The Role of [18F]Fluciclovine PET/CT in the Characterization of High-Risk Primary Prostate Cancer: Comparison with [11C]Choline PET/CT and Histopathological Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary and Secondary Aims
2.2. Study Design
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Radiotracer Production
2.4. PET/CT Protocol
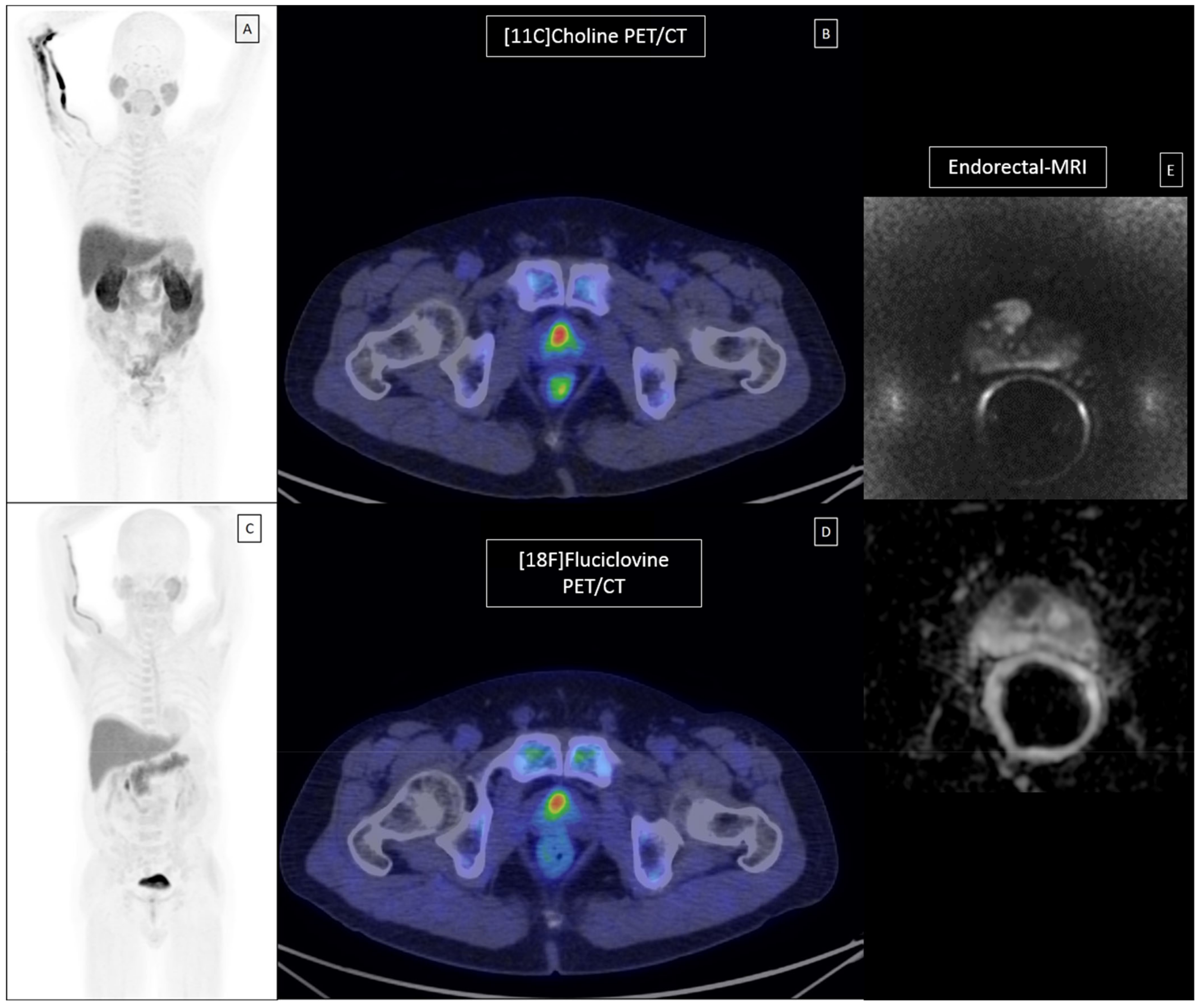
2.5. Imaging Interpretation
2.5.1. Patient-Based Interpretation
2.5.2. Lesion-Based Interpretation
- abdominal aorta (1 cm3-VOI within the vessel lumen), called TBR-AORTA;
- bone marrow (1 cm3-VOI at L3 vertebral body), called TBR-L3;
- liver (3 cm3-VOI in healthy hepatic parenchyma, when possible in the right lobe), called TBR-LIVER.
