Image-Guided Brachytherapy for Salvage Reirradiation: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
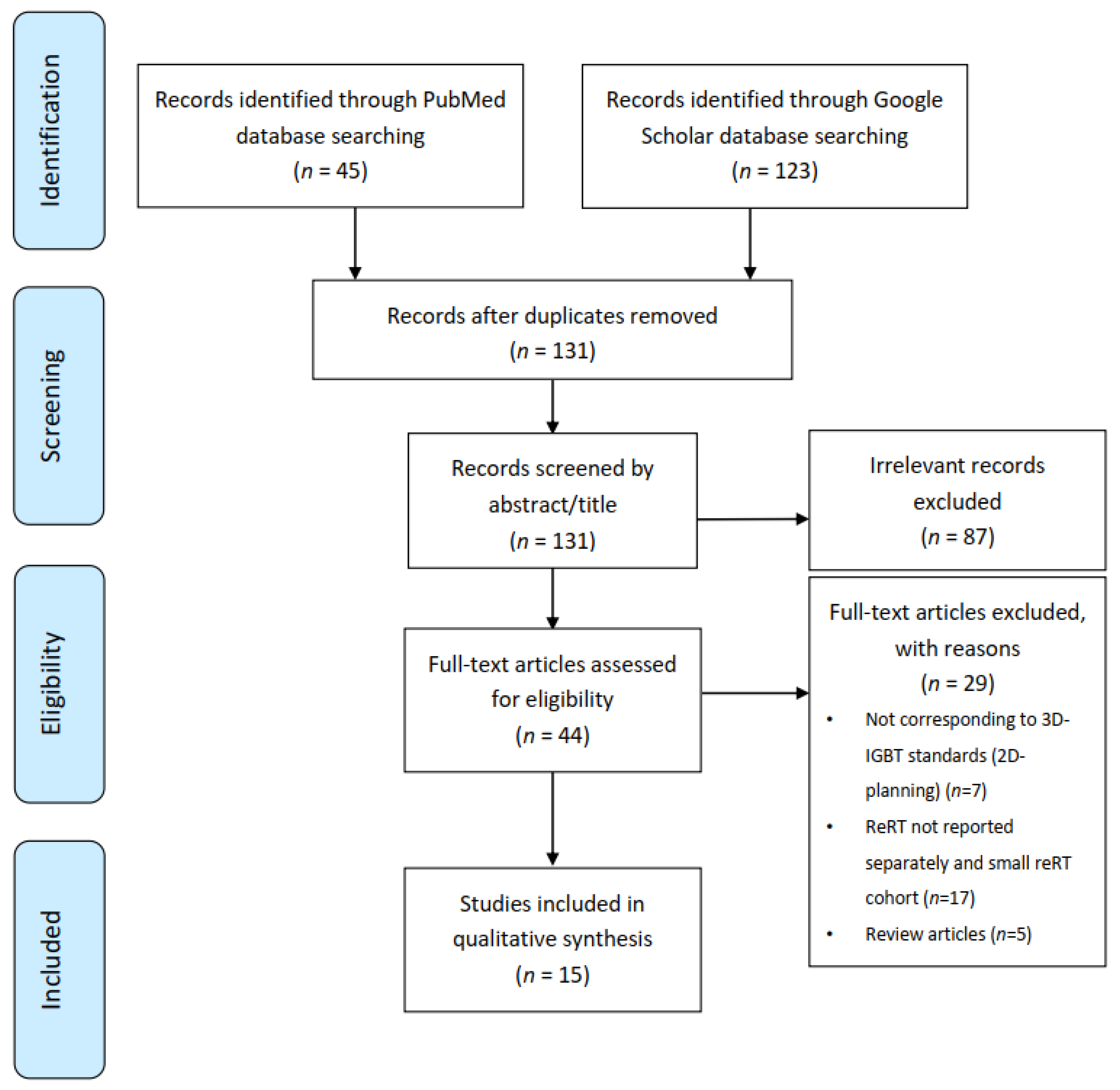
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
3. Results
3.1. General Aspects
3.2. Patient’s Selection and Population
3.2.1. Initial Work-Up
3.2.2. Type of Recurrence
3.2.3. Type of Primary Disease
3.2.4. Previous RT Treatment
3.2.5. Treatment-Free Interval
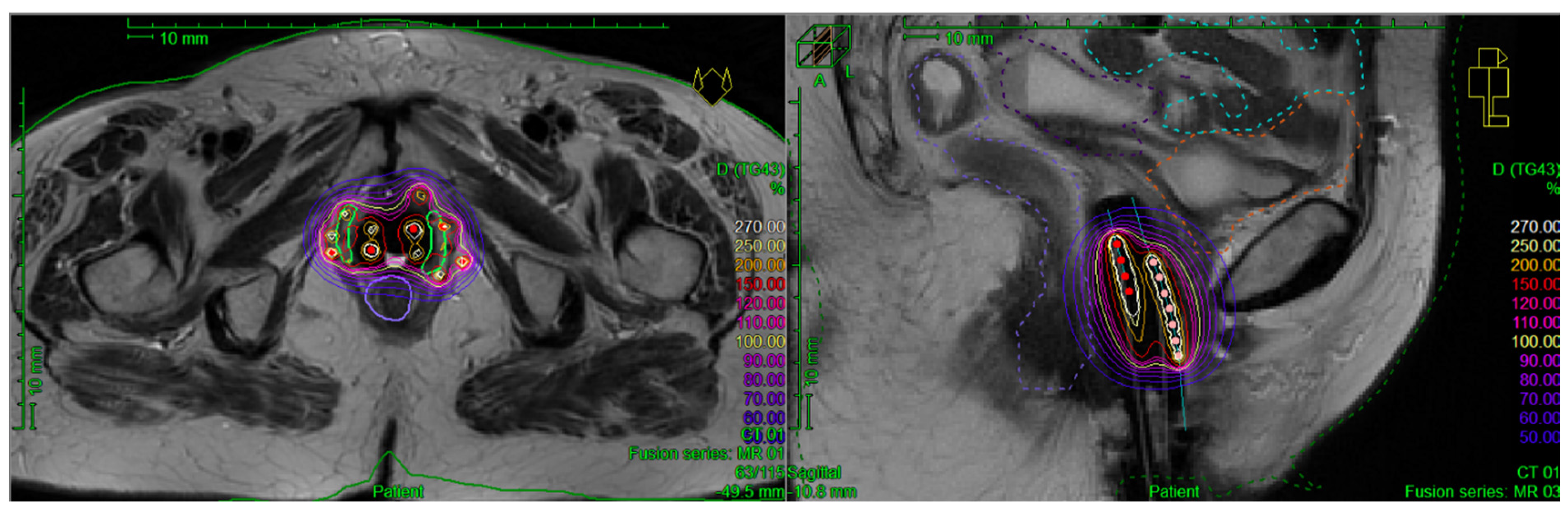
3.3. BT Specification
3.3.1. Implantation Procedure
3.3.2. BT Technique
3.3.3. Treatment Planning Modalities
3.3.4. Target Delineation
3.3.5. Dose Constraints
3.4. EBRT Doses and Techniques if Allowed before BT
3.5. Dosimetry
3.5.1. Target
3.5.2. OARs
3.6. Outcomes
3.7. Toxicity
3.8. Prognostic Factors
4. Discussion and Highlights
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Perez, C.A.; Grigsby, P.W.; Camel, H.M.; Galakatos, A.E.; Mutch, D.; Lockett, M.A. Irradiation Alone or Combined with Surgery in Stage IB, IIA, and IIB Carcinoma of Uterine Cervix: Update of a Nonrandomized Comparison. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 703–716. [Google Scholar] [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image Guided Brachytherapy in Locally Advanced Cervical Cancer: Improved Pelvic Control and Survival in RetroEMBRACE, a Multicenter Cohort Study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Tanderup, K.; Fokdal, L.U.; Sturdza, A.; Haie-Meder, C.; Mazeron, R.; van Limbergen, E.; Jürgenliemk-Schulz, I.; Petric, P.; Hoskin, P.; Dörr, W.; et al. Effect of Tumor Dose, Volume and Overall Treatment Time on Local Control after Radiochemotherapy Including MRI Guided Brachytherapy of Locally Advanced Cervical Cancer. Radiother. Oncol. 2016, 120, 441–446. [Google Scholar] [CrossRef]
- Schmid, M.P.; Kirisits, C.; Nesvacil, N.; Dimopoulos, J.C.A.; Berger, D.; Pötter, R. Local Recurrences in Cervical Cancer Patients in the Setting of Image-Guided Brachytherapy: A Comparison of Spatial Dose Distribution within a Matched-Pair Analysis. Radiother. Oncol. 2011, 100, 468–472. [Google Scholar] [CrossRef]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; et al. Ten-Year Results of the PORTEC-2 Trial for High-Intermediate Risk Endometrial Carcinoma: Improving Patient Selection for Adjuvant Therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef]
- Frank, S.J.; Jhingran, A.; Levenback, C.; Eifel, P.J. Definitive Radiation Therapy for Squamous Cell Carcinoma of the Vagina. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Nooij, L.S.; Brand, F.A.M.; Gaarenstroom, K.N.; Creutzberg, C.L.; de Hullu, J.A.; van Poelgeest, M.I.E. Risk Factors and Treatment for Recurrent Vulvar Squamous Cell Carcinoma. Crit. Rev. Oncol. Hematol. 2016, 106, 1–13. [Google Scholar] [CrossRef]
- Marnitz, S.; Köhler, C.; Müller, M.; Behrens, K.; Hasenbein, K.; Schneider, A. Indications for Primary and Secondary Exenterations in Patients with Cervical Cancer. Gynecol. Oncol. 2006, 103, 1023–1030. [Google Scholar] [CrossRef]
- Maggioni, A.; Roviglione, G.; Landoni, F.; Zanagnolo, V.; Peiretti, M.; Colombo, N.; Bocciolone, L.; Biffi, R.; Minig, L.; Morrow, C.P. Pelvic Exenteration: Ten-Year Experience at the European Institute of Oncology in Milan. Gynecol. Oncol. 2009, 114, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Ungar, L.; Palfalvi, L.; Novak, Z. Primary Pelvic Exenteration in Cervical Cancer Patients. Gynecol. Oncol. 2008, 111, S9–S12. [Google Scholar] [CrossRef] [PubMed]
