Are In Vitro Human Blood–Brain–Tumor-Barriers Suitable Replacements for In Vivo Models of Brain Permeability for Novel Therapeutics?
Abstract
Simple Summary
Abstract
1. Introduction
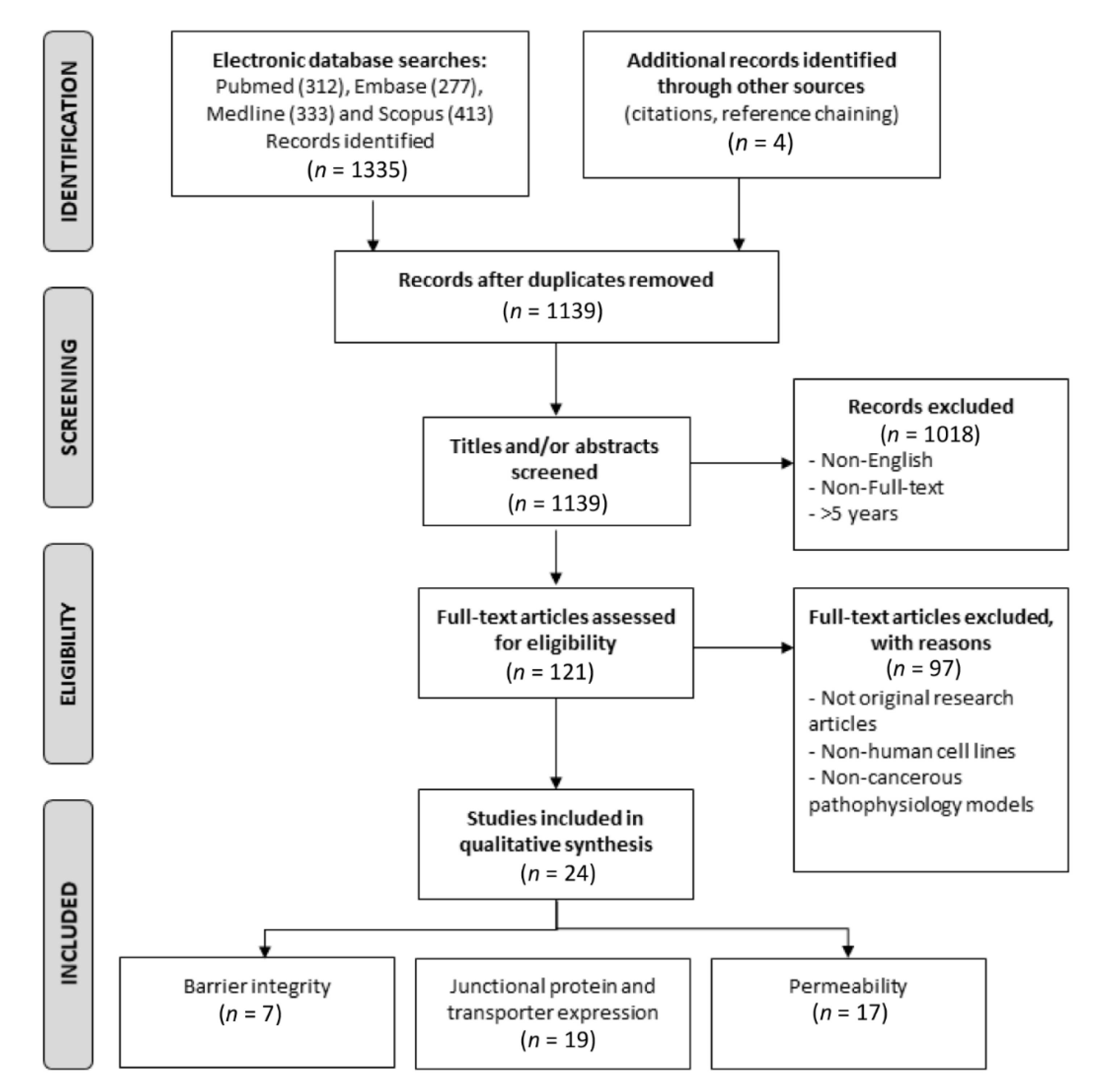
2. Materials and Methods
Caveat
3. Results
3.1. In Vitro BBB/BBTB Models
3.2. In Vivo BBB/BBTB Models
3.3. How Well Do In Vitro BBB/BBTB Mimic In Vivo and Clinical Physiology?
3.3.1. BBB Architecture
3.3.2. Barrier Integrity
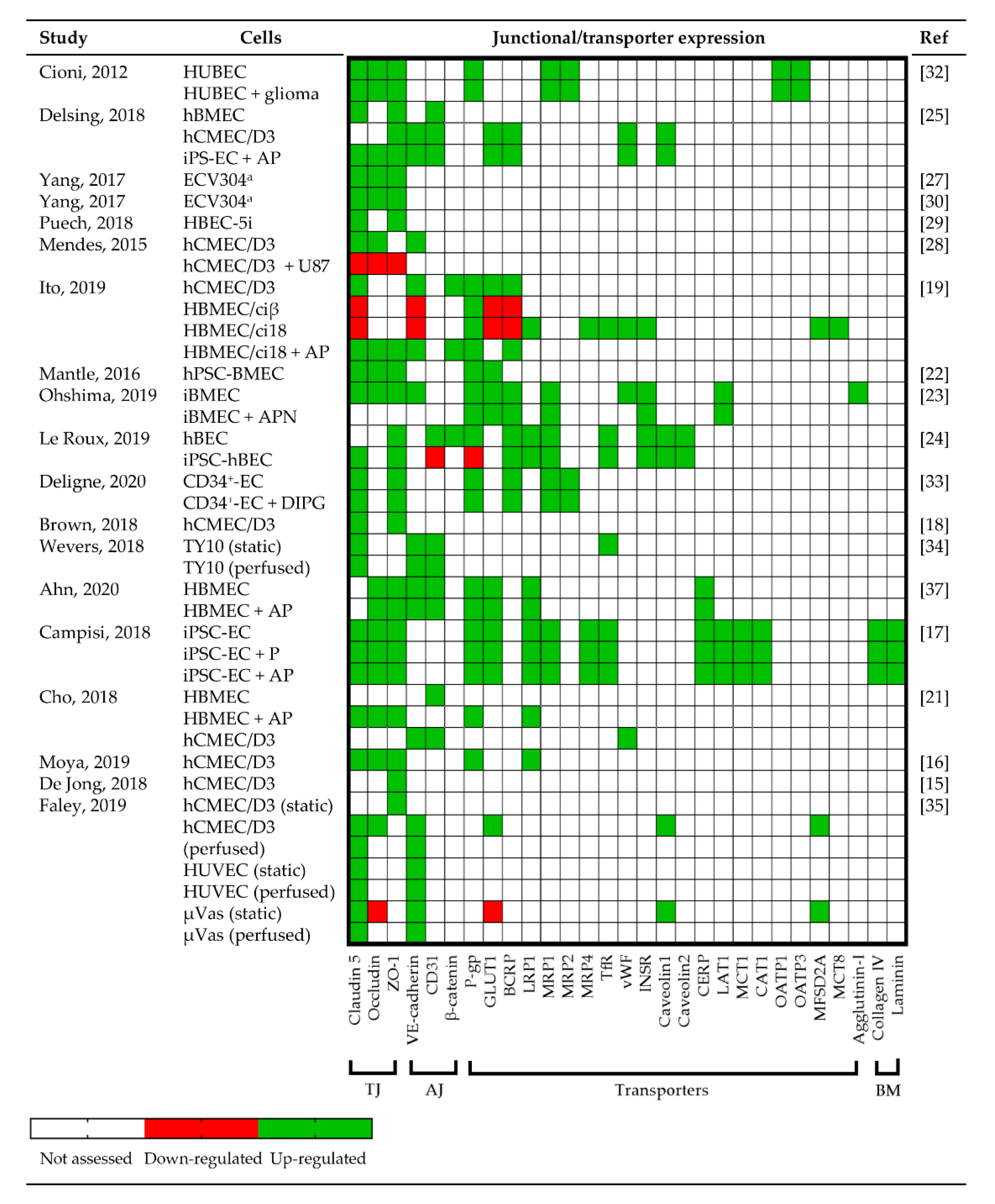
3.3.3. Junctional Protein and Transporter Expression
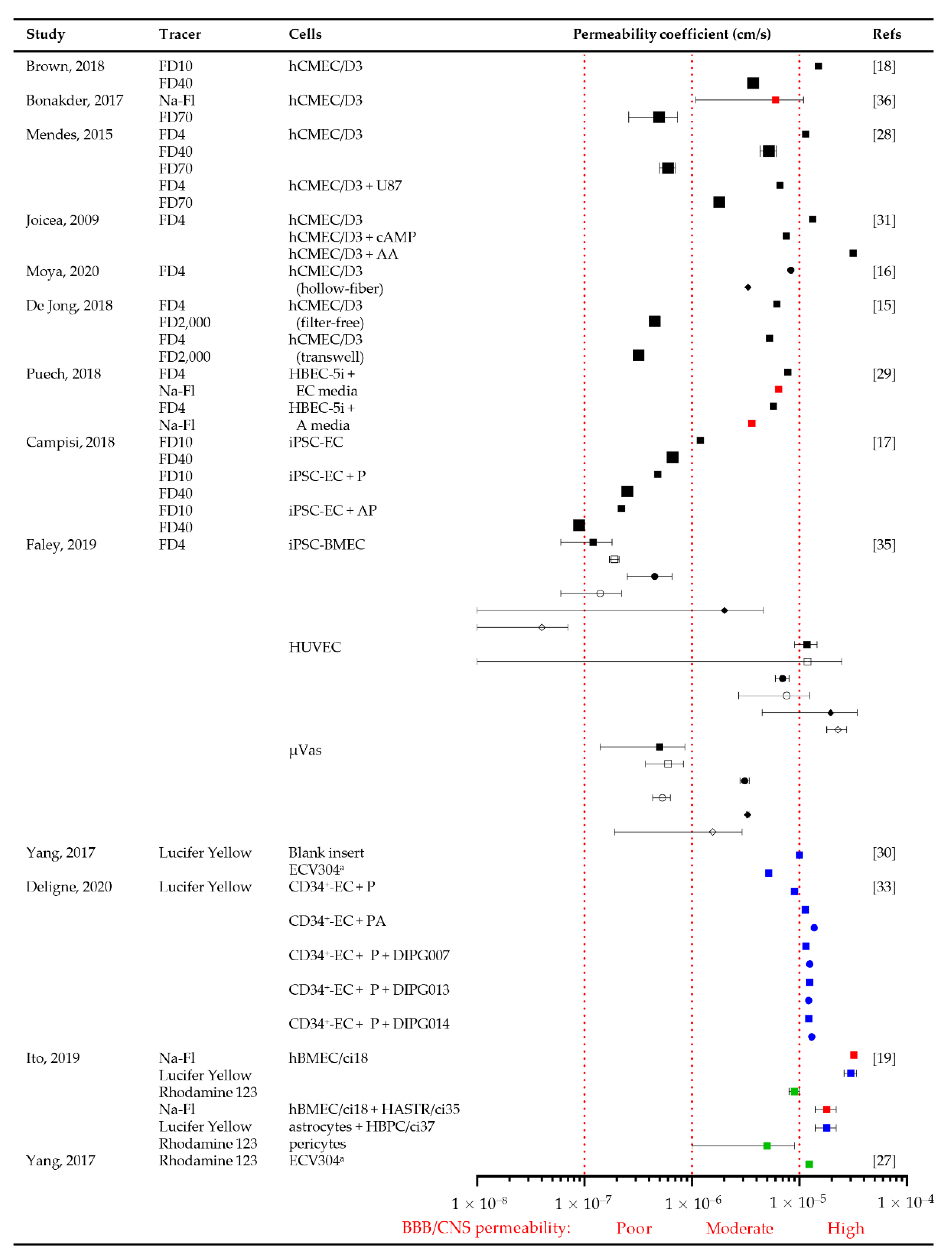
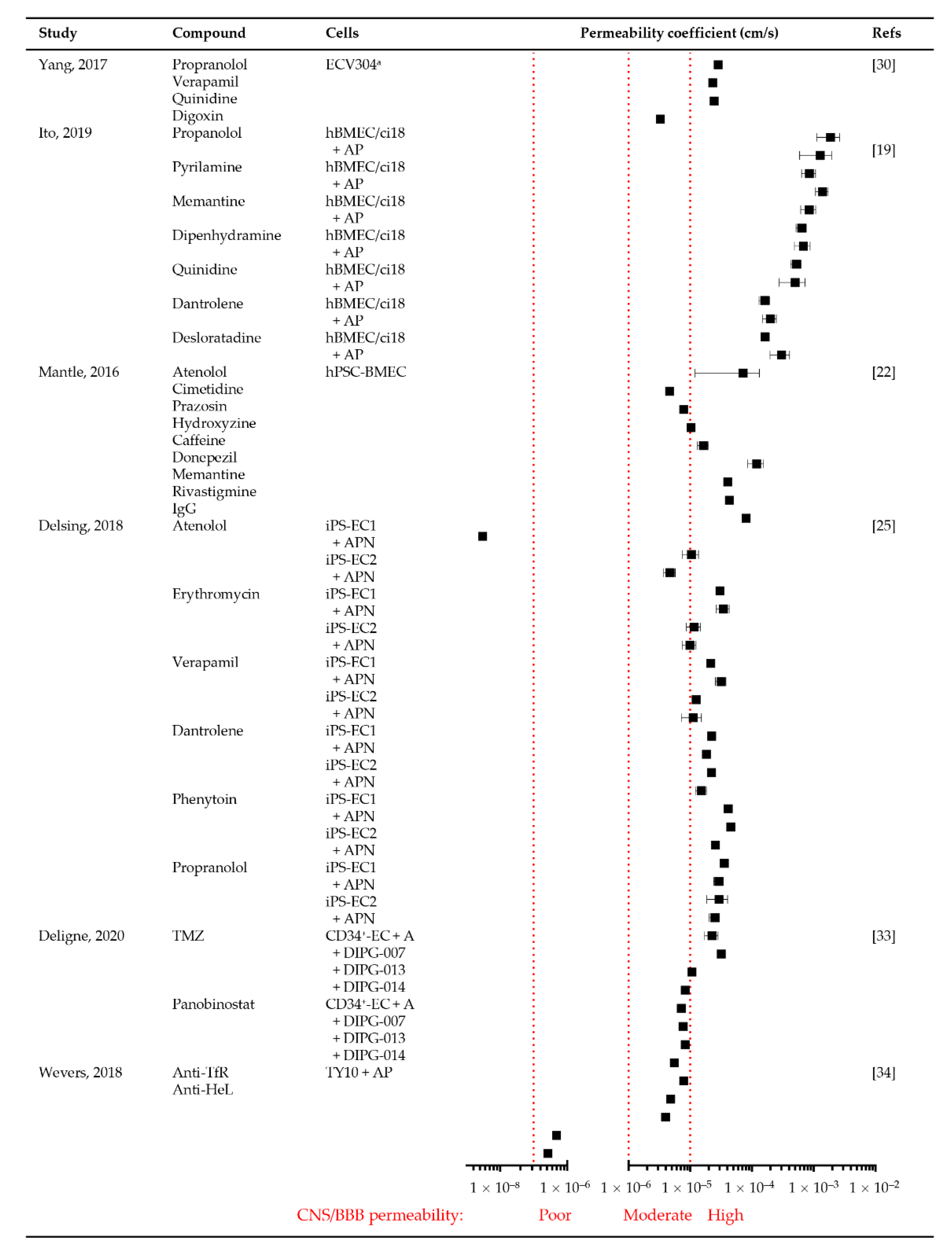
3.3.4. Permeability
3.4. Comparison of In Vitro BBB/BBTB Models with Clinical Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suryadevara, R.; Fadel, H.; Michelhaugh, S.K.; Mittal, S.; Parajuli, P. Tumors of the Central Nervous System: Anatomy and Interventional Considerations. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors, 1st ed.; Kesharwani, P., Gupta, U., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood Brain Barrier: A Challenge for Effectual Therapy of Brain Tumors. BioMed Res. Int. 2015, 2015, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Erhart, F.; Hackl, M.; Hahne, H.; Buchroithner, J.; Meng, C.; Klingenbrunner, S.; Reitermaier, R.; Fischhuber, K.; Skalicky, S.; Berger, W.; et al. Combined proteomics/miRNomics of dendritic cell immunotherapy-treated glioblastoma patients as a screening for survival-associated factors. npj Vaccines 2020, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Noureldine, M.H.A.; Shimony, N.; Jallo, G.I. Diffuse