Current Advances in Robotics for Head and Neck Surgery—A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
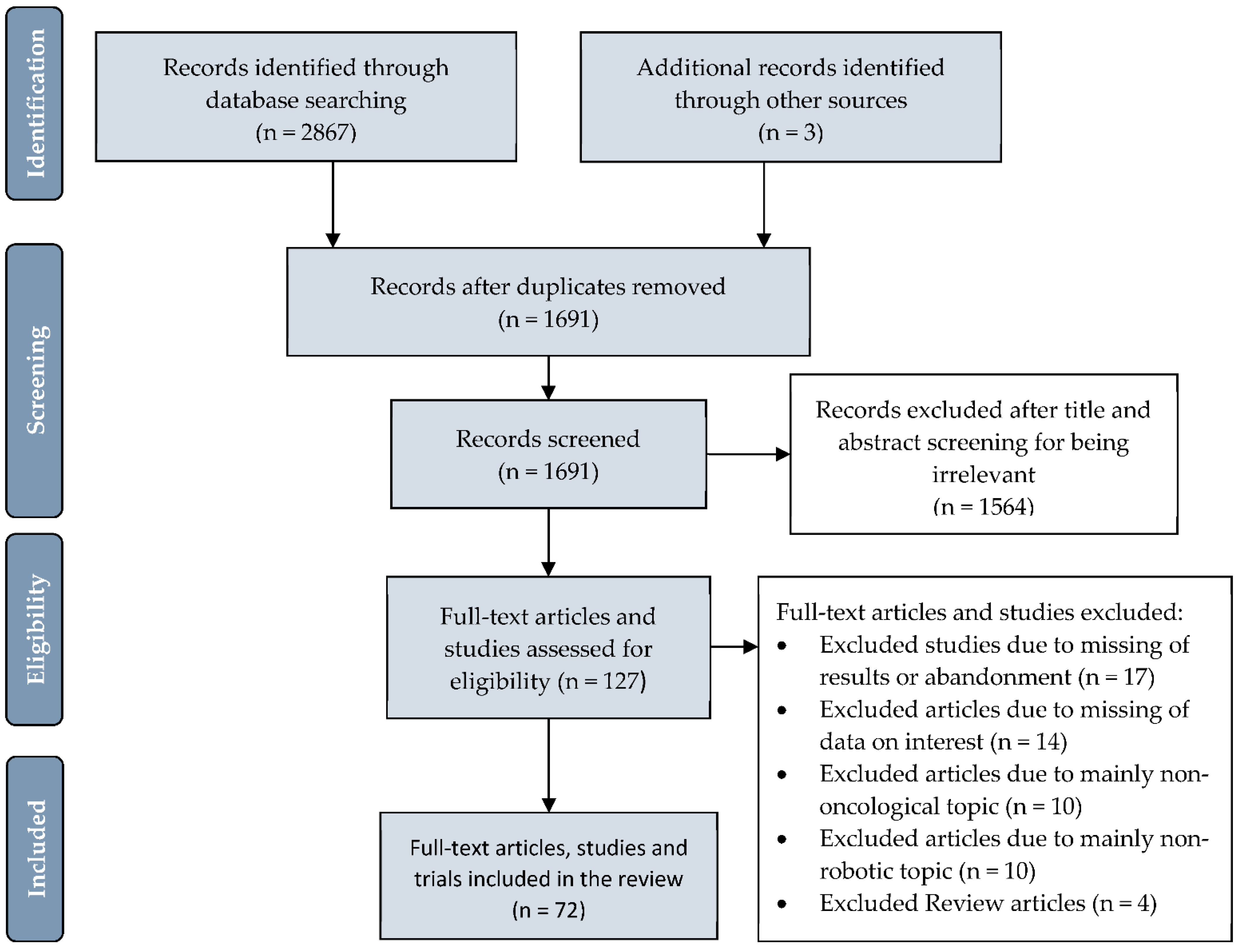
2. Methods
3. Results
3.1. Pharynx
3.2. Larynx
3.3. Paranasal Sinuses and Skull Base
3.4. Thyroid Gland and Neck Dissection
3.5. Costs
3.6. Clinical Trials
3.7. Robotic Research
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schuler, P.J.; Duvvuri, U.; Friedrich, D.T.; Rotter, N.; Scheithauer, M.O.; Hoffmann, T.K. First use of a computer-assisted operator-controlled flexible endoscope for transoral surgery. Laryngoscope 2015, 125, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Lee, S.C.; Lee, S.H.; Lee, K.Y.; Jeong, J.J.; Lee, Y.S.; Nam, K.H.; Chang, H.S.; Chung, W.Y.; Park, C.S. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: The operative outcomes of 338 consecutive patients. Surgery 2009, 146, 1048–1055. [Google Scholar] [CrossRef]
- Leal Ghezzi, T.; Campos Corleta, O. 30 Years of Robotic Surgery. World J. Surg. 2016, 40, 2550–2557. [Google Scholar] [CrossRef] [PubMed]
- Albus, J.S. NBS/RIA Robotics Research Workshop. In Proceedings of the NBS/RIA Workshop on Robotic Research, Gaithersburg, MD, USA, 13–15 November 1979. [Google Scholar]
- Carrau, R.L.; Prevedello, D.M.; de Lara, D.; Durmus, K.; Ozer, E. Combined transoral robotic surgery and endoscopic endonasal approach for the resection of extensive malignancies of the skull base. Head Neck 2013, 35, 351–358. [Google Scholar] [CrossRef]
- Parmar, A.; Grant, D.G.; Loizou, P. Robotic surgery in ear nose and throat. Eur. Arch. Otorhinolaryngol. 2010, 267, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.S.; O’Malley, B.W., Jr.; Magnuson, J.S.; Carroll, W.R.; Olsen, K.D.; Daio, L.; Moore, E.J.; Holsinger, F.C. Transoral robotic surgery: A multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 2012, 122, 1701–1707. [Google Scholar] [CrossRef]
- Hurtuk, A.M.; Marcinow, A.; Agrawal, A.; Old, M.; Teknos, T.N.; Ozer, E. Quality-of-life outcomes in transoral robotic surgery. Otolaryngol. Head Neck Surg. 2012, 146, 68–73. [Google Scholar] [CrossRef]
- Dziegielewski, P.T.; Teknos, T.N.; Durmus, K.; Old, M.; Agrawal, A.; Kakarala, K.; Marcinow, A.; Ozer, E. Transoral Robotic Surgery for Oropharyngeal Cancer: Long-term Quality of Life and Functional Outcomes. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1099–1108. [Google Scholar] [CrossRef]
- Park, Y.M.; Jung, C.M.; Cha, D.; Kim, S.H. The long-term oncological and functional outcomes of transoral robotic surgery in patients with hypopharyngeal cancer. Oral Oncol. 2017, 71, 138–143. [Google Scholar] [CrossRef]