- Mignot, F.; Gouy, S.; Schernberg, A.; Bockel, S.; Espenel, S.; Maulard, A.; Leary, A.; Genestie, C.; Annede, P.; Kissel, M.; et al. Comprehensive Analysis of Patient Outcome after Local Recurrence of Locally Advanced Cervical Cancer Treated with Concomitant Chemoradiation and Image-Guided Adaptive Brachytherapy. Gynecol. Oncol. 2020, 157, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Alcázar, J.L.; Martinez-Monge, R. Resectability Rates of Previously Irradiated Recurrent Cervical Cancer (PIRCC) Treated with Pelvic Exenteration: Is Still the Clinical Involvement of the Pelvis Wall a Real Contraindication? A Twenty-Year Experience. Gynecol. Oncol. 2010, 116, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Filleron, T.; Rouanet, P.; Méeus, P.; Lambaudie, E.; Classe, J.M.; Foucher, F.; Narducci, F.; Gouy, S.; Guyon, F.; et al. Prospective Assessment of First-Year Quality of Life After Pelvic Exenteration for Gynecologic Malignancy: A French Multicentric Study. Ann. Surg. Oncol. 2018, 25, 535–541. [Google Scholar] [CrossRef]
- Kamura, T.; Ushijima, K. Chemotherapy for Advanced or Recurrent Cervical Cancer. Taiwan. J. Obstet. Gynecol. 2013, 52, 161–164. [Google Scholar] [CrossRef]
- Kitagawa, R.; Katsumata, N.; Shibata, T.; Kamura, T.; Kasamatsu, T.; Nakanishi, T.; Nishimura, S.; Ushijima, K.; Takano, M.; Satoh, T.; et al. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J. Clin. Oncol. 2015, 33, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Uzan, C.; Goere, D.; Dumont, F.; Gouy, S.; Muret, J.; Hakime, A.; De Baere, T.; Bonvalot, S. Isolated Pelvic Perfusion in Irradiated Unresectable Recurrence of Pelvic Tumor: Preliminary Outcome and Ongoing Study. J. Visc. Surg. 2014, 151 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef]
- Sadozye, A.H. Re-Irradiation in Gynaecological Malignancies: A Review. Clin. Oncol. (R. Coll. Radiol.) 2018, 30, 110–115. [Google Scholar] [CrossRef]
- Yanez, L.; Ciudad, A.M.; Mehta, M.P.; Marsiglia, H. What Is the Evidence for the Clinical Value of SBRT in Cancer of the Cervix? Rep. Pr. Oncol. Radiother. 2018, 23, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.; Taylor, A. Re-Irradiation of Cervical and Endometrial Cancer. Curr. Opin. Oncol. 2017, 29, 343–350. [Google Scholar] [CrossRef]
- Wiebe, E.M.; Hajdok, G.; Hoover, D.; D’Souza, D.; Surry, K. Dosimetric Feasibility Of VMAT And SBRT versus Interstitial Brachytherapy Boost For Recurrent Endometrial Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, S476–S477. [Google Scholar] [CrossRef]
- Cengiz, M.; Dogan, A.; Ozyigit, G.; Erturk, E.; Yildiz, F.; Selek, U.; Ulger, S.; Colak, F.; Zorlu, F. Comparison of Intracavitary Brachytherapy and Stereotactic Body Radiotherapy Dose Distribution for Cervical Cancer. Brachytherapy 2012, 11, 125–129. [Google Scholar] [CrossRef]
- Chargari, C.; Renard, S.; Espenel, S.; Escande, A.; Buchheit, I.; Ducassou, A.; Peiffert, D.; Hannoun-Lévi, J.-M. Can stereotactic body radiotherapy replace brachytherapy for locally advanced cervical cancer? French society for radiation oncology statement. Cancer Radiother. 2020. [Google Scholar] [CrossRef]
- Gill, B.S.; Lin, J.F.; Krivak, T.C.; Sukumvanich, P.; Laskey, R.A.; Ross, M.S.; Lesnock, J.L.; Beriwal, S. National Cancer Data Base Analysis of Radiation Therapy Consolidation Modality for Cervical Cancer: The Impact of New Technological Advancements. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1083–1090. [Google Scholar] [CrossRef]
- Haie-Meder, C.; Pötter, R.; Limbergen, E.V.; Briot, E.; Brabandere, M.D.; Dimopoulos, J.; Dumas, I.; Hellebust, T.P.; Kirisits, C.; Lang, S.; et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group✩ (I): Concepts and Terms in 3D Image Based 3D Treatment Planning in Cervix Cancer Brachytherapy with Emphasis on MRI Assessment of GTV and CTV. Radiother. Oncol. 2005, 74, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.A.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical Outcome of Protocol Based Image (MRI) Guided Adaptive Brachytherapy Combined with 3D Conformal Radiotherapy with or without Chemotherapy in Patients with Locally Advanced Cervical Cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. Lancet Infect. Dis. 2010, 10, 226. [Google Scholar] [CrossRef]