2.6. Histopathological Analysis
2.7. Data Comparison and Validation
2.8. Statistical Analyses
2.8.1. Patient-Based Analyses
2.8.2. Lesion-Based Analyses
3. Results
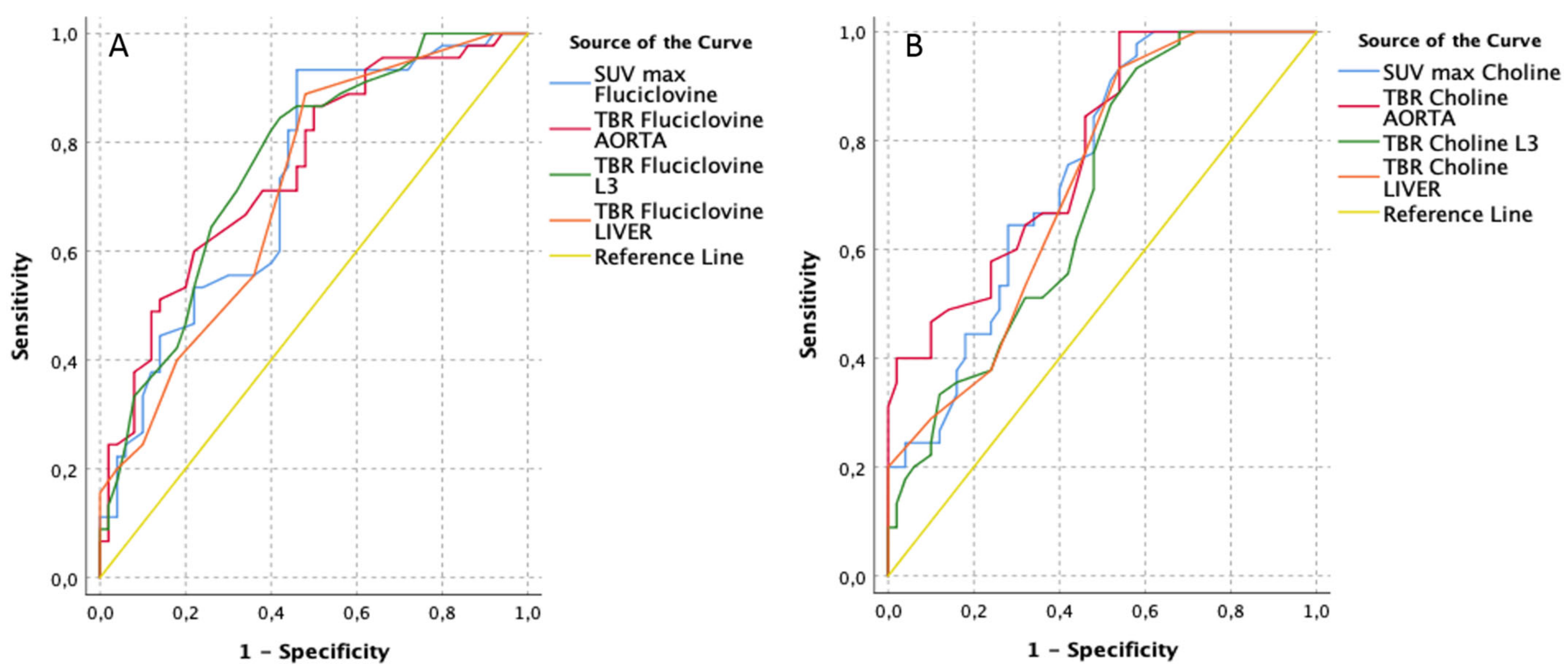
3.1. Patient-Based Analyses (Blinded to Histopathology)
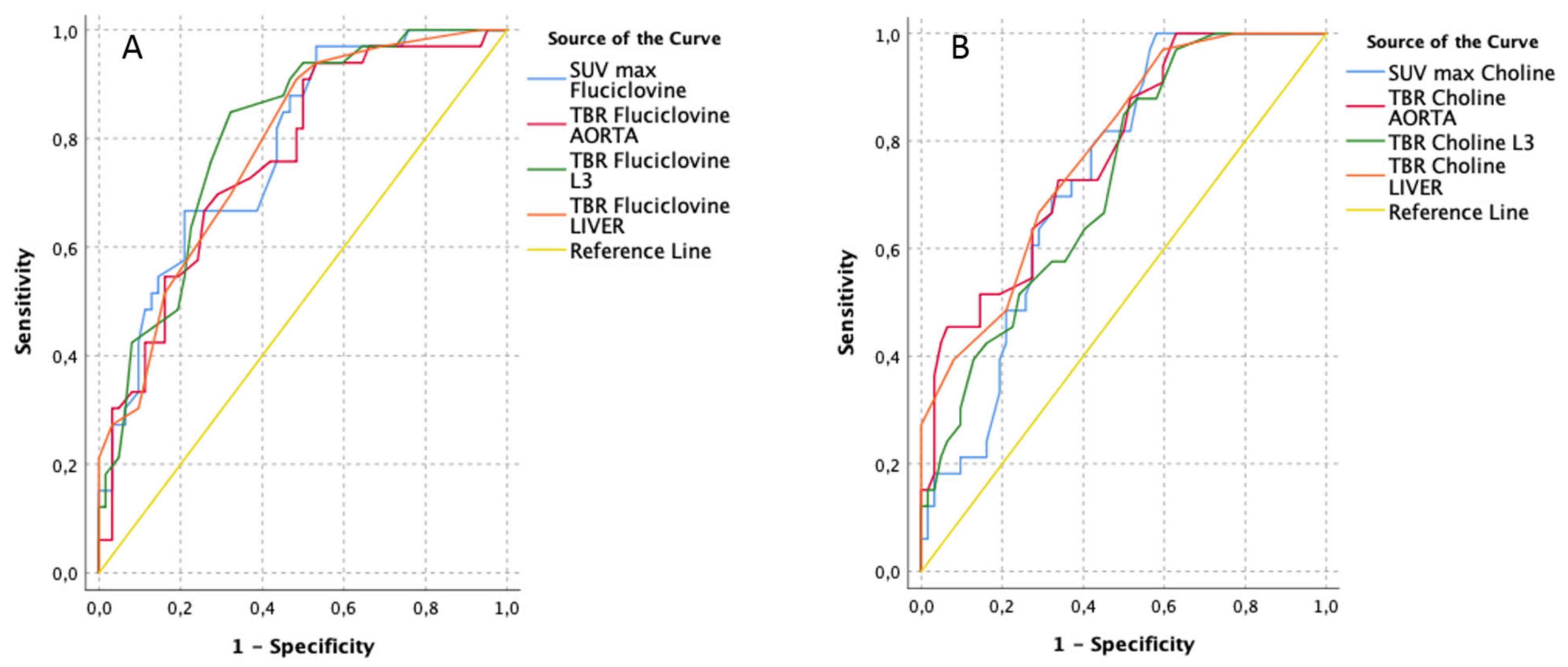
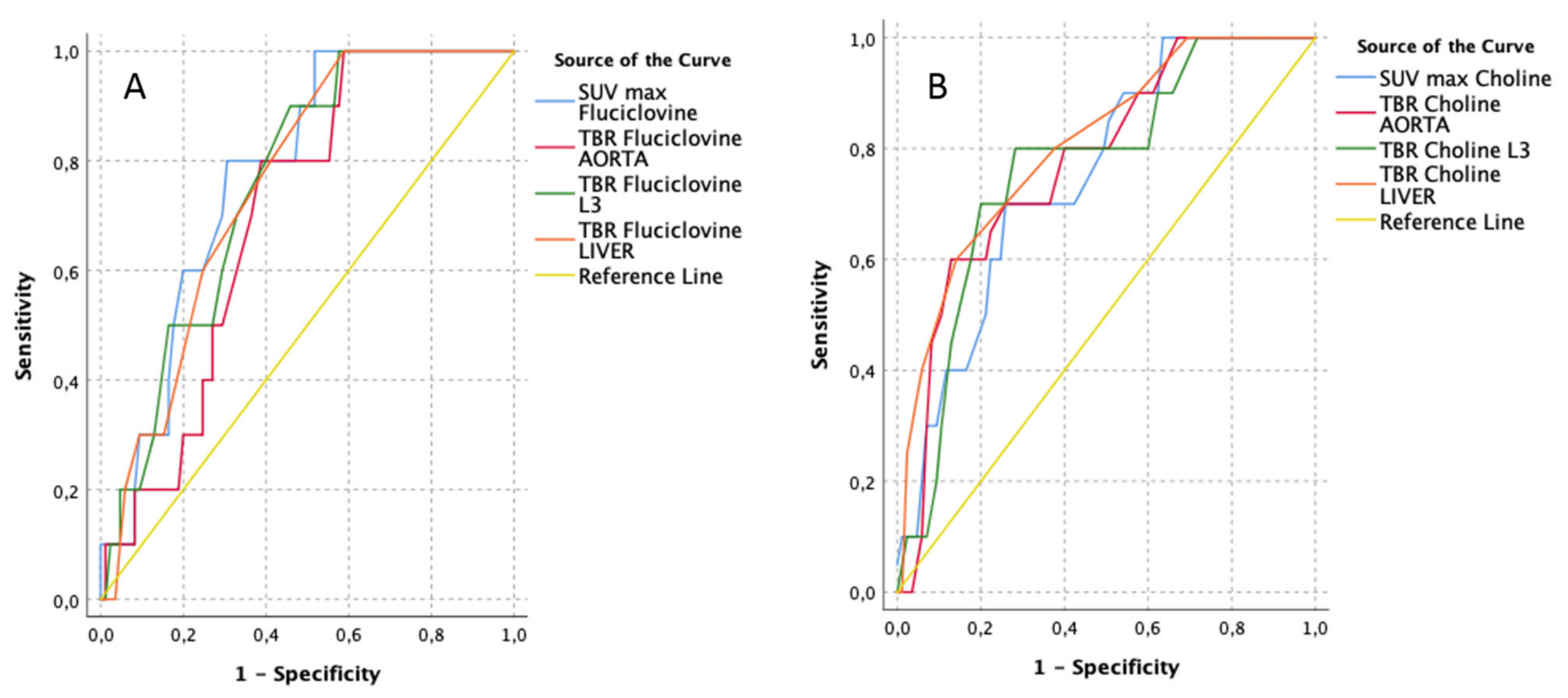
3.2. Lesion-Based Analyses (Unblinded to Histopathology, According to the Uro-Pathologist Prostate Template)
4. Discussion
4.1. Scientific Literature
4.2. Considerations on the Present Study
4.3. Limitations of the Present Study
4.4. Recent Research and Future Developments in Multimodality Imaging of Primary PCa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EAU Guidelines: Prostate Cancer|Uroweb. Available online: https://uroweb.org/guideline/prostate-cancer/ (accessed on 30 November 2020).
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Mottet, N.; Bellmunt, J.; Briers, E.; Bergh, R.C.N.; van den Bolla, M.; Casteren, N.J.; van Conford, P.; Culine, S.; Joniau, S.; Lam, T.; et al. Prostate Cancer 2020 Guidelines. Update 2020. Available online: https://uroweb.org/guideline/prostate-cancer/ (accessed on 1 February 2021).
- Pucar, D.; Hricak, H.; Shukla-Dave, A.; Kuroiwa, K.; Drobnjak, M.; Eastham, J.; Scardino, P.T.; Zelefsky, M.J. Clinically Significant Prostate Cancer Local Recurrence after Radiation Therapy Occurs at the Site of Primary Tumor: Magnetic Resonance Imaging and Step-Section Pathology Evidence. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 62–69. [Google Scholar] [CrossRef]
- Schiavina, R.; Bianchi, L.; Borghesi, M.; Dababneh, H.; Chessa, F.; Pultrone, C.V.; Angiolini, A.; Gaudiano, C.; Porreca, A.; Fiorentino, M.; et al. MRI Displays the Prostatic Cancer Anatomy and Improves the Bundles Management before Robot-Assisted Radical Prostatectomy. J. Endourol. 2018, 32, 315–321. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Mottrie, A.; Brunocilla, E. Response to Johnston re: MRI Displays the Prostatic Cancer Anatomy and Improves the Bundles Management Before Robot-Assisted Radical Prostatectomy by Bianchi et al. (From: Johnston WK, III. J Endourol 2018;32:322-323). J. Endourol. 2018, 32, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Drost, F.-J.H.; Osses, D.F.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Roobol, M.J.; Schoots, I.G. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Schiavina, R.; Chessa, F.; Borghesi, M.; Gaudiano, C.; Bianchi, L.; Corcioni, B.; Castellucci, P.; Ceci, F.; Ceravolo, I.; Barchetti, G.; et al. State-of-the-art imaging techniques in the management of preoperative staging and re-staging of prostate cancer. Int. J. Urol. 2019, 26, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Briganti, A.; Fanti, S.; Joniau, S.; Reske, S.; Schiavina, R.; Stief, C.; Thalmann, G.N.; Picchio, M. New