- Hussain, T.; Lang, S.; Haßkamp, P.; Holtmann, L.; Höing, B.; Mattheis, S. The Flex robotic system compared to transoral laser microsurgery for the resection of supraglottic carcinomas: First results and preliminary oncologic outcomes. Eur. Arch. Otorhinolaryngol. 2020, 277, 917–924. [Google Scholar] [CrossRef]
- Arshad, H.; Durmus, K.; Ozer, E. Transoral robotic resection of selected parapharyngeal space tumors. Eur. Arch. Otorhinolaryngol. 2013, 270, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, B.W., Jr.; Quon, H.; Leonhardt, F.D.; Chalian, A.A.; Weinstein, G.S. Transoral robotic surgery for parapharyngeal space tumors. ORL J. Otorhinolaryngol. Relat. Spec. 2010, 72, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sikka, K.; Thakar, A.; Sharma, S.C.; Krishnamurthy, P. Transoral robotic surgery for the parapharyngeal space: Expanding the transoral corridor. J. Robot. Surg. 2019, 14, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.; Jouffroy, T.; Veivers, D.; Hoffman, C.; Girod, A.; Badoual, C.; Rodriguez, J.; Brasnu, D. Transoral robotic-assisted free flap reconstruction after radiation therapy in hypopharyngeal carcinoma: Report of two cases. Eur. Arch. Otorhinolaryngol. 2013, 270, 2359–2364. [Google Scholar] [CrossRef]
- Turner, M.T.; Geltzeiler, M.; Albergotti, W.G.; Duvvuri, U.; Ferris, R.L.; Kim, S.; Wang, E.W. Reconstruction of TORS oropharyngectomy defects with the nasoseptal flap via transpalatal tunnel. J. Robot. Surg. 2019, 14, 311–316. [Google Scholar] [CrossRef]
- Turhan, M.; Bostanci, A. Robotic Tongue-Base Resection Combined with Tongue-Base Suspension for Obstructive Sleep Apnea. Laryngoscope 2019, 130, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Dallan, I.; Cristofani-Mencacci, L.; Seccia, V.; Cambi, C.; Fiacchini, G.; Berrettini, S.; Brevi, B. Transoral robotic tongue base reduction and supraglottoplasty combined with maxillomandibular advancement: A new option for selected sleep apnea patients? Preliminary report. Eur. Arch. Otorhinolaryngol. 2019, 276, 3543–3548. [Google Scholar] [CrossRef]
- Remacle, M.; MNPrasad, V.; Lawson, G.; Plisson, L.; Bachy, V.; Van der Vorst, S. Transoral robotic surgery (TORS) with the Medrobotics Flex System: First surgical application on humans. Eur. Arch. Otorhinolaryngol. 2015, 272, 1451–1455. [Google Scholar] [CrossRef]
- Sethi, N.; Gouzos, M.; Padhye, V.; Ooi, E.H.; Foreman, A.; Krishnan, S.; Hodge, J.C. Transoral robotic surgery using the Medrobotic Flex((R)) system: The Adelaide experience. J. Robot. Surg. 2019, 14, 109–113. [Google Scholar] [CrossRef]
