancer Res. Treat. 2012, 136, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.L.; Abramson, V.; Jankowitz, R.; Falkson, C.; Marcom, P.K.; Traina, T.; Carey, L.; Rimawi, M.; Specht, J.; Miller, K.; et al. TBCRC 030: A Phase II Study of Preoperative Cisplatin versus Paclitaxel in Triple-Negative Breast Cancer: Evaluating the Homologous Recombination Deficiency (HRD) Biomarker. Ann. Oncol. 2020, 31, 1518–1525. [Google Scholar] [CrossRef]
- Couch, F.J.; Hart, S.N.; Sharma, P.; Toland, A.E.; Wang, X.; Miron, P.; Olson, J.E.; Godwin, A.K.; Pankratz, V.S.; Olswold, C.; et al. Inherited Mutations in 17 Breast Cancer Susceptibility Genes among a Large Triple-Negative Breast Cancer Cohort Unselected for Family History of Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 304–311. [Google Scholar] [CrossRef]
- Alli, E.; Sharma, V.B.; Hartman, A.-R.; Lin, P.S.; McPherson, L.; Ford, J.M. Enhanced Sensitivity to Cisplatin and Gemcitabine in Brca1-Deficient Murine Mammary Epithelial Cells. BMC Pharmacol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Ear, U.S.; Koller, B.H.; Weichselbaum, R.R.; Bishop, D.K. The Breast Cancer Susceptibility Gene BRCA1 Is Required for Subnuclear Assembly of Rad51 and Survival Following Treatment with the DNA Cross-Linking Agent Cisplatin. J. Biol. Chem. 2000, 275, 23899–23903. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Quinn, J.E.; Mullan, P.B.; Johnston, P.G.; Harkin, D.P. The Role of BRCA1 in the Cellular Response to Chemotherapy. J. Natl. Cancer Inst. 2004, 96, 1659–1668. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lokuhetty, D.; White, V.A.; Watanabe, R.; Cree, I.A. Breast Tumours; WHO Classification of Tumours Editorial Board; International Agency for Research on Cancer: Lyon, France, 2019; ISBN 978-92-832-4500-1. [Google Scholar]
- Fujii, T.; Kogawa, T.; Dong, W.; Sahin, A.A.; Moulder, S.; Litton, J.K.; Tripathy, D.; Iwamoto, T.; Hunt, K.K.; Pusztai, L.; et al. Revisiting the Definition of Estrogen Receptor Positivity in HER2-Negative Primary Breast Cancer. Ann. Oncol. 2017, 28, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Er, T.-K.; Chang, J.-G. High-Resolution Melting: Applications in Genetic Disorders. Clin. Chim. Acta Int. J. Clin. Chem. 2012, 414, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of Age: Ten Years of next-Generation Sequencing Technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef]
- Soukupova, J.; Zemankova, P.; Lhotova, K.; Janatova, M.; Borecka, M.; Stolarova, L.; Lhota, F.; Foretova, L.; Machackova, E.; Stranecky, V.; et al. Validation of CZECANCA (CZEch CAncer PaNel for Clinical Application) for Targeted NGS-Based Analysis of Hereditary Cancer Syndromes. PLoS ONE 2018, 13, e0195761. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer. J. Mol. Diagn. JMD 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 380–391. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5; U.S. Department of Health and Human Services: Washington, DC, USA, 2017.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer Oxf. Engl. 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Cortazar, P.; Geyer, C.E. Pathological Complete Response in Neoadjuvant Treatment of Breast Cancer. Ann. Surg. Oncol. 2015, 22, 1441–1446. [Google Scholar] [CrossRef]
- R Core Team. European Environment Agency. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 8 February 2021).
