Which Is the Best Treatment in Recurrent Thymoma? A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
Study Characteristics
2.2. Report Characteristics
Information Sources, Study Selection and Data Collection
2.3. Statistical Analysis
3. Results
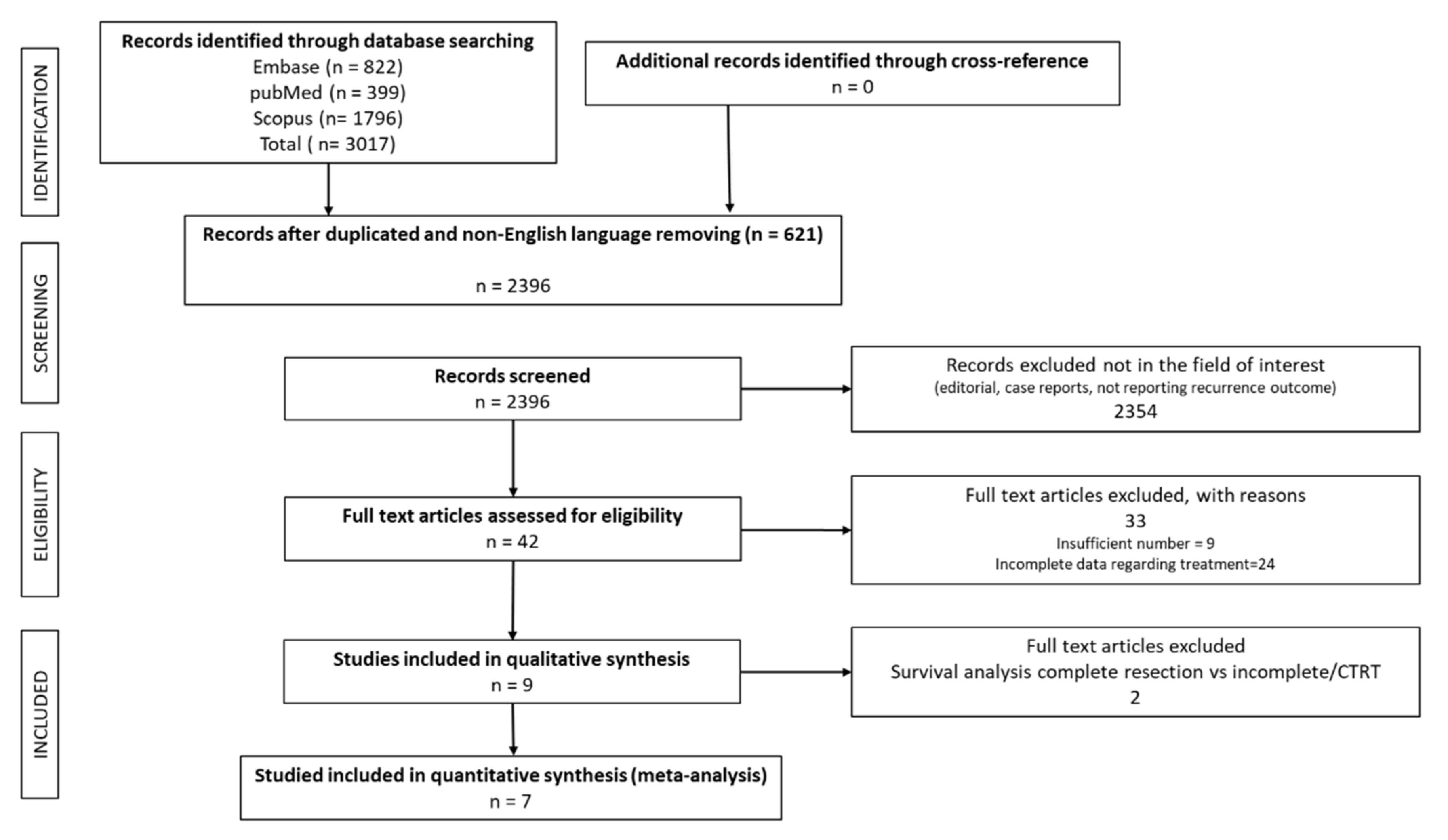
3.1. Literature Results
3.2. Recurrence Characteristics
3.3. Overall Survival
3.4. Surgery and Other Treatments
Meta-Analysis Results
3.5. Other Factors Affecting Survival
3.5.1. Myasthenia Gravis (MG)
3.5.2. Histology
3.5.3. Pattern of Recurrence
4. Discussion
Limitations of the Meta-Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- De Jong, W.K.; Blaauwgeers, J.L.; Schaapveld, M.; Timens, W.; Klinkenberg, T.J.; Groen, H.J. Thymic epithelial tumours: A population-based study of the incidence, diagnostic procedures and therapy. Eur. J. Cancer 2008, 44, 123–130. [Google Scholar] [CrossRef]
- Margaritora, S.; Cesario, A.; Cusumano, G.; Lococo, F.; Porziella, V.; Meacci, E.; Evoli, A.; Granone, P. Single-centre 40-year results of redo operation for recurrent thymomas. Eur. J. Cardiothorac. Surg. 2011, 40, 894–901. [Google Scholar] [CrossRef]
- Regnard, J.F.; Magdeleinat, P.; Dromer, C.; Dulmet, E.; De Montpreville, V.; Levi, J.F.; Levasseur, P. Prognostic factors and long-term results after thymoma resection: A series of 307 patients. J. Thorac. Cardiovasc. Surg. 1996, 112, 376–384. [Google Scholar] [CrossRef]