- Park, Y.M.; Kim, D.H.; Kang, M.S.; Lim, J.Y.; Choi, E.C.; Koh, Y.W.; Kim, S.H. The First Human Trial of Transoral Robotic Surgery Using a Single-Port Robotic System in the Treatment of Laryngo-Pharyngeal Cancer. Ann. Surg. Oncol. 2019, 26, 4472–4480. [Google Scholar] [CrossRef]
- Holsinger, F.C.; Magnuson, J.S.; Weinstein, G.S.; Chan, J.Y.K.; Starmer, H.M.; Tsang, R.K.Y.; Wong, E.W.Y.; Rassekh, C.H.; Bedi, N.; Hong, S.S.Y.; et al. A Next-Generation Single-Port Robotic Surgical System for Transoral Robotic Surgery: Results From Prospective Nonrandomized Clinical Trials. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Samalavicius, N.E.; Janusonis, V.; Siaulys, R.; Jasenas, M.; Deduchovas, O.; Venckus, R.; Ezerskiene, V.; Paskeviciute, R.; Klimaviciute, G. Robotic surgery using Senhance((R)) robotic platform: Single center experience with first 100 cases. J. Robot. Surg. 2019, 14, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, B.; Diana, M.; Ruurda, J.; Konstantinidis, K.; Marescaux, J.; Swanstrom, L. Enabling single-site laparoscopy: The SPORT platform. Surg. Endosc. 2019, 33, 3696–3703. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Costantino, A.; Ebm, C.; Conti, V.; Mondello, T.; Di Bari, M.; Cugini, G.; Mercante, G.; Spriano, G. High definition three-dimensional exoscope (VITOM 3D) for microsurgery training: A preliminary experience. Eur. Arch. Otorhinolaryngol. 2020, 277, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.I.; Mericli, A.F.; DeFazio, M.V.; Chang, E.I.; Hanasono, M.M.; Pederson, W.C.; Kaufman, M.; Selber, J.C. Application of the ORBEYE three-dimensional exoscope for microsurgical procedures. Microsurgery 2020, 40, 468–472. [Google Scholar] [CrossRef]
- Lawson, G.; Mendelsohn, A.; Fakhoury, R.; Van der Vorst, S.; Remacle, M.; Bachy, V.; Delahaut, G. Transoral Robotic Surgery Total Laryngectomy. ORL J. Otorhinolaryngol. Relat. Spec. 2018, 80, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.W.; Chan, R.C.L.; Chow, V.L.Y.; Tsang, R.K.Y.; Wong, S.T.S.; Wei, W.I. Transoral robotic total laryngopharyngectomy and free jejunal flap reconstruction for hypopharyngeal cancer. Oral. Oncol. 2017, 72, 194–196. [Google Scholar] [CrossRef]
- Morisod, B.; Guinchard, A.C.; Gorphe, P.; Schweizer, V.; Sandu, K.; Simon, C. Transoral robotic-assisted supracricoid partial laryngectomy with cricohyoidoepiglottopexy: Procedure development and outcomes of initial cases. Head Neck 2018, 40, 2254–2262. [Google Scholar] [CrossRef]