Midline Glioma—Diffuse Intrinsic Pontine Glioma. In Brainstem Tumors; Jallo, G.I., Noureldine, M.H.A., Shimony, N., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Tivnan, A.; Heilinger, T.; Lavelle, E.C.; Prehn, J.H.M. Advances in immunotherapy for the treatment of glioblastoma. J. Neuro-Oncology 2017, 131, 1–9. [Google Scholar] [CrossRef]
- Haddad-Tóvolli, R.; Dragano, N.R.V.; Ramalho, A.F.S.; Velloso, L.A. Development and Function of the Blood-Brain Barrier in the Context of Metabolic Control. Front. Neurosci. 2017, 11, 224. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit - concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.; Wesseling, P.; Wurdinger, T.; de Vries, H. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Passeleu-Le Bourdonnec, C.; Carrupt, P.-A.; Scherrmann, J.M.; Martel, S. Methodologies to Assess Drug Permeation Through the Blood–Brain Barrier for Pharmaceutical Research. Pharm. Res. 2013, 30, 2729–2756. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, 1–6. [Google Scholar] [CrossRef]
- De Jong, E.; Williams, D.S.; Abdelmohsen, L.K.E.A.; Van Hest, J.C.M.; Zuhorn, I.S. A filter-free blood-brain barrier model to quantitatively study transendothelial delivery of nanoparticles by fluorescence spectroscopy. J. Control. Release 2018, 289, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.L.; Triplett, M.; Simon, M.; Alvarado, J.; Booth, R.; Osburn, J.; Soscia, D.; Qian, F.; Fischer, N.O.; Kulp, K.; et al. A Reconfigurable In Vitro Model for Studying the Blood–Brain Barrier. Ann. Biomed. Eng. 2019, 48, 780–793. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Brown, T.D.; Nowak, M.; Bayles, A.V.; Prabhakarpandian, B.; Karande, P.; Lahann, J.; Helgeson, M.E.; Mitragotri, S. A microfluidic model of human brain (μHuB) for assessment of blood brain barrier. Bioeng. Transl. Med. 2018, 4, e10126. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Umehara, K.; Suzuki, S.; Kitamura, K.; Nunoya, K.-I.; Yamaura, Y.; Imawaka, H.; Izumi, S.; Wakayama, N.; Komori, T.; et al. A Human Immortalized Cell-Based Blood–Brain Barrier Triculture Model: Development and Characterization as a Promising Tool for Drug−Brain Permeability Studies. Mol. Pharm. 2019, 16, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef]
- Cho, C.-F.; Wolfe, J.M.; Fadzen, C.M.; Calligaris, D.; Hornburg, K.; Chiocca, E.A.; Agar, N.Y.R.; Pentelute, B.L.; Lawler, S.E. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017, 8, 15623. [Google Scholar] [CrossRef]
- Mantle, J.L.; Min, L.; Lee, K.H. Minimum Transendothelial Electrical Resistance Thresholds for the Study of Small and Large Molecule Drug Transport in a Human in Vitro Blood–Brain Barrier Model. Mol. Pharm. 2016, 13, 4191–4198. [Google Scholar] [CrossRef]
- Ohshima, M.; Kamei, S.; Fushimi, H.; Mima, S.; Yamada, T.; Yamamoto, T. Prediction of Drug Permeability Using In Vitro Blood–Brain Barrier Models with Human Induced Pluripotent Stem Cell-Derived Brain Microvascular Endothelial Cells. BioResearch Open Access 2019, 8, 200–209. [Google Scholar] [CrossRef]