- Sandri, A.; Cusumano, G.; Lococo, F.; Alifano, M.; Granone, P.; Margaritora, S.; Cesario, A.; Oliaro, A.; Filosso, P.; Regnard, J.F.; et al. Long-term results after treatment for recurrent thymoma: A multicenter analysis. J. Thorac. Oncol. 2014, 9, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Mancuso, M.; Oliaro, A.; Casadio, C.; Cavallo, A.; Cianci, R.; Filosso, P.L.; Molinatti, M.; Porrello, C.; Cappello, N.; et al. Recurrence of thymoma: Analysis of clinicopathologic features, treatment, and outcome. J. Thorac. Cardiovasc. Surg. 1997, 113, 55–63. [Google Scholar] [CrossRef]
- Huang, J.; Detterbeck, F.C.; Wang, Z.; Loehrer, P.J., Sr. Standard outcome measures for thymic malignancies. J. Thorac. Oncol. 2011, 6 (Suppl. S3), S1691–S1697. [Google Scholar] [CrossRef]
- Bott, M.; Wang, H.; Travis, W.; Riely, G.J.; Bains, M.; Downey, R.; Rusch, V.; Huang, J. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann. Thorac. Surg. 2011, 92, 1984–1992. [Google Scholar] [CrossRef]
- Giaccone, G.; Wilmink, H.; Paul, M.A.; van der Valk, P. Systemic treatment of malignant thymoma: A decade experience at a single institution. Am. J. Clin. Oncol. 2006, 29, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Critical Appraisal Tools. Available online: https://joannabriggs.org/critical-appraisal-tools (accessed on 23 March 2021).