- Doazan, M.; Hans, S.; Moriniere, S.; Lallemant, B.; Vergez, S.; Aubry, K.; De Mones, E.; Espitalier, F.; Jegoux, F.; Pradat, P.; et al. Oncologic outcomes with transoral robotic surgery for supraglottic squamous cell carcinoma: Results of the French Robotic Surgery Group of GETTEC. Head Neck 2018, 40, 2050–2059. [Google Scholar] [CrossRef]
- Orosco, R.K.; Tam, K.; Nakayama, M.; Holsinger, F.C.; Spriano, G. Transoral supraglottic laryngectomy using a next-generation single-port robotic surgical system. Head Neck 2019, 41, 2143–2147. [Google Scholar] [CrossRef]
- Remacle, M.; Prasad, V.M.N. Preliminary experience in transoral laryngeal surgery with a flexible robotic system for benign lesions of the vocal folds. Eur. Arch. Otorhinolaryngol. 2018, 275, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Tan Wen Sheng, B.; Wong, P.; Teo Ee Hoon, C. Transoral robotic excision of laryngeal papillomas with Flex(R) Robotic System -A novel surgical approach. Am. J. Otolaryngol. 2018, 39, 355–358. [Google Scholar] [CrossRef]
- Mattos, L.S.; Deshpande, N.; Barresi, G.; Guastini, L.; Peretti, G. A novel computerized surgeon-machine interface for robot-assisted laser phonomicrosurgery. Laryngoscope 2014, 124, 1887–1894. [Google Scholar] [CrossRef]
- Kundrat, D.; Schoob, A.; Piskon, T.; Grasslin, R.; Schuler, P.; Hoffmann, T.; Kahrs, L.A.; Ortmaier, T. Toward Assistive Technologies for Focus Adjustment in Teleoperated Robotic Non-Contact Laser Surgery. IEEE Trans. Med Robot. Bionics 2019, 1, 145–157. [Google Scholar] [CrossRef]
- Kundrat, D.; Graesslin, R.; Schoob, A.; Friedrich, D.T.; Scheithauer, M.O.; Hoffmann, T.K.; Ortmaier, T.; Kahrs, L.A.; Schuler, P.J. Preclinical Performance Evaluation of a Robotic Endoscope for Non-Contact Laser Surgery. Ann. Biomed. Eng. 2020, 49, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Schild, L.R.; Lemke, D.; Boehm, F.; Greve, J.; Dürselen, L.; Scheithauer, M.O.; Hoffmann, T.K.; Schuler, P.J. Force effects on anatomical structures in transoral surgery—videolaryngoscopic prototype vs. conventional direct microlaryngoscopy. Curr. Dir. Biomed. Eng. 2020, 6, 20200021. [Google Scholar] [CrossRef]
- Schild, L.R.; Böhm, F.; Boos, M.; Kahrs, L.A.; Coburger, J.; Greve, J.; Dürselen, L.; Hoffmann, T.K.; Schuler, P.J. Adding Flexible Instrumentation to a Curved Videolaryngoscope: A Novel Tool for Laryngeal Surgery. Laryngoscope 2021, 131, 561–568. [Google Scholar] [CrossRef]
- Messerklinger, W. Endoscopy of the Nose; Urban und Schwarzenberg: München, Germany, 1978. [Google Scholar]