Clinical Indications for 18F/11C-choline, New Tracers for Positron Emission Tomography and a Promising Hybrid Device for Prostate Cancer Staging: A Systematic Review of the Literature [Figure presented]. Eur. Urol. 2016, 70, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, P.; Ceci, F.; Fanti, S. Imaging of Prostate Cancer Using 11C-Choline PET/Computed Tomography. Urol. Clin. N. Am. 2018, 45, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Bianchi, L.; Borghesi, M.; Polverari, G.; Farolfi, A.; Briganti, A.; Schiavina, R.; Brunocilla, E.; Castellucci, P.; Fanti, S. Prediction nomogram for 68Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Morigi, J.J.; Anderson, J.; De Nunzio, C.; Fanti, S. PSMA PET/CT and staging high risk prostate cancer: A non-systematic review of high clinical impact literature. Minerva Urol. Nefrol. 2020. [Google Scholar] [CrossRef]
- Schuster, D.M.; Votaw, J.R.; Nieh, P.T.; Yu, W.; Nye, J.A.; Master, V.; Bowman, F.D.B.; Issa, M.M.; Goodman, M.M. Initial experience with the radiotracer anti-1-amino-3-18F- fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J. Nucl. Med. 2007, 48, 56–63. [Google Scholar] [PubMed]
- Oka, S.; Hattori, R.; Kurosaki, F.; Toyama, M.; Williams, L.A.; Yu, W.; Votaw, J.R.; Yoshida, Y.; Goodman, M.M.; Ito, O. A Preliminary Study of Anti-1-Amino-3-18F-Fluorocyclobutyl-1-Carboxylic Acid for the Detection of Prostate Cancer. J. Nucl. Med. 2007, 48, 46–55. [Google Scholar] [PubMed]
- Nye, J.A.; Schuster, D.M.; Yu, W.; Camp, V.M.; Goodman, M.M.; Votaw, J.R. Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti- 18F-FACBC in humans. J. Nucl. Med. 2007, 48, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Owenius, R.; Lax, M.; Johansson, S. Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 394–402. [Google Scholar] [CrossRef]
- Schuster, D.M.; Taleghani, P.A.; Nieh, P.T.; Master, V.A.; Amzat, R.; Savir-Baruch, B.; Halkar, R.K.; Fox, T.; Osunkoya, A.O.; Moreno, C.S.; et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F] -fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 85–96. [Google Scholar] [PubMed]
- Schiavina, R.; Concetti, S.; Brunocilla, E.; Nanni, C.; Borghesi, M.; Gentile, G.; Cevenini, M.; Bianchi, L.; Molinaroli, E.; Fanti, S.; et al. First case of F-FACBC PET/CT-guided salvage retroperitoneal lymph node dissection for disease relapse after radical prostatectomy for prostate cancer and negative 11C-Choline PET/CT: New imaging techniques may expand pioneering approaches. Urol. Int. 2014, 92, 242–245. [Google Scholar] [CrossRef]
- Nanni, C.; Zanoni, L.; Pultrone, C.; Schiavina, R.; Brunocilla, E.; Lodi, F.; Malizia, C.; Ferrari, M.; Rigatti, P.; Fonti, C.; et al. 18F-FACBC (anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET/CT in prostate cancer relapse: Results of a prospective trial. Eur. J. Nucl. Med. Mol. Imaging 2016, 43. [Google Scholar] [CrossRef]
