Role of Repeat PET/CT Imaging in Head and Neck Cancer Following Initial Incomplete PET/CT Response to Chemoradiation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility
2.2. Data Collection, Treatment, and Follow Up
2.3. Treatment Response Assessment with PET/CT Imaging
2.4. Statistical Analyses
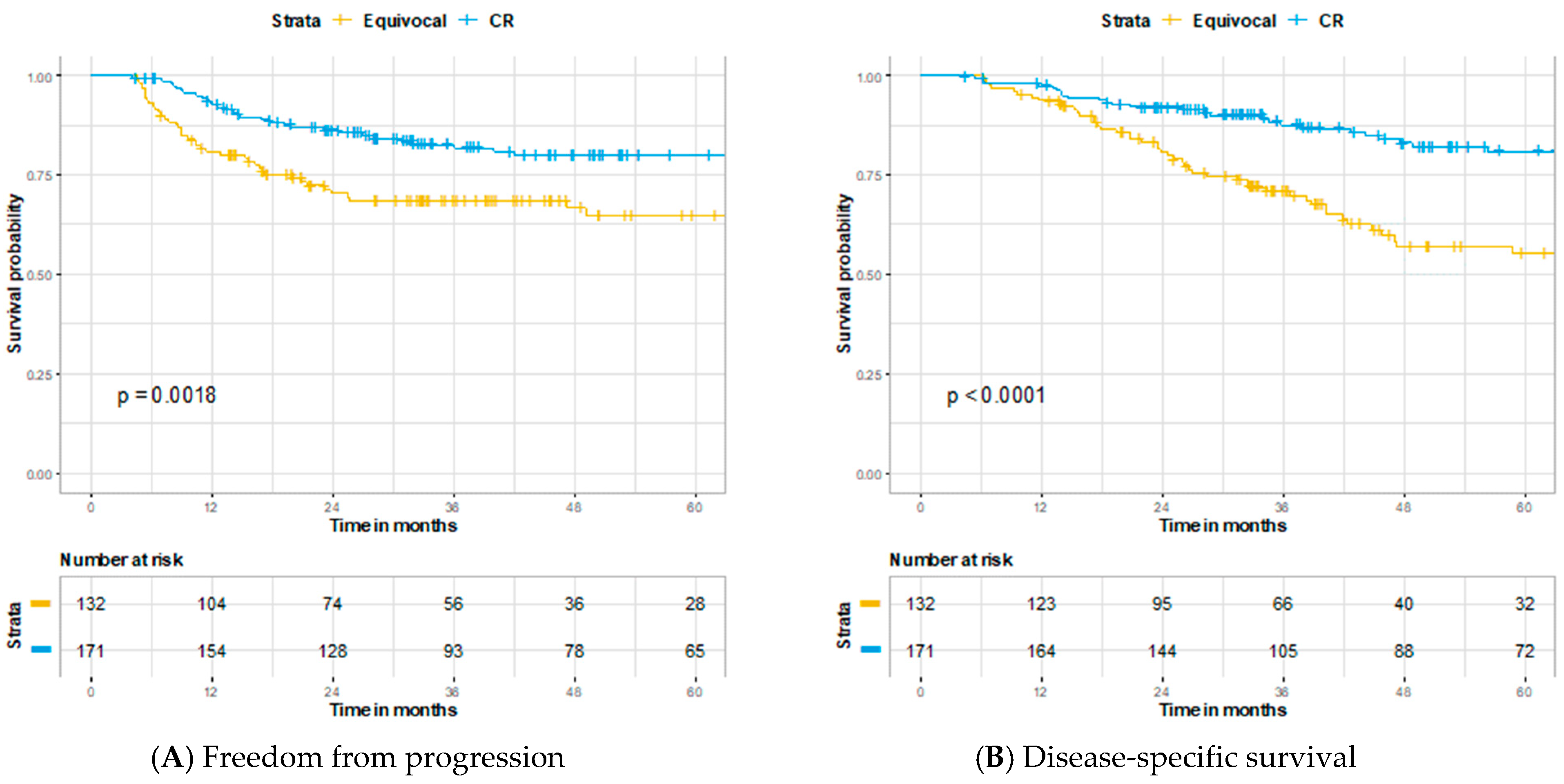
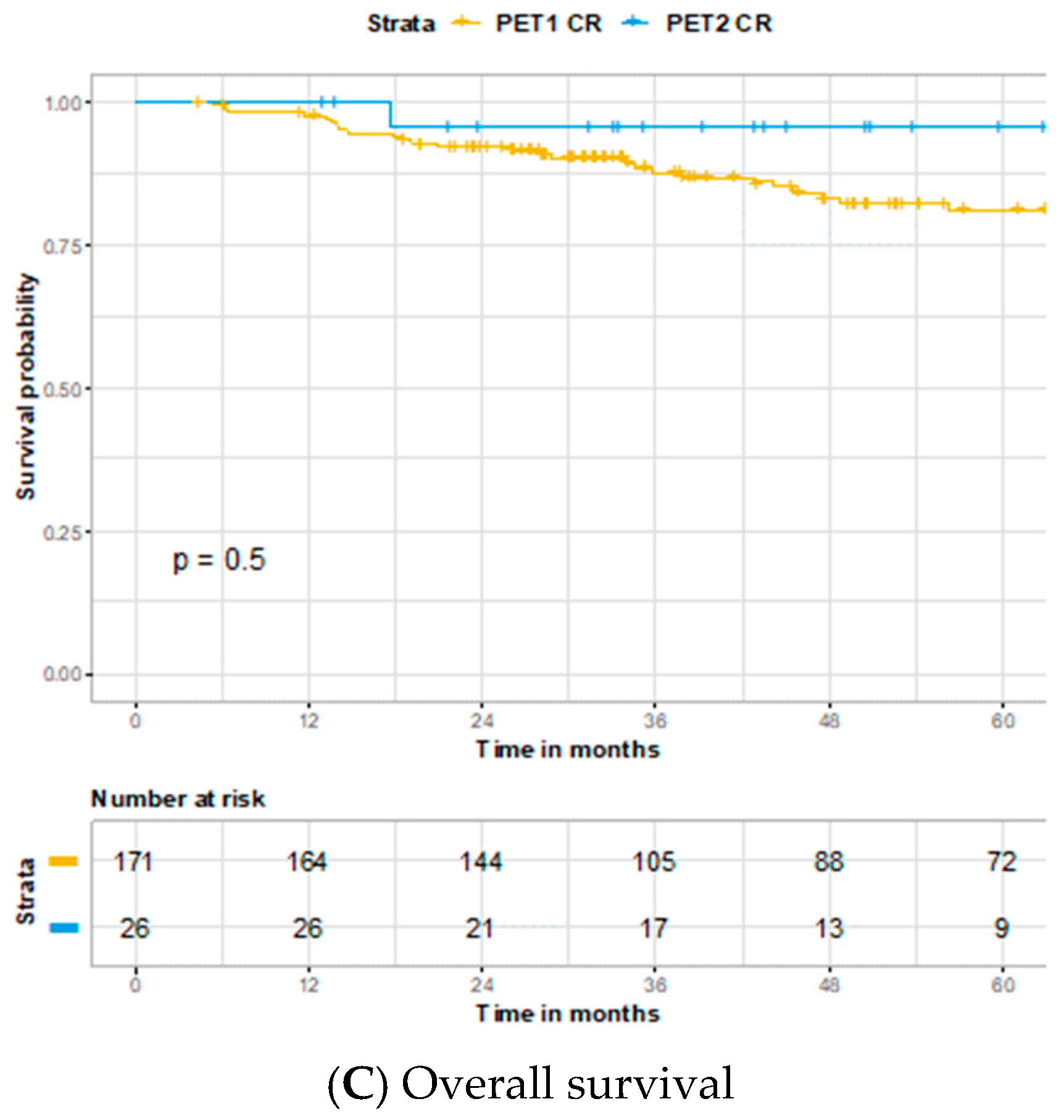
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terhaard, C.H.; Bongers, V.; Van Rijk, P.P.; Hordijk, G.-J. F-18-fluoro-deoxy-glucose positron-emission tomography scanning in detection of local recurrence after radiotherapy for laryngeal/pharyngeal cancer. Head Neck 2001, 23, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Adelstein, D.J.; Rybicki, L.A.; Saxton, J.P.; Esclamado, R.M.; Wood, B.G.; Lorenz, R.R.; Strome, M.; Carroll, M.A. Ability of Positron Emission Tomography to Detect Residual Neck Node Disease in Patients With Head and Neck Squamous Cell Carcinoma After Definitive Chemoradiotherapy. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 435. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Yokoyama, J.; Yamaguchi, K.; Ono, S.; Qureshy, A.; Itoh, M.; Fukuda, H. FDG-PET delayed imaging for the detection of head and neck cancer recurrence after radio-chemotherapy: Comparison with MRI/CT. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 590–595. [Google Scholar] [CrossRef]