- Wu, K.; Yang, Q.; Liu, Y.; Wu, A.; Yang, Z. Meta-Analysis on the Association between Pathologic Complete Response and Triple-Negative Breast Cancer after Neoadjuvant Chemotherapy. World J. Surg. Oncol. 2014, 12, 95. [Google Scholar] [CrossRef]
- Pandy, J.G.P.; Balolong-Garcia, J.C.; Cruz-Ordinario, M.V.B.; Que, F.V.F. Triple Negative Breast Cancer and Platinum-Based Systemic Treatment: A Meta-Analysis and Systematic Review. BMC Cancer 2019, 19, 1065. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Kümmel, S.; Vogel, P.; Hanusch, C.; Eidtmann, H.; Hilfrich, J.; Gerber, B.; Huober, J.; Costa, S.D.; Jackisch, C.; et al. Intensified Neoadjuvant Chemotherapy in Early-Responding Breast Cancer: Phase III Randomized GeparTrio Study. J. Natl. Cancer Inst. 2008, 100, 552–562. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Kümmel, S.; Vogel, P.; Hanusch, C.; Eidtmann, H.; Hilfrich, J.; Gerber, B.; Huober, J.; Costa, S.D.; Jackisch, C.; et al. Neoadjuvant Vinorelbine-Capecitabine versus Docetaxel-Doxorubicin-Cyclophosphamide in Early Nonresponsive Breast Cancer: Phase III Randomized GeparTrio Trial. J. Natl. Cancer Inst. 2008, 100, 542–551. [Google Scholar] [CrossRef]
- Fasching, P.A.; Heusinger, K.; Haeberle, L.; Niklos, M.; Hein, A.; Bayer, C.M.; Rauh, C.; Schulz-Wendtland, R.; Bani, M.R.; Schrauder, M.; et al. Ki67, Chemotherapy Response, and Prognosis in Breast Cancer Patients Receiving Neoadjuvant Treatment. BMC Cancer 2011, 11, 486. [Google Scholar] [CrossRef]
- Del Prete, S.; Caraglia, M.; Luce, A.; Montella, L.; Galizia, G.; Sperlongano, P.; Cennamo, G.; Lieto, E.; Capasso, E.; Fiorentino, O.; et al. Clinical and Pathological Factors Predictive of Response to Neoadjuvant Chemotherapy in Breast Cancer: A Single Center Experience. Oncol. Lett. 2019, 18, 3873–3879. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, M.; Zhou, H.; Chen, K.; Jin, J.; Wu, Y.; Ying, L.; Ding, X.; Su, D.; Zou, D. A Nomogram to Predict the Pathologic Complete Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Based on Simple Laboratory Indicators. Ann. Surg. Oncol. 2019, 26, 3912–3919. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Huang, X.-Y.; Mo, M.; Li, J.-W.; Jia, X.-Q.; Shao, Z.-M.; Shen, Z.-Z.; Wu, J.; Liu, G.-Y. Serum Tumor Marker Levels Might Have Little Significance in Evaluating Neoadjuvant Treatment Response in Locally Advanced Breast Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4603–4608. [Google Scholar] [CrossRef]