- Nanni, C.; Schiavina, R.; Brunocilla, E.; Borghesi, M.; Ambrosini, V.; Zanoni, L.; Gentile, G.; Vagnoni, V.; Romagnoli, D.; Martorana, G.; et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: A prospective study in 28 patients. Clin. Genitourin. Cancer 2014, 12. [Google Scholar] [CrossRef]
- Nanni, C.; Schiavina, R.; Brunocilla, E.; Boschi, S.; Borghesi, M.; Zanoni, L.; Pettinato, C.; Martorana, G.; Fanti, S. 18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT. Clin. Nucl. Med. 2015, 40. [Google Scholar] [CrossRef]
- Zanoni, L.; Bossert, I.; Matti, A.; Schiavina, R.; Pultrone, C.; Fanti, S.; Nanni, C. A review discussing fluciclovine (18F) PET/CT imaging in the detection of recurrent prostate cancer. Future Oncol. 2018, 14. [Google Scholar] [CrossRef]
- Bach-Gansmo, T.; Nanni, C.; Nieh, P.T.; Zanoni, L.; Bogsrud, T.V.; Sletten, H.; Korsan, K.A.; Kieboom, J.; Tade, F.I.; Odewole, O.; et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (18F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J. Urol. 2017, 197. [Google Scholar] [CrossRef]
- Farsad, M.; Schiavina, R.; Castellucci, P.; Nanni, C.; Corti, B.; Martorana, G.; Canini, R.; Grigioni, W.; Boschi, S.; Marengo, M.; et al. Detection and Localization of Prostate Cancer: Correlation of 11C-Choline PET/CT with Histopathologic Step-Section Analysis. J. Nucl. Med. 2005, 46, 1642–1649. [Google Scholar]
- FDA Approves New Diagnostic Imaging Agent to Detect Recurrent Prostate Cancer|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-diagnostic-imaging-agent-detect-recurrent-prostate-cancer (accessed on 7 February 2021).
- Axumin|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/axumin (accessed on 7 February 2021).
- Turkbey, B.; Mena, E.; Shih, J.; Pinto, P.A.; Merino, M.J.; Lindenberg, M.L.; Bernardo, M.; McKinney, Y.L.; Adler, S.; Owenius, R.; et al. Localized prostate cancer detection with 18F FACBC PET/CT: Comparison with MR imaging and histopathologic analysis. Radiology 2014, 270, 849–856. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nanni, C.; Fanti, S.; Oka, S.; Okudaira, H.; Inoue, Y.; Sörensen, J.; Owenius, R.; Choyke, P.; Turkbey, B.; et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: Physiologic uptake patterns, incidental findings, and variants that may simulate disease. J. Nucl. Med. 2014, 55, 1986–1992. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph. Eur.) 10th Edition|EDQM—European Directorate for the Quality of Medicines. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 7 February 2021).
- AIMN—Linee-Guida. Available online: https://www.aimn.it/site/page/attivita/linee-guida (accessed on 7 February 2021).