- Le Roux, G.; Jarray, R.; Guyot, A.-C.; Pavoni, S.; Costa, N.; Théodoro, F.; Nassor, F.; Pruvost, A.; Tournier, N.; Kiyan, Y.; et al. Proof-of-Concept Study of Drug Brain Permeability Between in Vivo Human Brain and an in Vitro iPSCs-Human Blood-Brain Barrier Model. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Delsing, L.; Dönnes, P.; Sánchez, J.; Clausen, M.; Voulgaris, D.; Falk, A.; Herland, A.; Brolén, G.; Zetterberg, H.; Hicks, R.; et al. Barrier Properties and Transcriptome Expression in Human iPSC-Derived Models of the Blood-Brain Barrier. STEM CELLS 2018, 36, 1816–1827. [Google Scholar] [CrossRef]
- Kulczar, C.; Lubin, K.E.; Lefebvre, S.; Miller, D.W.; Knipp, G.T. Development of a direct contact astrocyte-human cerebral microvessel endothelial cells blood–brain barrier coculture model. J. Pharm. Pharmacol. 2017, 69, 1684–1696. [Google Scholar] [CrossRef]
- Yang, S.; Mei, S.; Jin, H.; Zhu, B.; Tian, Y.; Huo, J.; Cui, X.; Guo, A.; Zhao, Z. Identification of two immortalized cell lines, ECV304 and bEnd3, for in vitro permeability studies of blood-brain barrier. PLoS ONE 2017, 12, e0187017. [Google Scholar] [CrossRef]
- Mendes, B.; Marques, C.; Carvalho, I.; Costa, P.; Martins, S.; Ferreira, D.; Sarmento, B. Influence of glioma cells on a new co-culture in vitro blood–brain barrier model for characterization and validation of permeability. Int. J. Pharm. 2015, 490, 94–101. [Google Scholar] [CrossRef]
- Puech, C.; Hodin, S.; Forest, V.; He, Z.; Mismetti, P.; Delavenne, X.; Perek, N. Assessment of HBEC-5i endothelial cell line cultivated in astrocyte conditioned medium as a human blood-brain barrier model for ABC drug transport studies. Int. J. Pharm. 2018, 551, 281–289. [Google Scholar] [CrossRef]
