Preoperative Radiochemotherapy in Esophageal Squamous Cell Cancer with 5-Fluorouracil/Cisplatin or Carboplatin/Paclitaxel: Treatment Practice over a 20-Year Period and Implications for the Individual Treatment Modalities
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Baseline, Radiochemotherapy, Surgical, and Histopathological Characteristics
2.2. Toxicity, Treatment Compliance and Surgical Outcomes
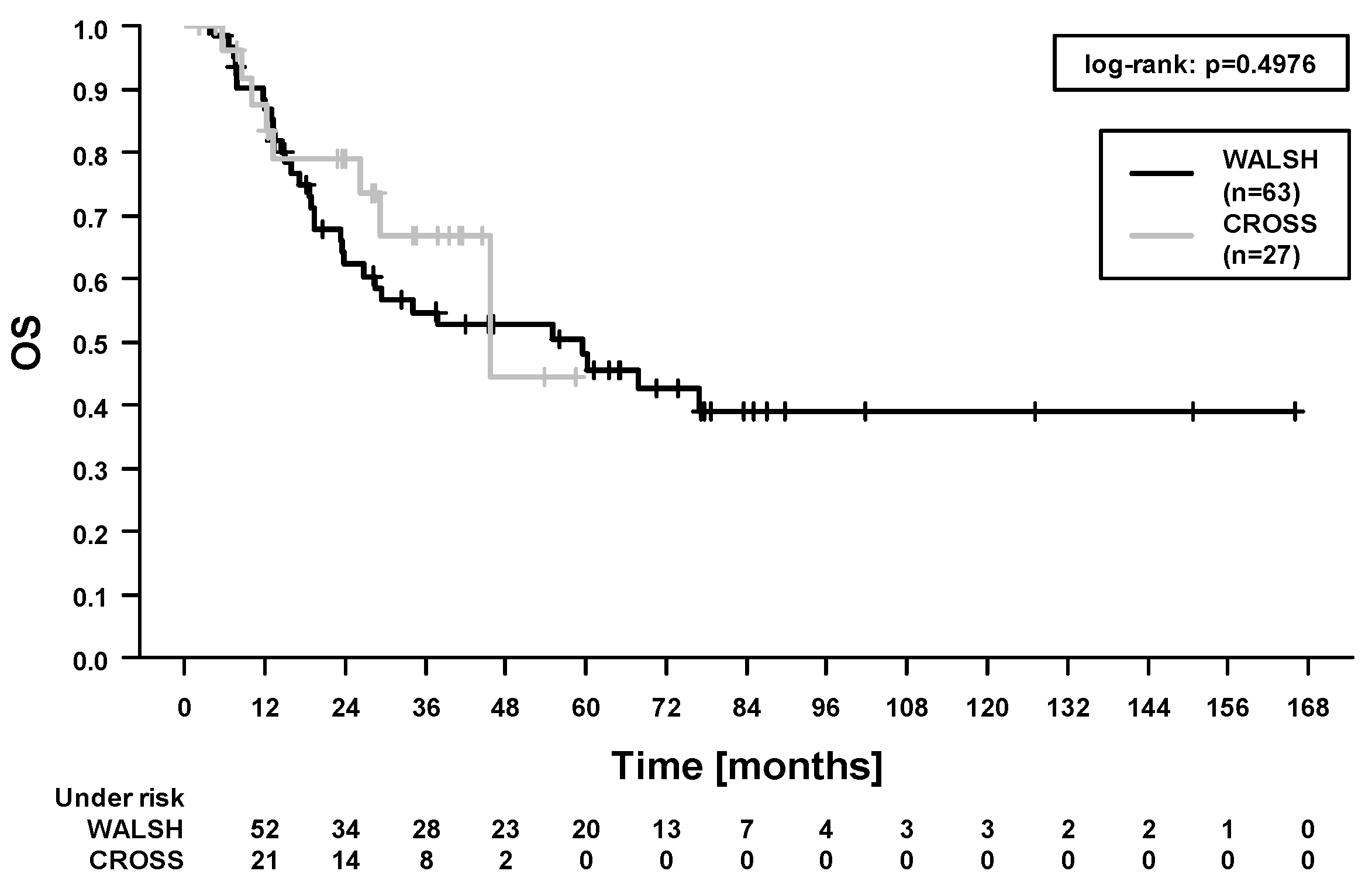
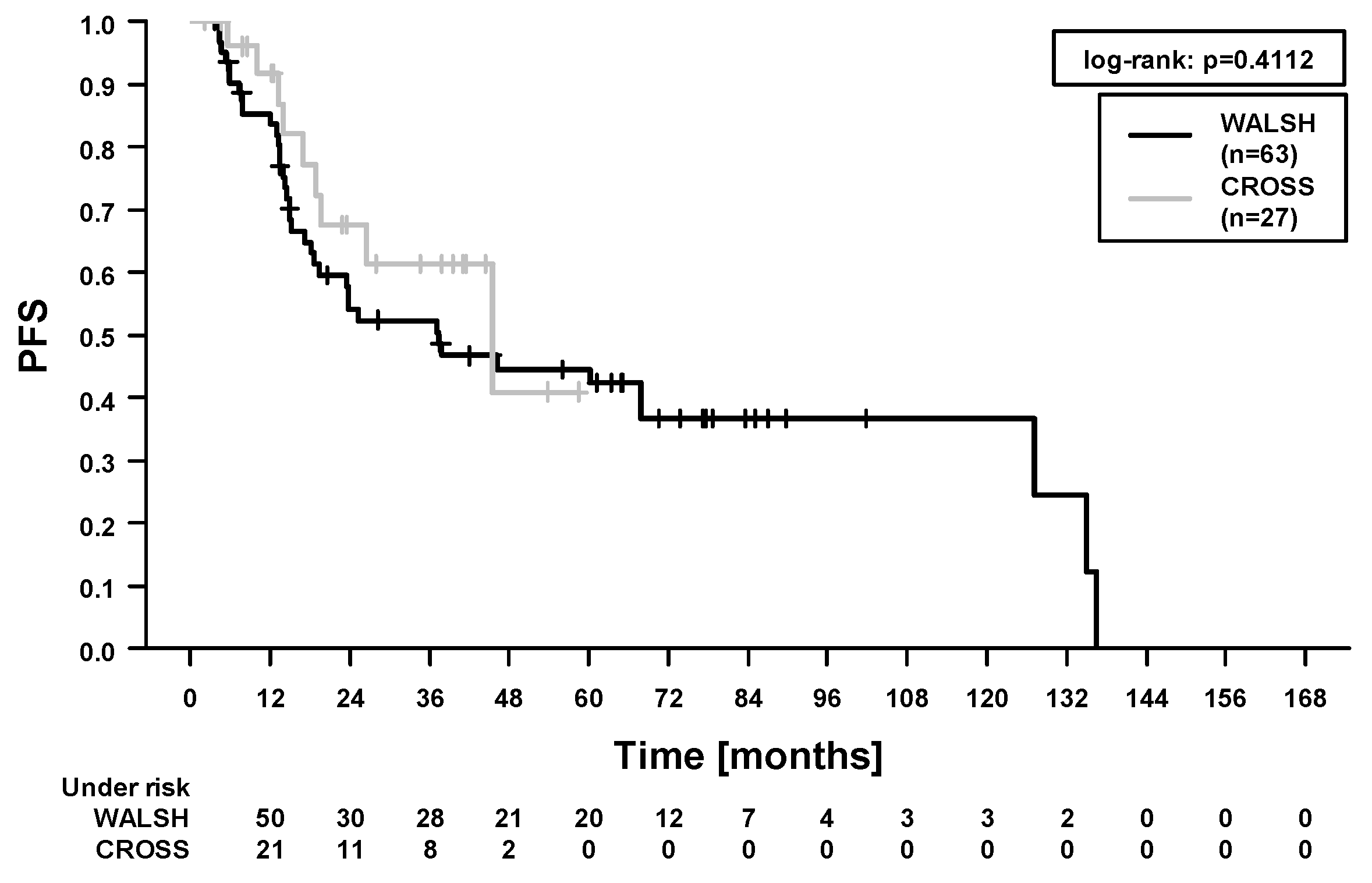
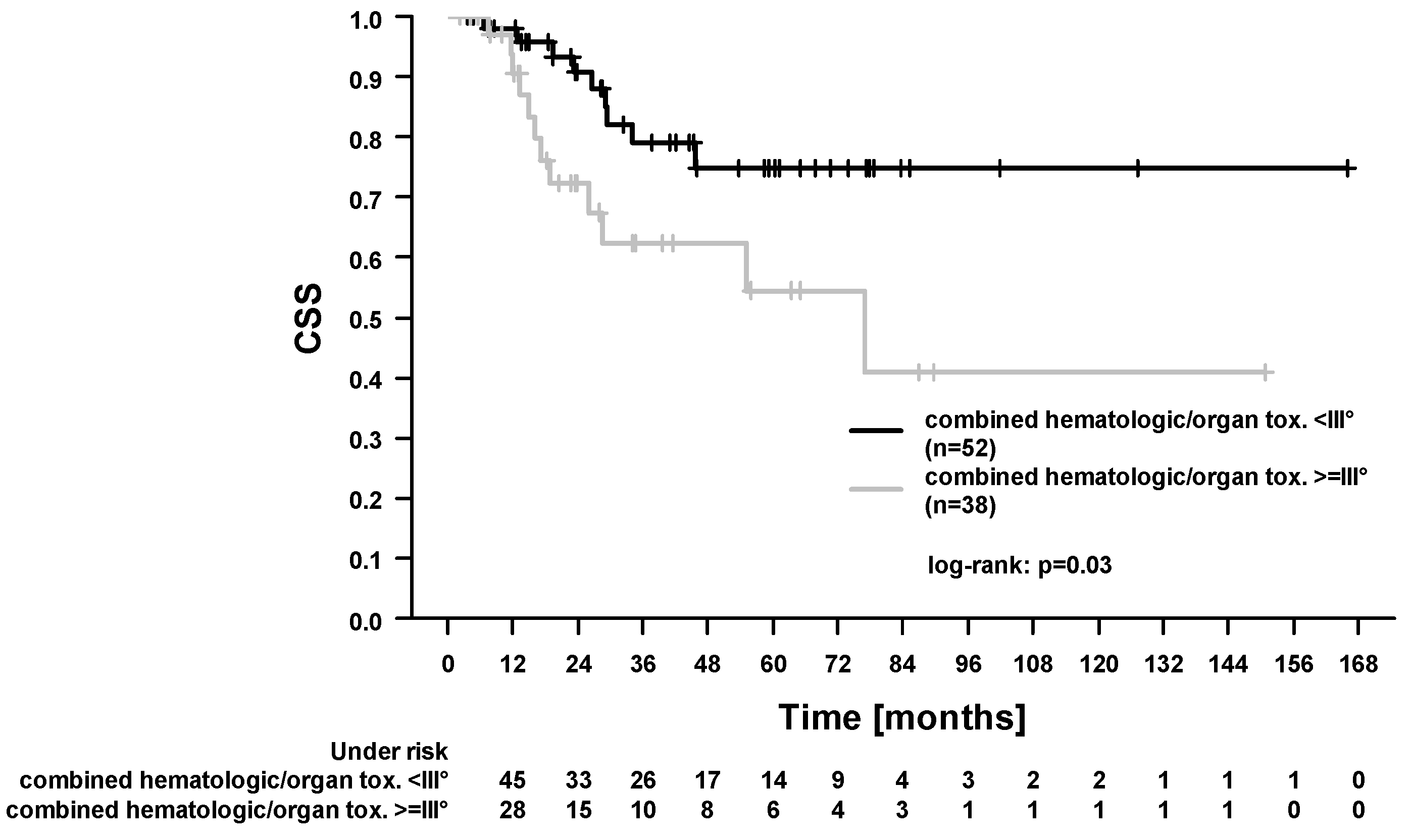
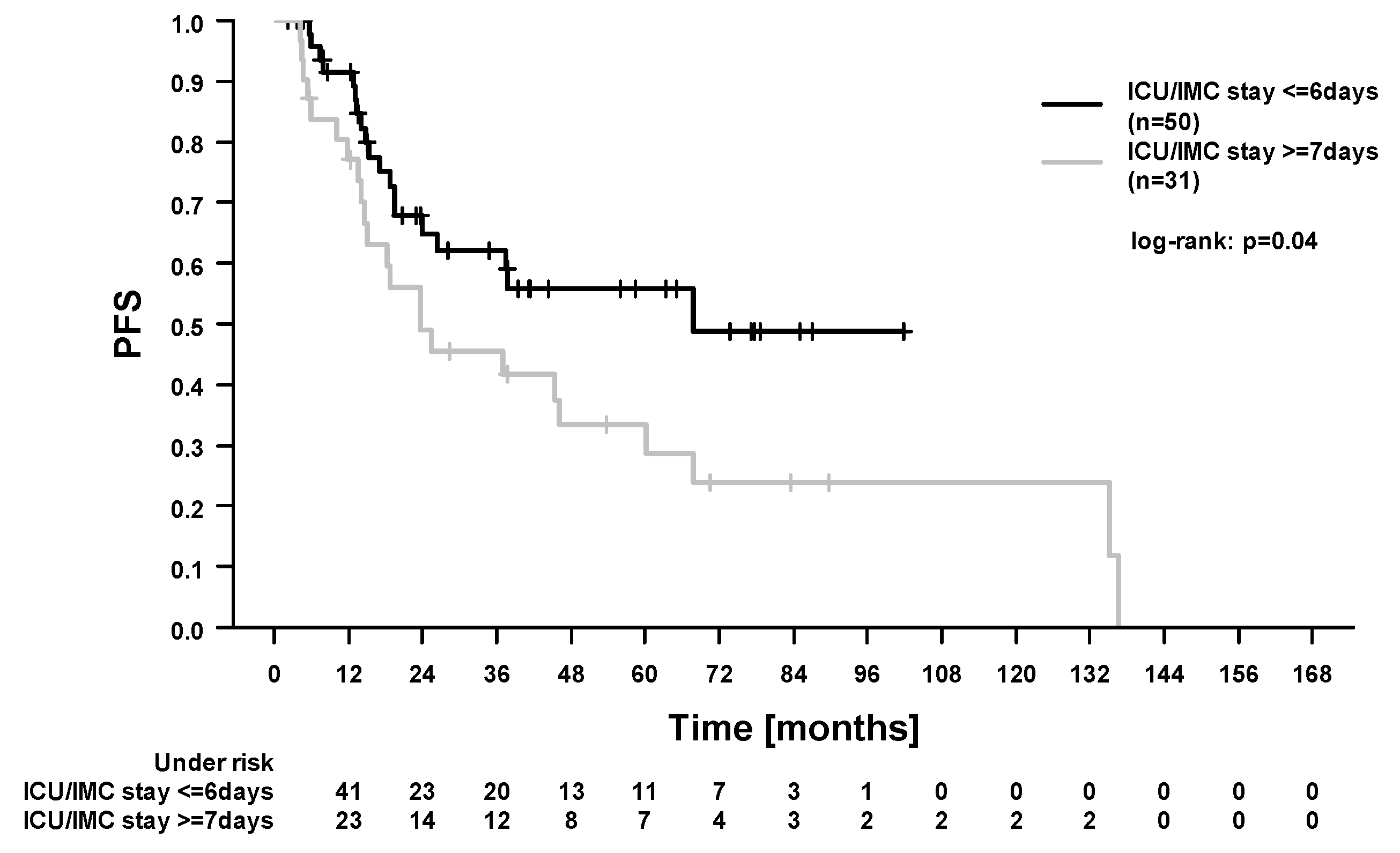
2.3. Survival Outcomes and Prognostic Factors
3. Discussion
4. Patients and Methods
4.1. Study Design and Patient Eligibility
4.2. Multimodal Treatment: Preoperative Radiochemotherapy and Surgical Procedures
4.3. Patient Monitoring during RCT and Follow-up
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaatsch, P.; Spix, C.; Katalinic, A.; Hentschel, S.; Luttmann, S.; Waldeyer-Sauerland, M.; Waldmann, A.; Christ, M.; Folkerts, J.; Hansmann, J.; et al. Cancer in Germany in 2015/2016; Robert Koch Institute and the Association of Population-Based Cancer Registries in Germany: Berlin, Germany, 2020; Volume 12, pp. 28–31. [Google Scholar]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef]