- Bianchi, L.; Schiavina, R.; Borghesi, M.; Casablanca, C.; Chessa, F.; Bianchi, F.M.; Puitrone, C.; Vagnoni, V.; Ercolino, A.; Dababneh, H.; et al. Patterns of positive surgical margins after open radical prostatectomy and their association with clinical recurrence. Minerva Urol. Nefrol. 2020, 72, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Schiavina, R.; Bianchi, L.; Lodi, S.; Cercenelli, L.; Chessa, F.; Bortolani, B.; Gaudiano, C.; Casablanca, C.; Droghetti, M.; Porreca, A.; et al. Real-time Augmented Reality Three-dimensional Guided Robotic Radical Prostatectomy: Preliminary Experience and Evaluation of the Impact on Surgical Planning. Eur. Urol. Focus 2020. [Google Scholar] [CrossRef]
- Elschot, M.; Selnaes, K.M.; Sandsmark, E.; Krüger-Stokke, B.; Størkersen, Ø.; Giskeødegård, G.F.; Tessem, M.-B.; Moestue, S.A.; Bertilsson, H.; Bathen, T.F. Combined 18 F-Fluciclovine PET/MRI Shows Potential for Detection and Characterization of High-Risk Prostate Cancer. J. Nucl. Med. 2018, 59, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Jambor, I.; Kuisma, A.; Kähkönen, E.; Kemppainen, J.; Merisaari, H.; Eskola, O.; Teuho, J.; Montoya Perez, I.; Pesola, M.; Aronen, H.J.; et al. Prospective evaluation of 18 F-FACBC PET/CT and PET/MRI versus multiparametric MRI in intermediate-to high-risk prostate cancer patients (FLUCIPRO trial). Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.W. The role of 18F-fluciclovine PET in the management of prostate cancer: A systematic review and meta-analysis. Clin. Radiol. 2019, 74, 886–892. [Google Scholar] [CrossRef]
- Hernández-Argüello, M.; Quiceno, H.; Pascual, I.; Solorzano, J.L.; Benito, A.; Collantes, M.; Rodríguez-Fraile, M.; Pardo, J.; Richter, J.A. Index lesion characterization by 11C-Choline PET/CT and Apparent Diffusion Coefficient parameters at 3 Tesla MRI in primary prostate carcinoma. Prostate 2016, 76, 3–12. [Google Scholar] [CrossRef]
- Wetter, A.; Nensa, F.; Schenck, M.; Heusch, P.; Pöppel, T.; Bockisch, A.; Forsting, M.; Schlosser, T.W.; Lauenstein, T.C.; Nagarajah, J. Combined PET imaging and diffusion-weighted imaging of intermediate and high-risk primary prostate carcinomas with simultaneous [18F] choline PET/MRI. PLoS ONE 2014, 9, e101571. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong Cheon, G.; Chul Paeng, J.; Yeon Cho, J.; Kwak, C.; Wook Kang, K.; Chung, J.-K.; Edmund Kim, E.; Soo Lee, D.; Cheon, G.J.; et al. Usefulness of MRI-assisted metabolic volumetric parameters provided by simultaneous 18 F-fluorocholine PET/MRI for primary prostate cancer characterization. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1247–1256. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yang, J.; Noworolski, S.M.; Behr, S.; Chang, A.J.; Simko, J.P.; Nguyen, H.G.; Carroll, P.R.; Kurhanewicz, J.; Seo, Y. 18F fluorocholine dynamic timeof-flight PeT/Mr imaging in patients with newly diagnosed intermediate-to high-risk prostate cancer: Initial clinicalpathologic comparisons. Radiology 2017, 282, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Schaefferkoetter, J.D.; Wang, Z.; Stephenson, M.C.; Roy, S.; Conti, M.; Eriksson, L.; Townsend, D.W.; Thamboo, T.; Chiong, E. Quantitative 18F-fluorocholine positron emission tomography for prostate cancer: Correlation between kinetic parameters and Gleason scoring. EJNMMI Res. 2017, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, B.; Giorgio, T.; Rasoul, Z.S.; Werner, L.; Ali, G.R.M.; Reza, D.K.V.; Ramin, S. Application of 11C-acetate positron-emission tomography (PET) imaging in prostate cancer: Systematic review and meta-analysis of the literature. BJU Int. 2013, 112, 1062–1072. [Google Scholar] [CrossRef]
- Beauregard, J.-M.; Blouin, A.-C.; Fradet, V.; Caron, A.; Fradet, Y.; Lemay, C.; Lacombe, L.; Dujardin, T.; Tiguert, R.; Rimac, G.; et al. FDG-PET/CT for pre-operative staging and prognostic stratification of patients with high-grade prostate cancer at biopsy. Cancer Imaging 2015, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Kanagawa, M.; Doi, Y.; Oka, S.; Kobayashi, R.; Nakata, N.; Toyama, M.; Shirakami, Y. Comparison of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid (anti-[18F]FACBC) accumulation in lymph node prostate cancer metastasis and lymphadenitis in rats. Nucl. Med. Biol. 2014, 41, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Savir-Baruch, B.; Zanoni, L.; Schuster, D.M. Imaging of Prostate Cancer Using Fluciclovine. Urol. Clin. N. Am. 2018, 45. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Zanoni, L.; Bach-Gansmo, T.; Minn, H.; Willoch, F.; Bogsrud, T.V.; Edward, E.P.; Savir-Baruch, B.; Teoh, E.; Ingram, F.; et al. [18F]Fluciclovine PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging—Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47. [Google Scholar] [CrossRef]
