Thyroid Dysfunction in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors (ICIs): Outcomes in a Multiethnic Urban Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Definitions
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Study Population
3.2. Evaluation of Thyroid Dysfunction
3.3. Clinical Presentation
3.4. Association of Thyroid Dysfunction and Survival Outcomes
4. Discussion
4.1. Biology and Epidemiology
4.2. Clinical Impact
4.3. Recommendations for Monitoring and Management
4.4. Directions for Future Research
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated analysis of Keynote-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; de Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef]
- Michot, J.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Thompson, J.A. Management of immune-related adverse events in patients treated with immune Checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline summary. J. Oncol. Pract. 2018, 14, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Nguyen, H.; Chambers, C.; Kang, J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl. Acad. Sci. USA 2010, 107, 1524–1528. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yoshida, T.; Nakaki, F.; Hiai, H.; Okazaki, T.; Honjo, T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc. Natl. Acad. Sci. USA 2005, 102, 11823–11828. [Google Scholar] [CrossRef]
- Lucas, J.A.; Menke, J.; Rabacal, W.A.; Schoen, F.J.; Sharpe, A.H.; Kelley, V.R. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J. Immunol. 2008, 181, 2513–2521. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Msc, E.H.B.; Fisher, D.E. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 2017, 123, 2143–2153. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Abdel-Ghani, A.; Yadav, S.; Hoversten, K.P.; Reed, C.T.; Sitek, A.N.; Enninga, E.A.L.; Paludo, J.; Aguilera, J.V.; Leventakos, K.; et al. Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non-small cell lung cancer: Are we all equal? Oncologist 2019, 24, e1148–e1155. [Google Scholar] [CrossRef]
- Kartolo, A.; Sattar, J.; Sahai, V.; Baetz, T.; Lakoff, J.M. Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol. 2018, 25, e403–e410. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Şenler, F.Ç.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Albarel, F.; Gaudy, C.; Castinetti, F.; Carré, T.; Morange, I.; Conte-Devolx, B.; Grob, J.-J.; Brue, T. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur. J. Endocrinol. 2015, 172, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; ElHalawani, H.; Fouad, M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: A meta-analysis. Futur. Oncol. 2016, 12, 413–425. [Google Scholar] [CrossRef]