- Kapoor, V.; Fukui, M.B.; McCook, B.M. Role of 18F FDG PET/CT in the Treatment of Head and Neck Cancers: Posttherapy Evaluation and Pitfalls. Am. J. Roentgenol. 2005, 184, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Rana, V.; Cannon, B.A.; Williams, M.D.; Sturgis, E.M.; Ginsberg, L.E.; Macapinlac, H.A.; Lee, J.J.; Ang, K.K.; Chao, K.C.; et al. Prospective Risk-Adjusted [18F]Fluorodeoxyglucose Positron Emission Tomography and Computed Tomography Assessment of Radiation Response in Head and Neck Cancer. J. Clin. Oncol. 2009, 27, 2509–2515. [Google Scholar] [CrossRef]
- Gupta, T.; Master, Z.; Kannan, S.; Agarwal, J.P.; Ghsoh-Laskar, S.; Rangarajan, V.; Murthy, V.; Budrukkar, A. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Isles, M.; McConkey, C.; Mehanna, H. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin. Otolaryngol. 2008, 33, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, C.A.; Pruim, J.; Dijkstra, P.U.; Balink, H.; Van Der Laan, B.F.; De Visscher, J.G.; Roodenburg, J.L. 18F-FDG PET as a Routine Posttreatment Surveillance Tool in Oral and Oropharyngeal Squamous Cell Carcinoma: A ProspectiveStudy. J. Nucl. Med. 2009, 50, 1940–1947. [Google Scholar] [CrossRef]
- Greven, K.M.; Williams, D.W., III; McGuirt Sr, W.F.; Harkness, B.A.; D’Agostino, R.B., Jr.; Keyes, J.W., Jr.; Watson, N.E., Jr. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck 2001, 23, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-A.; Roh, J.-L.; Kim, J.S.; Lee, J.H.; Lee, S.H.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. 18F-FDG PET/CT surveillance for the detection of recurrence in patients with head and neck cancer. Eur. J. Cancer 2017, 72, 62–70. [Google Scholar] [CrossRef]