- Campbell, R.G. Robotic surgery of the anterior skull base. Int. Forum Allergy Rhinol. 2019, 9, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.W.; Zanation, A.M.; Gardner, P.A.; Schwartz, T.H.; Eloy, J.A.; Adappa, N.D.; Bettag, M.; Bleier, B.S.; Cappabianca, P.; Carrau, R.L.; et al. ICAR: Endoscopic skull-base surgery. Int. Forum Allergy Rhinol. 2019, 9, 145–365. [Google Scholar] [CrossRef]
- Ozer, E.; Waltonen, J. Transoral robotic nasopharyngectomy: A novel approach for nasopharyngeal lesions. Laryngoscope 2008, 118, 1613–1616. [Google Scholar] [CrossRef]
- Yin Tsang, R.K.; Ho, W.K.; Wei, W.I. Combined transnasal endoscopic and transoral robotic resection of recurrent nasopharyngeal carcinoma. Head Neck 2012, 34, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.E.; Haugen, T.W.; Rassekh, C.H.; Adappa, N.D.; Weinstein, G.S.; O’Malley, B.W., Jr. A novel transpalatal-transoral robotic surgery approach to clival chordomas extending into the nasopharynx. Head Neck 2019, 41, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bly, R.A.; Su, D.; Lendvay, T.S.; Friedman, D.; Hannaford, B.; Ferreira, M.; Moe, K.S. Multiportal robotic access to the anterior cranial fossa: A surgical and engineering feasibility study. Otolaryngol. Head Neck Surg. 2013, 149, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.K.; To, V.S.; Ho, A.C.; Ho, W.K.; Chan, J.Y.; Wei, W.I. Early results of robotic assisted nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck 2015, 37, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Burgner-Kahrs, J.; Rucker, D.C.; Choset, H. Continuum Robots for Medical Applications: A Survey. IEEE Trans. Robot. 2015, 31, 1261–1280. [Google Scholar] [CrossRef]
- Boehm, F.; Friedrich, D.T.; Sommer, F.; Scheithauer, M.O.; Greve, J.; Hoffmann, T.K.; Schuler, P.J. Nasolacrimal duct stenosis-Surgery with a novel robotic endoscope positioning system. Int. J. Med. Robot. 2020, 16, 1–5. [Google Scholar] [CrossRef]
- Lobe, T.E.; Wright, S.K.; Irish, M.S. Novel uses of surgical robotics in head and neck surgery. J. Laparoendosc. Adv. Surg. Tech. A 2005, 15, 647–652. [Google Scholar] [CrossRef]
- Song, C.M.; Cho, Y.H.; Ji, Y.B.; Jeong, J.H.; Kim, D.S.; Tae, K. Comparison of a gasless unilateral axillo-breast and axillary approach in robotic thyroidectomy. Surg. Endosc. 2013, 27, 3769–3775. [Google Scholar] [CrossRef]
- Chai, Y.J.; Lee, K.E.; Youn, Y.K. Can robotic thyroidectomy be performed safely in thyroid carcinoma patients? Endocrinol. Metab. 2014, 29, 226–232. [Google Scholar] [CrossRef]
- Hoffmann, T.; Friedrich, D.; Schuler, P. Robot-assisted surgery in the head and neck region. HNO 2016, 64, 658–666. [Google Scholar] [CrossRef]