- Abid, H.; Khavandi, M.; Siddiqui, N.; Panjawatanan, P.; Patel, A. Incidence and risk of thyroid dysfunction in advanced or metastatic non-small cell lung cancer patients treated with pembrolizumab: A meta-analysis. Cureus 2019, 11, e5997. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Conner, S.C.; Trinquart, L. Survivorship bias in analyses of immune checkpoint inhibitor trials. JAMA Oncol. 2019, 5, 1226. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, M.; Lee, S.H.; Park, S.Y.; Kim, Y.N.; Kim, H.; Jeon, M.J.; Kim, T.Y.; Kim, S.W.; Kim, W.B.; et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. OncoImmunology 2017, 7, e1375642. [Google Scholar] [CrossRef]
- Peiró, I.; Palmero, R.; Iglesias, P.; Díez, J.J.; Simó-Servat, A.; Marín, J.A.; Jiménez, L.; Domingo-Domenech, E.; Mancho-Fora, N.; Nadal, E.; et al. Thyroid dysfunction induced by nivolumab: Searching for disease patterns and outcomes. Endocr. 2019, 64, 605–613. [Google Scholar] [CrossRef]
- Tomer, Y. Mechanisms of autoimmune thyroid diseases: From genetics to epigenetics. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Gilkeson, G. Estrogen receptors in immunity and autoimmunity. Clin. Rev. Allergy Immunol. 2011, 40, 66–73. [Google Scholar] [CrossRef]
- Jacobson, D.L.; Gange, S.J.; Rose, N.R.; Graham, N.M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 1997, 84, 223–243. [Google Scholar] [CrossRef]
- Kanda, N.; Tamaki, K. Estrogen enhances immunoglobulin production by human PBMCs. J. Allergy Clin. Immunol. 1999, 103, 282–288. [Google Scholar] [CrossRef]
- Li, C.W.; Concepcion, E.; Tomer, Y. Dissecting the role of the foxp3 gene in the joint genetic susceptibility to autoimmune thyroiditis and diabetes: A genetic and functional analysis. Gene 2015, 556, 142–148. [Google Scholar] [CrossRef][Green Version]
- Aoki, Y.; Belin, R.M.; Clickner, R.; Jeffries, R.; Phillips, L.; Mahaffey, K.R. Serum TSH and Total T4 in the United States population and their association with participant characteristics: National health and Nutrition examination survey (NHANES 1999–2002). Thyroid 2007, 17, 1211–1223. [Google Scholar] [CrossRef]
- McLeod, D.S.A.; Caturegli, P.; Cooper, D.S.; Matos, P.G.; Hutfless, S. Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel. JAMA 2014, 311, 1563–1565. [Google Scholar] [CrossRef]
- McLeod, D.S.; Cooper, D.S.; Ladenson, P.W.; Whiteman, D.C.; Jordan, S.J. Race/ethnicity and the prevalence of thyrotoxicosis in young americans. Thyroid 2015, 25, 621–628. [Google Scholar] [CrossRef]