- Yang, S.; Jin, H.; Zhao, Z. An ECV304 monoculture model for permeability assessment of blood–brain barrier. Neurol. Res. 2017, 40, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Joice, S.L.; Mydeen, F.; Couraud, P.-O.; Weksler, B.B.; Romero, I.A.; Fraser, P.A.; Easton, A.S. Modulation of blood–brain barrier permeability by neutrophils: In vitro and in vivo studies. Brain Res. 2009, 1298, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Cioni, C.; Turlizzi, E.; Zanelli, U.; Oliveri, G.; Annunziata, P. Expression of Tight Junction and Drug Efflux Transporter Proteins in an in vitro Model of Human Blood–Brain Barrier. Front. Psychiatry 2012, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Deligne, C.; Hachani, J.; Duban-Deweer, S.; Meignan, S.; Leblond, P.; Carcaboso, A.M.; Sano, Y.; Shimizu, F.; Kanda, T.; Gosselet, F.; et al. Development of a human in vitro blood–brain tumor barrier model of diffuse intrinsic pontine glioma to better understand the chemoresistance. Fluids Barriers CNS 2020, 17, 37. [Google Scholar] [CrossRef]
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; Van Vught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G.; et al. A perfused human blood–brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Faley, S.L.; Neal, E.H.; Wang, J.X.; Bosworth, A.M.; Weber, C.M.; Balotin, K.M.; Lippmann, E.S.; Bellan, L.M. iPSC-Derived Brain Endothelium Exhibits Stable, Long-Term Barrier Function in Perfused Hydrogel Scaffolds. Stem Cell Rep. 2019, 12, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, M.; Graybill, P.M.; Davalos, R.V. A microfluidic model of the blood–brain barrier to study permeabilization by pulsed electric fields. RSC Adv. 2017, 7, 42811–42818. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; Macdonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Luhach, K.; Kulkarni, G.T. 4—In vitro and in vivo models of BBB to evaluate brain targeting drug delivery. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 53–101. [Google Scholar] [CrossRef]
- Elbakary, B.; Badhan, R.K.S. A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Sci. Rep. 2020, 10, 3788. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Rosa, S.; Praça, C.; Pitrez, P.R.; Gouveia, P.J.; Aranguren, X.L.; Ricotti, L.; Ferreira, L.S. Functional characterization of iPSC-derived arterial- and venous-like endothelial cells. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.V.; Garwood, C.J.; Jennings, L.; Simpson, J.E.; Castelli, L.M.; Heath, P.R.; Mihaylov, S.R.; Vaquéz-Villaseñor, I.; Minshull, T.C.; Ince, P.G.; et al. Proteomic and cellular localisation studies suggest non-tight junction cytoplasmic and nuclear roles for occludin in astrocytes. Eur. J. Neurosci. 2018, 47, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zeng, M.; Sun, Y.; Fu, B.M. Quantification of Blood-Brain Barrier Solute Permeability and Brain Transport by Multiphoton Microscopy. J. Biomech. Eng. 2014, 136, 031005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Lv, Y.; Zeng, M.; Fu, B.M. Non-invasive measurement of solute permeability in cerebral microvessels of the rat. Microvasc. Res. 2009, 77, 166–173. [Google Scholar] [CrossRef]
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef]