- Walsh, T.N.; Noonan, N.; Hollywood, D.; Kelly, A.; Keeling, N.; Hennessy, T.P. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N. Engl. J. Med. 1996, 335, 462–467. [Google Scholar] [CrossRef]
- Burmeister, B.H.; Smithers, B.M.; Gebski, V.; Fitzgerald, L.; Simes, R.J.; Devitt, P.; Ackland, S.; Gotley, D.C.; Joseph, D.; Millar, J.; et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol. 2005, 6, 659–668. [Google Scholar] [CrossRef]
- Stahl, M. Is there any role for surgery in the multidisciplinary treatment of esophageal cancer? Ann. Oncol. 2010, 21 (Suppl. 7), vii283–vii285. [Google Scholar] [CrossRef]
- Ilson, D.H. Esophageal cancer chemotherapy: Recent advances. Gastrointest Cancer Res. 2008, 2, 85–92. [Google Scholar]
- Münch, S.; Pigorsch, S.U.; Feith, M.; Slotta-Huspenina, J.; Weichert, W.; Friess, H.; Combs, S.E.; Habermehl, D. Comparison of neoadjuvant chemoradiation with carboplatin/ paclitaxel or cisplatin/ 5-fluoruracil in patients with squamous cell carcinoma of the esophagus. Radiat. Oncol. 