- Kang, S.W.; Lee, S.H.; Ryu, H.R.; Lee, K.Y.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; Park, C.S. Initial experience with robot-assisted modified radical neck dissection for the management of thyroid carcinoma with lateral neck node metastasis. Surgery 2010, 148, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, W.S.; Hong, H.J.; Ban, M.J.; Lee, D.; Koh, Y.W.; Choi, E.C. Robot-assisted Supraomohyoid neck dissection via a modified face-lift or retroauricular approach in early-stage cN0 squamous cell carcinoma of the oral cavity: A comparative study with conventional technique. Ann. Surg. Oncol. 2012, 19, 3871–3878. [Google Scholar] [CrossRef]
- Tae, K.; Ji, Y.B.; Song, C.M.; Min, H.J.; Kim, K.R.; Park, C.W. Robotic selective neck dissection using a gasless postauricular facelift approach for early head and neck cancer: Technical feasibility and safety. J. Laparoendosc. Adv. Surg. Tech. A 2013, 23, 240–245. [Google Scholar] [CrossRef]
- Park, Y.M.; Cha, D.; Koh, Y.W.; Choi, E.C.; Kim, S.H. Transoral Robotic Surgery with Transoral Retropharyngeal Lymph Node Dissection in Patients with Tonsillar Cancer: Anatomical Points, Surgical Techniques, and Clinical Usefulness. J. Craniofac. Surg. 2019, 30, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Troob, S.; Givi, B.; Hodgson, M.; Mowery, A.; Gross, N.D.; Andersen, P.E.; Clayburgh, D. Transoral robotic retropharyngeal node dissection in oropharyngeal squamous cell carcinoma: Patterns of metastasis and functional outcomes. Head Neck 2017, 39, 1969–1975. [Google Scholar] [CrossRef]
- Hoffmann, T.K.; Schuler, P.J.; Bankfalvi, A.; Greve, J.; Heusgen, L.; Lang, S.; Mattheis, S. Comparative analysis of resection tools suited for transoral robot-assisted surgery. Eur. Arch. Otorhinolaryngol. 2014, 271, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Dombree, M.; Crott, R.; Lawson, G.; Janne, P.; Castiaux, A.; Krug, B. Cost comparison of open approach, transoral laser microsurgery and transoral robotic surgery for partial and total laryngectomies. Eur. Arch. Otorhinolaryngol. 2014, 271, 2825–2834. [Google Scholar] [CrossRef]
- Tam, K.; Orosco, R.K.; Dimitrios Colevas, A.; Bedi, N.; Starmer, H.M.; Beadle, B.M.; Christopher Holsinger, F. Cost comparison of treatment for oropharyngeal carcinoma. Laryngoscope 2019, 129, 1604–1609. [Google Scholar] [CrossRef]
- Sher, D.J.; Fidler, M.J.; Tishler, R.B.; Stenson, K.; al-Khudari, S. Cost-Effectiveness Analysis of Chemoradiation Therapy Versus Transoral Robotic Surgery for Human Papillomavirus-Associated, Clinical N2 Oropharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 512–522. [Google Scholar] [CrossRef]