- Hamaji, M.; Allen, M.S.; Cassivi, S.D.; Nichols, F.C., III; Wigle, D.A.; Deschamps, C.; Shen, K.R. The role of surgical management in recurrent thymic tumors. Ann. Thorac. Surg. 2012, 94, 247–254. [Google Scholar] [CrossRef]
- Fiorelli, A.; D’Andrilli, A.; Vanni, C.; Cascone, R.; Anile, M.; Diso, D.; Tassi, V.; Vannucci, J.; Serra, N.; Puma, F.; et al. Iterative Surgical Treatment for Repeated Recurrences After Complete Resection of Thymic Tumors. Ann. Thorac. Surg. 2017, 103, 422–431. [Google Scholar] [CrossRef]
- Mizuno, T.; Okumura, M.; Asamura, H.; Yoshida, K.; Niwa, H.; Kondo, K.; Horio, H.; Matsumura, A.; Yokoi, K. Surgical management of recurrent thymic epithelial tumors a retrospective analysis based on the Japanese nationwide database. J. Thorac. Oncol. 2015, 10, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Marulli, G.; Margaritora, S.; Lucchi, M.; Cardillo, G.; Granone, P.; Mussi, A.; Carleo, F.; Perissinotto, E.; Rea, F. Surgical treatment of recurrent thymoma: Is it worthwhile? Eur. J. Cardiothorac. Surg. 2016, 49, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, M.; Zanfrini, E.; Giraldi, L.; Mastromarino, M.G.; Petracca-Ciavarella, L.; Nachira, D.; Congedo, M.T.; Aprile, V.; Ambrogi, M.C.; Lucchi, M.; et al. Prognostic factors after treatment for iterative thymoma recurrences: A multicentric experience. Lung Cancer 2019, 138, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.K.; Byun, C.S.; Lee, C.Y.; Lee, J.G.; Park, I.K.; Kim, D.J.; Yang, W.I.; Chung, K.Y. Clinical outcomes and prognosis of recurrent thymoma management. J. Thorac. Oncol. 2012, 7, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Maury, J.M.; Girard, N.; Tabutin, M.; Grima, R.; Chalabreysse, L.; Pavlakovic, I.; Sayag-Beaujard, A.; Leroux, C.; Souquet, P.J.; Glehen, O.; et al. Intra-thoracic chemo-hyperthermia for pleural recurrence of thymoma. Lung Cancer 2017, 108, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aprile, V.; Bacchin, D.; Korasidis, S.; Nesti, A.; Marrama, E.; Ricciardi, R.; Petrini, I.; Ambrogi, M.C.; Paladini, P.; Lucchi, M. Surgical treatment of pleural recurrence of thymoma: Is hyperthermic intrathoracic chemotherapy worthwhile? Interact. Cardiovasc. Thorac. Surg. 2020, 30, 765–772. [Google Scholar] [CrossRef]
- Detterbeck, F. International thymic malignancies interest group: A way forward. J. Thorac. Oncol. 2010, 5 (Suppl. S4), S365–S370. [Google Scholar] [CrossRef]
- Ciccone, A.M.; Rendina, E.A. Treatment of recurrent thymic tumors. Semin. Thorac. Cardiovasc. Surg. 2005, 17, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.D.; Wain, J.C.; Wong, D.R.; Donahue, D.M.; Gaissert, H.A.; Grillo, H.C.; Mathisen, D.J. Predictors of recurrence in thymic tumors: Importance of invasion, World Health Organization histology, and size. J. Thorac. Cardiovasc. Surg. 2005, 130, 1413–1421. [Google Scholar] [CrossRef]