- Boucai, L.; Surks, M.I. Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin. Endocrinol. 2009, 70, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Sridama, V.; Hara, Y.; Fauchet, R.; DeGroot, L.J. HLA immunogenetic heterogeneity in black American patients with graves’ disease. Arch. Intern. Med. 1987, 147, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.A.; Hammond, M.G.; Desai, R.K.; Motala, A.A.; Aboo, N.; Seedat, M.A. HLA class I and II antigens in South African Blacks with Graves’ disease. Clin. Immunol. Immunopathol. 1990, 54, 98–102. [Google Scholar] [CrossRef]
- Ofosu, M.H.; Dunston, G.; Henry, L.; Ware, D.; Cheatham, W.; Brembridge, A.; Brown, C.; Alarif, L. HLA-DQ3 is Associated with Graves’ Disease in African-Americans. Immunol. Investig. 1996, 25, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, T.; DeGroot, L.J. HLA class II associations in African-American female patients with Graves’ disease. Thyroid 1996, 6, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Nadell, D.; Zhang, X.Y.; Kukreja, A.; Huang, Y.J.; Wise, J.; Svec, F.; Richards, R.; Friday, K.E.; Vargas, A.; et al. The human leukocyte antigen HLA DRB3?0202/DQA1?0501 haplotype is associated with Graves’ disease in African Americans. J. Clin. Endocrinol. Metab. 2000, 85, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Smikle, M.F.; Pascoe, R.W.; Barton, E.; Morgan, O.; Christian, N.; Dowe, G.; Roye-Green, K.; Bailey, V.; James, O. HLA-DRB3 *0101 is associated with Graves’ disease in Jamaicans. Clin. Endocrinol. 2001, 55, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Cabanillas, M.E.; Waguespack, S.G.; Hu, M.I.; Thosani, S.; Lavis, V.R.; Busaidy, N.L.; Subudhi, S.K.; Diab, A.; Dadu, R. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018, 28, 1243–1251. [Google Scholar] [CrossRef]
- Olsson-Brown, A.; Lord, R.; Sacco, J.; Wagg, J.; Coles, M.; Pirmohamed, M. Two distinct clinical patterns of checkpoint inhibitor-induced thyroid dysfunction. Endocr. Connect. 2020, 9, 318–325. [Google Scholar] [CrossRef]
- Azmat, U.; Liebner, D.; Joehlin-Price, A.; Agrawal, A.; Nabhan, F. Treatment of ipilimumab induced Graves’ disease in a patient with metastatic melanoma. Case Rep. Endocrinol. 2016, 2016, 2087525. [Google Scholar] [CrossRef]
- Delivanis, D.A.; Gustafson, P.M.P.; Bornschlegl, S.; Merten, A.M.M.; Kottschade, A.L.; Withers, S.; Dietz, P.A.B.; Ryder, M. Pembrolizumab-induced thyroiditis: Comprehensive clinical review and insights into underlying involved mechanisms. J. Clin. Endocrinol. Metab. 2017, 102, 2770–2780. [Google Scholar] [CrossRef]