- Benito, D.; Michel, M.C.; Thakkar, P.G.; Goodman, J.F.; Sadeghi, N.; Joshi, A.S. A cost effective custom dental guard for transoral robotic surgery. J. Robot. Surg. 2019, 14, 91–94. [Google Scholar] [CrossRef]
- Richmon, J.D.; Quon, H.; Gourin, C.G. The effect of transoral robotic surgery on short-term outcomes and cost of care after oropharyngeal cancer surgery. Laryngoscope 2014, 124, 165–171. [Google Scholar] [CrossRef]
- Mattheis, S.; Mandapathil, M.; Rothmeier, N.; Lang, S.; Dominas, N.; Hoffmann, T.K. Transoral robotic surgery for head and neck tumors: A series of 17 patients. Laryngorhinootologie 2012, 91, 768–773. [Google Scholar] [CrossRef]
- Dean, N.R.; Rosenthal, E.L.; Carroll, W.R.; Kostrzewa, J.P.; Jones, V.L.; Desmond, R.A.; Clemons, L.; Magnuson, J.S. Robotic-assisted surgery for primary or recurrent oropharyngeal carcinoma. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Mattheis, S.; Hasskamp, P.; Lawson, G.; Guldner, C.; Mandapathil, M.; Schuler, P.; Hoffmann, T.; Scheithauer, M.; Remacle, M. A european multicenter study evaluating the flex robotic system in transoral robotic surgery. Laryngoscope 2017, 127, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): An open-label, phase 2, randomised trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Hoffmann, T.K. ORATOR study: Surgery or radiotherapy for oropharyngeal carcinoma in the context of HPV? HNO 2020, 68, 278–279. [Google Scholar] [CrossRef] [PubMed]
- International Federation of Robotics. World Robotics R&D Programs; International Federation of Robotics: Frankfurt, Germany, 2020; pp. 1–77. [Google Scholar]
- Su, H.; Yang, C.; Ferrigno, G.; Momi, E.D. Improved Human–Robot Collaborative Control of Redundant Robot for Teleoperated Minimally Invasive Surgery. IEEE Robot. Autom. Lett. 2019, 4, 1447–1453. [Google Scholar] [CrossRef]

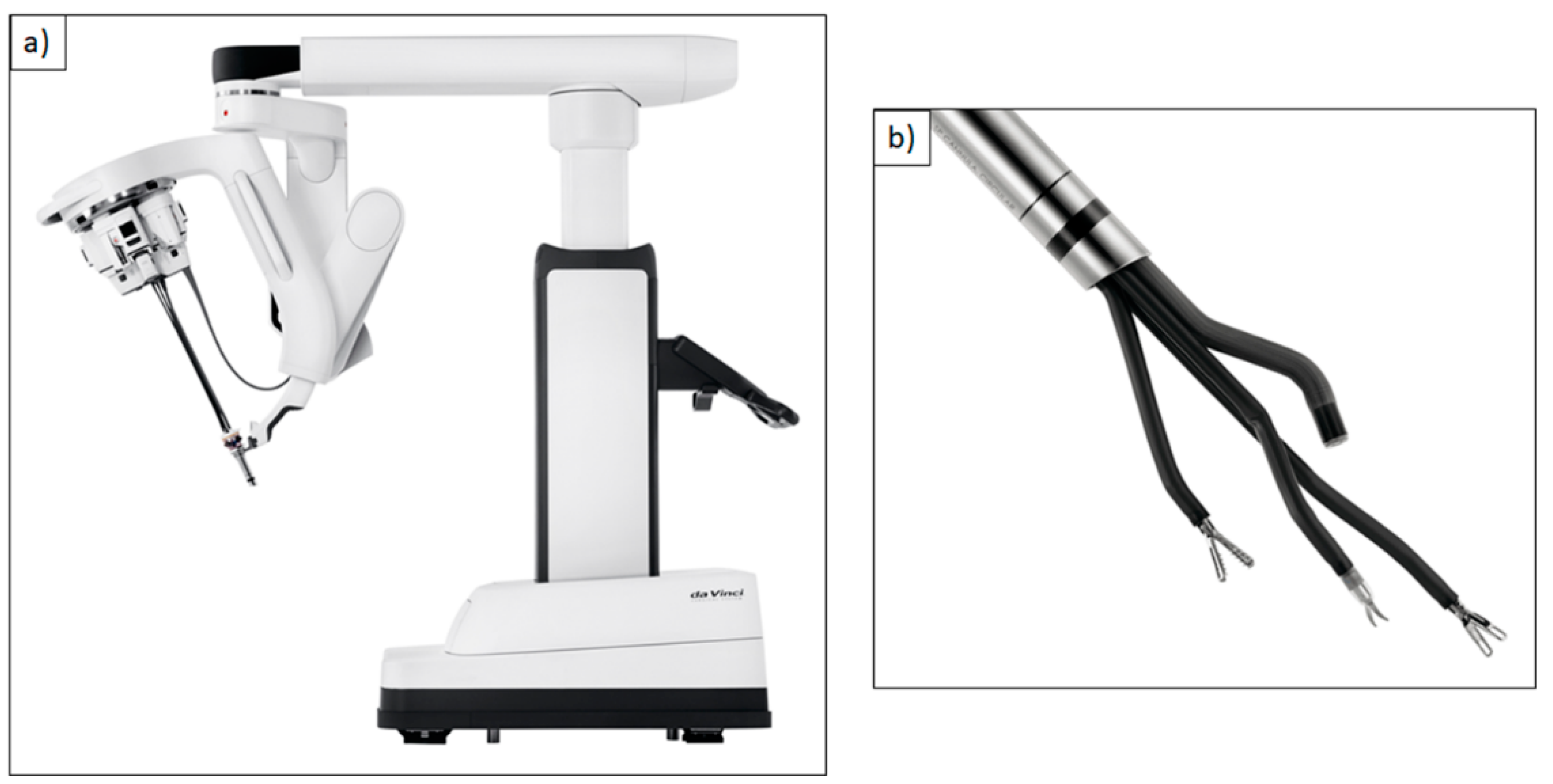

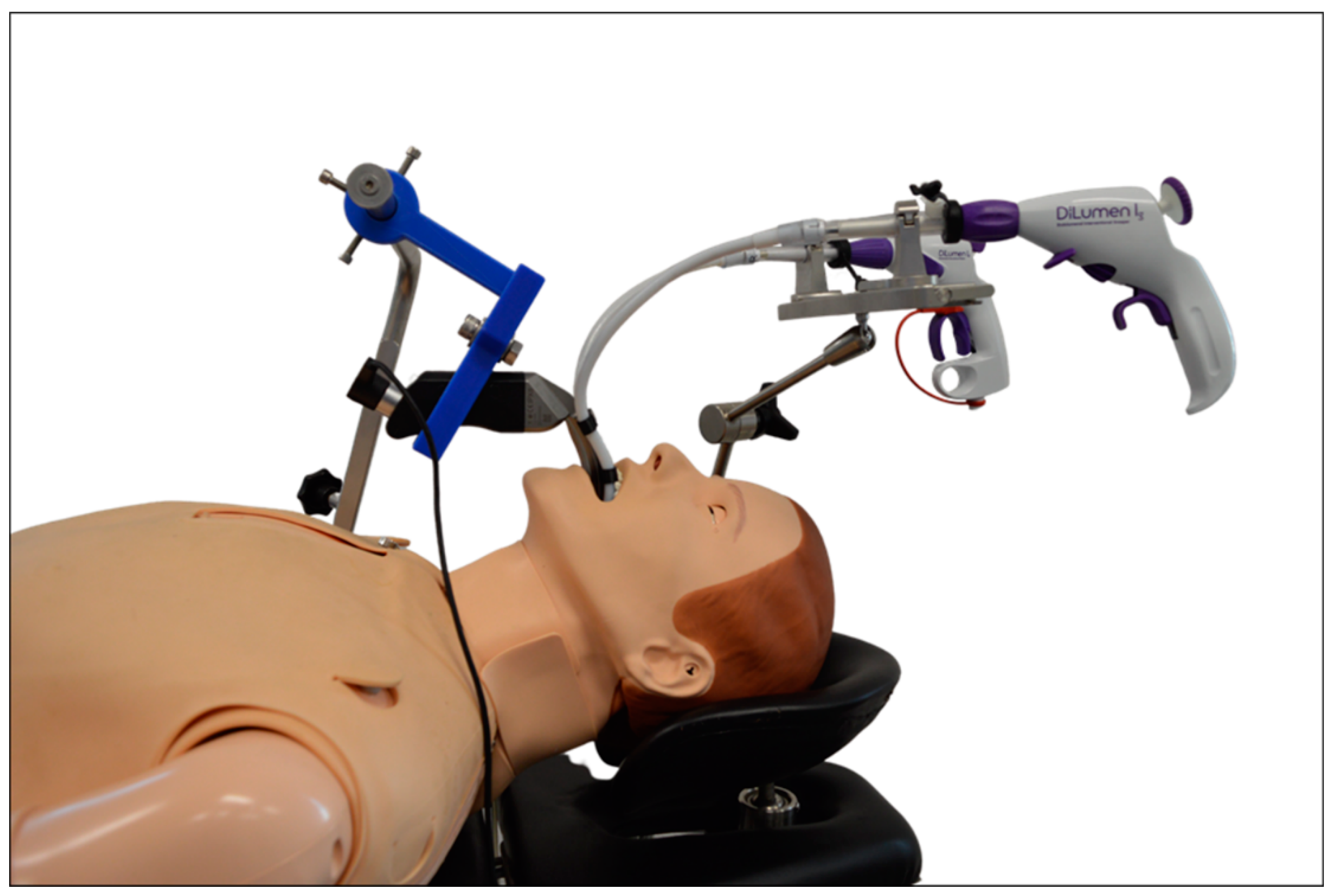

| DaVinci Xi® Multi-Port Robotic System | DaVinci Single-Port (SP)® Single-Port Robotic System | Flex® Robotic System Flexible Single-Port Robotic System | Versius®, Senhance® Surgical System Multi-Port Robotic Systems |
| Fields of application | |||
|
|
|
|
| Advantages | |||
|
|
|
|
| Disadvantages | |||
|
|
|
|
| RoboticScope® Robotic Exoscope | VITOM® 3D HD Manual or Robotic Exoscope | ORBEYE® Manual Exoscope with Multispectral Imaging | Cirq® Robot-Assisted Endoscope Guidance System |
| Fields of application | |||
|
|
|
|
| Advantages | |||
|
|
|
|
| Disadvantages | |||
|
|
|
|
