Neoadjuvant Chemoradiotherapy Followed by Esophagectomy with Three-Field Lymph Node Dissection for Thoracic Esophageal Squamous Cell Carcinoma Patients with Clinical Stage III and with Supraclavicular Lymph Node Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
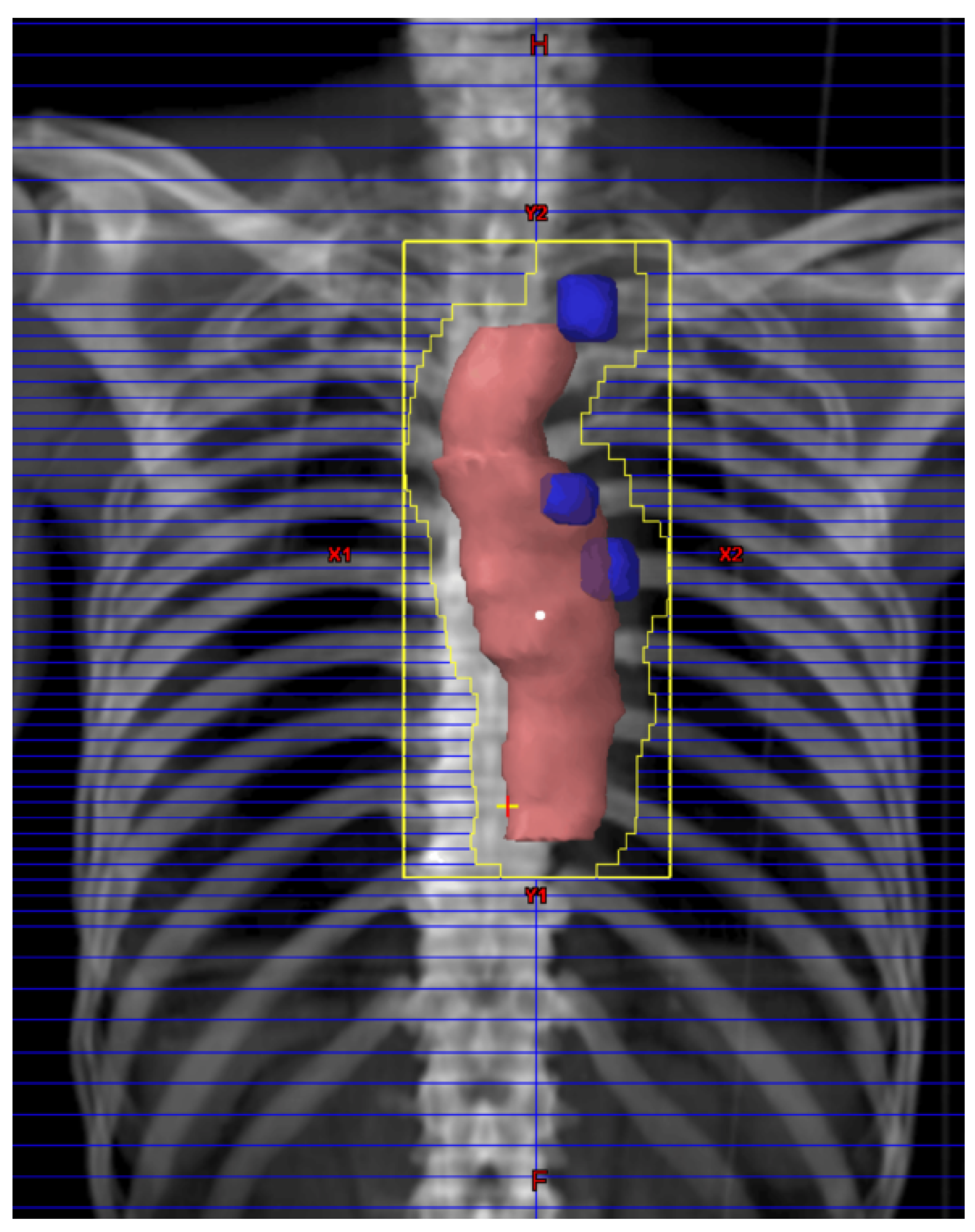
2.2. Neoadjuvant Chemoradiotherapy
2.3. Esophagectomy
2.4. Pathological Response
2.5. Statistical Analysis
3. Results
3.1. Neoadjuvant CRT Grade and Pathological Stage of Clinical Stage III and IVB Patients
3.2. Adverse Events and Reasons for Discontinuation during NACRT
3.3. Esophagectomy with Three-Field LN Dissection after Neoadjuvant CRT
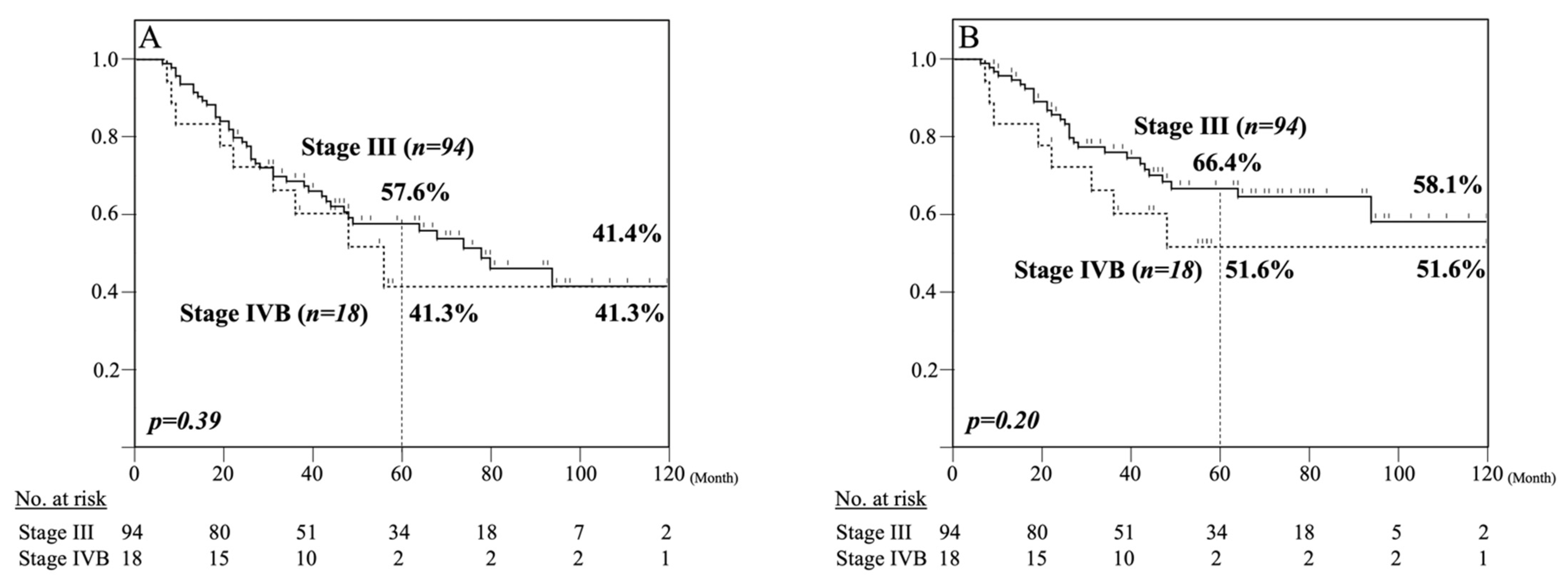
3.4. Five-Year Survival Analysis of Clinical Stage III and IVB Patients Treated with NACRT Followed by Esophagectomy with Three-Field LN Dissection
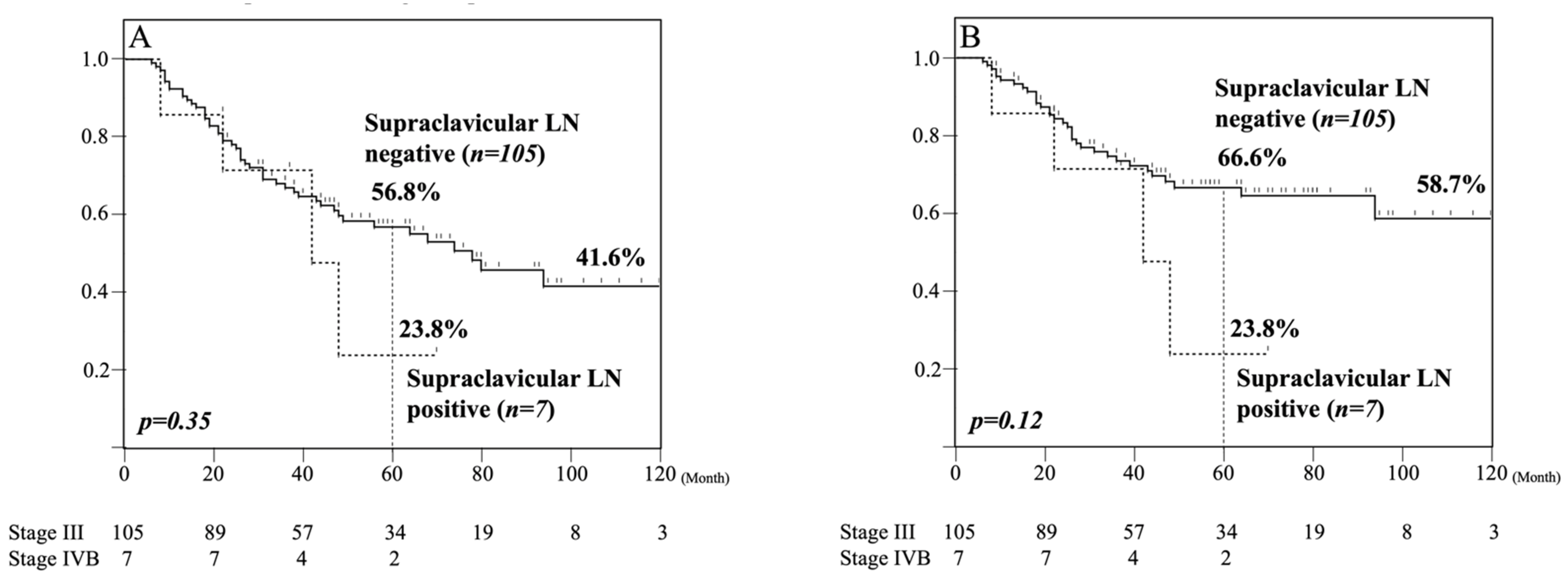
3.5. Five-Year Survival Analysis of Pathologically Supraclavicular LN Metastasis-Positive and -Negative Patients
3.6. Pattern of Recurrence in 47 Patients after NACRT Followed by Esophagectomy with Three-Field LN Dissection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EAC | esophageal adenocarcinoma |
| ESCC | esophageal squamous cell carcinoma |
| OS | overall survival |
| DSS | disease specific survival |
| LN | lymph node |
| NAC | neoadjuvant chemotherapy |
| NACRT | neoadjuvant chemoradiotherapy |
| CF | cisplatin + fluorouracil |
| DCF | docetaxel + cisplatin + fluorouracil |
| ICI | immune checkpoint inhibitor |
References
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Walsh, T.N.; Noonan, N.; Hollywood, D.; Kelly, A.; Keeling, N.; Hennessy, T.P.J. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N. Engl. J. Med. 1996, 335, 462–467. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J. Clin. Oncol. 2018, 36, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381–387. [Google Scholar] [CrossRef]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: Part I. Esophagus 2017, 14, 1–36. [Google Scholar] [CrossRef]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: Part II and III. Esophagus 2017, 14, 37–65. [Google Scholar] [CrossRef]
- Nishihira, T.; Hirayama, K.; Mori, S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am. J. Surg. 1998, 175, 47–51. [Google Scholar] [CrossRef]
