Phase Ib Trial of Copanlisib, A Phosphoinositide-3 Kinase (PI3K) Inhibitor, with Trastuzumab in Advanced Pre-Treated HER2-Positive Breast Cancer “PantHER”
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objectives
2.2. Eligibility
2.3. Study Design and Treatment
Dosing and Administration
2.4. Study Assessments
2.4.1. Safety
2.4.2. Efficacy
2.4.3. Tumour Tissue Mutational Status
2.4.4. Circulating Tumour PIK3CA Mutation Status
2.4.5. Pre and Post Treatment Biopsies
3. Results
3.1. Safety
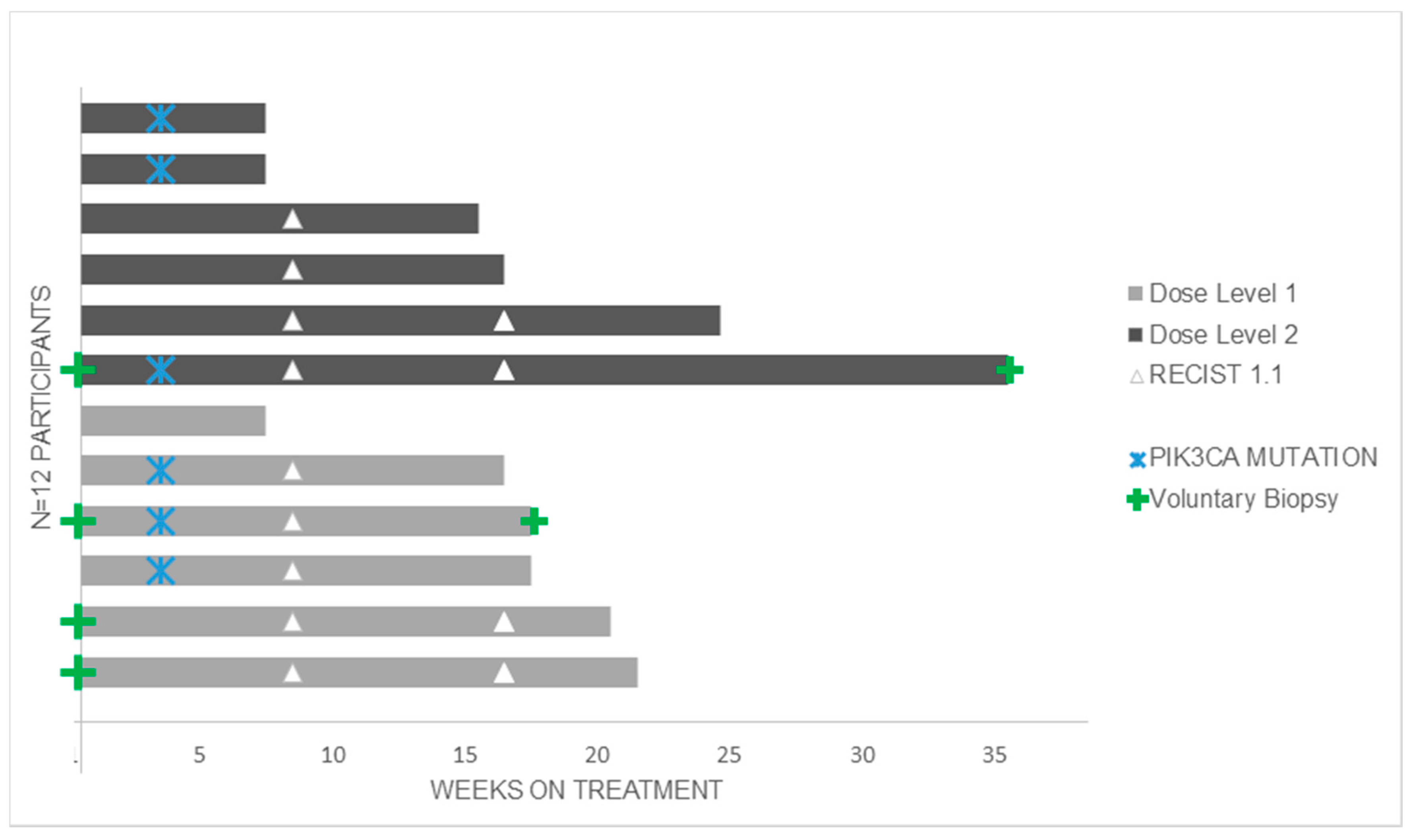
3.2. Anti-Tumour Activity
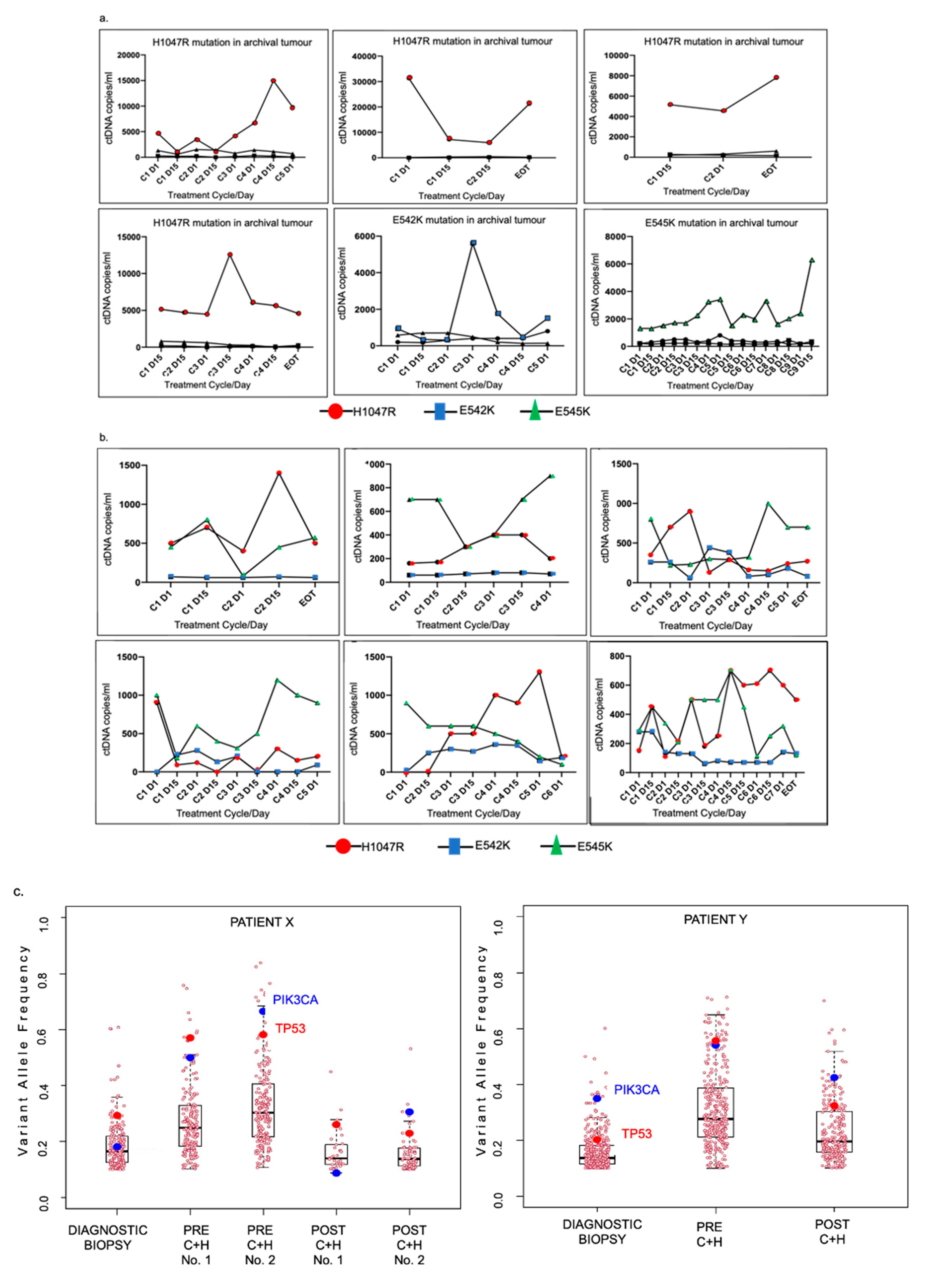
3.3. PIK3CA Mutational Status in Tumour and Circulating Tumour DNA (ctDNA)
3.4. Serial Tumour Biopsy Sequencing
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| ANC | Absolute Neutrophil Count |
| CISH | Chromogenic In Situ Hybridisation |
| CMV | Cytomegalovirus |
| CT | Computed Tomography |
| ctDNA | Circulating Tumour DNA |
| CYP3A4 | Cytochrome P450 3A4 |
| ddPCR | Droplet Digital PCR |
| DLT | Dose Limiting Toxicity |
| DNA | Deoxyribonucleic Acid |
| ECOG | Eastern Cooperative Oncology Group |
| FFPE | Formalin Fixed Paraffin Embedded |
| FISH | Fluorescence In Situ Hybridisation |
| GCP | Good Clinical Practice |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HIV | Human Immunodeficiency Virus |
| HPRA | Health Products Regulatory Authority of Ireland |
| IHC | Immunohistochemistry |
| IV | Intravenous |
| MTD | Maximum Tolerated Dose |
| NGS | Next Generation Sequencing |
| PI3K | Phosphoinositide-3 Kinase |
| RECIST | Response Evaluation Criteria in Solid Tumours |
| SAE | Serious Adverse Event |
| T-DM1 | Trastuzumab Emtansine |
| WES | Whole Exome Sequencing |
| WT | Wildtype |
| VAF | Variant Allele Frequency |
References
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002, 20, 719–726. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.G.; Morris, P.G. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anti Cancer Drugs 2012, 23, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Jungsil CLEOPATRA Study Group; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.