- Ferris, R.; Gillison, M.L. Nivolumab for squamous-cell cancer of head and neck. N. Engl. J. Med. 2017, 376, 595–596. [Google Scholar] [CrossRef]
- Ferreira, J.L.; Costa, C.; Marques, B.; Castro, S.; Victor, M.; Oliveira, J.; Santos, A.P.; Sampaio, I.L.; Duarte, H.; Marques, A.P.; et al. Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol. Immunother. 2020, 70, 299–309. [Google Scholar] [CrossRef]
- Basak, E.A.; Van Der Meer, J.W.; Hurkmans, D.P.; Schreurs, M.W.; Hoop, E.O.-D.; Van Der Veldt, A.A.; Bins, S.; Joosse, A.; Koolen, S.L.; Debets, R.; et al. Overt thyroid dysfunction and anti-thyroid antibodies predict response to Anti-PD-1 immunotherapy in cancer patients. Thyroid 2020, 30, 966–973. [Google Scholar] [CrossRef]
- Kotwal, A.; Kottschade, L.; Ryder, M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid 2020, 30, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sakakida, T.; Ishikawa, T.; Uchino, J.; Chihara, Y.; Komori, S.; Asai, J.; Narukawa, T.; Arai, A.; Kobayashi, T.; Tsunezuka, H.; et al. Clinical features of immune-related thyroid dysfunction and its association with outcomes in patients with advanced malignancies treated by PD-1 blockade. Oncol. Lett. 2019, 18, 2140–2147. [Google Scholar] [CrossRef]
- Grangeon, M.; Tomasini, P.; Chaleat, S.; Jeanson, A.; Souquet-Bressand, M.; Khobta, N.; Bermudez, J.; Trigui, Y.; Greillier, L.; Blanchon, M.; et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin. Lung Cancer 2019, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Mishra, M.; Pentz, R.; Owonikoko, T.K. Enrollment of racial minorities in clinical trials: Old problem assumes new urgency in the age of immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Toi, Y.; Sugawara, S.; Sugisaka, J.; Ono, H.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Aso, M.; Tsurumi, K.; et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019, 5, 376–383. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Menzies, A.M. Immune checkpoint inhibitors in challenging populations. Cancer 2017, 123, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Perez-Gracia, J.L.; Han, J.Y.; Arvis, C.D.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-Positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J. Clin. Oncol. 2020, 38, 1580–1590. [Google Scholar] [CrossRef] [PubMed]

| Category | n = 205 |
|---|---|
| Age (years) at treatment start, median (range) | 66 (33–87) |
| Sex, n (%) | |
| Male | 103 (50.2) |
| Female | 102 (49.8) |
| Ethnicity, n (%) | |