- Kato, H.; Watanabe, H.; Tachimori, Y.; Iizuka, T. Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. Ann. Thorac. Surg. 1991, 51, 931–935. [Google Scholar] [CrossRef]
- Fujita, H.; Sueyoshi, S.; Tanaka, T.; Fujii, T.; Toh, U.; Mine, T.; Sasahara, H.; Sudo, T.; Matono, S.; Yamana, H.; et al. Optimal Lymphadenectomy for Squamous Cell Carcinoma in the Thoracic Esophagus: Comparing the Short- and Long-term Outcome among the Four Types of Lymphadenectomy. World J. Surg. 2003, 27, 571–579. [Google Scholar] [CrossRef]
- Igaki, H.; Tachimori, Y.; Kato, H. Improved Survival for Patients With Upper and/or Middle Mediastinal Lymph Node Metastasis of Squamous Cell Carcinoma of the Lower Thoracic Esophagus Treated With 3-Field Dissection. Ann. Surg. 2004, 239, 483–490. [Google Scholar] [CrossRef]
- Tachimori, Y.; Ozawa, S.; Numasaki, H.; Matsubara, H.; Shinoda, M.; Toh, Y.; Udagawa, H. Supraclavicular node metastasis from thoracic esophageal carcinoma: A surgical series from a Japanese multi-institutional nationwide registry of esophageal cancer. J. Thorac. Cardiovasc. Surg. 2014, 148, 1224–1229. [Google Scholar] [CrossRef]
- Ando, N.; Iizuka, T.; Ide, H.; Ishida, K.; Shinoda, M.; Takiyama, T.N.; Watanabe, H.; Isono, K.; Aoyama, N.; Makuuchi, H.; et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: A Japan Clinical Oncology Group Study--JCOG9204. J. Clin. Oncol. 2003, 21, 4592–4596. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A Randomized Trial Comparing Postoperative Adjuvant Chemotherapy with Cisplatin and 5-Fluorouracil Versus Preoperative Chemotherapy for Localized Advanced Squamous Cell Carcinoma of the Thoracic Esophagus (JCOG9907). Ann. Surg. Oncol. 2011, 19, 68–74. [Google Scholar] [CrossRef]
- Nakamura, K.; Kato, K.; Igaki, H.; Ito, Y.; Mizusawa, J.; Ando, N.; Udagawa, H.; Tsubosa, Y.; Daiko, H.; Hironaka, S.; et al. Three-arm Phase III Trial Comparing Cisplatin Plus 5-FU (CF) Versus Docetaxel, Cisplatin Plus 5-FU (DCF) Versus Radiotherapy with CF (CF-RT) as Preoperative Therapy for Locally Advanced Esophageal Cancer (JCOG1109, NExT Study). Jpn. J. Clin. Oncol. 2013, 43, 752–755. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Fukushima, H.; Sasaki, T.; Shimbashi, W.; Seto, Y.; Kitano, M.; Koizumi, Y.; Ueki, Y.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Sato, Y.; Motoyama, S.; Wakita, A.; Kawakita, Y.; Liu, J.; Nagaki, Y.; Nanjo, H.; Ito, S.; Terata, K.; Imai, K.; et al. High TLR4 expression predicts a poor prognosis after esophagectomy for advanced thoracic esophageal squamous cell carcinoma. Esophagus 2020, 17, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Motoyama, S.; Wakita, A.; Kawakita, Y.; Liu, J.; Nagaki, Y.; Nanjo, H.; Terata, K.; Imai, K.; Saito, H.; et al. TLR3 expression status predicts prognosis in patients with advanced thoracic esophageal squamous cell carcinoma after esophagectomy. Am. J. Surg. 2018, 216, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Sato, Y.; Sasaki, T.; Wakita, A.; Kawakita, Y.; Liu, J.; Nagaki, Y.; Saito, H.; Imai, K.; Konno, H.; et al. Efficacy and Safety of Neoadjuvant Chemoradiotherapy Following Esophagectomy with Japanese-style Extended 3-Field Lymphadenectomy for Thoracic Esophageal Cancer. Anticancer. Res. 2017, 37, 5837–5843. [Google Scholar]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’Journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef]
- Sun, H.B.; Xing, W.Q.; Liu, X.B.; Johannessen, H.-O.; Nielsen, N.H.; Johnsen, G.; Hatlevoll, I.; Glenjen, N.I.; Friesland, S.; Lundell, L.; et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for locally advanced oesophageal squamous cell carcinoma: A single-Centre, open-label, randomized, controlled, clinical trial (HCHTOG1903). BMC Cancer 2020, 20, 303. [Google Scholar] [CrossRef]
- Wakita, A.; Motoyama, S.; Sato, Y.; Nagaki, Y.; Fujita, H.; Terata, K.; Imai, K.; Minamiya, Y. Verification of the Optimal Interval Before Esophagectomy after Preoperative Neoadjuvant Chemoradiotherapy for Locally Advanced Thoracic Esophageal Cancer. In Ann. Surg. Oncol.; 2020. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Chao, Y.-K.; Chang, H.-K.; Tseng, C.-K.; Chan, S.-C.; Liu, Y.-H.; Chen, W.-H. Interval Between Neoadjuvant Chemoradiotherapy and Surgery for Esophageal Squamous Cell Carcinoma: Does Delayed Surgery Impact Outcome? Ann. Surg. Oncol. 2013, 20, 4245–4251. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, L.R.; Dikken, J.L.; van der Willik, E.M.; van Berge Henegouwen, M.I.; Nieuwenhuijzen, G.A.P.; Wijnhoven, B.P.L. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer: A nationwide study. Eur. J. Cancer 2018, 91, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today 2020, 50, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Kato, K.; Ando, N.; Ohtsu, A.; Muro, K.; Igaki, H.; Abe, T.; Takeuchi, H.; Daiko, H.; Gotoh, M.; et al. Comparison between neoadjuvant chemotherapy followed by surgery and definitive chemoradiotherapy for overall survival in patients with clinical Stage II/III esophageal squamous cell carcinoma (JCOG1406-A). Jpn. J. Clin. Oncol. 2017, 47, 480–486. [Google Scholar] [CrossRef]
- Kato, K.; Muro, K.; Minashi, K.; Ohtsu, A.; Ishikura, S.; Boku, N.; Takiuchi, H.; Komatsu, Y.; Miyata, Y.; Fukuda, H. Phase II Study of Chemoradiotherapy With 5-Fluorouracil and Cisplatin for Stage II–III Esophageal Squamous Cell Carcinoma: JCOG Trial (JCOG 9906). Int. J. Radiat. Oncol. 2011, 81, 684–690. [Google Scholar] [CrossRef]
- Taniyama, Y.; Sakurai, T.; Heishi, T.; Okamoto, H.; Sato, C.; Maruyama, S.; Onodera, Y.; Ishida, H.; Unno, M.; Kamei, T. Different strategy of salvage esophagectomy between residual and recurrent esophageal cancer after definitive chemoradiotherapy. J. Thorac. Dis. 2018, 10, 1554–1562. [Google Scholar] [CrossRef]
- Watanabe, M.; Mine, S.; Nishida, K.; Yamada, K.; Shigaki, H.; Matsumoto, A.; Sano, T. Salvage Esophagectomy after Definitive Chemoradiotherapy for Patients with Esophageal Squamous Cell Carcinoma: Who Really Benefits from this High-Risk Surgery? Ann. Surg. Oncol. 2015, 22, 4438–4444. [Google Scholar] [CrossRef]
- Ariga, H.; Nemoto, K.; Miyazaki, S.; Yoshioka, T.; Ogawa, Y.; Sakayauchi, T.; Jingu, K.; Miyata, G.; Onodera, K.; Ichikawa, H.; et al. Prospective Comparison of Surgery Alone and Chemoradiotherapy With Selective Surgery in Resectable Squamous Cell Carcinoma of the Esophagus. Int. J. Radiat. Oncol. 2009, 75, 348–356. [Google Scholar] [CrossRef]