| Title | Country/Time | Budget in Million USD |
|---|---|---|
| Development Plan of the Robot Industry | China 2016–2020 | 577 total |
| Key Special Program on Intelligent Robots | China 2019 | 577 total |
| New Robot Strategy | Japan 2016–2020 | 351 total (53.6 *) |
| Implementation Plan for Intelligent Robots | Korea 2018 | 150 total (0.84 *) |
| The 3rd Basic Plan on Intelligent Robots | Korea 2019–2023 | 126 for 2020 |
| Horizon 2020 ICT Robotics Work Program | EU 2014–2020 | 780 total (5 *) |
| National Robotics Initiative 2.0 | The United States since 2016 | 35 for 2019 |
| Title | Form of Research Promotion | Speaker and Web Page |
|---|---|---|
| SARAS project | EU-Promotion Horizon 202 (project 779813) | Riccardo Muradore, Verona, Italy www.saras-project.eu † |
| Robotics Technology Development and Deployment | National Institutes of Health * (Funding No. PAR-10-279) since 2011 | www.grants.nih.gov/grants/guide/pa-files/PAR-10-279.html † |
| Development of Single Port Surgical Robot for Flexible Joints for Light Oral or Laparoscopic Surgery | Ministry of Trade, Industry and Energy, Korea 2018 | www.motie.go.kr/www/main.do † |
| SMARTsurg | EU-Promotion Horizon 2020 (project 732515) since 2017 | Sanja Dogramadzi, Bristol, UK www.smartsurg-project.eu † |
| Soft tissue robotics | DFG post-graduate program (GRK 2198) since 2017 | Oliver Röhrle, Stuttgart www.str.uni-stuttgart.de † |
| Soft material robotics | DFG priority program (SPP 2100) since 2019 | Annika Raatz, Hannover www.spp2100.de † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boehm, F.; Graesslin, R.; Theodoraki, M.-N.; Schild, L.; Greve, J.; Hoffmann, T.K.; Schuler, P.J. Current Advances in Robotics for Head and Neck Surgery—A Systematic Review. Cancers 2021, 13, 1398. https://doi.org/10.3390/cancers13061398
Boehm F, Graesslin R, Theodoraki M-N, Schild L, Greve J, Hoffmann TK, Schuler PJ. Current Advances in Robotics for Head and Neck Surgery—A Systematic Review. Cancers. 2021; 13(6):1398. https://doi.org/10.3390/cancers13061398
Chicago/Turabian StyleBoehm, Felix, Rene Graesslin, Marie-Nicole Theodoraki, Leon Schild, Jens Greve, Thomas K. Hoffmann, and Patrick J. Schuler. 2021. "Current Advances in Robotics for Head and Neck Surgery—A Systematic Review" Cancers 13, no. 6: 1398. https://doi.org/10.3390/cancers13061398
APA StyleBoehm, F., Graesslin, R., Theodoraki, M.-N., Schild, L., Greve, J., Hoffmann, T. K., & Schuler, P. J. (2021). Current Advances in Robotics for Head and Neck Surgery—A Systematic Review. Cancers, 13(6), 1398. https://doi.org/10.3390/cancers13061398