| Black | 79 (38.5) |
| Hispanic | 63 (30.7) |
| White | 42 (20.5) |
| Asian | 2 (1.9) |
| Other or Unknown | 19 (9.3) |
| Stage, n (%) | |
| II | 1 (0.5) |
| III | 30 (14.6) |
| IV | 174 (84.9) |
| Lung cancer type, n (%) | |
| NSCLC | 187 (91.2) |
| SCLC | 18 (8.8) |
| Treatment, n (%) | |
| Immunotherapy only | 142 (69.3) |
| Immunotherapy + chemotherapy | 63 (30.7) |
| Immunotherapy type, n (%) | |
| Pembrolizumab | 101 (49.3) |
| Nivolumab | 65 (31.7) |
| Durvalumab | 26 (12.7) |
| Atezolizumab | 20 (9.8) |
| Duration of treatment (weeks), median (range) | |
| Overall | 15.0 (0.1–145.0) |
| Immunotherapy only | 15.2 (0.1–145.0) |
| Immunotherapy + chemotherapy | 15.1 (0.1–135.0) |
| Category | Thyroid Dysfunction | No Thyroid Dysfunction | p-Value | Hypothyroidism | No Hypothyroidism | p-Value | Thyrotoxicosis | No Thyrotoxicosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Age (years), mean ± STD | 65.6 ± 10.5 | 65.7 ± 9.6 | 0.96 | 67.8 ± 10.1 | 65.1 ± 9.8 | 0.089 | 63.5 ± 10.0 | 66.3 ± 9.8 | 0.106 |
| Male, n (%) | 39 (51.3) | 64 (49.6) | 0.814 | 28 (58.3) | 75 (47.8) | 0.200 | 20 (47.6) | 83 (50.9) | 0.703 |
| Female, n (%) | 37 (48.7) | 65 (50.3) | 20 (41.7) | 82 (52.2) | 22 (52.4) | 80 (49.1) | |||
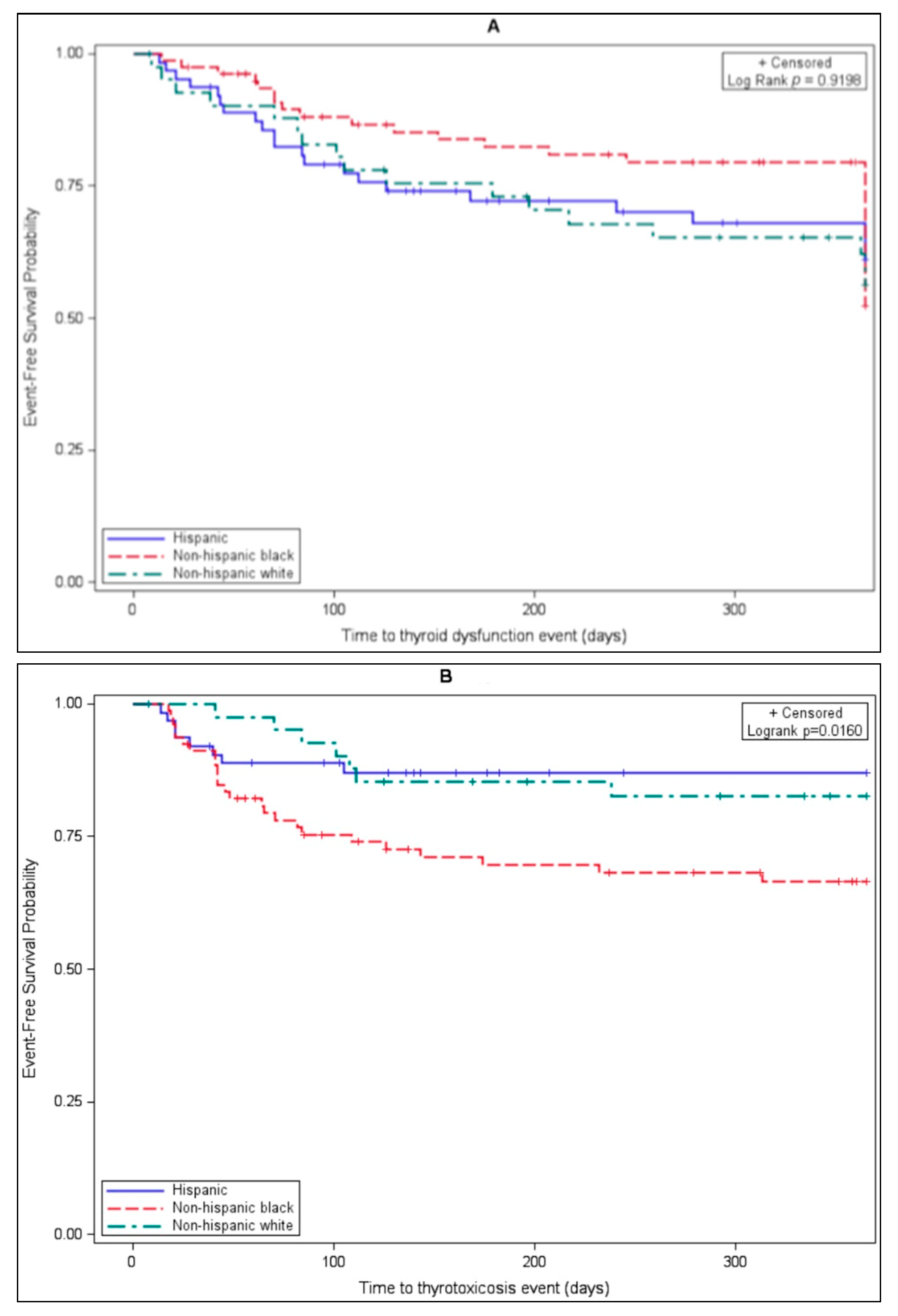
| Black, n (%) | 31 (40.8) | 48 (37.2) | 0.792 | 12 (25.0) | 67 (42.7) | 0.135 | 25 (59.5) | 54 (33.1) | 0.034 |
| Hispanic, n (%) | 22 (29.0) | 41 (31.8) | 18 (37.5) | 45 (28.7) | 8 (19.0) | 55 (33.7) | |||
| White, n (%) | 17 (22.4) | 25 (19.4) | 13 (27.1) | 29 (18.5) | 7 (16.7) | 35 (21.5) | |||
| Asian, n (%) | 1 (1.3) | 1 (0.8) | 1 (2.1) | 1 (0.6) | 0 (0) | 2 (1.23) | |||
| Other, n (%) | 5 (6.6) | 14 (10.9) | 4 (8.3) | 15 (9.6) | 2 (4.8) | 17 (10.4) | |||
| BMI normal, n (%) | 28 (36.8) | 49 (38.0) | 0.196 | 13 (27.1) | 64 (40.8) | 0.356 | 18 (42.9) | 59 (36.2) | 0.097 |
| Underweight, n (%) | 7 (9.2) | 11 (8.5) | 5 (10.4) | 13 (8.3) | 5 (11.9) | 13 (8.0) | |||
| Overweight, n (%) | 31 (40.8) | 38 (29.5) | 20 (41.7) | 49 (31.2) | 16 (38.1) | 53 (32.5) | |||
| Obese, n (%) | 10 (13.16) | 31 (24.0) | 10 (20.8) | 31 (19.8) | 3 (7.1) | 38 (23.3) | |||
| ICIs, n (%) | 51 (67.1) | 85 (66.0) | 0.671 | 34 (70.8) | 102 (65.0) | 0.325 | 25 (59.5) | 111 (68.1) | 0.432 |
| ICIs + CTX, n (%) | 25 (32.9) | 44 (34.1) | 14 (29.2) | 55 (35.0) | 17 (40.5) | 52 (31.9) | |||
| ICIs duration (weeks), median (IQR) | 21.6 (10.0–44.0) | 13.1 (5.8–31.0) | 0.002 | 23.5 (12.1–47.4) | 14.0 (6.0–31.0) | 0.001 | 21.6 (10.0–43.1) | 15.0 (6.0–33.0) | 0.067 |