- Wong, R.J.; Lin, D.T.; Schöder, H.; Patel, S.G.; Gonen, M.; Wolden, S.; Pfister, D.G.; Shah, J.P.; Larson, S.M.; Kraus, D.H. Diagnostic and Prognostic Value of [18F]Fluorodeoxyglucose Positron Emission Tomography for Recurrent Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2002, 20, 4199–4208. [Google Scholar] [CrossRef]
- Mehanna, H.; Wong, W.-L.; McConkey, C.C.; Rahman, J.K.; Robinson, M.; Hartley, A.G.J.; Nutting, C.; Powell, N.; Al-Booz, H.; Robinson, M.; et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N. Engl. J. Med. 2016, 374, 1444–1454. [Google Scholar] [CrossRef]
- Smith, A.; Hall, P.; Hulme, C.; Dunn, J.; McConkey, C.; Rahman, J.; McCabe, C.; Mehanna, H. Cost-effectiveness analysis of PET-CT-guided management for locally advanced head and neck cancer. Eur. J. Cancer 2017, 85, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Homer, J.; Clarke, P.; MacKenzie, K.; Paleri, V.; Pracy, P.; Roland, N. Follow-up after treatment for head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S208–S211. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Tsao, G.J.; Chen, F.W.; Shen, T.; Kaplan, M.J.; Colevas, A.D.; Fischbein, N.J.; Quon, A.; Le, Q.-T.; Pinto, H.A.; et al. Impact of positron emission tomography/computed tomography surveillance at 12 and 24 months for detecting head and neck cancer recurrence. Cancer 2013, 119, 1349–1356. [Google Scholar] [CrossRef]
- Perie, S.; Hugentobler, A.; Susini, B.; Balogova, S.; Grahek, D.; Kerrou, K.; Montravers, F.; El Chater, P.; Guily, J.L.S.; Talbot, J.N. Impact of FDG-PET to detect recurrence of head and neck squamous cell carcinoma. Otolaryngol. Neck Surg. 2007, 137, 647–653. [Google Scholar] [CrossRef]