- Gamucci, T.; Pizzuti, L.; Sperduti, I.; Mentuccia, L.; Vaccaro, A.; Moscetti, L.; Marchetti, P.; Carbognin, L.; Michelotti, A.; Iezzi, L.; et al. Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: A Multicentric Retrospective Observational Study in Real-Life Setting. J. Cell. Physiol. 2018, 233, 2313–2323. [Google Scholar] [CrossRef]
- Alba, E.; Lluch, A.; Ribelles, N.; Anton-Torres, A.; Sanchez-Rovira, P.; Albanell, J.; Calvo, L.; García-Asenjo, J.A.L.; Palacios, J.; Chacon, J.I.; et al. High Proliferation Predicts Pathological Complete Response to Neoadjuvant Chemotherapy in Early Breast Cancer. Oncologist 2016, 21, 778. [Google Scholar] [CrossRef][Green Version]
- Brown, J.R.; DiGiovanna, M.P.; Killelea, B.; Lannin, D.R.; Rimm, D.L. Quantitative Assessment Ki-67 Score for Prediction of Response to Neoadjuvant Chemotherapy in Breast Cancer. Lab. Investig. J. Tech. Methods Pathol. 2014, 94, 98–106. [Google Scholar] [CrossRef]
- Loibl, S.; Jackisch, C.; Gade, S.; Untch, M.; Paepke, S.; Kuemmel, S.; Schneeweiss, A.; Jackisch, C.; Huober, J.; Hilfrich, J.; et al. Abstract S3-1: Neoadjuvant Chemotherapy in the Very Young 35 Years of Age or Younger. Cancer Res. 2012, 72, S3-1. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, K.; Yang, H.; He, X.; Mo, W.; Ding, X. Does Dose-Dense Neoadjuvant Chemotherapy Have Clinically Significant Prognostic Value in Breast Cancer?: A Meta-Analysis of 3,724 Patients. PLoS ONE 2020, 15, e0234058. [Google Scholar] [CrossRef]
- Reinisch, M.; Ataseven, B.; Kümmel, S. Neoadjuvant Dose-Dense and Dose-Intensified Chemotherapy in Breast Cancer—Review of the Literature. Breast Care Basel Switz. 2016, 11, 13–20. [Google Scholar] [CrossRef]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.-O.; Calogrias, D.; Buraimoh, A.; et al. Efficacy of Neoadjuvant Cisplatin in Triple-Negative Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1145–1153. [Google Scholar] [CrossRef]
- Arun, B.; Bayraktar, S.; Liu, D.D.; Gutierrez Barrera, A.M.; Atchley, D.; Pusztai, L.; Litton, J.K.; Valero, V.; Meric-Bernstam, F.; Hortobagyi, G.N.; et al. Response to Neoadjuvant Systemic Therapy for Breast Cancer in BRCA Mutation Carriers and Noncarriers: A Single-Institution Experience. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3739–3746. [Google Scholar] [CrossRef]
- Hahnen, E.; Lederer, B.; Hauke, J.; Loibl, S.; Kröber, S.; Schneeweiss, A.; Denkert, C.; Fasching, P.A.; Blohmer, J.U.; Jackisch, C.; et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1378–1385. [Google Scholar] [CrossRef]