- Okumura, M.; Shiono, H.; Inoue, M.; Tanaka, H.; Yoon, H.E.; Nakagawa, K.; Matsumura, A.; Ohta, M.; Iuchi, K.; Matsuda, H. Outcome of surgical treatment for recurrent thymic epithelial tumors with reference to world health organization histologic classification system. J. Surg. Oncol. 2007, 95, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Hamaji, M.; Ali, S.O.; Burt, B.M. A meta-analysis of surgical versus nonsurgical management of recurrent thymoma. Ann. Thorac. Surg. 2014, 98, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. ESMO Clinical PracticeGuidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v40–v55. [Google Scholar]

| Study | Number of Patients | Who Upstaging | Myasthenia Gravis | DFI (Months) | Surgery | Other Treatments | Complete Resection |
|---|---|---|---|---|---|---|---|
| RUFFINI (1997) | 30 | NR | 22 (73.3%) | Mean 86 ± 45 (range 4–192) | 16 (54%) | 14 (46%) | 10 (62.5%) |
| MARGARITORA (2011) | 43 | 18 (60%) | 40 (93%) | Mean 92.7 ± 77.8 | 30 (69.7%) | 13 (30.3%) CT/RT 12 None 1 | 22 (73%) |
| HAMAJI (2012) | 30 | NR | 13 (43%) | Median 61 (range 9–242) | 20 (66.6%) | 10 (33.4%) | 18 (90%) |
| BAE (2012) | 41 | 6 (40%) | 22 (53.6%) | Median 52 (range 6–234 | 15 (36.6%) | 26 (63.4%) CT/RT 25 Other 1 | 13 (87%) |
| SANDRI (2014) | 81 | 25 (40.9%) | 54 (66.7%) | Mean 86.5 ± 72.1 | 61 (61.3%) | 20 (32.7%) CT/RT 14 Other 6 | 45 (72.5%) |
| MIZUNO (2015) | 242 | NR | 54 (13.3%) | Mean 2.7 ± 2.3 (years) | 119 (49.1%) | 122 (50.9%) | Not reported for thymomas only |
| MARULLI (2016) | 103 | NR | 63 (61.2%) | Median 50 (range 10–301) | 73 (70.8%) | 30 (29.2%) CT/RT 30 | 50 (68.5%) |
| FIORELLI (2017) | 53 | 14 (37%) | 30 (56%) | Mean 55 (range 38–69) | 38 (71.7%) | 15 (28.3%) CT/RT 13 Other 1 | 32 (60%) |
| CHIAPPETTA (2019) | 155 | 24 (15.5%) | 107 (69%) | Mean 78 ± 102 | 135 (87.1%) | 20 (12.9%) CT/RT 19 Radio frequency 1 | 109 (70.4%) |
| Study | Recurrence Site (Number of Patients) | Surgical Procedure | Adjuvant Treatments (Number of Patients) | Favourable Prognostic Factors |
|---|---|---|---|---|
| RUFFINI (1997) | Loco-regional (26) Distant (4) | Resection Thoracotomy Sternotomy | Not reported | Local Recurrence Complete Resection |
| MARGARITORA (2011) | Pleura (25) Mediastinum (12) Lung (5), Liver And Bone (1) | Resection Thoracotomy Sternotomy | Not significant (p = 0.25) | Surgical Treatment Complete Resection |
| HAMAJI (2012) | Loco-Regional (28) Distant 2 (1 Liver, 1 Brain) | Resection | 2 patients | Surgical Treatment Initial Masaoka Stage Complete Resection |
| BAE (2012) | Local (11) Regional (29) Distant (7) | Resection Thoracotomy Sternotomy | Radiotherapy (4) Chemoterapy (5) Chemoradiotherapy (2) | Complete Resection Histology (AB, B1) |
| SANDRI (2014) | Mediastinum (15) Pleura/Pericardium (47) Lung (13) Other Site (6) | Resection Thoracotomy Sternotomy | Pre or post operative chemo/radiotherapy: 15 | Histology |
| MIZUNO (2015) | Pleura Lung Local Other | Resection | Not reported | Surgical Treatment Complete Resection |
| MARULLI (2016) | Local (17) Regional (63) Distant (14) Combined-Distant (9) | Resection Thoracotomy Sternotomy Laparotomy Single Pleural: Limited Pleural Resection Multiple Pleural Relapses: Partial Or Total Pleurectomy | Not significant (p = 0.87) | Complete Resection * Single Relapse * Initial Masaoka I-II * Loco-Regional Relapse * AB-B1-B2 Histology |