- Vivaldi, C.; Catanese, S.; Massa, V.; Pecora, I.; Salani, F.; Santi, S.; Lencioni, M.; Vasile, E.; Falcone, A.; Fornaro, L. Immune Checkpoint Inhibitors in Esophageal Cancers: Are We Finally Finding the Right Path in the Mist? Int. J. Mol. Sci. 2020, 21, 1658. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, C.; He, J.; Wang, L.; Zhang, T. Combination of checkpoint inhibitors with radiotherapy in esophageal squamous cell carcinoma treatment: A novel strategy (Review). Oncol. Lett. 2019, 18, 5011–5021. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients (n = 112) | Clinical Stage III (n = 94) | Clinical Stage IVB (n = 18) | p Value |
|---|---|---|---|---|
| Sex | 0.2479 | |||
| Female | 16 (14.3%) | 15 (16.0%) | 1 (5.6%) | |
| Male | 96 (85.7%) | 79 (85.7%) | 17 (94.4%) | |
| Age at surgery | 63.0 | 63.0 | 64.0 | 0.5895 |
| (41–77) | (43–75) | (41–77) | ||
| Tumor location | 0.0086 | |||
| Upper | 27 (24.1%) | 22 (23.4%) | 5 (27.8%) | |
| Middle | 52 (46.4%) | 39 (41.5%) | 13 (41.5%) | |
| Lower | 33 (29.5%) | 33 (35.1%) | 0 | |
| Differentiation | 0.3871 | |||
| Well | 25 (22.3%) | 23 (24.5%) | 2 (11.1%) | |
| Moderate | 72 (64.3%) | 58 (61.7%) | 14 (77.8%) | |
| Poor | 15 (13.4%) | 13 (13.8%) | 2 (11.1%) | |
| cT | 0.0049 | |||
| 1 | 1 (0.9%) | 0 | 1 (5.6%) | |
| 2 | 1 (0.9%) | 0 | 1 (5.6%) | |
| 3 | 110 (98.2%) | 94 (100%) | 16 (88.8%) | |
| cN | 0.0431 | |||
| 1 | 71 (63.4%) | 62 (66.0%) | 9 (50.0 %) | |
| 2 | 40 (35.7%) | 32 (34.0%) | 8 (44.4%) | |
| 3 | 1 (0.9%) | 0 | 1 (5.6%) | |
| cM (supraclavicular LN metastasis) | <0.0001 | |||
| Positive | 18 (16.1%) | 0 | 18 (100%) | |
| Negative | 94 (83.9%) | 94 (100%) | 0 |
| Characteristics | All Patients (n = 112) | Clinical Stage III (n = 94) | Clinical Stage IVB (n = 18) | p Value |
|---|---|---|---|---|
| Neoadjuvant treatment grade | 0.7371 | |||
| 1 | 40 (35.7%) | 35 (37.2%) | 5 (27.8%) | |
| 2 | 51 (45.5%) | 42 (44.7%) | 9 (50.0 %) | |
| 3 (complete response) | 21 (18.8%) | 17 (18.1%) | 4 (22.2%) | |
| ypT | 0.3414 | |||
| 0 | 29 (25.9%) | 23 (24.5%) | 6 (33.2%) | |
| 1 | 17 (15.2%) | 12 (12.8%) | 5 (27.8%) | |
| 2 | 15 (13.4%) | 14 (14.9%) | 1 (5.6%) | |
| 3 | 46 (41.0%) | 41 (43.6%) | 5 (27.8%) | |
| 4a | 3 (2.7%) | 2 (2.1%) | 1 (5.6%) | |
| 4b | 2 (1.8%) | 2 (2.1%) | 0 | |
| ypN | 0.0132 | |||
| 0 | 63 (56.2%) | 54 (57.4%) | 9 (50.0%) | |
| 1 | 31 (27.7%) | 26 (27.7%) | 5 (27.8%) | |