- Caramelo, O.; Silva, C.; Caramelo, F.; Frutuoso, C.; Almeida-Santos, T. The Effect of Neoadjuvant Platinum-Based Chemotherapy in BRCA Mutated Triple Negative Breast Cancers -Systematic Review and Meta-Analysis. Hered. Cancer Clin. Pract. 2019, 17, 11. [Google Scholar] [CrossRef]
- Sharma, P.; Kimler, B.F.; O’Dea, A.; Nye, L.; Wang, Y.Y.; Yoder, R.; Staley, J.M.; Prochaska, L.; Wagner, J.; Amin, A.L.; et al. Randomized Phase II Trial of Anthracycline-Free and Anthracycline-Containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-Negative Breast Cancer (NeoSTOP). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 975–982. [Google Scholar] [CrossRef]
- Wang, D.; Feng, J.; Xu, B. A Meta-Analysis of Platinum-Based Neoadjuvant Chemotherapy versus Standard Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer. Future Oncol. 2019, 15, 2779–2790. [Google Scholar] [CrossRef]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Somlo, G.; Port, E.R.; Qamar, R.; Sturtz, K.; et al. Abstract S2-05: Event-Free and Overall Survival Following Neoadjuvant Weekly Paclitaxel and Dose-Dense AC +/- Carboplatin and/or Bevacizumab in Triple-Negative Breast Cancer: Outcomes from CALGB 40603 (Alliance). Cancer Res. 2016, 76, S2-05. [Google Scholar] [CrossRef]
- Engel, C.; Rhiem, K.; Hahnen, E.; Loibl, S.; Weber, K.E.; Seiler, S.; Zachariae, S.; Hauke, J.; Wappenschmidt, B.; Waha, A.; et al. Prevalence of Pathogenic BRCA1/2 Germline Mutations among 802 Women with Unilateral Triple-Negative Breast Cancer without Family Cancer History. BMC Cancer 2018, 18. [Google Scholar] [CrossRef]
- Kurbel, S.; Dmitrović, B.; Marjanović, K.; Vrbanec, D.; Juretić, A. Distribution of Ki-67 Values within HER2 & ER/PgR Expression Variants of Ductal Breast Cancers as a Potential Link between IHC Features and Breast Cancer Biology. BMC Cancer 2017, 17, 231. [Google Scholar] [CrossRef]

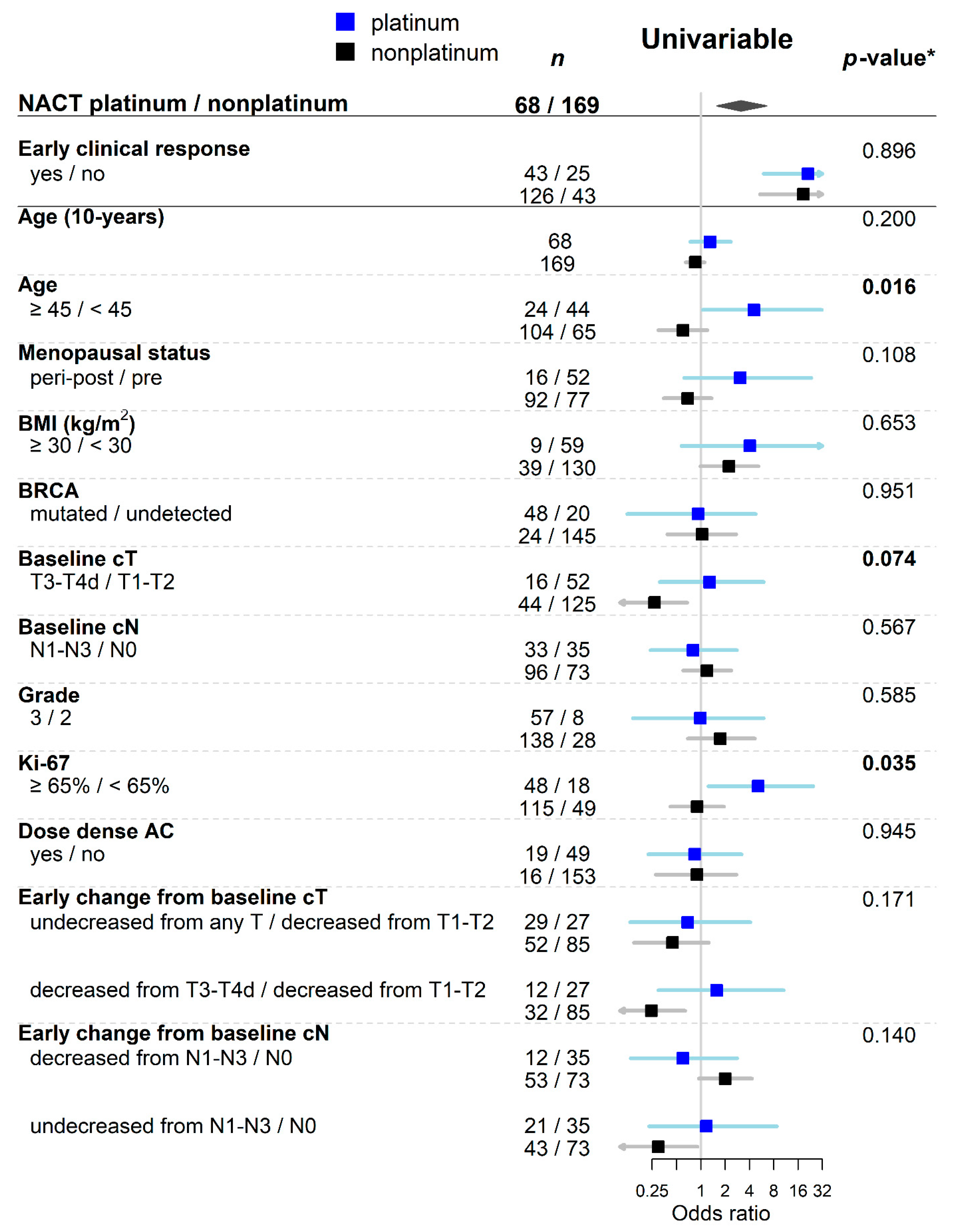

| Variables | Values | Overall | Early Clinical Response | Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 237 | No n = 68 | Yes n = 169 | OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Age (years) | Median (IQR) | 46 (37, 58) | 49 (39, 59) | 45 (37, 57) | 0.98 | 0.96, 1.01 | 0.140 | |||
| Range | 17, 78 | 23, 77 | 17, 78 | |||||||
| Age | <45 | 109 (46%) | 28 (26%) | 81 (74%) | — | — | 0.344 | |||
| ≥45 | 128 (54%) | 40 (31%) | 88 (69%) | 0.76 | 0.43, 1.34 | |||||