- Miller, M.P.; Kostakoglu, L.; Pryma, D.; Yu, J.Q.; Chau, A.; Perlman, E.; Clarke, B.; Rosen, D.; Ward, P. Reader training for the restaging of biochemically recurrent prostate cancer using 18F-Fluciclovine PET/CT. J. Nucl. Med. 2017, 58, 1596–1602. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Zhao, Y.; Li, X.; Sun, P.; Wang, M.; Wang, R.; Jin, X. Imaging primary prostate cancer with 11C-Choline PET/CT: Relation to tumour stage, Gleason score and biomarkers of biologic aggressiveness. Radiol. Oncol. 2012, 46, 179–188. [Google Scholar] [CrossRef]
- Elschot, M.; Selnaes, K.M.; Sandsmark, E.; Krüger-Stokke, B.; Størkersen, Ø.; Tessem, M.-B.; Moestue, S.A.; Bertilsson, H.; Bathen, T.F. A PET/MRI study towards finding the optimal [18F]Fluciclovine PET protocol for detection and characterisation of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Ma, B.; Kunju, L.P.; Davenport, M.; Piert, M. Challenges in accurate registration of 3-D medical imaging and histopathology in primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 72. [Google Scholar] [CrossRef] [PubMed]
- Sutinen, E.; Nurmi, M.; Roivainen, A.; Varpula, M.; Tolvanen, T.; Lehikoinen, P.; Minn, H. Kinetics of [11C]choline uptake in prostate cancer: A PET stydy. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kwee, S.A.; Coel, M.N.; Lim, J.; Ko, J.P. Prostate cancer localization with 18fluorine fluorocholine positron emission tomography. J. Urol. 2005, 173, 252–255. [Google Scholar] [CrossRef]
- Grosu, A.L.; Weirich, G.; Wendl, C.; Prokic, V.; Kirste, S.; Geinitz, H.; Souvatzoglou, M.; Gschwend, J.E.; Schwaiger, M.; Molls, M.; et al. 11C-Choline PET/pathology image coregistration in primary localized prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2242–2248. [Google Scholar] [CrossRef]
- Zamboglou, C.; Schiller, F.; Fechter, T.; Wieser, G.; Jilg, C.A.; Chirindel, A.; Salman, N.; Drendel, V.; Werner, M.; Mix, M.; et al. Ga-HBED-CC-PSMA PET/CT Versus Histopathology in Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef]
- Giesel, F.L.; Sterzing, F.; Schlemmer, H.P.; Holland-Letz, T.; Mier, W.; Rius, M.; Afshar-Oromieh, A.; Kopka, K.; Debus, J.; Haberkorn, U.; et al. Intra-individual comparison of 68Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2016, 43, 1400–1406. [Google Scholar] [CrossRef]
- Schmuck, S.; Mamach, M.; Wilke, F.; von Klot, C.A.; Henkenberens, C.; Thackeray, J.T.; Sohns, J.M.; Geworski, L.; Ross, T.L.; Wester, H.-J.; et al. Multiple Time-Point 68Ga-PSMA I&T PET/CT for Characterization of Primary Prostate Cancer. Clin. Nucl. Med. 2017, 42, e286–e293. [Google Scholar] [CrossRef]
- Amin, A.; Blazevski, A.; Thompson, J.; Scheltema, M.J.; Hofman, M.S.; Murphy, D.; Lawrentschuk, N.; Sathianathen, N.; Kapoor, J.; Woo, H.H.; et al. Protocol for the PRIMARY clinical trial, a prospective, multicentre, cross-sectional study of the additive diagnostic value of gallium-68 prostate-specific membrane antigen positron-emission tomography/computed tomography to multiparametric magnetic reson. BJU Int. 2020, 125, 515–524. [Google Scholar] [CrossRef]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Bodar, Y.J.L.; Jansen, B.H.E.; Van Der Voorn, J.P.; Zwezerijnen, G.J.C.; Meijer, D.; Nieuwenhuijzen, J.A.; Boellaard, R.; Hendrikse, N.H.; Hoekstra, O.S.; Van Moorselaar, R.J.A.; et al. Detection of prostate cancer with 18F-DCFPyL PET/CT compared to final histopathology of radical prostatectomy specimens: Is PSMA-targeted biopsy feasible? The DeTeCT trial. World J. Urol. 2020. [Google Scholar] [CrossRef]
- Koseoglu, E.; Kordan, Y.; Kilic, M.; Sal, O.; Seymen, H.; Can Kiremit, M.; Armutlu, A.; Ertoy Baydar, D.; Altinmakas, E.; Vural, M.; et al. Prostate Cancer and Prostatic Diseases Diagnostic ability of Ga-68 PSMA PET to detect dominant and non-dominant tumors, upgrading and adverse pathology in patients with PIRADS 4-5 index lesions undergoing radical prostatectomy. Prostate Cancer Prostatic Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ergül, N.; Yilmaz Güneş, B.; Yücetaş, U.; Toktaş, M.G.; Çermik, T.F. 68Ga-PSMA-11 PET/CT in Newly Diagnosed Prostate Adenocarcinoma. Clin. Nucl. Med. 2018, 43, E422–E427. [Google Scholar] [CrossRef]
- Demirci, E.; Kabasakal, L.; Şahin, O.E.; Akgün, E.; Gültekin, H.; Doğanca, T.; Tuna, M.B.; Kılıç, M.; Esen, T.; Kural, A.R. Can SUVmax values of Ga-68-PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl. Med. 2018, 40, 86. [Google Scholar] [CrossRef]
- Wang, L.; Yu, F.; Yang, L.; Zang, S.; Xue, H.; Yin, X.; Guo, H.; Sun, H.; Wang, F. 68Ga-PSMA-11 PET/CT combining ADC value of MRI in the diagnosis of naive prostate cancer. Medicine 2020, 99, e20755. [Google Scholar] [CrossRef]