-H.; Conte, P.; Martin, M.; Pienkowski, T.; Pivot, X.; Burris, H.A.; et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2–Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J. Clin. Oncol. 2017, 35, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Chandarlapaty, S.; Sakr, R.A.; Giri, D.; Patil, S.; Heguy, A.; Morrow, M.; Modi, S.; Norton, L.; Rosen, N.; Hudis, C.; et al. Frequent Mutational Activation of the PI3K-AKT Pathway in Trastuzumab-Resistant Breast Cancer. Clin. Cancer Res. 2012, 18, 6784–6791. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chinratanalab, W.; A Ritter, C.; King, W.; Seelig, S.; Arteaga, C.L. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002, 62, 4132–4141. [Google Scholar]
- Junttila, T.T.; Akita, R.W.; Parsons, K.; Fields, C.; Phillips, G.D.L.; Friedman, L.S.; Sampath, D.; Sliwkowski, M.X. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 2009, 15, 429–440. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.-L.; Davies, M.; Carey, M.; Yinghui, G.; Guan, Y.; Sahin, A.; et al. An Integrative Genomic and Proteomic Analysis of PIK3CA, PTEN, and AKT Mutations in Breast Cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef]
- Berns, K.; Horlings, H.M.; Hennessy, B.T.; Madiredjo, M.; Hijmans, E.M.; Beelen, K.; Linn, S.C.; Gonzalez-Angulo, A.M.; Stemke-Hale, K.; Hauptmann, M.; et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef]
- Baselga, J.; Im, S.A.S.; Iwata, H.; Cortés, J.; De Laurentiis, M.; Jiang, Z.; Arteaga, C.C.; Jonat, W.; Clemons, M.J.; Ito, Y.Y.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.A.; Kalev., D.; Egle, D.; et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 1, 87–100. [Google Scholar] [CrossRef]
- Liu, N.; Rowley, B.R.; Bull, C.O.; Schneider, C.; Haegebarth, A.; Schatz, C.A.; Fracasso, P.R.; Wilkie, D.P.; Hentemann, M.; Wilhelm, S.M.; et al. BAY 80-6946 Is a Highly Selective Intravenous PI3K Inhibitor with Potent p110α and p110δ Activities in Tumor Cell Lines and Xenograft Models. Mol. Cancer Ther. 2013, 12, 2319–2330. [Google Scholar] [CrossRef]