| Menopausal status | pre | 129 (54%) | 34 (26%) | 95 (74%) | — | — | 0.386 | |||
| peri-post | 108 (46%) | 34 (31%) | 74 (69%) | 0.78 | 0.44, 1.37 | |||||
| BMI (kg/m²) | <30 | 189 (80%) | 53 (28%) | 136 (72%) | — | — | 0.663 | |||
| ≥30 | 48 (20%) | 15 (31%) | 33 (69%) | 0.86 | 0.44, 1.74 | |||||
| BRCA1/2 | undetected 1 | 165 (70%) | 53 (32%) | 112 (68%) | — | — | 0.072 | — | — | 0.152 |
| mutated | 72 (30%) | 15 (21%) | 57 (79%) | 1.80 | 0.95, 3.56 | 1.63 | 0.84, 3.33 | |||
| Baseline cT | T1-T2 | 177 (75%) | 41 (23%) | 136 (77%) | — | — | 0.002 | — | — | 0.011 |
| T3-T4d | 60 (25%) | 27 (45%) | 33 (55%) | 0.37 | 0.20, 0.68 | 0.43 | 0.22, 0.82 | |||
| Focality | multi-centric/focal | 10 (4%) | 6 (60%) | 4 (40%) | — | — | 0.035 | — | — | 0.090 |
| unifocal | 227 (96%) | 62 (27%) | 165 (73%) | 3.99 | 1.10, 16.1 | 3.44 | 0.82, 15.4 | |||
| Baseline cN | N0 | 108 (46%) | 26 (24%) | 82 (76%) | — | — | 0.149 | |||
| N1–N3 | 129 (54%) | 42 (33%) | 87 (67%) | 0.66 | 0.37, 1.16 | |||||
| Grade | 2 | 36 (16%) | 10 (28%) | 26 (72%) | — | — | 0.859 | |||
| 3 | 195 (84%) | 57 (29%) | 138 (71%) | 0.93 | 0.41, 2.00 | |||||
| Unknown | 6 | 1 | 5 | |||||||
| Ki-67 (%) | Median (IQR) | 76 (60, 90) | 74 (52, 90) | 77 (64, 90) | 1.01 | 0.99, 1.02 | 0.386 | |||
| Range | 15, 100 | 38, 98 | 15, 100 | |||||||
| Unknown | 7 | 3 | 4 | |||||||
| Ki-67 | <65% | 67 (29%) | 25 (37%) | 42 (63%) | — | — | 0.054 | — | — | 0.079 |
| ≥65% | 163 (71%) | 40 (25%) | 123 (75%) | 1.83 | 0.99, 3.37 | 1.77 | 0.94, 3.34 | |||
| Unknown | 7 | 3 | 4 | |||||||
| Histology | IBC-NST | 226 (95%) | 64 (28%) | 162 (72%) | ||||||
| Other | 11 (4.6%) | 4 (36%) | 7 (64%) | |||||||
| Laboratory parameters * | ||||||||||
| LDH (μkat/L) | <3.8 | 170 (80%) | 46 (27%) | 124 (73%) | — | — | 0.275 | |||
| ≥3.8 | 42 (20%) | 15 (36%) | 27 (64%) | 0.67 | 0.33, 1.39 | |||||
| CRP (mg/L) | <10 | 171 (90%) | 43 (25%) | 128 (75%) | — | — | <0.001 | |||
| ≥10 | 18 (9.5%) | 12 (67%) | 6 (33%) | 0.17 | 0.06, 0.46 | |||||
| CAR | <0.095 | 144 (76%) | 35 (24%) | 109 (76%) | — | — | 0.011 | |||
| ≥0.095 | 45 (24%) | 20 (44%) | 25 (56%) | 0.40 | 0.20, 0.81 | |||||
| CEA (μg/L) | <2.8 | 176 (86%) | 46 (26%) | 130 (74%) | — | — | 0.101 | |||
| ≥2.8 | 29 (14%) | 12 (41%) | 17 (59%) | 0.50 | 0.22, 1.15 | |||||
| CA 15-3 (kU/L) | <18.4 | 86 (43%) | 17 (20%) | 69 (80%) | — | — | 0.020 | |||
| ≥18.4 | 116 (57%) | 40 (34%) | 76 (66%) | 0.47 | 0.24, 0.89 | |||||