| Pembrolizumab, n (%) | 36 (47.3) | 65 (50.4) | 0.676 | 20 (41.7) | 81 (51.6) | 0.229 | 22 (52.4) | 79 (48.5) | 0.660 |
| No pembrolizumab, n (%) | 40 (52.6) | 64 (49.6) | 28 (58.3) | 76 (48.4) | 20 (47.6) | 84 (51.5) | |||
| Nivolumab, n (%) | 23 (30.3) | 42 (32.6) | 0.733 | 17 (35.4) | 48 (30.6) | 0.528 | 12 (28.6) | 53 (32.5) | 0.624 |
| No nivolumab, n (%) | 53 (69.7) | 87 (67.4) | 31 (64.6) | 109 (69.4) | 30 (71.4) | 110 (67.5) | |||
| Atezolizumab, n (%) | 10 (13.1) | 10 (7.6) | 0.208 | 6 (12.5) | 14 (8.9) | 0.464 | 6 (14.3) | 14 (8.6) | 0.267 |
| No atezolizumab, n (%) | 66 (86.8) | 119 (92.3) | 42 (87.5) | 143 (91.1) | 36 (85.7) | 149 (91.4) | |||
| Durvalumab, n (%) | 10 (13.1) | 16 (12.4) | 0.875 | 8 (16.7) | 18 (11.5) | 0.343 | 4 (9.5) | 22 (13.5) | 0.609 |
| No durvalumab, n (%) | 66 (86.8) | 113 (87.6) | 40 (83.3) | 139 (88.5) | 38 (90.5) | 141 (86.5) |
| Thyroid Dysfunction | p-Value | Hypothyroid | p-Value | Thyrotoxicosis | p-Value | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Race/ethnicity | ||||||
| Black | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| Hispanic | 1.047 (0.5993–1.8289) | 0.8720 | 2.466 (1.1596–5.2451) | 0.0191 | 0.360 (0.1609–0.8052) | 0.0129 |
| White | 1.140 (0.6292–2.0671) | 0.6651 | 2.182 (0.9883–4.8185) | 0.0535 | 0.484 (0.2082–1.1236) | 0.0912 |
| Asian or other | 0.709 (0.2901–1.7306) | 0.4495 | 1.386 (0.4782–4.0152) | 0.5478 | 0.273 (0.0632–1.1751) | 0.0812 |
| Treatment (CTX-ICIs vs. ICIs only) | 0.879 (0.519–1.442) | 0.6183 | 0.816 (0.415–1.604) | 0.5552 | 1.098 (0.573–2.106) | 0.7773 |
| Gender (M v F) | 1.056 (0.667–1.653) | 0.8342 | 0.7704 (0.724–2.330) | 0.3801 | 0.982 (0.532–1.811) | 0.982 |
| ICIs duration (for every 3 week increase) | 1.050 (0.994–1.1036) | 0.1641 | 1.011 (1.007–1.058) | 0.0122 | 1.001 (0.974–1.031) | 0.8802 |
| Age | 1.005 (0.983–1.029) | 0.6509 | 1.032 (1.000–1.065) | 0.0476 | 0.975 (0.946–1.004) | 0.0939 |
| Reference | Cancer Type(s) | Agent(s) | No. Participants | Key Results |
|---|---|---|---|---|
| Ferreira 2020 [48] | Melanoma, NSCLC, head and neck, Hodgkin’s lymphoma, urothelial | Pembrolizumab, nivolumab, ipilimumab | 161 | OS 3.27 vs. 1.76 years (p = 0.030) for subjects with and without thyroid dysfunction, respectively |
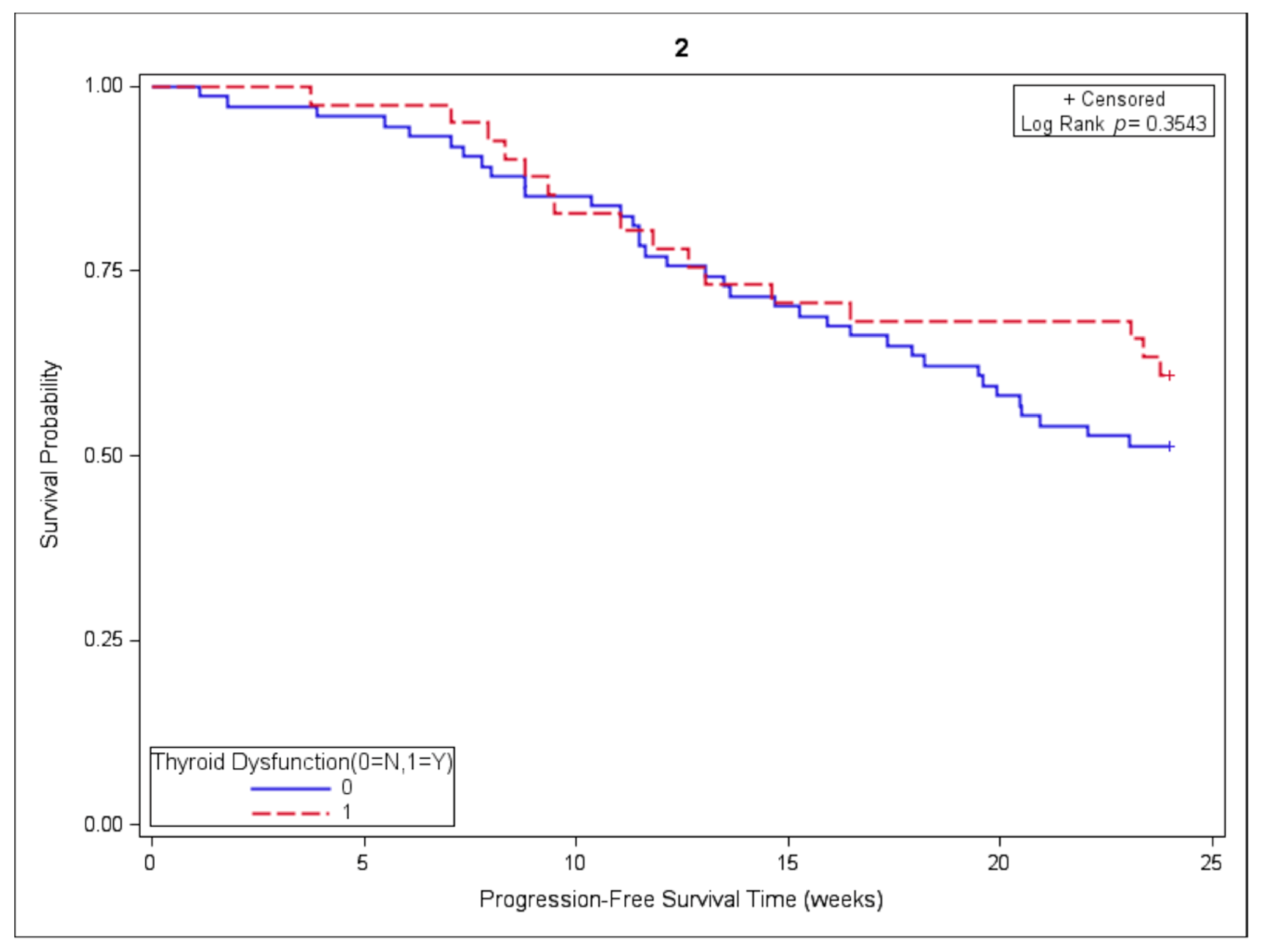
| Basak 2020 [49] | NSCLC, RCC, metastatic melanoma | Pembrolizumab, nivolumab | 168 | OS HR 0.18, p = 0.020 for subjects with thyroid dysfunction compared to those without; PFS5 HR6 0.39, p = 0.005 for subjects with thyroid dysfunction compared to those without |