- Elster, N.; Cremona, M.; Morgan, C.; Toomey, S.; Carr, A.; O’Grady, A.; Hennessy, B.T.; Eustace, A.J. A preclinical evaluation of the PI3K alpha/delta dominant inhibitor BAY 80-6946 in HER2-positive breast cancer models with acquired resistance to the HER2-targeted therapies trastuzumab and lapatinib. Breast Cancer Res. Treat. 2015, 149, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Patnaik, A.; Appleman, L.J.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Weiss, G.J.; Sachdev, J.C.; Chadha, M.; Fulk, M.; et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann. Oncol. 2016, 27, 1928–1940. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Depristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; Del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Zhang, Q.; Larson, D.E.; Shen, D.; McLellan, M.D.; Lin, L.; Miller, C.A.; Mardis, E.R.; Ding, L.; Wilson, R.K. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012, 22, 568–576. [Google Scholar] [CrossRef]
- McLaren, W.; Pritchard, B.; Rios, D.; Chen, Y.; Flicek, P.; Cunningham, F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 2010, 26, 2069–2070. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Seshan, V.E. FACETS: Allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 2016, 44, e131. [Google Scholar] [CrossRef]
- McMahon, S.B.; A Van Buskirk, H.; A Dugan, K.; Copeland, T.D.; Cole, M.D. The Novel ATM-Related Protein TRRAP Is an Essential Cofactor for the c-Myc and E2F Oncoproteins. Cell 1998, 94, 363–374. [Google Scholar] [CrossRef]
- Dreyling, M.; Morschhauser, F.; Bouabdallah, K.; Bron, D.; Cunningham, D.; Assouline, S.E.; Verhoef, G.; Linton, K.; Thieblemont, C.; Vitolo, U. Faculty Opinions recommendation of Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2017, 28, 2169–2178. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Pauli, C.; Du, X.; Wang, D.G.; Li, X.; Wu, D.; Amadiume, S.C.; Goncalves, M.D.; Hodakoski, C.; Lundquist, M.R.; et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nat. Cell Biol. 2018, 560, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shan, M.; Liu, T.; Shi, Q.; Zhong, Z.; Wei, W.; Pang, D. Analysis of TRRAP as a Potential Molecular Marker and Therapeutic Target for Breast Cancer. J. Breast Cancer 2016, 19, 61–67. [Google Scholar] [CrossRef]