- Liu, H.Y.; Milne, R.; Lock, G.; Panizza, B.J.; Bernard, A.; Foote, M.; McGrath, M.; Brown, E.; Gandhi, M.; Porceddu, S.V. Utility of a repeat PET/CT scan in HPV-associated Oropharyngeal Cancer following incomplete nodal response from (chemo)radiotherapy. Oral Oncol. 2019, 88, 153–159. [Google Scholar] [CrossRef]
- Prestwich, R.J.D.; Arunsingh, M.; Zhong, J.; Dyker, K.E.; Vaidyanathan, S.; Scarsbrook, A.F. Second-look PET-CT following an initial incomplete PET-CT response to (chemo)radiotherapy for head and neck squamous cell carcinoma. Eur. Radiol. 2020, 30, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Fung-Kee-Fung, S.D. A prospective trial of volumetric intensity-modulated arc therapyvsconventional intensity modulated radiation therapy in advanced head and neck cancer. World J. Clin. Oncol. 2012, 3, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Platek, M.E.; McCloskey, S.A.; Cruz, M.; Burke, M.S.; Reid, M.E.; Wilding, G.E.; Rigual, N.R.; Popat, S.R.; Loree, T.R.; Gupta, V.; et al. Quantification of the effect of treatment duration on local-regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Head Neck 2013, 35, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, J.M.; Spector, M.E.; Stenmark, M.H.; Bradford, C.R.; Wolf, G.T.; Worden, F.P.; Chepeha, U.B.; McHugh, J.B.; Carey, T.; Wong, K.K.; et al. Reliability of post-chemoradiotherapy F-18-FDG PET/CT for prediction of locoregional failure in human papillomavirus-associated oropharyngeal cancer. Oral Oncol. 