2017, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Hennies, S.; Hermann, R.M.; Gaedcke, J.; Grade, M.; Hess, C.F.; Christiansen, H.; Wolff, H.A. Increasing toxicity during neoadjuvant radiochemotherapy as positive prognostic factor for patients with esophageal carcinoma. Dis. Esophagus 2014, 27, 146–151. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Paireder, M.; Jomrich, G.; Kristo, I.; Asari, R.; Rieder, E.; Beer, A.; Ilhan-Mutlu, A.; Preusser, M.; Schmid, R.; Schoppmann, S.F. Modification of preoperative radiochemotherapy for esophageal cancer (CROSS protocol) is safe and efficient with no impact on surgical morbidity. Strahlenther Onkol. 2020, 196, 779–786. [Google Scholar] [CrossRef]
- Münch, S.; Aichmeier, S.; Hapfelmeier, A.; Duma, M.N.; Oechsner, M.; Feith, M.; Combs, S.E.; Habermehl, D. Comparison of dosimetric parameters and toxicity in esophageal cancer patients undergoing 3D conformal radiotherapy or VMAT. Strahlenther Onkol. 2016, 192, 722–729. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrere, N.; et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Liao, X.Y.; Liu, C.Y.; He, J.F.; Wang, L.S.; Zhang, T. Combination of checkpoint inhibitors with radiotherapy in esophageal squamous cell carcinoma treatment: A novel strategy. Oncol. Lett. 2019, 18, 5011–5021. [Google Scholar] [CrossRef]
- Abdo, J.; Agrawal, D.K.; Mittal, S.K. “Targeted” Chemotherapy for Esophageal Cancer. Front. Oncol. 2017, 7, 63. [Google Scholar] [CrossRef]
- Li, C.; Zhao, S.; Zheng, Y.; Han, Y.; Chen, X.; Cheng, Z.; Wu, Y.; Feng, X.; Qi, W.; Chen, K.; et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur. J. Cancer 2021, 144, 232–241. [Google Scholar] [CrossRef]
- Lindenmann, J.; Fediuk, M.; Fink-Neuboeck, N.; Porubsky, C.; Pichler, M.; Brcic, L.; Anegg, U.; Balic, M.; Dandachi, N.; Maier, A.; et al. Hazard Curves for Tumor Recurrence and Tumor-Related Death Following Esophagectomy for Esophageal Cancer. Cancers 2020, 12, 2066. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- NCCN. Esophageal and Esophagogastric Junction Cancers. Available online: https://www.nccn.org/professionals/physician_gls/PDF/esophageal.pdf (accessed on 15 February 2021).