- Sturdza, A.; Viswanathan, A.N.; Erickson, B.; Yashar, C.; Bruggeman, A.; Feddock, J.; Klopp, A.; Beriwal, S.; Gaffney, D.; Han, K.; et al. American Brachytherapy Society Working Group Report on the Patterns of Care and a Literature Review of Reirradiation for Gynecologic Cancers. Brachytherapy 2020, 19, 127–138. [Google Scholar] [CrossRef]
- Kamrava, M.; Beriwal, S.; Erickson, B.; Gaffney, D.; Jhingran, A.; Klopp, A.; Park, S.J.; Viswanathan, A.; Yashar, C.; Lin, L. American Brachytherapy Society Recurrent Carcinoma of the Endometrium Task Force Patterns of Care and Review of the Literature. Brachytherapy 2017, 16, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Goyal, S.; Kataria, T.; Gupta, D.; Patro, K.C.; Malik, A.; Bisht, S.S.; Abhishek, A. Re-Irradiation in Gynecological Cancers, Present Experiences and Future Hopes. J. Radiat. Oncol. 2018, 7, 205–211. [Google Scholar] [CrossRef]
- Martínez-Monge, R.; Cambeiro, M.; Rodríguez-Ruiz, M.E.; Olarte, A.; Ramos, L.I.; Villafranca, E.; Bascón, N.; Jurado, M. Phase II Trial of Image-Based High-Dose-Rate Interstitial Brachytherapy for Previously Irradiated Gynecologic Cancer. Brachytherapy 2014, 13, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Raziee, H.; D’Souza, D.; Velker, V.; Barnes, E.; Taggar, A.; Mendez, L.; Leung, E. Salvage Re-Irradiation With Single-Modality Interstitial Brachytherapy for the Treatment of Recurrent Gynaecological Tumours in the Pelvis: A Multi-Institutional Study. Clin. Oncol. 2020, 32, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, S.; Takahashi, R.; Isohashi, F.; Yokoi, T.; Okazawa, M.; Sasano, T.; Maruoka, S.; Anzai, M.; Yoshioka, Y.; Ogawa, K.; et al. Reirradiation Using High-Dose-Rate Interstitial Brachytherapy for Locally Recurrent Cervical Cancer: A Single Institutional Experience. Int. J. Gynecol. Cancer 2014, 24, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Amsbaugh, M.J.; Bhatt, N.; Hunter, T.; Gaskins, J.; Parker, L.; Metzinger, D.; Amsbaugh, A.; Sowards, K.; El-Ghamry, M. Computed Tomography Planned Interstitial Brachytherapy for Recurrent Gynecologic Cancer. Brachytherapy 2015, 14, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; D’Souza, D.; Patil, N.; Velker, V.; Leung, E.; Stitt, L.; Whiston, F.; Sugimoto, A.; McGee, J.; Prefontaine, M. High-Dose-Rate Interstitial Brachytherapy for the Treatment of High-Volume Locally Recurrent Endometrial Carcinoma. Brachytherapy 2016, 15, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-S.; Guo, J.; Zhao, Y.-Z.; Lin, X.; Chen, B.; Zhang, M.; Li, J.-M.; Ren, X.-J.; Zhang, B.-Y.; Wang, T.-J. Salvage Interstitial Brachytherapy Based on Computed Tomography for Recurrent Cervical Cancer after Radical Hysterectomy and Adjuvant Radiation Therapy: Case Presentations and Introduction of the Technique. J. Contemp. Brachytherapy 2016, 8, 415–421. [Google Scholar] [CrossRef]
- Murakami, N.; Kasamatsu, T.; Sumi, M.; Yoshimura, R.; Harada, K.; Kitaguchi, M.; Sekii, S.; Takahashi, K.; Yoshio, K.; Inaba, K.; et al. Vaginal Tolerance of CT Based Image-Guided High-Dose Rate Interstitial Brachytherapy for Gynecological Malignancies. Radiat. Oncol. 2014, 9, 31. [Google Scholar] [CrossRef][Green Version]
- Aridgides, P.; Onderdonk, B.; Cunningham, M.; Daugherty, E.; Du, L.; Bunn, W.D.; Agarwal, R.; Hahn, S.S. Institutional Experience Using Interstitial Brachytherapy for the Treatment of Primary and Recurrent Pelvic Malignancies. J. Contemp. Brachytherapy 2016, 8, 173–180. [Google Scholar] [CrossRef]