2014, 50, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Rulach, R.; Zhou, S.; Hendry, F.; Stobo, D.; James, A.; Dempsey, M.-F.; Grose, D.; Lamb, C.; Schipani, S.; Rizwanullah, M.; et al. 12 week PET-CT has low positive predictive value for nodal residual disease in human papillomavirus-positive oropharyngeal cancers. Oral Oncol. 2019, 97, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Aiken, A.H.; Rath, T.J.; Anzai, Y.; Branstetter, B.F.; Hoang, J.K.; Wiggins, R.H.; Juliano, A.F.; Glastonbury, C.; Phillips, C.D.; Brown, R.; et al. ACR Neck Imaging Reporting and Data Systems (NI-RADS): A White Paper of the ACR NI-RADS Committee. J. Am. Coll. Radiol. 2018, 15, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Sundersingh, M.; Dyker, K.; Currie, S.; Vaidyanathan, S.; Prestwich, R.; Scarsbrook, A. Post-treatment FDG PET-CT in head and neck carcinoma: Comparative analysis of 4 qualitative interpretative criteria in a large patient cohort. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]

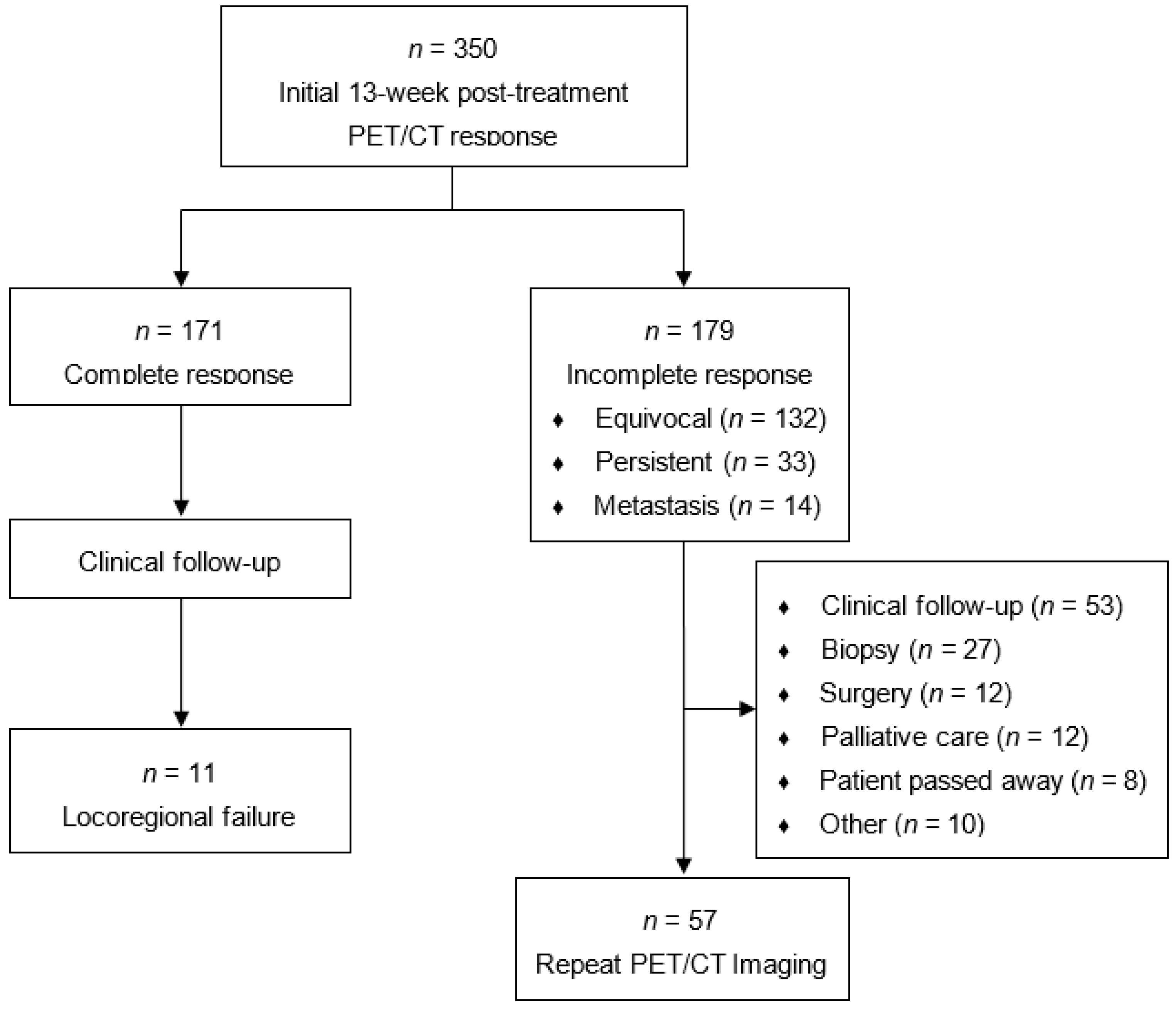

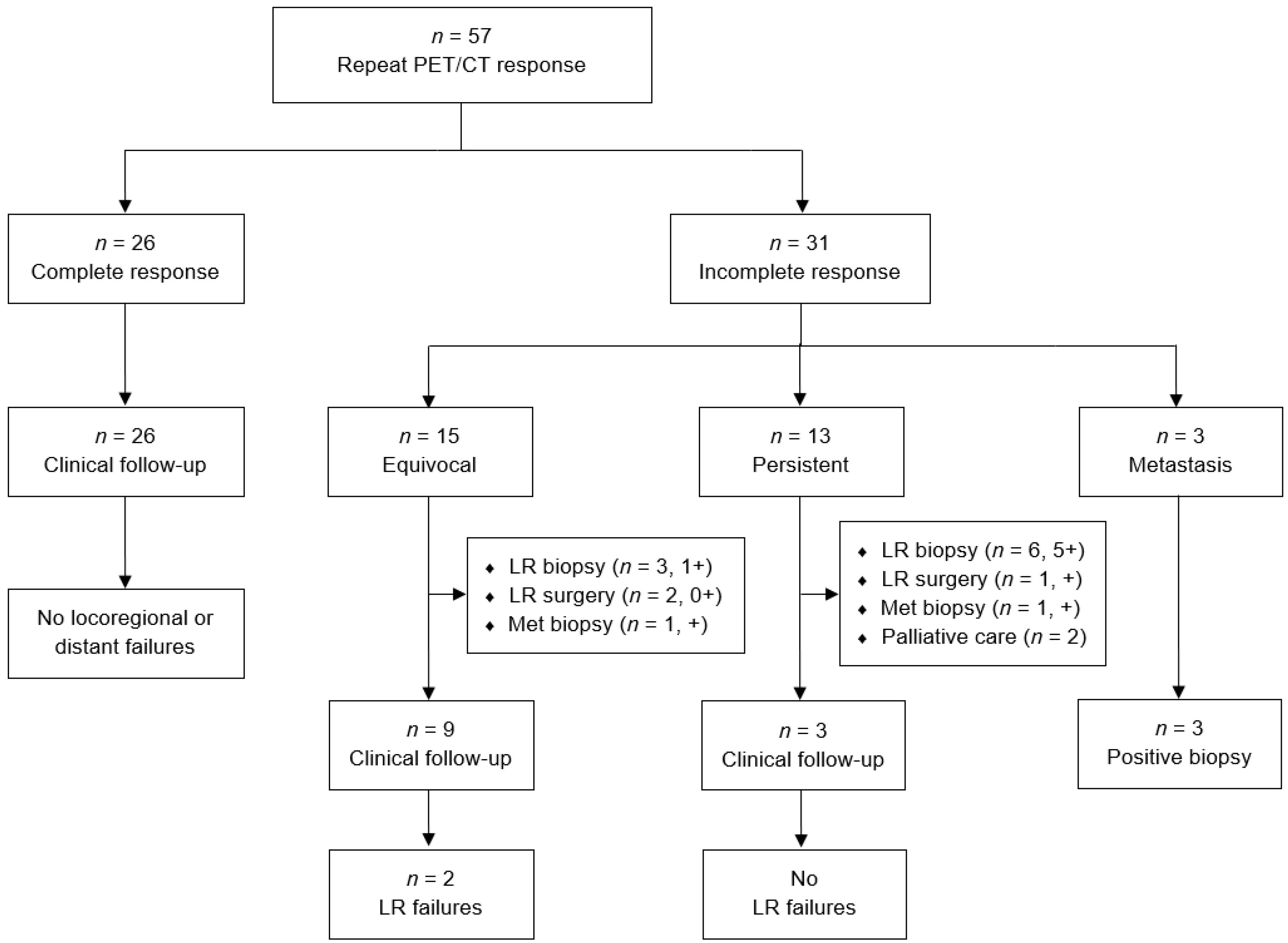

| Total Cohort (n = 350) | Repeat PET/CT (n = 57) | |
|---|---|---|
| Age (y) | ||
| Median +/−SD | 58 ± 9.6 | 56 ± 10.4 |
| Range | 18–90 | 18–88 |
| Gender, n (%) | ||
| Male | 295 (84.3) | 48 (84.2) |
| Female | 55 (15.7) | 9 (15.8) |
| Smoking, n (%) | ||
| Never | 84 (24.0) | 10 (17.5) |
| Former | 174 (49.7) | 30 (52.6) |
| Current | 92 (26.3) | 17 (29.8) |
| T Stage *, n (%) | ||