- Takata, F.; Dohgu, S.; Yamauchi, A.; Matsumoto, J.; Machida, T.; Fujishita, K.; Shibata, K.; Shinozaki, Y.; Sato, K.; Kataoka, Y.; et al. In Vitro Blood-Brain Barrier Models Using Brain Capillary Endothelial Cells Isolated from Neonatal and Adult Rats Retain Age-Related Barrier Properties. PLoS ONE 2013, 8, e55166. [Google Scholar] [CrossRef]
- Brighi, C.; Reid, L.; Genovesi, L.A.; Kojic, M.; Millar, A.; Bruce, Z.; White, A.L.; Day, B.W.; Rose, S.; Whittaker, A.K.; et al. Comparative study of preclinical mouse models of high-grade glioma for nanomedicine research: The importance of producing blood-brain barrier heterogeneity. Theranostics 2020, 10, 6361–6371. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Houghton, S.; Magdeldin, T.; Durán, J.G.B.; Minotti, A.P.; Snead, A.; Sproul, A.; Nguyen, D.-H.T.; Xiang, J.; Fine, H.A.; et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc. Natl. Acad. Sci. 2021, 118, e2016950118. [Google Scholar] [CrossRef]
- Jiang, L.; Li, S.; Zheng, J.; Li, Y.; Huang, H. Recent Progress in Microfluidic Models of the Blood-Brain Barrier. Micromachines 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Song, H.W.; Foreman, K.L.; Gastfriend, B.D.; Kuo, J.S.; Palecek, S.P.; Shusta, E.V. Transcriptomic comparison of human and mouse brain microvessels. Sci. Rep. 2020, 10, 12358. [Google Scholar] [CrossRef] [PubMed]
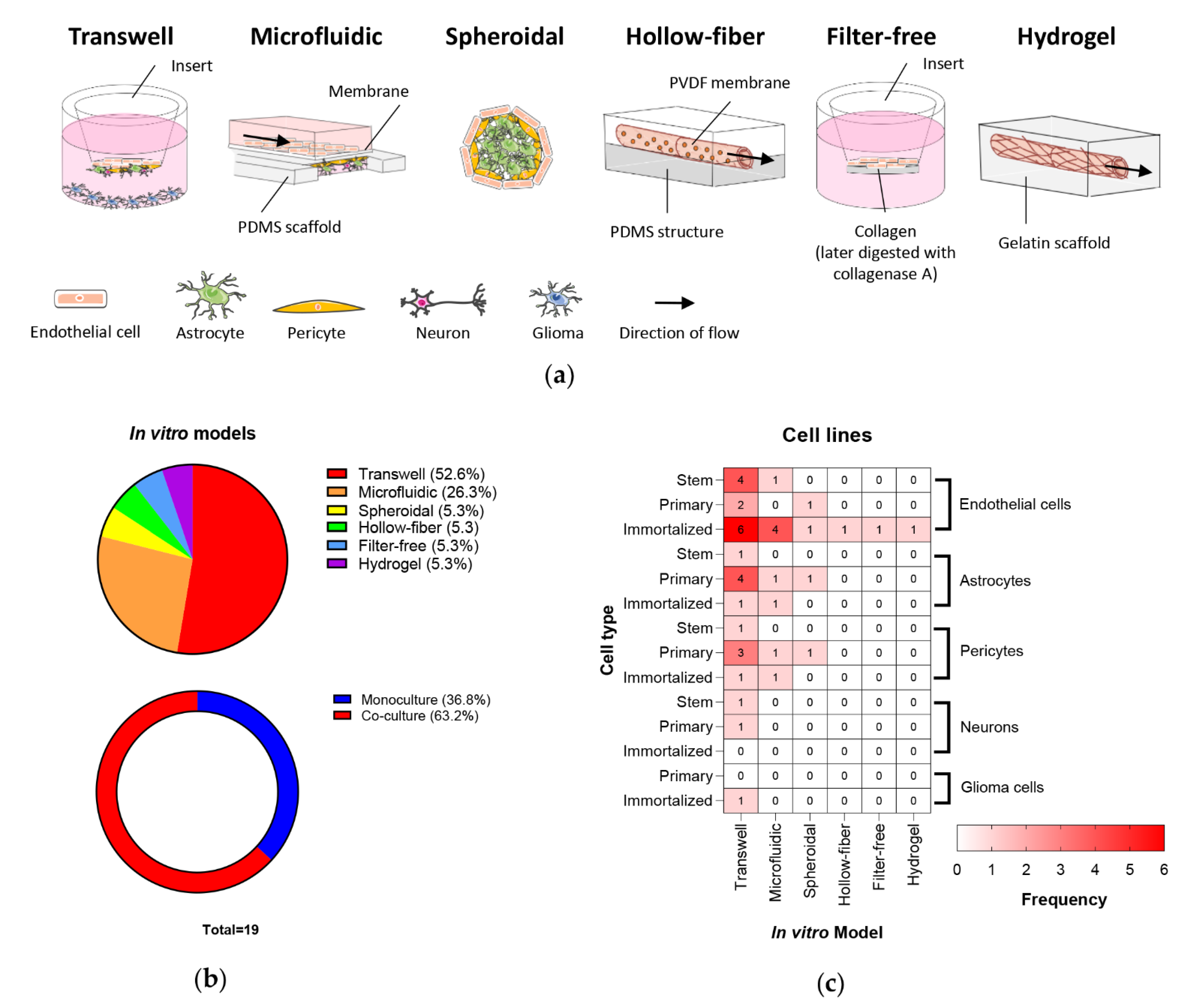

| Model | Advantages | Disadvantages | Refs |
|---|---|---|---|
| Transwell |
|
| [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] |
| Microfluidic |
|
| [17,18,20,21,34,35,36,37] |