- Huang, C.; Huang, D.; Zhu, Y.; Xie, G.; Wang, H.; Shi, J.; Jia, B.; Yuan, Y.; Zhang, W. Comparison of a Concurrent Fluorouracil-Based Regimen and a Taxane-Based Regimen Combined with Radiotherapy in Elderly Patients with Esophageal Squamous Cell Carcinoma. Transl. Oncol. 2020, 13, 100736. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Sim, H.W.; Espin-Garcia, O.; Chan, B.A.; Natori, A.; Lim, C.H.; Moignard, S.; Chen, E.X.; Liu, G.; Darling, G.; et al. Chemoradiotherapy Using Carboplatin plus Paclitaxel versus Cisplatin plus Fluorouracil for Esophageal or Gastroesophageal Junction Cancer. Oncology. 2021, 99, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Haisley, K.R.; Hart, K.D.; Nabavizadeh, N.; Bensch, K.G.; Vaccaro, G.M.; Thomas, C.R., Jr.; Schipper, P.H.; Hunter, J.G.; Dolan, J.P. Neoadjuvant chemoradiotherapy with concurrent cisplatin/5-fluorouracil is associated with increased pathologic complete response and improved survival compared to carboplatin/paclitaxel in patients with locally advanced esophageal cancer. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Wikman, A. Toward improved survivorship: Supportive care needs of esophageal cancer patients, a literature review. Dis. Esophagus 2016, 29, 1081–1089. [Google Scholar] [CrossRef]
- Watanabe, M.; Okamura, A.; Toihata, T.; Yamashita, K.; Yuda, M.; Hayami, M.; Fukudome, I.; Imamura, Y.; Mine, S. Recent progress in perioperative management of patients undergoing esophagectomy for esophageal cancer. Esophagus 2018, 15, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.R.; Markar, S.R.; Baker, C.R.; Soon, Y.; Singh, S.; Low, D.E. Impact of a multidisciplinary standardized clinical pathway on perioperative outcomes in patients with oesophageal cancer. Br. J. Surg. 2013, 100, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’Journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, J.; Plum, P.S.; Buhr, H.; Gockel, I.; Lorenz, D.; Ghadimi, M.; Bruns, C.; Qualitätskommission der Deutschen Gesellschaft für Allgemein- und Viszeralchirurgie. Surgical treatment of esophageal cancer-Indicators for quality in diagnostics and treatment. Chirurg 2021, 92, 350–360. [Google Scholar] [CrossRef]
- Sanford, N.N.; Catalano, P.J.; Enzinger, P.C.; King, B.L.; Bueno, R.; Martin, N.E.; Hong, T.S.; Wo, J.Y.; Mamon, H.J. A retrospective comparison of neoadjuvant chemoradiotherapy regimens for locally advanced esophageal cancer. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Mariette, C.; Haustermans, K.; Obermannova, R.; Arnold, D.; Committee, E.G. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v50–v57. [Google Scholar] [CrossRef]
- Dähn, D.; Martell, J.; Vorwerk, H.; Hess, C.F.; Becker, H.; Jung, K.; Hilgers, R.; Wolff, H.A.; Hermann, R.M.; Christiansen, H. Influence of irradiated lung volumes on perioperative morbidity and mortality in patients after neoadjuvant radiochemotherapy for esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 44–52. [Google Scholar] [CrossRef]
- Wang, S.L.; Liao, Z.; Vaporciyan, A.A.; Tucker, S.L.; Liu, H.; Wei, X.; Swisher, S.; Ajani, J.A.; Cox, J.D.; Komaki, R. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 692–699. [Google Scholar] [CrossRef]
- Martini, S.; Arcadipane, F.; Strignano, P.; Spadi, R.; Contu, V.; Fiandra, C.; Ragona, R.; Catalano, G.; Satolli, M.A.; Camandona, M.; et al. Volumetric modulated arc therapy (VMAT) in the treatment of esophageal cancer patients. Med. Oncol. 2018, 35, 150. [Google Scholar] [CrossRef]
- Minami, H.; Ohe, Y.; Niho, S.; Goto, K.; Ohmatsu, H.; Kubota, K.; Kakinuma, R.; Nishiwaki, Y.; Nokihara, H.; Sekine, I.; et al. Comparison of pharmacokinetics and pharmacodynamics of docetaxel and Cisplatin in elderly and non-elderly patients: Why is toxicity increased in elderly patients? J. Clin. Oncol. 2004, 22, 2901–2908. [Google Scholar] [CrossRef]