- Eiber, M.; Nekolla, S.G.; Maurer, T.; Weirich, G.; Wester, H.J.; Schwaiger, M. 68Ga-PSMA PET/MR with multimodality image analysis for primary prostate cancer. Abdom. Imaging 2015, 40, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Zattoni, F.; Cassarino, G.; Artioli, P.; Cecchin, D.; dal Moro, F.; Zucchetta, P. PET/MRI in prostate cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Fang, M.; Zou, J.; Yang, S.; Yu, D.; Zhong, L.; Hu, C.; Zang, Y.; Dong, D.; Tian, J.; et al. Using biparametric MRI radiomics signature to differentiate between benign and malignant prostate lesions. Eur. J. Radiol. 2019, 114, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ibarrola, R.; Hein, S.; Reis, G.; Gratzke, C.; Miernik, A. Current and future applications of machine and deep learning in urology: A review of the literature on urolithiasis, renal cell carcinoma, and bladder and prostate cancer. World J. Urol. 2020, 38, 2329–2347. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.; Spielvogel, C.P.; Grubmüller, B.; Grahovac, M.; Krajnc, D.; Ecsedi, B.; Sareshgi, R.A.M.; Mohamad, D.; Hamboeck, M.; Rausch, I.; et al. Supervised machine learning enables non-invasive lesion characterization in primary prostate cancer with [68Ga] Ga-PSMA-11 PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
| Age (years) | Mean | 63.4 |
| SD | 5.8 | |
| Median | 64 | |
| Range | 51–72 | |
| iPSA (ng/mL) | Mean | 9.1 |
| SD | 6.1 | |
| Median | 6.6 | |
| Range | 4.2–25 | |
| DRE | Negative | 6/19 (32%) |
| Positive | 2/19 (10%) | |
| n/a | 11/19 (58%) | |
| TRUS | Negative | 4/19 (21%) |
| Positive | 12/19 (63%) | |
| n/a | 3/19 (16%) | |
| PI-RADS v.2 | 3 | 3/19 (16%) |
| 4 | 2/19 (10%) | |
| 5 | 8/19 (26%) | |
| n/a | 6/19 (32%) | |
| Positive cores | Mean | 54% |
| SD | 18% | |
| Median | 0.5% | |
| Range | 25–100% | |
| cGS | 3 + 4 | 2/19 (10%) |
| 4 + 3 | 1/19 (1%) | |
| 4 + 4 | 10/19 (53%) | |
| 4 + 5 | 6/19 (32%) | |
| cT | 1 | 2/19 (10%) |
| 2 | 17/19 (89%) | |
| pT | 2c | 5/19 (26%) |
| 3a | 10/19 (53%) | |
| 3b | 4/19 (21%) | |
| pGS | 4 + 3 | 5/19 (26%) |
| 4 + 4 | 5/19 (26%) | |
| 3 + 5 | 1/19 (5%) | |
| 4 + 5 | 7/19 (37%) | |
| 5 + 4 | 1/19 (1%) | |
| R1 | No | 10/19 (53%) |
| Yes | 9/19 (47%) | |
| ECE | Yes | 14/19 (74%) |
| No | 5/19 (26%) | |
| SVI | Yes | 4/19 (21%) |
| No | 15/19 (79%) | |
| Perineural Invasion | Yes | 16/19 (84%) |
| No | 3/19 (16%) | |
| pN | 0 | 14/19 (74%) |
| 1 | 5/19 (26%) |
| Spearman Correlations (n = 45) | SUV Max Choline | SUV Max Fluciclovine | TBR Choline L3 | TBR Fluciclovine L3 | TBR Choline LIVER | TBR Fluciclovine LIVER | TBR Choline AORTA | TBR Fluciclovine AORTA | |
|---|---|---|---|---|---|---|---|---|---|
| pISUP | Coeff | 0.22 | 0.46 | 0.28 | 0.37 | 0.45 | 0.47 | 0.22 | 0.26 |
| p | 0.15 | <0.001 | 0.06 | 0.01 | <0.001 | <0.001 | 0.15 | 0.08 | |
| PET Parameters | Malignant (n = 45) | Non-Malignant (n = 50) | p Value | Malignant (n = 45) | Benign (n = 31) | Normal Prostatic Tissue (n = 19) | p Value |
|---|---|---|---|---|---|---|---|
| SUV max Choline Median (IQR) | 5 (3.6–6.2) | 3.2 (1.9–5.2) | 0.001 | 5 (3.6–6.2) | 4.5 (3.1–5.8) | 2.1 (1.7–2.6) | <0.001 |
| SUV max Fluciclovine Median (IQR) | 5.1 (3.9–6.3) | 2.8 (2.4–4.8) | <0.001 | 5.1 (3.9–6.3) | 4.7 (2.8–5.2) | 2.3 (2–2.8) | <0.001 |
| TBR aorta Choline Median (IQR) | 3.7 (2.6–5.4) | 2.4 (1.5–3.3) | <0.001 | 3.7 (2.6–5.4) | 3.1 (2.4–3.7) | 1.6 (1.3–2.3) | <0.001 |
| TBR aorta Fluciclovine Median (IQR) | 4 (3.1–5.4) | 2.5 (1.5–3.7) | <0.001 | 4 (3.1–5.4) | 3.6 (2.2–3.9) | 1.6 (1.3–2.3) | <0.001 |
| TBR L3 Choline Median (IQR) | 1.7 (1.2–2.2) | 1.1 (0.6–1.8) | <0.001 | 1.7 (1.2–2.2) | 1.7 (0.9–2) | 0.8 (0.6–0.9) | <0.001 |
| TBR L3 Fluciclovine Median (IQR) | 1.8 (1.5–2.2) | 1.1 (0.6–1.7) | <0.001 | 1.8 (1.5–2.2) | 1.5 (1.1–2) | 0.8 (0.5–0.9) | <0.001 |
| TBR Liver Choline Median (IQR) | 0.6 (0.5–0.8) | 0.4 (0.2–0.6) | <0.001 | 0.6 (0.5–0.8) | 0.6 (0.4–0.7) | 0.3 (0.2–0.3) | <0.001 |
| TBR Liver Fluciclovine Median (IQR) | 0.7 (0.6–0.9) | 0.4 (0.3–0.7) | <0.001 | 0.7 (0.6–0.9) | 0.7 (0.4–0.8) | 0.3 (0.3–0.3) | <0.001 |
| [18F]Fluciclovine PET/CT Parameters | AUC | Std. Error | Asymptotic Sig. | Asymptotic 95% Confidence Interval | Best Cut-Off (Youden-Index) | [11C]Choline PET/CT Parameters | AUC | Std. Error | Asymptotic Sig. | Asymptotic 95% Confidence Interval | Best Cut-Off (Youden-Index) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Value | Sens | Spec | Lower Bound | Upper Bound | Value | Sens | Spec | ||||||||
| TBR Fluciclovine L3 | 0.76 | 0.05 | 0.00 | 0.66 | 0.86 | 1.35 | 0.84 | 0.58 | TBR Choline AORTA | 0.78 | 0.05 | 0.00 | 0.69 | 0.87 | 2.00 | 1.00 | 0.46 |
| TBR Fluciclovine AORTA | 0.75 | 0.05 | 0.00 | 0.65 | 0.85 | 3.75 | 0.60 | 0.78 | SUV max Choline | 0.74 | 0.05 | 0.00 | 0.65 | 0.84 | 2.75 | 0.93 | 0.46 |
| SUV max Fluciclovine | 0.73 | 0.05 | 0.00 | 0.63 | 0.83 | 3.05 | 0.93 | 0.54 | TBR Choline LIVER | 0.72 | 0.05 | 0.00 | 0.62 | 0.82 | 0.35 | 0.93 | 0.46 |
| TBR Fluciclovine LIVER | 0.71 | 0.05 | 0.00 | 0.61 | 0.82 | 0.45 | 0.89 | 0.52 | TBR Choline L3 | 0.70 | 0.05 | 0.00 | 0.59 | 0.80 | 0.85 | 0.93 | 0.42 |