| 2 | 16 (14.3%) | 14 (14.9%) | 2 (11.1%) | |
| 3 | 2 (1.8%) | 0 | 2 (11.1%) | |
| ypM (supraclavicular LN metastasis) | <0.0001 | |||
| positive | 7 (6.3%) | 2 (2.1%) | 5 (27.8%) | |
| negative | 105 (93.7%) | 92 (97.9%) | 13 (72.2%) | |
| ypStage | 0.0039 | |||
| I | 39 (34.8%) | 34 (36.2%) | 5 (27.8%) | |
| II | 23 (20.5%) | 20 (21.3%) | 3 (16.7%) | |
| IIIA | 15 (13.4%) | 13 (13.8%) | 2 (11.1%) | |
| IIIB | 25 (22.3%) | 22 (23.4%) | 3 (16.7%) | |
| IVA | 3 (2.7%) | 3 (3.2%) | 0 | |
| IVB | 7 (6.3%) | 2 (2.1%) | 5 (27.8%) |
| Characteristic | All Patients (n = 112) | Clinical Stage III (n = 94) | Clinical Stage IVB (n = 18) | p Value |
|---|---|---|---|---|
| Leukopenia | 0.7311 | |||
| Grade 3 | 43 (38.4%) | 38 (40.4%) | 5 (27.8%) | |
| Grade 4 | 5 (4.5%) | 4 (4.3%) | 1 (5.6%) | |
| Neutropenia | 0.5537 | |||
| Grade 3 | 16 (14.3%) | 13 (13.8%) | 3 (16.7%) | |
| Grade 4 | 3 (2.7%) | 2 (2.1%) | 1 (5.6%) | |
| Anemia | 0.6495 | |||
| Grade 3 | 3 (2.7%) | 2 (2.1%) | 1 (5.6%) | |
| Grade 4 | 0 | 0 | 0 | |
| Thrombopenia | 0.1121 | |||
| Grade 3 | 1 (0.9%) | 1 (1.1%) | 0 | |
| Grade 4 | 3 (2.7%) | 3 (3.2%) | 0 | |
| Hyponatremia | 0.7753 | |||
| Grade 3 | 8 (7.1%) | 7 (7.5%) | 1 (5.6%) | |
| Grade 4 | 0 | 0 | 0 | |
| Neoadjuvant treatment completion | 0.6562 | |||
| Completed | 97 (86.6%) | 82 (87.2%) | 15 (83.3) | |
| Not completed | 15 (13.4%) | 12 (12.8%) | 3 (16.7%) | |
| Reason of discontinuation | - | |||
| Leukopenia | 5 (4.5%) | 4 (4.3%) | 1 (5.6%) | |
| Renal function deterioration | 4 (3.6%) | 2 (2.1%) | 2 (11.1%) | |
| Hyponatremia | 2 (1.8%) | 2 (2.1%) | ||
| Thrombopenia | 1 (0.9%) | 1 (1.1%) | ||
| Sepsis | 1 (0.9%) | 1 (1.1%) | ||
| Osteomyelitis | 1 (0.9%) | 1 (1.1%) | ||
| Rejection | 1 (0.9%) | 1 (1.1%) |
| Characteristics | All Patients (n = 112) | Clinical Stage III (n = 94) | Clinical Stage IVB (n = 18) | p Value |
|---|---|---|---|---|
| LN dissection | 0.1990 | |||
| 2-field | 8 (7.1%) | 8 (8.5%) | 0 | |
| 3-field | 104 (92.9%) | 86 (91.5%) | 18 (100%) | |
| Operative procedure | 0.6980 | |||
| Open | 83 (74.1%) | 69 (73.4%) | 14 (77.8%) | |
| Thoracoscopic/robot-assisted | 29 (25.9%) | 25 (26.6%) | 4 (22.2%) | |
| Organ for reconstruction | 0.3816 | |||