- Wakui, R.; Yamashita, H.; Okuma, K.; Kobayashi, S.; Shiraishi, K.; Terahara, A.; Sasano, N.; Ohtomo, K.; Nakagawa, K. Esophageal cancer: Definitive chemoradiotherapy for elderly patients. Dis. Esophagus 2010, 23, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Meadows, H.M.; Lopes, A.; Muirhead, R.; Sebag-Montefiore, D.; Adams, R.; ACTII Study Group. Impact of compliance to chemoradiation on long-term outcomes in squamous cell carcinoma of the anus: Results of a post hoc analysis from the randomised phase III ACT II trial. Ann. Oncol. 2020, 31, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Zhang, W.W.; Sun, J.Y.; Li, F.Y.; Lin, Q.; He, Z.Y. Patterns of Distant Metastasis Between Histological Types in Esophageal Cancer. Front. Oncol. 2018, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, K.; Wang, T.; Li, M.; Li, B.; Li, S.; Yuan, L. The Combination Options and Predictive Biomarkers of PD-1/PD-L1 Inhibitors in Esophageal Cancer. Front. Oncol. 2020, 10, 300. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.X. Prognostic and predictive significance of tumor length in patients with esophageal squamous cell carcinoma undergoing radical resection. BMC Cancer 2016, 16, 394. [Google Scholar] [CrossRef] [PubMed]
- Werner-Wasik, M.; Yorke, E.; Deasy, J.; Nam, J.; Marks, L.B. Radiation dose-volume effects in the esophagus. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S86–S93. [Google Scholar] [CrossRef] [PubMed]
- van der Schaaf, M.K.; Tilanus, H.W.; van Lanschot, J.J.; Johar, A.M.; Lagergren, P.; Lagergren, J.; Wijnhoven, B.P. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J. Thorac. Cardiovasc. Surg. 2014, 147, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Baba, Y.; Shigaki, H.; Harada, K.; Iwatsuki, M.; Kurashige, J.; Sakamoto, Y.; Miyamoto, Y.; Ishimoto, T.; Kosumi, K.; et al. Preoperative Nutritional Assessment by Controlling Nutritional Status (CONUT) is Useful to estimate Postoperative Morbidity After Esophagectomy for Esophageal Cancer. World J. Surg. 2016, 40, 1910–1917. [Google Scholar] [CrossRef]
- Rasmussen, S.R.; Nielsen, R.V.; Fenger, A.S.; Siemsen, M.; Ravn, H.B. Postoperative complications and survival after surgical resection of esophageal squamous cell carcinoma. J. Thorac. Dis. 2018, 10, 4052–4060. [Google Scholar] [CrossRef]
- Meredith, K.L.; Weber, J.M.; Turaga, K.K.; Siegel, E.M.; McLoughlin, J.; Hoffe, S.; Marcovalerio, M.; Shah, N.; Kelley, S.; Karl, R. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann. Surg. Oncol. 2010, 17, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Malthaner, R.A.; Wong, R.K.; Rumble, R.B.; Zuraw, L.; Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based, C. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: A clinical practice guideline. BMC Cancer 2004, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Porschen, R.; Fischbach, W.; Gockel, I.; Hollerbach, S.; Holscher, A.; Jansen, P.L.; Miehlke, S.; Pech, O.; Stahl, M.; Thuss-Patience, P.; et al. S3-Leitlinie—Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus. Z. Gastroenterol. 2019, 57, 336–418. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.M.; Horstmann, O.; Haller, F.; Perske, C.; Christiansen, H.; Hille, A.; Schmidberger, H.; Fuzesi, L. Histomorphological tumor regression grading of esophageal carcinoma after neoadjuvant radiochemotherapy: Which score to use? Dis. Esophagus 2006, 19, 329–334. [Google Scholar] [CrossRef]
- Roila, F.; Herrstedt, J.; Aapro, M.; Gralla, R.J.; Einhorn, L.H.; Ballatori, E.; Bria, E.; Clark-Snow, R.A.; Espersen, B.T.; Feyer, P.; et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann. Oncol. 2010, 21 (Suppl. 5), v232–v243. [Google Scholar] [CrossRef]
- Mandard, A.M.; Dalibard, F.; Mandard, J.C.; Marnay, J.; Henry-Amar, M.; Petiot, J.F.; Roussel, A.; Jacob, J.H.; Segol, P.; Samama, G.; et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, N.I.o.H., National Cancer Institute. Common Terminology Criteria for Adverse Evens (CTCAE), Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 11 February 2021).
- Rubin, P.; Constine, L.S.; Fajardo, L.F.; Phillips, T.L.; Wasserman, T.H. RTOG Late Effects Working Group. Overview. Late Effects of Normal Tissues (LENT) scoring system. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1041–1042. [Google Scholar] [CrossRef]