- Kamran, S.C.; Manuel, M.M.; Catalano, P.; Cho, L.; Damato, A.L.; Lee, L.J.; Schmidt, E.J.; Viswanathan, A.N. MR- versus CT-Based High-Dose-Rate Interstitial Brachytherapy for Vaginal Recurrence of Endometrial Cancer. Brachytherapy 2017, 16, 1159–1168. [Google Scholar] [CrossRef]
- Mahantshetty, U.; Kalyani, N.; Engineer, R.; Chopra, S.; Jamema, S.; Ghadi, Y.; Deshpande, D.; Shrivastava, S. Reirradiation Using High-Dose-Rate Brachytherapy in Recurrent Carcinoma of Uterine Cervix. Brachytherapy 2014, 13, 548–553. [Google Scholar] [CrossRef]
- Umezawa, R.; Murakami, N.; Nakamura, S.; Wakita, A.; Okamoto, H.; Tsuchida, K.; Kashihara, T.; Kobayashi, K.; Harada, K.; Takahashi, K.; et al. Image-Guided Interstitial High-Dose-Rate Brachytherapy for Locally Recurrent Uterine Cervical Cancer: A Single-Institution Study. Brachytherapy 2018, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamazaki, H.; Kotsuma, T.; Takenaka, T.; Masui, K.; Yoshioka, Y.; Uesugi, Y.; Shimbo, T.; Yoshikawa, N.; Yoshioka, H.; et al. Treatment Results of Image-Guided High-Dose-Rate Interstitial Brachytherapy for Pelvic Recurrence of Uterine Cancer. Brachytherapy 2015, 14, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Zolciak-Siwinska, A.; Bijok, M.; Jonska-Gmyrek, J.; Kawczynska, M.; Kepka, L.; Bujko, K.; Michalski, W. HDR Brachytherapy for the Reirradiation of Cervical and Vaginal Cancer: Analysis of Efficacy and Dosage Delivered to Organs at Risk. Gynecol. Oncol. 2014, 132, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.C.; Vargo, J.A.; Glaser, S.M.; Kim, H.; Beriwal, S. Outcomes after Definitive Re-Irradiation with 3D Brachytherapy with or without External Beam Radiation Therapy for Vaginal Recurrence of Endometrial Cancer. Gynecol. Oncol. 2019, 152, 581–586. [Google Scholar] [CrossRef]
- Feddock, J.; Cheek, D.; Steber, C.; Edwards, J.; Slone, S.; Luo, W.; Randall, M. Reirradiation Using Permanent Interstitial Brachytherapy: A Potentially Durable Technique for Salvaging Recurrent Pelvic Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1225–1233. [Google Scholar] [CrossRef]
- Pötter, R.; Haie-Meder, C.; Limbergen, E.V.; Barillot, I.; Brabandere, M.D.; Dimopoulos, J.; Dumas, I.; Erickson, B.; Lang, S.; Nulens, A.; et al. Recommendations from Gynaecological (GYN) GEC ESTRO Working Group (II): Concepts and Terms in 3D Image-Based Treatment Planning in Cervix Cancer Brachytherapy—3D Dose Volume Parameters and Aspects of 3D Image-Based Anatomy, Radiation Physics, Radiobiology. Radiother. Oncol. 2006, 78, 67–77. [Google Scholar] [CrossRef]
- Westin, S.N.; Rallapalli, V.; Fellman, B.; Urbauer, D.L.; Pal, N.; Frumovitz, M.M.; Ramondetta, L.M.; Bodurka, D.C.; Ramirez, P.T.; Soliman, P.T. Overall Survival after Pelvic Exenteration for Gynecologic Malignancy. Gynecol. Oncol. 2014, 134, 546–551. [Google Scholar] [CrossRef]
- Lawhead, R.A.; Clark, D.G.; Smith, D.H.; Pierce, V.K.; Lewis, J.L. Pelvic Exenteration for Recurrent or Persistent Gynecologic Malignancies: A 10-Year Review of the Memorial Sloan-Kettering Cancer Center Experience (1972–1981). Gynecol. Oncol. 1989, 33, 279–282. [Google Scholar] [CrossRef]
- Coleman, R.L.; Keeney, E.D.; Freedman, R.S.; Burke, T.W.; Eifel, P.J.; Rutledge, F.N. Radical Hysterectomy for Recurrent Carcinoma of the Uterine Cervix after Radiotherapy. Gynecol. Oncol. 1994, 55, 29–35. [Google Scholar] [CrossRef]
- Rubin, S.C.; Hoskins, W.J.; Lewis, J.L. Radical Hysterectomy for Recurrent Cervical Cancer Following Radiation Therapy. Gynecol. Oncol. 1987, 27, 316–324. [Google Scholar] [CrossRef]
- Yoo, H.J.; Lim, M.C.; Seo, S.-S.; Kang, S.; Yoo, C.W.; Kim, J.-Y.; Park, S.-Y. Pelvic Exenteration for Recurrent Cervical Cancer: Ten-Year Experience at National Cancer Center in Korea. J. Gynecol. Oncol. 2012, 23, 242–250. [Google Scholar] [CrossRef]