| Stomach | 99 (88.4%) | 82 (87.2%) | 17 (94.4%) | |
| Colon | 13 (11.6%) | 12 (12.8%) | 1 (5.6%) | |
| Reconstructive route | 0.8281 | |||
| Posterior mediastinal | 97 (86.6%) | 82 (87.2%) | 15 (83.3%) | |
| Subcutaneous | 15 (13.4%) | 12 (12.8%) | 3 (16.7%) | |
| Surgical time (min) | 0.3724 | |||
| 575 | 578 | 552 | ||
| (386–928) | (386–928) | (407–704) | ||
| Blood loss (mL) | 0.5458 | |||
| 542.5 | 550 | 535 | ||
| (86–3366) | (86–3366) | (195–1833) | ||
| Number of all dissected lymph nodes | 0.8991 | |||
| 49 | 49.5 | 49 | ||
| (12–97) | (16–97) | (12–80) | ||
| Cervical paraesophageal (101RL) | 0.1498 | |||
| 3 | 3 | 2 | ||
| (0–13) | (0–13) | (0–6) | ||
| Supraclavicular (104RL) | 0.9389 | |||
| 14 | 14 | 12.5 | ||
| (1–35) | (1–35) | (3–32) | ||
| Upper mediastinal (105, 106, 107, 109) | 0.6829 | |||
| 14 | 14 | 12.5 | ||
| (3–50) | (3–50) | (4–44) | ||
| Lower mediastinal (108, 110, 111, 112) | 0.4759 | |||
| 5 | 5 | 5 | ||
| (0–39) | (0–39) | (0–13) | ||
| Abdominal (1, 2, 3, 4, 7, 8, 9, 11) | 0.1276 | |||
| 13 | 13 | 9.5 | ||
| (0–45) | (0–45) | (0–27) | ||
| Days between neoadjuvant CRT and esophagectomy | 0.8889 | |||
| 40 | 40 | 39 | ||
| (21–92) | (21–92) | (27–77) | ||
| Days of hospital stay after esophagectomy | 0.6059 | |||
| 29 | 29 | 29 | ||
| (15–168) | (15–168) | (16–111) | ||
| Anastomotic leakage (Type I or more) | 16 (14.3%) | 14 (14.9%) | 2 (11.1%) | 0.6744 |
| Respiratory failure requiring reintubation | 6 (5.4%) | 6 (6.4%) | 0 | 0.2705 |
| Recurrent laryngeal nerve palsy (Type Ia or more) | 26 (23.2%) | 22 (23.4%) | 4 (22.2%) | 0.9133 |
| 30-day mortality | 0 | 0 | 0 | |
| 90-day mortality | 0 | 0 | 0 | |
| Recurrence of ESCC | 47 (42.0%) | 39 (41.5%) | 8 (44.4%) | 0.8160 |
| Prognosis | 0.3151 | |||
| Alive | 57 (50.9%) | 47 (50.0%) | 10 (55.6%) | |
| Alive after recurrence | 8 (7.1%) | 8 (8.5%) | 0 | |
| Deceased with ESCC | 36 (32.2%) | 28 (29.8%) | 8 (44.4%) | |
| Deceased with other cancer | 3 (2.7%) | 3 (3.2%) | 0 | |
| Deceased with other diseases | 8 (7.1%) | 8 (8.5%) | 0 |
| Pattern of Recurrence | All Patients (n = 47/112, 42.0%) | Clinical Stage III (n = 39/94, 41.5%) | Clinical Stage IVB (n = 8/18, 44.4%) | p Value |
|---|---|---|---|---|