| Hemoglobin (g/L) | <120 | 17 (8.0%) | 9 (53%) | 8 (47%) | — | — | 0.032 | |||
| ≥120 | 196 (92%) | 53 (27%) | 143 (73%) | 3.04 | 1.11, 8.48 | |||||
| LMR | <5.53 | 197 (92%) | 55 (28%) | 142 (72%) | — | — | 0.195 | |||
| ≥5.53 | 16 (7.5%) | 7 (44%) | 9 (56%) | 0.50 | 0.18, 1.45 | |||||
| NLR | <2.58 | 129 (61%) | 35 (27%) | 94 (73%) | — | — | 0.433 | |||
| ≥2.58 | 84 (39%) | 27 (32%) | 57 (68%) | 0.79 | 0.43, 1.44 | |||||
| SII | <774 | 151 (71%) | 39 (26%) | 112 (74%) | — | — | 0.104 | |||
| ≥774 | 62 (29%) | 23 (37%) | 39 (63%) | 0.59 | 0.31, 1.12 | |||||
| Variables | Values | Overall | Early Clinical Response | ||
|---|---|---|---|---|---|
| n = 237 | No n = 68 | Yes n = 169 | p-Value | ||
| Regimens of NACT | A-based only | 12 (5.1%) | 4 (5.9%) | 8 (4.7%) | |
| A → T | 154 (65%) | 37 (54%) | 117 (69%) | ||
| T-based only | 2 (0.8%) | 1 (1.5%) | 1 (0.6%) | ||
| CMF | 1 (0.4%) | 1 (1.5%) | 0 (0%) | ||
| A → T + CBDCA | 42 (18%) | 22 (32%) | 20 (12%) | ||
| A → CDDP | 26 (11%) | 3 (4.4%) | 23 (14%) | ||
| Dose dense AC | 35 (15%) | 9 (13%) | 26 (15%) | 0.673 | |
| NACT | nonplatinum | 169 (71%) | 43 (63%) | 126 (75%) | 0.081 |
| platinum | 68 (29%) | 25 (37%) | 43 (25%) | ||
| Platinum salts | CBDCA | 42 (62%) | 22 (88%) | 20 (47%) | <0.001 |
| CDDP | 26 (38%) | 3 (12%) | 23 (53%) | ||
| Time from diagnosis to NACT (days) 1 | Median (IQR) | 20 (14, 29) | 20 (13, 32) | 21 (14, 29) | 0.500 |
| Range | 0, 130 | 0, 113 | 2, 130 | ||
| Unknown | 18 | 2 | 16 | ||
| NACT | |||||
| Overall n = 237 | Nonplatinum n = 169 | Platinum n = 68 | p-Value | ||
| Time from NACT to surgery (days) 2 | Median (IQR) | 30 (23, 38) | 29 (22, 37) | 33 (26, 41) | 0.158 |
| Range | 3, 159 | 3, 159 | 3, 81 | ||
| Unknown | 16 | 15 | 1 | ||
| Toxicity | Nonplatinum NACT n = 145 * | Platinum NACT n = 68 | p-Value Any Grade | p-Value Grade 3–4 | ||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |||
| Overall toxicity | 38 (26%) | 68 (47%) | 23 (34%) | 39 (57%) | 0.003 | 0.155 |
| Myelotoxicity | 19 (13%) | 54 (37%) | 16 (24%) | 32 (47%) | 0.005 | 0.173 |
| Leukopenia/Neutropenia | 17 (12%) | 52 (36%) | 13 (19%) | 31 (46%) | ||
| Anaemia | 3 (2.1%) | 0 (0%) | 3 (4.4%) | 2 (2.9%) | ||
| Thrombocytopenia | 1 (0.7%) | 1 (0.7%) | 2 (2.9%) | 0 (0%) | ||
| Febrile neutropenia | 0 (0%) | 2 (1.4%) | 0 (0%) | 1 (1.5%) | ||
| Nonhaematological toxicity | 67 (46%) | 23 (16%) | 36 (53%) | 11 (16%) | 0.317 | 0.953 |
| Skin and mucosal toxicity | 19 (13%) | 5 (3.4%) | 2 (2.9%) | 3 (4.4%) | ||