- Skliarenko, J.; Barnes, E.A. Palliative Pelvic Radiotherapy for Gynaecologic Cancer. J. Radiat. Oncol. 2012, 1, 239–244. [Google Scholar] [CrossRef]
- Fuccio, L.; Guido, A.; Andreyev, H.J.N. Management of Intestinal Complications in Patients with Pelvic Radiation Disease. Clin. Gastroenterol. Hepatol. 2012, 10, 1326–1334.e4. [Google Scholar] [CrossRef]
- Weitmann, H.D.; Knocke, T.H.; Waldhäusl, C.; Pötter, R. Ultrasound-Guided Interstitial Brachytherapy in the Treatment of Advanced Vaginal Recurrences from Cervical and Endometrial Carcinoma. Strahlenther. Onkol. 2006, 182, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Badakh, D.K.; Grover, A.H. Reirradiation with High-Dose-Rate Remote Afterloading Brachytherapy Implant in Patients with Locally Recurrent or Residual Cervical Carcinoma. J. Cancer Res. 2009, 5, 24–30. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II Study: The Outcome and Prospect of Two Decades of Evolution within the GEC-ESTRO GYN Working Group and the EMBRACE Studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.P.; Fokdal, L.; Westerveld, H.; Chargari, C.; Rohl, L.; Morice, P.; Nesvacil, N.; Mazeron, R.; Haie-Meder, C.; Pötter, R.; et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group—ACROP: Target Concept for Image Guided Adaptive Brachytherapy in Primary Vaginal Cancer. Radiother. Oncol. 2020, 145, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Thomadsen, B.R.; Shahabi, S.; Stitt, J.A.; Buchler, D.A.; Fowler, J.F.; Paliwal, B.R.; Kinsella, T.J. High Dose Rate Intracavitary Brachytherapy for Carcinoma of the Cervix: The Madison System: II. Procedural and Physical Considerations. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 349–357. [Google Scholar] [CrossRef]
- Eifel, P.J. High-Dose-Rate Brachytherapy for Carcinoma of the Cervix: High Tech or High Risk? Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 383–386; discussion 383–388. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J.; Huang, Y.; Sachs, R.K. Potential Reduced Late Effects for Pulsed Brachytherapy Compared with Conventional LDR. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 201–202. [Google Scholar] [CrossRef]
- Skowronek, J. Pulsed Dose Rate Brachytherapy—Is It the Right Way? J. Contemp. Brachytherapy 2010, 2, 107–113. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Erickson, B.; Gaffney, D.K.; Beriwal, S.; Bhatia, S.K.; Lee Burnett, O.; D’Souza, D.P.; Patil, N.; Haddock, M.G.; Jhingran, A.; et al. Comparison and Consensus Guidelines for Delineation of Clinical Target Volume for CT- and MR-Based Brachytherapy in Locally Advanced Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 320–328. [Google Scholar] [CrossRef]
- Eskander, R.N.; Scanderbeg, D.; Saenz, C.C.; Brown, M.; Yashar, C. Comparison of Computed Tomography and Magnetic Resonance Imaging in Cervical Cancer Brachytherapy Target and Normal Tissue Contouring. Int. J. Gynecol. Cancer 2010, 20, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Harkenrider, M.M.; Alite, F.; Silva, S.R.; Small, W. Image-Based Brachytherapy for the Treatment of Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Damato, A.L.; Nguyen, P.L. Novel Use of a Hydrogel Spacer Permits Reirradiation in Otherwise Incurable Recurrent Gynecologic Cancers. J. Clin. Oncol. 2013, 31, e446–e447. [Google Scholar] [CrossRef]
- Lux, F.; Detappe, A.; Dufort, S.; Sancey, L.; Louis, C.; Carme, S.; Tillement, O. Ultrasmall nanoparticles for radiotherapy: AGuIX. Cancer Radiother. 2015, 19, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Sancey, L.; Dufort, S.; Le Duc, G.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.L.; Villa, J.; et al. Treatment of Multiple Brain Metastases Using Gadolinium Nanoparticles and Radiotherapy: NANO-RAD, a Phase I Study Protocol. BMJ Open 2019, 9, e023591. [Google Scholar] [CrossRef]