| T0 | 23 (6.6) | 3 (5.3) |
| Tis | 1 (0.3) | 1 (1.8) |
| T1 | 46 (13.1) | 6 (10.5) |
| T2 | 107 (30.6) | 14 (24.6) |
| T3 | 123 (35.1) | 22 (38.6) |
| T4 | 50 (14.3) | 11 (19.3) |
| N Stage *, n (%) | ||
| N0 | 77 (22.0) | 17 (29.8) |
| N1 | 39 (11.1) | 1 (1.8) |
| N2 | 199 (56.9) | 31 (54.5) |
| N3 | 35 (10.0) | 8 (14.0) |
| Overall Clinical Stage *, n (%) | ||
| I | 2 (0.6) | 0 (0) |
| II | 23 (6.6) | 7 (12.3) |
| III | 73 (20.9) | 6 (10.5) |
| IVA | 219 (62.6) | 38 (66.7) |
| IVB | 33 (9.4) | 6 (15.8) |
| Primary Tumor Site, n (%) | ||
| Nasopharynx | 13 (3.7) | 4 (7.0) |
| Oropharynx | 187 (53.4) | 27 (47.4) |
| Oral cavity | 10 (2.9) | 2 (3.5) |
| Larynx | 85 (24.3) | 16 (28.1) |
| Hypopharynx | 32 (9.1) | 5 (8.8) |
| Unknown primary | 23 (6.6) | 3 (5.3) |
| HPV Status, n (%) | ||
| Negative | 73 (20.9) | 12 (21.1) |
| Positive | 145 (41.4) | 18 (31.6) |
| Unknown | 132 (37.7) | 27 (47.4) |
| Treatment, n (%) | ||
| RT alone | 13 (3.7) | 2 (3.5) |
| ICT + CCRT | 38 (10.9) | 7 (12.3) |
| CCRT | 299 (85.4) | 48 (84.2) |
| Relapses, n (%) | ||
| Local | 41 (11.7) | 11 (19.3) |
| Regional | 26 (7.4) | 2 (3.5) |
| Distant | 62 (17.7) | 7 (12.3) |
| Deaths, n (%) | ||
| Total | 128 (36.6) | 23 (40.4) |
| Locoregional Status | Disease Recurrence | Disease Controlled | - |
|---|---|---|---|
| Initial PET CR | 26 | 145 | NPV: 85% |
| Initial PET IR | 77 | 102 | PPV: 43% |
| - | Sensitivity: 75% | Specificity: 59% | - |
| Locoregional Status | Disease Recurrence | Disease Controlled | - |
|---|---|---|---|
| Repeat PET CR | 0 | 26 | NPV: 100% |