| Characteristic | Copanlisib + Trastuzumab |
|---|---|
| N = 12 | |
| Age at Therapy Start | 53 (42–72) |
| Histology | |
| Ductal | 11 (92%) |
| Lobular | 1 (8%) |
| Receptor Status | |
| HER2 Positive | 12 (100%) |
| Oestrogen Receptor Positive | 9 (75%) |
| Prior Neo–or Adjuvant Chemotherapy | 10 (83%) |
| Sites of Disease | |
| Liver Lung Bone Other | 3 (25%) 3 (25%) 6 (50%) 6 (50%) |
| Time from Metastatic Disease to Trial Registration | |
| Median (range) | 31 (10–140) |
| Prior Lines of Chemotherapy in the Metastatic Setting | |
| Median (range) Trastuzumab Pertuzumab TDM1 Lapatinib | 3 (1–9) 12 (100%) 10 (83%) 8 (67%) 4 (33%) |
| Adverse Event | Any Grade n = 12 | Grade 3 or Higher n = 12 | Any Grade Dose Level 1 n = 6 | Grade 3 or Higher Dose Level 1 n = 6 | Any Grade Dose Level 2 n = 6 | Grade 3 or Higher Dose Level 2 n = 6 |
|---|---|---|---|---|---|---|
| Any Adverse Event n (%) | 12 (100%) | 7 (58%) | 6 (100%) | 4 (67%) | 6 (100%) | 3 (50%) |
| Hyperglycaemia | 7 (58%) | 0 | 4 (67%) | 0 | 3 (50%) | 0 |
| Constipation | 7 (58%) | 0 | 3 (50%) | 0 | 4 (67%) | 0 |
| Fatigue | 7 (58%) | 1 (8%) | 4 (67%) | 1 (17%) | 3 (50%) | 0 |
| Hypertension | 6 (50%) | 4 (33%) | 5 (83%) | 3 (50%) | 1 (17%) | 1 (17%) |
| Nausea | 7 (58%) | 0 | 3 (50%) | 0 | 4 (67%) | 0 |
| Diarrhea | 6 (50%) | 0 | 3 (50%) | 0 | 3 (50%) | 0 |
| Rash | 6 (50%) | 0 | 3 (50%) | 0 | 3 (50%) | 0 |
| Vomiting | 7 (58%) | 0 | 4 (67%) | 0 | 3 (50%) | 0 |
| Cough | 4 (33%) | 0 | 3 (50%) | 0 | 1 (17%) | 0 |
| Mucositis | 5 (42%) | 0 | 4 (67%) | 0 | 1 (17%) | 0 |
| Decreased appetite | 4 (33%) | 0 | 3 (50%) | 0 | 1 (17%) | 0 |
| Dry skin | 4 (33%) | 0 | 3 (50%) | 0 | 1 (17%) | 0 |
| Fever | 4 (33%) | 0 | 3 (50%) | 0 | 1 (17%) | 0 |
| Headache | 4 (33%) | 0 | 1 (17%) | 0 | 3 (50%) | 0 |
| Paresthesia | 3 (25%) | 0 | 1 (17%) | 0 | 2 (33%) | 0 |
| Weight decreased | 3 (25%) | 0 | 3 (50%) | 0 | 0 | 0 |
| Anemia | 2 (17%) | 0 | 1 (17%) | 0 | 1 (17%) | 0 |
| Dehydration | 2 (17%) | 0 | 2 (33%) | 0 | 0 | 0 |
| Dyspnea | 2 (17%) | 1 (8%) | 1 (17%) | 0 | 1 (17%) | 1 (17%) |
| Oedema peripheral | 1 (8%) | 0 | 1 (17%) | 0 | 0 | 0 |