| Study | Type of Study | No. of Reirradiated Pts/No. of Pts Included | Primary Disease | Treatment Free Interval (Range) | Median Follow-Up | Outcome | Toxicity |
|---|---|---|---|---|---|---|---|
| Raziee, 2020 [32] | R | 26/26 (100%) | Endometrium (76.9%) Cervix (15.3%) Vagina (3.9%) Vulva (3.9%) | 20.3 months (9.9–30.5) | 24 months (12.7–30.8) | 2-year LC 50% 2-year PFS 38% 2-year OS 78% CR: 65% | No gr 4–5 Gr 3: 7.7%, Gr 1–2: 38.5% |
| Ling, 2019 [44] | R | 22/22 (100%) | Endometrium | 26.6 months (17.2–54.4) | 27.6 months | 3-year LC 65.8% 3-year DFS 40.8% 3-year OS 68.1% | No gr 4–5 Gr 3: 4.5% (ureteral stricture) |
| Umezawa, 2018 [41] | R | 18/18 (100%) | Cervix | 14.9 months (3.1–53.6) | 18.1 months (8.4–75.4) | 2-year LC 51% 2-year PFS 20% 2-year OS 61% CR: 66.6% PR: 33.3% | No Gr 5 Gr ≥ 3: 17% Gr 4 vagina fistula: 11.1% Gr 3 vagina fistula: 5.5% |
| Martinez- Monge, 2014 [31] | Phase II | 15/15 (100%) | Endometrium (40%) Cervix (40%) Vagina (20%) | NR | 2.9 years (1.2–9.2) | 2-year DFS without gr ≥ 3 toxicity 40% 2-year LC 71.4%, 5y LC 71.4% 2-year PFS 42.9%, 5y PFS 21.4% 2-year OS 59.3%, 5y 0S 39.5% | Gr 5: 6.6% (n = 1, bowel obstruction) Gr ≥ 3: 20% |
| Feddock, 2017 [45] | R | 42/42 (100%) | Cervix (28.6%) Endometrium (26.2%) Vagina (21.4%) Vulva (11.9%) Fallopian tube, rectal, anal (11.9%) | 26.1 months (1.4–8.1 years) | 16.3 months | 73% LC at death 52% OS at last follow-up | No Grade 5 Gr ≥ 3: 16.7% (vaginal necrosis: 9.5%, vaginal fistula: 7.1%) |
| Liu, 2016 [36] | R | 16/16 (100%) | Cervix | NR | NR | At 3 months: CR: 37.5%, PR: 43.8%, SD: 18.7%, No PD | NR |
| Mahantshetty, 2014 [40] | R | 30/30 (100%) | Cervix | 25 months (7–227) | 25 months (3–96) | 2-year LC 44% 2-year DFS 42% 2-year OS 52% CR: 76%, PR: 18%, SD: 3%, PD: 3% | No gr 4–5 Gr 3 rectal & urinary: 10% Gr 3 vagina: 10% Gr 2 small bowel: 10% |
| Zolciak-Siwinska, 2014 [43] | R | 20/20 (100%) | Cervix (70%) Vagina (30%) | 23 months (3–76) | 31 months | 3-year LC 45% 3-year DFS 42% 3-year OS 68% CR: 95%, LR: 45%, DR: 45% | No gr 5 Gr 3: urinary10%, Gi 5% Gr 3–4 vagina: 40% (obliteration of vagina) |
| Mabuchi, 2014 [33] | R | 52/52 (100%) | Cervix | 13 months | 55.6 months | 5-year OS 52.6% CR: 59.6%, PR: 17.3%, SD: 15.4% PD: 53.8%, LR: 35.7%, RR: 17.9% (pelvic sidewall) DR: 39.3%, DR + LR: 7.1% | No gr 5 Gr ≥ 3: 25% (fistula: 17.3%) |
| Kamran, 2017 [39] | R | 24/66 (36%) | Endometrium | 20 months | 33 months | 3-year LC 71% 3-year DFS 52% 3-year OS 54% | Gr 3: 33% (urinary 16.7%, rectal 25%) No gr 4–5 |
| Murakami, 2016 [37] | R | 8/44 (18%) | Cervix | NR | NR | 3-year LC 45% 3-year OS 51% | Gr 4: 27% (vagina fistula) Gr 2: 12.5% (vagina necrosis) |
| Amsbaugh, 2015 [34] | R | 18/21 (86%) | Endometrium (52.4%) Cervix (33.3%) Vulva (14.3%) | NR | 16.5 months | 1-year LC 71.5% 1-year PFS 66% 1-year OS 82.2% 2-year OS 52.5% 52.4% relapses LR: 23.8%, RR: 14.3%, DR: 14.3% | No gr 4–5 Gr 3: Vaginal (28.6%) urinary (9.5%), rectal (19%) Gr 1–2: Vaginal (71.4%), urinary (47.6%), rectal (23.8%) |
| Yoshida, 2015 [42] | R | 21/56 (38%) | Cervix (80.4%) Endometrium (19.6%) | NR | 33 months | Initial radical hysterctomy + adjuvant RT: 3-year LC 75% 3-year OS 88% Initial definitive RT: 3-year LC 46% 3-year OS 42% | Gr ≥3: 4.8% (vaginal fistula) |
| Aridgides, 2016 [38] | R | 6/60 (10%) | Endometrium (26.7%) Cervix (8.3%) Vulva (5%) Ovary (3.3%) Rectal (1.7%) | NR | 36.9 months (4–234) | Overall LC: 66.7% Local Failure: 33.3% Distant Relapse: 33.3% | Gr 3 soft tissue necrosis: 1.7% |