| Repeat PET IR | 13 | 18 | PPV: 42% |
| - | Sensitivity: 100% | Specificity: 59% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iovoli, A.J.; Farrugia, M.K.; Ma, S.J.; Chan, J.M.; Markiewicz, M.R.; McSpadden, R.; Wooten, K.E.; Gupta, V.; Kuriakose, M.A.; Hicks, W.L., Jr.; et al. Role of Repeat PET/CT Imaging in Head and Neck Cancer Following Initial Incomplete PET/CT Response to Chemoradiation. Cancers 2021, 13, 1461. https://doi.org/10.3390/cancers13061461
Iovoli AJ, Farrugia MK, Ma SJ, Chan JM, Markiewicz MR, McSpadden R, Wooten KE, Gupta V, Kuriakose MA, Hicks WL Jr., et al. Role of Repeat PET/CT Imaging in Head and Neck Cancer Following Initial Incomplete PET/CT Response to Chemoradiation. Cancers. 2021; 13(6):1461. https://doi.org/10.3390/cancers13061461
Chicago/Turabian StyleIovoli, Austin J., Mark K. Farrugia, Sung Jun Ma, Jon M. Chan, Michael R. Markiewicz, Ryan McSpadden, Kimberly E. Wooten, Vishal Gupta, Moni A. Kuriakose, Wesley L. Hicks, Jr., and et al. 2021. "Role of Repeat PET/CT Imaging in Head and Neck Cancer Following Initial Incomplete PET/CT Response to Chemoradiation" Cancers 13, no. 6: 1461. https://doi.org/10.3390/cancers13061461
APA StyleIovoli, A. J., Farrugia, M. K., Ma, S. J., Chan, J. M., Markiewicz, M. R., McSpadden, R., Wooten, K. E., Gupta, V., Kuriakose, M. A., Hicks, W. L., Jr., & Singh, A. K. (2021). Role of Repeat PET/CT Imaging in Head and Neck Cancer Following Initial Incomplete PET/CT Response to Chemoradiation. Cancers, 13(6), 1461. https://doi.org/10.3390/cancers13061461