| Nausea, Vomiting | 21 (14%) | 10 (6.9%) | 20 (29%) | 4 (5.9%) | ||
| Diarrhoea | 4 (2.8%) | 0 (0%) | 2 (2.9%) | 0 (0%) | ||
| Neurotoxicity | 29 (20%) | 9 (6.2%) | 14 (21%) | 4 (5.9%) | ||
| Hepatotoxicity | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | ||
| Premature Termination | 19 (13%) | 9 (13%) | ||||
| Variables | Values | OS | RFS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| ECR | no/yes | 2.66 | 1.47, 4.83 | 0.001 | 2.43 | 1.43, 4.13 | 0.001 |
| NACT | platinum/nonplatinum | 0.58 | 0.29, 1.19 | 0.139 | 0.74 | 0.40, 1.36 | 0.331 |
| pCR | no/yes | 3.36 | 1.36, 8.28 | 0.008 | 6.47 | 2.51, 16.7 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holanek, M.; Selingerova, I.; Bilek, O.; Kazda, T.; Fabian, P.; Foretova, L.; Zvarikova, M.; Obermannova, R.; Kolouskova, I.; Coufal, O.; et al. Neoadjuvant Chemotherapy of Triple-Negative Breast Cancer: Evaluation of Early Clinical Response, Pathological Complete Response Rates, and Addition of Platinum Salts Benefit Based on Real-World Evidence. Cancers 2021, 13, 1586. https://doi.org/10.3390/cancers13071586
Holanek M, Selingerova I, Bilek O, Kazda T, Fabian P, Foretova L, Zvarikova M, Obermannova R, Kolouskova I, Coufal O, et al. Neoadjuvant Chemotherapy of Triple-Negative Breast Cancer: Evaluation of Early Clinical Response, Pathological Complete Response Rates, and Addition of Platinum Salts Benefit Based on Real-World Evidence. Cancers. 2021; 13(7):1586. https://doi.org/10.3390/cancers13071586
Chicago/Turabian StyleHolanek, Milos, Iveta Selingerova, Ondrej Bilek, Tomas Kazda, Pavel Fabian, Lenka Foretova, Maria Zvarikova, Radka Obermannova, Ivana Kolouskova, Oldrich Coufal, and et al. 2021. "Neoadjuvant Chemotherapy of Triple-Negative Breast Cancer: Evaluation of Early Clinical Response, Pathological Complete Response Rates, and Addition of Platinum Salts Benefit Based on Real-World Evidence" Cancers 13, no. 7: 1586. https://doi.org/10.3390/cancers13071586
APA StyleHolanek, M., Selingerova, I., Bilek, O., Kazda, T., Fabian, P., Foretova, L., Zvarikova, M., Obermannova, R., Kolouskova, I., Coufal, O., Petrakova, K., Svoboda, M., & Poprach, A. (2021). Neoadjuvant Chemotherapy of Triple-Negative Breast Cancer: Evaluation of Early Clinical Response, Pathological Complete Response Rates, and Addition of Platinum Salts Benefit Based on Real-World Evidence. Cancers, 13(7), 1586. https://doi.org/10.3390/cancers13071586