| Huang, 2016 [35] | R | 16/40 (40%) | Endometrium | 61 months | 18 months | 2-year LC 53% 2-year PFS 44% 2-year OS 67% | No Gr 5. Gr 1–2: 52.2% Gr ≥ 3: 12.5% (Rectal bleeding, Rectovaginal fistula, Radiation necrosis, cystitis) |
| Study | BT Technique: HDR/LDR | BT Technique: IC/IS | BT Technique: Dose Schedule (Physical Dose) | Treatment Planning: 2D-Planning Allowed? | 3D-IGBT: MRI/CT |
|---|---|---|---|---|---|
| Raziee, 2020 [32] | HDR | IS | Median 30 Gy, in 3 to 6 Fr, BID Median HR-CTV D90: 29.1 Gy (16.1–64.6) | No | CT-planned |
| Ling, 2019 [44] | HDR | IC and IS (IC if tumor thickness ≤ 5 mm) | Median 28.75 Gy (range, 24.8–30) In 4 to 7 Fr, BID | No | CT- and MRI-planned (MRI in 16 patients (72.7%)) |
| Umezawa, 2018 [41] | HDR | IS | Median 48 Gy (range, 24–50) in 8 Fr (range, 4–20), BID | No | CT-planned |
| Martinez-Monge, 2014 [31] | HDR | IS | 38 Gy in 8 Fr, BID | No | CT-planned |
| Feddock, 2017 [45] | LDR | IS | Template-guided median dose: 47.5 Gy (range, 25–55) Free-hand median dose: 50 Gy (range, 22–75) | No | CT-planned |
| Liu, 2016 [36] | HDR | IS | 36 Gy in 6 Fr | No | CT-planned |
| Mahantshetty, 2014 [40] | HDR | IS | 3 to 4 Gy/Fr, in 6 to 13 Fr, BID | No | CT- and MRI-planned (MRI in 6 patients (20%)) |
| Zolciak-Siwinska, 2014 [43] | HDR | IC and IS (IC if tumor thickness ≤5 mm) | 10 to 15 daily Fr of 3 Gy, or 4 to 6 once a week Fr of 5 to 7.5 Gy | Yes 8 patients (40%) | CT-planned |
| Mabuchi, 2014 [33] | HDR | IS | 42 Gy in 7 Fr, BID | Yes 40 patients (77%) | CT-planned |
| Kamran, 2017 [39] | HDR and LDR | IS | Median 22.5 Gy in 5 Fr, BID | No | CT- and MRI-planned (MRI: 18 patients (75%)) |
| Murakami, 2016 [37] | HDR | IS | Median 32 Gy (range, 36–48), Median dose/Fr 5.3 Gy (range, 4–6) * | No | CT-planned |
| Amsbaugh, 2015 [34] | HDR and LDR | IS | HDR: Median 22.5 Gy (range, 13.5–30), 3 to 5 Fr, BID LDR: Median 41.5 Gy (range, 28.5–55) | No | CT-planned |
| Yoshida, 2015 [42] | HDR | IS | Median 48 Gy (range, 42–51), in 7 to 8 Fr, BID | No | CT-planned |
| Aridgides, 2016 [38] | HDR and LDR | IS | HDR: Median 20 Gy (range, 13.5–30), in 4 Fr, BID LDR: Median 30 Gy (20–40) | No | CT-planned |
| Huang, 2016 [35] | HDR | IS | Median 21 Gy (range, 15–27.5), in 2 to 5 Fr | No | CT-planned |
| Study | Doses to Target Volume (EQD2) | Doses to OARs | Tumor Volume | Dose Constraints |
|---|---|---|---|---|
| Raziee, 2020 [32] | NR | Not considering prior RT Bladder D2cc: 15.5 Gy (11–23.2) Rectum D2cc: 18.7 Gy (15.8–22.5) Sigmoid D2cc 3.7 Gy (1.9–5.6) | HR-CTV in cm3: 34.6 (4.8–96) | No |
| Ling, 2019 [44] | Median prescribed dose 56 Gy Not considering prior RT CTV D90 72.2 Gy (63.9–78.1) | Not considering prior RT Bladder D2cc: 54.3 Gy (EQD2) (23.9–93.4) Rectum D2cc: 50.6 Gy (EQD2) (34.6–82) | Median tumor diameter 23 mm (3–33) | Lifetime bladder D2cc (EQD2) < 90 Gy Lifetime rectosigmoid D2cc (EQD2) < 75 Gy Ideal HR-CTV D90 (EQD2) > 60 Gy |
| Umezawa, 2018 [41] | Not considering prior RT HR-CTV D90: 62.6 Gy (48.6–82.5) | Not considering prior RT Sigmoid D2cc: 39.9 Gy (EQD2) (3–60) Rectum and bladder D2cc: 36.8 Gy (EQD2) (12–62) | Median CTV volume 50.35 cm3 (2.1–129.2) | No |
| Martinez-Monge, 2014 [31] | Median prescribed dose to CTV D90: 46.7 Gy Not considering prior RT CTV D90: 43 Gy ± 7.4 GTV D90: 54.2 Gy ± 9.4 | Not considering prior RT Bladder D2cc: 37.4 Gy (EQD2) ± 15.1 Rectum D2cc: 27.5 Gy (EQD2) ± 8.5 Lifetime EQD2: Bladder D2cc: 120.8 Gy ± 50 Rectum D2cc: 110.8 Gy ± 48.2 | Average CTV volume 60.9 cm3 (14.8–165.3) | Average urethral dose < 5.5 Gy/Fr (<15% of prescription dose) Rectal D10 < 3.3 Gy (<70% of the prescription dose) Bladder D10 < 3.8 Gy (<80% of the prescription dose) |