| [18F]Fluciclovine PET/CT Parameters | AUC | Std. Error | Asymptotic Sig. | Asymptotic 95% Confidence Interval | Best Cut-Off (Youden-Index) | [11C]Choline PET/CT Parameters | AUC | Std. Error | Asymptotic Sig. | Asymptotic 95% Confidence Interval | Best Cut-Off (Youden-Index) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Value | Sens | Spec | Lower Bound | Upper Bound | Value | Sens | Spec | ||||||||
| TBR Fluciclovine L3 | 0.80 | 0.04 | 0.00 | 0.72 | 0.89 | 1.55 | 0.85 | 0.68 | TBR Choline LIVER | 0.78 | 0.05 | 0.00 | 0.69 | 0.87 | 0.55 | 0.67 | 0.71 |
| SUV max Fluciclovine | 0.78 | 0.05 | 0.00 | 0.69 | 0.88 | 3.05 | 0.97 | 0.47 | TBR Choline AORTA | 0.77 | 0.05 | 0.00 | 0.68 | 0.87 | 4.60 | 0.45 | 0.94 |
| TBR Fluciclovine LIVER | 0.78 | 0.05 | 0.00 | 0.69 | 0.87 | 0.55 | 0.91 | 0.52 | SUV max Choline | 0.73 | 0.05 | 0.00 | 0.63 | 0.83 | 2.75 | 1.00 | 0.42 |
| TBR Fluciclovine AORTA | 0.76 | 0.05 | 0.00 | 0.66 | 0.86 | 2.70 | 0.91 | 0.50 | TBR Choline L3 | 0.72 | 0.05 | 0.00 | 0.62 | 0.83 | 1.15 | 0.85 | 0.50 |
| [18F]Fluciclovine PET/CT Parameters | AUC | Std. Error | Asymptotic Sig. | Asymptotic 95% Confidence Interval | Best Cut-Off (Youden-Index) | [11C]Choline PET/CT Parameters | AUC | Std. Error | Asymptotic Sig. | Asymptotic 95% Confidence Interval | Best Cut-Off (Youden-Index) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Value | Sens | Spec | Lower Bound | Upper Bound | Value | Sens | Spec | ||||||||
| SUV max Fluciclovine | 0.77 | 0.06 | 0.00 | 0.66 | 0.89 | 4.00 | 1.00 | 0.52 | TBR Choline LIVER | 0.81 | 0.07 | 0.00 | 0.67 | 0.94 | 0.75 | 0.60 | 0.86 |
| TBR Fluciclovine LIVER | 0.76 | 0.06 | 0.00 | 0.64 | 0.88 | 0.55 | 1.00 | 0.59 | TBR Choline AORTA | 0.78 | 0.07 | 0.00 | 0.63 | 0.92 | 4.85 | 0.60 | 0.87 |
| TBR Fluciclovine L3 | 0.76 | 0.06 | 0.00 | 0.63 | 0.88 | 1.55 | 0.90 | 0.46 | TBR Choline L3 | 0.76 | 0.08 | 0.00 | 0.62 | 0.91 | 1.75 | 0.80 | 0.72 |
| TBR Fluciclovine AORTA | 0.70 | 0.07 | 0.00 | 0.57 | 0.83 | 2.90 | 1.00 | 0.59 | SUV max Choline | 0.75 | 0.07 | 0.00 | 0.61 | 0.89 | 5.45 | 0.70 | 0.74 |
| Clinical and PET Parameters | UNIVARIATE | MULTIVARIATE 1 | MULTIVARIATE 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| iPSA | 0.98 (0.87–1.03) | 0.2 | - | - | - | - |
| Clinic stage | 0.6 | - | - | - | - | |
| cT1 | 1.0→(Ref) | |||||
| cT2 | 1.56 (0.35–6.91) | |||||
| Clinic ISUP | - | - | - | - | ||
| 2 | 1.0→(Ref) | 0.8 | ||||
| 3 | 1.84 (0.42–8.22) | 0.4 | ||||
| 4 | 4.00 (0.25–63.95) | 0.3 | ||||
| 5 | 1.89 (0.41–8.78) | 0.4 | ||||
| PIRADS v.2 | - | - | - | - | ||
| 1–2 | 1.0→(Ref) | 0.2 | ||||
| 3 | 3.50 81.04–11.71) | 0.04 | ||||
| 4 | 1.90 (0.27–4.55) | 0.9 | ||||
| 5 | 1.40 (0.47–4.13) | 0.5 | ||||
| SUV max Choline PET/CT | 1.65 (1.27–2.14) | <0.001 | 1.57 (0.93–2.63) | 0.09 | - | - |
| TBR aorta Choline PET/CT | 2.36 (1.61–3.48) | <0.001 | 6.12 (1.51–24.79) | 0.01 | - | - |
| TBR L3 Choline PET/CT | 2.60 (1.41–4.81) | 0.002 | 0.2 (0.04–1.03) | 0.05 | - | - |
| TBR Liver Choline PET/CT | 47.00 (5.85–377.63) | <0.001 | 0.02 (0.00–51.22) | 0.3 | - | - |
| SUV max Fluciclovine PET/CT | 1.58 (1.22–2.04) | 0.001 | - | - | 1.34 (0.75–2.37) | 0.3 |
| TBR aorta Fluciclovine PET/CT | 1.81 (1.33–2.46) | <0.001 | - | - | 1.30 (0.81–2.10) | 0.3 |
| TBR L3 Fluciclovine PET/CT | 4.77 (2.19–10.38) | <0.001 | - | - | 3.96 (0.93–16.87) | 0.06 |
| TBR Liver Fluciclovine PET/CT | 32 (4.89–221.90) | <0.001 | - | - | 0.07 (0.00–19.72) | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanoni, L.; Mei, R.; Bianchi, L.; Giunchi, F.; Maltoni, L.; Pultrone, C.V.; Nanni, C.; Bossert, I.; Matti, A.; Schiavina, R.; et al. The Role of [18F]Fluciclovine PET/CT in the Characterization of High-Risk Primary Prostate Cancer: Comparison with [11C]Choline PET/CT and Histopathological Analysis. Cancers 2021, 13, 1575. https://doi.org/10.3390/cancers13071575
Zanoni L, Mei R, Bianchi L, Giunchi F, Maltoni L, Pultrone CV, Nanni C, Bossert I, Matti A, Schiavina R, et al. The Role of [18F]Fluciclovine PET/CT in the Characterization of High-Risk Primary Prostate Cancer: Comparison with [11C]Choline PET/CT and Histopathological Analysis. Cancers. 2021; 13(7):1575. https://doi.org/10.3390/cancers13071575
Chicago/Turabian StyleZanoni, Lucia, Riccardo Mei, Lorenzo Bianchi, Francesca Giunchi, Lorenzo Maltoni, Cristian Vincenzo Pultrone, Cristina Nanni, Irene Bossert, Antonella Matti, Riccardo Schiavina, and et al. 2021. "The Role of [18F]Fluciclovine PET/CT in the Characterization of High-Risk Primary Prostate Cancer: Comparison with [11C]Choline PET/CT and Histopathological Analysis" Cancers 13, no. 7: 1575. https://doi.org/10.3390/cancers13071575
APA StyleZanoni, L., Mei, R., Bianchi, L., Giunchi, F., Maltoni, L., Pultrone, C. V., Nanni, C., Bossert, I., Matti, A., Schiavina, R., Fiorentino, M., Fonti, C., Lodi, F., D’Errico, A., Brunocilla, E., & Fanti, S. (2021). The Role of [18F]Fluciclovine PET/CT in the Characterization of High-Risk Primary Prostate Cancer: Comparison with [11C]Choline PET/CT and Histopathological Analysis. Cancers, 13(7), 1575. https://doi.org/10.3390/cancers13071575