| Kotwal 2020 [50] | Lung, uroepithelial, Merkel, prostate, penile | Atezolizumab, nivolumab, avelumab | 91 | Mortality 43.5% vs. 79.4% for subjects with and without thyroid dysfunction, respectively; median OS not reached vs. 9.8 (p = 0.027) for subjects with and without thyroid dysfunction, respectively |
| Sakakida 2019 [51] | Metastatic or unresectable malignancies | Pembrolizumab, nivolumab | 150 | Median OS 156 weeks vs. 59 weeks (HR 0.32, p = 0.001) for subjects with and without thyroid dysfunction, respectively; median PFS 66 weeks vs. 27 (HR 0.50, p = 0.02) for subjects with and without thyroid dysfunction, respectively |
| Grangeon 2019 [52] | Advanced NSCLC | Anti-PD-1 or anti-PD-L1 | 270 | OS not reached vs. 18.2 months (HR 0.46, p = 0.01) for subjects with and without thyroid dysfunction, respectively; PFS 8.05 vs. 2.59 months (HR 0.56, p = 0.005) for subjects with and without thyroid dysfunction, respectively |
| Peiro 2019 [27] | NSCLC, melanoma, Hodgkin’s lymphoma | Nivolumab | 73 | OS HR 0.4, p = 0.035 in NSCLC patients, specifically with thyroid dysfunction compared to those without |
| Kim 2017 [26] | Stage IV NSCLC | Pembrolizumab | 58 | OS HR 0.11, p = 0.041 for subjects with thyroid dysfunction compared to those without it; PFS HR 0.38, p = 0.018 for subjects with thyroid dysfunction compared to those without |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aiello, A.; Lin, J.; Gucalp, R.; Tabatabaie, V.; Cheng, H.; Bloomgarden, N.A.; Tomer, Y.; Halmos, B. Thyroid Dysfunction in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors (ICIs): Outcomes in a Multiethnic Urban Cohort. Cancers 2021, 13, 1464. https://doi.org/10.3390/cancers13061464
D’Aiello A, Lin J, Gucalp R, Tabatabaie V, Cheng H, Bloomgarden NA, Tomer Y, Halmos B. Thyroid Dysfunction in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors (ICIs): Outcomes in a Multiethnic Urban Cohort. Cancers. 2021; 13(6):1464. https://doi.org/10.3390/cancers13061464
Chicago/Turabian StyleD’Aiello, Angelica, Juan Lin, Rasim Gucalp, Vafa Tabatabaie, Haiying Cheng, Noah A. Bloomgarden, Yaron Tomer, and Balazs Halmos. 2021. "Thyroid Dysfunction in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors (ICIs): Outcomes in a Multiethnic Urban Cohort" Cancers 13, no. 6: 1464. https://doi.org/10.3390/cancers13061464
APA StyleD’Aiello, A., Lin, J., Gucalp, R., Tabatabaie, V., Cheng, H., Bloomgarden, N. A., Tomer, Y., & Halmos, B. (2021). Thyroid Dysfunction in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors (ICIs): Outcomes in a Multiethnic Urban Cohort. Cancers, 13(6), 1464. https://doi.org/10.3390/cancers13061464