- Gross, A.; Ziepert, M.; Scholz, M. KMWin—A convenient tool for graphical presentation of results from Kaplan-Meier survival time analysis. PLoS ONE 2012, 7, e38960. [Google Scholar] [CrossRef]
| Characteristics | 5‑Fluorouracil/ Cisplatin (n = 63) | Carboplatin/ Paclitaxel (n = 27) | p-Value |
|---|---|---|---|
| Age, years, median (min, max) | 61.1 (42.3–77.7) | 70.1 (43.7–79.5) | <0.01 |
| ECOG performance status | 0.96 | ||
| 0 | 47 (74.6) | 20 (74.1) | |
| 1 | 16 (25.4) | 7 (25.9) | |
| Charlson Comorbidity Index | 0.02 | ||
| <4 | 23 (36.5) | 3 (11.1) | |
| ≥4 | 40 (63.5) | 24 (88.9) | |
| Female | 13 (20.6) | 10 (37.0) | 0.10 |
| Follow-up, months, median (min, max) | 28.6 (3.6–165.9) | 26.2 (2.1–58.4) | 0.12 |
| Behavioral factors | 0.09 | ||
| Smoking w/o regular alcohol | 22 (34.9) | 14 (51.9) | |
| Alcohol abuse w/o smoking | 6 (9.5) | 1 (3.7) | |
| Smoking and alcohol abuse | 18 (28.6) | 6 (22.2) | |
| Neither smoking nor regular alcohol | 15 (23.8) | 5 (18.5) | |
| Undetermined | 2 (3.2) | 1 (3.7) | |
| Tumor localization | 0.28 | ||
| Upper third | 2 (3.2) | 1 (3.7) | |
| Middle third | 29 (46.0) | 12 (44.4) | |
| Lower third | 32 (50.8) | 14 (51.9) | |
| T category clinical/ultrasound | 0.77 | ||
| T1 | 1 (1.6) | 1 (3.7) | |
| T2 | 6 (9.5) | 8 (29.6) | |
| T3 | 51 (81.0) | 17 (63.0) | |
| T4 | 5 (7.9) | 1 (3.7) | |
| Nodal status clinical/ultrasound | 0.76 | ||
| N0 | 11 (17.5) | 4 (14.8) | |
| N+ | 52 (82.5) | 23 (85.2) | |
| UICC stage [AJCC 8th edition, 2017] | 0.1 | ||
| II | 14 (22.2) | 12 (44.4) | |
| III | 44 (70.0) | 14 (51.9) | |
| IVA | 5 (7.8) | 1 (3.7) | |
| Radiotherapy dose, median (min, max) | 40.0 (39.6–41.4) | 41.4 (41.4–41.4) | |
| Radiotherapy technique | <0.01 | ||
| 3DCRT | 63 (100.0) | 24 (88.9) | |
| VMAT | 0 (0.0) | 3 (11.1) | |
| Surgical technique | 0.73 | ||
| Abdominothoracic esophagus resection | 52 (82.5) | 23 (85.2) | |
| Esophagectomy with cervical anastomosis | 11 (17.5) | 4 (14.8) | |
| ypT stage | 0.28 | ||
| ypT0 | 28 (44.4) | 12 (44.4) | |
| ypT1 | 2 (3.2) | 4 (14.9) | |
| ypT2 | 11 (17.5) | 5 (18.5) | |
| ypT3 | 21 (33.3) | 6 (22.2) | |
| ypT4 | 1 (1.6) | 0 (0.0) | |
| ypN stage | 0.09 | ||
| ypN0 | 48 (76.2) | 16 (59.3) | |
| ypN1 | 13 (20.6) | 7 (25.9) | |
| ypN2 | 2 (3.2) | 4 (14.8) | |
| Resection status: R0 | 63 (100) | 27 (100) | |
| Pathological complete response | 26 (41.3) | 9 (33.3) | 0.48 |
| Characteristics | 5‑Fluorouracil/ Cisplatin (n = 63) | Carboplatin/ Paclitaxel (n = 27) | p-Value |
|---|---|---|---|
| Radiotherapy | - | ||
| Received the planned dose without treatment breaks | 63 (100) | 27 (100) | |
| Chemotherapy | 0.01 | ||
| Received 100% of the planned cycles and dose | 51 (81.0) | 15 (55.6) | |
| Received < 100% of the planned cycles and/or dose | 12 (19.0) | 12 (44.4) | |
| Acute toxicity | |||
| Overall acute organ toxicity | 0.26 | ||
| ≥grade 3 | 16 (25.4) | 10 (37.0) | |
| <grade 3 | 47 (74.6) | 17 (63.0) | |
| Mucositis | 0.74 | ||
| 0 | 61 (96.8) | 24 (88.9) | |
| 1 | 1 (1.6) | 1 (3.7) | |
| 2 | 0 | 1 (3.7) | |
| 3 | 1 (1.6) | 1 (3.7) | |
| Dermatitis | 0.58 | ||
| 0 | 49 (77.8) | 25 (92.6) | |
| 1 | 13 (20.6) | 2 (7.4) | |
| 2 | 1 (1.6) | 0 | |
| Nausea | 0.75 | ||
| 0 | 46 (73.0) | 21 (77.8) | |
| 1 | 11 (17.5) | 2 (7.4) | |
| 2 | 6 (9.5) | 3 (11.1) | |
| 3 | 0 | 1 (3.7) | |
| Dysphagia | 0.18 | ||
| 0 | 2 (3.2) | 1 (3.7) | |
| 1 | 13 (20.6) | 8 (29.6) | |
| 2 | 30 (47.6) | 7 (25.9) | |
| 3 | 15 (23.8) | 11 (40.7) | |
| 4 | 3 (4.8) | 0 | |
| Hematologic toxicity | |||
| Overall hematologic toxicity | 0.04 | ||
| ≥grade 3 | 11 (17.5) | 10 (37.0) | |
| <grade 3 | 52 (82.5) | 17 (63.0) | |
| Anaemia | 0.40 | ||
| 0 | 10 (15.9) | 4 (14.8) | |
| 1 | 43 (68.3) | 18 (66.7) | |
| 2 | 6 (9.5) | 4 (14.8) | |
| 3 | 4 (6.3) | 1 (3.7) | |
| Leukopenia | 0.29 | ||
| 0 | 18 (28.6) | 3 (11.1) | |
| 1 | 21 (33.3) | 7 (25.9) | |
| 2 | 17 (27.0) | 7 (25.9) | |
| 3 | 6 (9.5) | 9 (33.3) | |
| 4 | 1 (1.6) | 1 (3.7) | |
| Thrombopenia | 0.53 | ||
| 0 | 40 (63.5) | 17 (63.0) | |
| 1 | 20 (31.7) | 8 (29.6) | |