| Feddock, 2017 [45] | Not considering prior RT -At first salvage: median 44.3 Gy (17.1–81.5) -At 2nd salvage: median 39.4 Gy (21.5–49.1) Lifetimes doses: -At first salvage: median 112.6 Gy (75.7–144) -At 2nd salvage: median 152.2 Gy (115.2–172.2) | Not considering prior RT Rectum D2cc: 27.8 Gy (12.9–43.6) Bladder D2cc: 34.2 Gy (21.3–46.1). | -Free-hand implant: Median GTV: 6 cm3 (2.25–44.5) -Template-guided implant Median GTV: 27.9 cm3 (12.2–44.6) | No |
| Liu, 2016 [36] | Median prescribed dose to the HR-CTV: 48 Gy Not considering prior RT Median HR-CTV D90: 52.5 ± 3.3 Gy | Taking prior RT into account Median bladder D2cc: 85.6 Gy (EQD2) ± 5.8 Gy Median rectum D2cc: 71.6 Gy (EQD2) ± 6.4 Gy Median sigmoid D2cc: 69.6 Gy (EQD2) ± 5.9 Gy | NR | HR-CTV D90 (EQD2) ≥ 50 Gy Bladder D2cc (EQD2) ≤ 90 Gy Rectum D2cc (EQD2) ≤ 75 Gy Sigmoid D2cc (EQD2) ≤ 75 Gy |
| Mahant-shetty, 2014 [40] | Median prescription dose: 42 Gy (31.5–54.4) | Not considering prior RT (DVH available n = 6) Mean bladder D2cc: 42.1 Gy (EQD2) ± 13.3 Mean rectum D2cc: 25.1 Gy (EQD2) ± 8.9 Mean sigmoid D2cc: 25.1 Gy (EQD2) ± 12.1 | Median tumor diameter: <20 mm: 46% (n = 14) 20–40 mm: 40% (n = 12) >40 mm: 14% (n = 4) | No |
| Zolciak-Siwinska, 2014 [43] | Median prescription dose: 48.8 Gy (16–91) Not considering prior RT Median D100 31.75 Gy (10–69.8) Median lifetime prescribed dose 133.5 Gy (96.8–164.2) | Not considering prior RT Median rectum D2cc: 42.4 Gy (EQD2) (7.4–78.8 Gy) Median bladder D2cc: 42.7 Gy (EQD2) (6.4–84.8 Gy) Median lifetime doses at OARs, if 3D planned: Rectum D2cc: 94.4 Gy (EQD2) (67.1–118.8 Gy) Bladder D2cc: 99.3 Gy (EQD2) (70.4–122.3) Median lifetime doses at OARs, if 2D planned: Rectal point: 119.75 Gy (EQD2) (82–138.3) Bladder point: 113.5 Gy (EQD2) (75–139) | Median tumor diameter: ≤30 mm: 60% (n = 12) >30 mm: 40% (n = 8) | No |
| Mabuchi, 2014 [33] | Median prescribed dose: 56 Gy Not considering prior RT CTV D90: 72.2 Gy (63.9–78.1) | Not considering prior RT Bladder D2cc: 54 Gy (EQD2) (23.9–93.4) Rectum D2cc 50.6 Gy (EQD2) (34.6–82) | Median tumor diameter 22.5 mm | No |
| Kamran, 2017 [39] | Not considering prior RT: Median HR-CTV D90 41.8 Gy (10.4–77.3) Lifetime doses: Median cumulative dose to HR-CTV D90 78.1 Gy (37–108.7 Gy) | NR | Median tumor diameter 20.5 mm (5–84) | No |
| Murakami, 2016 [37] | Mean prescribed dose 54 Gy (42–64) | Lifetime doses: Rectum D2cc: 91.1 Gy (EQD2) (71–114.3) Bladder D2cc: 100.9 Gy (EQD2) (69.7–120.3) Vaginal wall D2cc: 170.1 Gy (EQD2) (56.6–247.5) | Median tumor diameter 36 mm (10–80) | No |
| Amsbaugh, 2015 [34] | NR | NR | Median tumor diameter 30 mm (15–100) | No |
| Yoshida, 2015 [42] | Median planning aim dose 64 Gy | NR | Median tumor diameter 25 mm (5–79) | No |
| Aridgides, 2016 [38] | Median prescribed dose 48.7 Gy | NR | NR | Rectum Dmax (EQD2) < 70 Gy Urethra Dmax (EQD2) < 70 Gy Bladder Dmax (EQD2) < 75 Gy |
| Huang, 2016 [35] | NR | NR | NR | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bockel, S.; Espenel, S.; Sun, R.; Dumas, I.; Gouy, S.; Morice, P.; Chargari, C. Image-Guided Brachytherapy for Salvage Reirradiation: A Systematic Review. Cancers 2021, 13, 1226. https://doi.org/10.3390/cancers13061226
Bockel S, Espenel S, Sun R, Dumas I, Gouy S, Morice P, Chargari C. Image-Guided Brachytherapy for Salvage Reirradiation: A Systematic Review. Cancers. 2021; 13(6):1226. https://doi.org/10.3390/cancers13061226
Chicago/Turabian StyleBockel, Sophie, Sophie Espenel, Roger Sun, Isabelle Dumas, Sébastien Gouy, Philippe Morice, and Cyrus Chargari. 2021. "Image-Guided Brachytherapy for Salvage Reirradiation: A Systematic Review" Cancers 13, no. 6: 1226. https://doi.org/10.3390/cancers13061226
APA StyleBockel, S., Espenel, S., Sun, R., Dumas, I., Gouy, S., Morice, P., & Chargari, C. (2021). Image-Guided Brachytherapy for Salvage Reirradiation: A Systematic Review. Cancers, 13(6), 1226. https://doi.org/10.3390/cancers13061226