| FIORELLI (2017) | Local (13) Regional (26) Lung/Extrathoracic (11/3) | Resection VATS Thoracotomy | 32 patients Chemotherapy alone 11 Radiotherapy alone 2 Chemotherapy/radiotherapy 19 Adjuvant therapy: 152 (115–161) months vs. 70 (28–149) months without (p = 0.03) | Ab-B1 Histology Complete Resection * Myasthenia Gravis Adjuvant Therapy |
| CHIAPPETTA (2019) | Local 21 Regional 111 Distant 23 | Thoracotomy Sternotomy VATS Single Pleural: Limited Pleural Resection Multiple Pleural Relapses: Partial or Total Pleurectomy + HITOC | Adjuvant CT/RT 78 Not significant | Female Gender Myasthenia Gravis * Age Single Localization DFS > 36 Months * |
| Author | OS | OS Surgery | OS Other Treatments | p Value | HR | 95% CI | Note | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 5Y | 10Y | 5Y | 10Y | 5Y | 10Y | |||||
| RUFFINI (1997) | 48 | 24 | NR | NR | NR | NR | 0.008 | NR | NR | complete resection vs incomplete resection + other treatments |
| MARGARITORA (2011) | 64 | 51 | 77 | 59 | 35 | 0 | 0.001 | 0.22 | 0.08—0.59 | |
| HAMAJI (2012) * | 50 | NR | 75.7 | 29.9 | 0 | 0 | 0.0002 | NR | NR | Data reported in their successive meta-analysis |
| BAE (2012) | 59.7 | 33.2 | 90.0 | NR | 40.7 | NR | 0.088 | 6.075 | 0.763–48.33 | complete resection vs incomplete resection + other treatments |
| SANDRI (2014) | 94.4 | 71.7 | 70.2 | 54.1 | 64.3 | 46.9 | 0.19 | 0.417 | 0.186–0.933 | |
| MIZUNO (2015) | 76.2 | 50 | 90.5 | 72.5 | 63.3 | 31.4 | 0.001 | 0.272 | 0.142– 0.521 | |
| MARULLI (2016) | 63 | 37 | 81 | 60 | 36 | 20 | 0.0001 | 7.65 | 3.07–19.10 | |
| FIORELLI (2017) | 52 | 32 | 92% | NR | 15.3% | NR | 0.0001 | 4.29 | 1.29–14.2 | |
| CHIAPPETTA (2019) | 70.2 | 44.4 | 70.5 | 49.1 | 67.3 | 15.1 | 0.064 | 1.91 | 0.96–3.79 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappetta, M.; Grossi, U.; Sperduti, I.; Margaritora, S.; Marulli, G.; Fiorelli, A.; Sandri, A.; Mizuno, T.; Cusumano, G.; Hamaji, M.; et al. Which Is the Best Treatment in Recurrent Thymoma? A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1559. https://doi.org/10.3390/cancers13071559
Chiappetta M, Grossi U, Sperduti I, Margaritora S, Marulli G, Fiorelli A, Sandri A, Mizuno T, Cusumano G, Hamaji M, et al. Which Is the Best Treatment in Recurrent Thymoma? A Systematic Review and Meta-Analysis. Cancers. 2021; 13(7):1559. https://doi.org/10.3390/cancers13071559
Chicago/Turabian StyleChiappetta, Marco, Ugo Grossi, Isabella Sperduti, Stefano Margaritora, Giuseppe Marulli, Alfonso Fiorelli, Alberto Sandri, Tetsuya Mizuno, Giacomo Cusumano, Masatsugu Hamaji, and et al. 2021. "Which Is the Best Treatment in Recurrent Thymoma? A Systematic Review and Meta-Analysis" Cancers 13, no. 7: 1559. https://doi.org/10.3390/cancers13071559
APA StyleChiappetta, M., Grossi, U., Sperduti, I., Margaritora, S., Marulli, G., Fiorelli, A., Sandri, A., Mizuno, T., Cusumano, G., Hamaji, M., Cesario, A., & Lococo, F. (2021). Which Is the Best Treatment in Recurrent Thymoma? A Systematic Review and Meta-Analysis. Cancers, 13(7), 1559. https://doi.org/10.3390/cancers13071559