| Insomnia | 2 (17%) | 0 | 1 (17%) | 0 | 1 (17%) | 0 |
| Paresthesia Oral | 2 (17%) | 0 | 0 | 0 | 2 (33%) | 0 |
| Peripheral neuropathy | 2 (17%) | 0 | 1 (17%) | 0 | 1 (17%) | 0 |
| Blood Bilirubin increased | 1 (8%) | 1 (8%) | 0 | 0 | 1 (17%) | 1 (17%) |
| Gamma GT increased | 1 (8%) | 1 (8%) | 0 | 0 | 1 (17%) | 1 (17%) |
| Bile duct obstruction | 1 (8%) | 1 (8%) | 0 | 0 | 1 (17%) | 1 (17%) |
| Lymphangitis carcinomatosis | 1 (8%) | 1 (8%) | 0 | 0 | 1 (17%) | 1 (17%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keegan, N.M.; Furney, S.J.; Walshe, J.M.; Gullo, G.; Kennedy, M.J.; Smith, D.; McCaffrey, J.; Kelly, C.M.; Egan, K.; Kerr, J.; et al. Phase Ib Trial of Copanlisib, A Phosphoinositide-3 Kinase (PI3K) Inhibitor, with Trastuzumab in Advanced Pre-Treated HER2-Positive Breast Cancer “PantHER”. Cancers 2021, 13, 1225. https://doi.org/10.3390/cancers13061225
Keegan NM, Furney SJ, Walshe JM, Gullo G, Kennedy MJ, Smith D, McCaffrey J, Kelly CM, Egan K, Kerr J, et al. Phase Ib Trial of Copanlisib, A Phosphoinositide-3 Kinase (PI3K) Inhibitor, with Trastuzumab in Advanced Pre-Treated HER2-Positive Breast Cancer “PantHER”. Cancers. 2021; 13(6):1225. https://doi.org/10.3390/cancers13061225
Chicago/Turabian StyleKeegan, Niamh M., Simon J. Furney, Janice M. Walshe, Giuseppe Gullo, M. John Kennedy, Diarmuid Smith, John McCaffrey, Catherine M. Kelly, Keith Egan, Jennifer Kerr, and et al. 2021. "Phase Ib Trial of Copanlisib, A Phosphoinositide-3 Kinase (PI3K) Inhibitor, with Trastuzumab in Advanced Pre-Treated HER2-Positive Breast Cancer “PantHER”" Cancers 13, no. 6: 1225. https://doi.org/10.3390/cancers13061225
APA StyleKeegan, N. M., Furney, S. J., Walshe, J. M., Gullo, G., Kennedy, M. J., Smith, D., McCaffrey, J., Kelly, C. M., Egan, K., Kerr, J., Given, M., O’Donovan, P., Hernando, A., Teiserskiene, A., Parker, I., Kay, E., Farrelly, A., Carr, A., Calzaferri, G., ... Hennessy, B. T. (2021). Phase Ib Trial of Copanlisib, A Phosphoinositide-3 Kinase (PI3K) Inhibitor, with Trastuzumab in Advanced Pre-Treated HER2-Positive Breast Cancer “PantHER”. Cancers, 13(6), 1225. https://doi.org/10.3390/cancers13061225