| distant metastasis | 23 (48.9%) | 21 (53.8%) | 2 (25.0%) | 0.1264 |
| lung | 12 (25.4%) | 11 (28.2%) | 1 (5.6%) | |
| liver | 3 (6.4%) | 3 (7.7%) | ||
| kidney | 3 (6.4%) | 3 (7.7%) | ||
| brain | 2 (4.3%) | 2 (5.1%) | ||
| bone | 2 (4.3%) | 1 (2.6%) | 1 (5.6%) | |
| skin | 1 (2.1%) | 1 (2.6%) | ||
| dissemination | 6 (12.8%) | 6 (15.4%) | ||
| non-regional LN | 10 (21.3%) | 7 (17.9%) | 3 (37.5%) | |
| regional LN | 7 (14.9%) | 4 (10.3%) | 3 (37.5%) | |
| intramural | 1 (2.1%) | 1 (2.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Motoyama, S.; Wada, Y.; Wakita, A.; Kawakita, Y.; Nagaki, Y.; Terata, K.; Imai, K.; Anbai, A.; Hashimoto, M.; et al. Neoadjuvant Chemoradiotherapy Followed by Esophagectomy with Three-Field Lymph Node Dissection for Thoracic Esophageal Squamous Cell Carcinoma Patients with Clinical Stage III and with Supraclavicular Lymph Node Metastasis. Cancers 2021, 13, 983. https://doi.org/10.3390/cancers13050983
Sato Y, Motoyama S, Wada Y, Wakita A, Kawakita Y, Nagaki Y, Terata K, Imai K, Anbai A, Hashimoto M, et al. Neoadjuvant Chemoradiotherapy Followed by Esophagectomy with Three-Field Lymph Node Dissection for Thoracic Esophageal Squamous Cell Carcinoma Patients with Clinical Stage III and with Supraclavicular Lymph Node Metastasis. Cancers. 2021; 13(5):983. https://doi.org/10.3390/cancers13050983
Chicago/Turabian StyleSato, Yusuke, Satoru Motoyama, Yuki Wada, Akiyuki Wakita, Yuta Kawakita, Yushi Nagaki, Kaori Terata, Kazuhiro Imai, Akira Anbai, Manabu Hashimoto, and et al. 2021. "Neoadjuvant Chemoradiotherapy Followed by Esophagectomy with Three-Field Lymph Node Dissection for Thoracic Esophageal Squamous Cell Carcinoma Patients with Clinical Stage III and with Supraclavicular Lymph Node Metastasis" Cancers 13, no. 5: 983. https://doi.org/10.3390/cancers13050983
APA StyleSato, Y., Motoyama, S., Wada, Y., Wakita, A., Kawakita, Y., Nagaki, Y., Terata, K., Imai, K., Anbai, A., Hashimoto, M., & Minamiya, Y. (2021). Neoadjuvant Chemoradiotherapy Followed by Esophagectomy with Three-Field Lymph Node Dissection for Thoracic Esophageal Squamous Cell Carcinoma Patients with Clinical Stage III and with Supraclavicular Lymph Node Metastasis. Cancers, 13(5), 983. https://doi.org/10.3390/cancers13050983