| 2 | 2 (3.2) | 1 (3.7) | |
| 3 | 1 (1.6) | 1 (3.7) |
| Characteristics | 5‑Fluorouracil/ Cisplatin (n = 63) | Carboplatin/ Paclitaxel (n = 27) | p-Value |
|---|---|---|---|
| Surgical outcomes | |||
| Surgical complications | 0.43 | ||
| Yes | 30 (48.4) | 15 (57.7) | |
| No | 32 (51.6) | 11 (42.3) | |
| Hospital stay, days, median (min, max) | 19 (7–87) | 15 (8–140) | 0.24 |
| Intensive/intermediate care unit stay, days, median (min, max) | 7 (1–54) | 4 (1–115) | 0.01 |
| Death within 30 days post-surgery | 1 (1.6) | 1 (3.7) | 0.53 |
| Late toxicity | |||
| Late dysphagia | 0.58 | ||
| 0 | 55 (87.3) | 18 (85.7) | |
| 1 | 6 (9.5) | 3 (11.1) | |
| 2 | 2 (3.2) | 0 | |
| Dermatitis | 0.52 | ||
| 0 | 62 (98.4) | 27 (100.0) | |
| 1 | 1 (1.6) | 0 |
| Variable | LRC | PFS | OS | CSS | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age (per year) | 1.04 (0.98–1.12) | 0.23 | 1.00 (0.97–1.03) | 0.88 | 1.01 (0.98–1.04) | 0.55 | 1.0 (0.96–1.05) | 0.85 |
| T stage: u/cT3-4 (74) vs. u/cT1-2 (16) | 0.88 (1.87–4.16) | 0.87 | 1.36 (0.57–3.22) | 0.49 | 1.89 (0.67–5.31) | 0.23 | 0.93 (0.31–2.76) | 0.89 |
| cN stage: cN+ (75) vs. cN0 (15) | 1.69 (0.21–13.39) | 0.62 | 0.92 (0.41–2.08) | 0.85 | 0.87 (0.38–1.97) | 0.74 | 0.46 (0.18–1.19) | 0.11 |
| Chemotherapy | ||||||||
| Carboplatin/paclitaxel (27) vs. 5-fluorouracil/cisplatin (63) | 2.19 (0.59–8.18) | 0.24 | 0.73 (0.35–1.54) | 0.41 | 0.76 (0.35–1.68) | 0.50 | 0.50 (0.01–1.71) | 0.27 |
| Chemotherapy complete: yes (66) vs. no (24) | 1.46 (0.38–5.69) | 0.58 | 1.49 (0.78–2.86) | 0.23 | 1.49 (0.76–2.95) | 0.25 | 1.73 (0.69–4.31) | 0.24 |
| pCR: yes (35) vs. no (55) | 0.08 (0.01–0.66) | 0.02 | 0.25 (0.13–0.50) | <0.01 | 0.19 (0.08–0.42) | <0.01 | 0.14 (0.04–0.48) | <0.01 |
| Acute organ toxicity ≥ III°: yes (26) vs. no (64) | 2.43 (0.68–8.71) | 0.17 | 1.94 (1.06–3.56) | 0.03 | 1.78 (0.93–3.42) | 0.08 | 2.58 (1.08–6.15) | 0.03 |
| Maximal dysphagia ≥ III° during RCT: yes (29) vs. no (61) | 2.75 (0.79–9.64) | 0.11 | 1.67 (0.91–3.04) | 0.09 | 1.36 (0.71–2.63) | 0.35 | 3.47 (1.45–8.29) | <0.01 |
| Acute hematologic/organ toxicity ≥ III°: yes (38) vs. no (52) | 1.30 (0.37–4.64) | 0.69 | 1.54 (0.86–2.76) | 0.15 | 1.52 (0.82–2.85) | 0.19 | 2.58 (1.06–6.13) | 0.03 |
| ICU/IMC stay: ≥7 (31) vs. ≤6 (50) days | 1.34 (0.36–4.99) | 0.67 | 1.87 (1.01–3.49) | <0.05 | 1.78 (0.93–3.39) | 0.08 | 2.56 (1.05–6.28) | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dröge, L.H.; Karras, P.J.; Guhlich, M.; Schirmer, M.A.; Ghadimi, M.; Rieken, S.; Conradi, L.-C.; Leu, M. Preoperative Radiochemotherapy in Esophageal Squamous Cell Cancer with 5-Fluorouracil/Cisplatin or Carboplatin/Paclitaxel: Treatment Practice over a 20-Year Period and Implications for the Individual Treatment Modalities. Cancers 2021, 13, 1834. https://doi.org/10.3390/cancers13081834
Dröge LH, Karras PJ, Guhlich M, Schirmer MA, Ghadimi M, Rieken S, Conradi L-C, Leu M. Preoperative Radiochemotherapy in Esophageal Squamous Cell Cancer with 5-Fluorouracil/Cisplatin or Carboplatin/Paclitaxel: Treatment Practice over a 20-Year Period and Implications for the Individual Treatment Modalities. Cancers. 2021; 13(8):1834. https://doi.org/10.3390/cancers13081834
Chicago/Turabian StyleDröge, Leif Hendrik, Philipp Johannes Karras, Manuel Guhlich, Markus Anton Schirmer, Michael Ghadimi, Stefan Rieken, Lena-Christin Conradi, and Martin Leu. 2021. "Preoperative Radiochemotherapy in Esophageal Squamous Cell Cancer with 5-Fluorouracil/Cisplatin or Carboplatin/Paclitaxel: Treatment Practice over a 20-Year Period and Implications for the Individual Treatment Modalities" Cancers 13, no. 8: 1834. https://doi.org/10.3390/cancers13081834
APA StyleDröge, L. H., Karras, P. J., Guhlich, M., Schirmer, M. A., Ghadimi, M., Rieken, S., Conradi, L.-C., & Leu, M. (2021). Preoperative Radiochemotherapy in Esophageal Squamous Cell Cancer with 5-Fluorouracil/Cisplatin or Carboplatin/Paclitaxel: Treatment Practice over a 20-Year Period and Implications for the Individual Treatment Modalities. Cancers, 13(8), 1834. https://doi.org/10.3390/cancers13081834