| Spheroid |
|
| [17,20,21] |
| Hollow-fiber |
|
| [16] |
| Filter-free |
|
| [15] |
| Hydrogel scaffold |
|
| [35] |
| Model | Advantages | Disadvantages | Refs |
|---|---|---|---|
| Single/Internal Carotid Artery Perfusion |
|
| [18,25] |
| Intravenous Tail or Cannulated Femoral Vein Injections |
|
| [18,25] |
| Intracerebral Microdialysis or Cerebral Open Flow Microperfusion |
|
| [38] |
| Primary Cell-Derived Xenograft |
|
| [25,33] |
| Genetically Engineered Mouse Model |
|
| [33] |
| Culture Type | Lateral Vessel Diameter (μm) | Transverse Vessel Diameter (μm) | Vascular Branch Length (μm) |
|---|---|---|---|
| EC | 108 ± 14 | 29 ± 10 | 226 ± 40 |
| EC + A | 64 ± 13 | 27 ± 7 | 179 ± 31 |
| EC + AP | 42 ± 13 | 25 ± 6 | 136 ± 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prashanth, A.; Donaghy, H.; Stoner, S.P.; Hudson, A.L.; Wheeler, H.R.; Diakos, C.I.; Howell, V.M.; Grau, G.E.; McKelvey, K.J. Are In Vitro Human Blood–Brain–Tumor-Barriers Suitable Replacements for In Vivo Models of Brain Permeability for Novel Therapeutics? Cancers 2021, 13, 955. https://doi.org/10.3390/cancers13050955
Prashanth A, Donaghy H, Stoner SP, Hudson AL, Wheeler HR, Diakos CI, Howell VM, Grau GE, McKelvey KJ. Are In Vitro Human Blood–Brain–Tumor-Barriers Suitable Replacements for In Vivo Models of Brain Permeability for Novel Therapeutics? Cancers. 2021; 13(5):955. https://doi.org/10.3390/cancers13050955
Chicago/Turabian StylePrashanth, Archana, Heather Donaghy, Shihani P. Stoner, Amanda L. Hudson, Helen R. Wheeler, Connie I. Diakos, Viive M. Howell, Georges E. Grau, and Kelly J. McKelvey. 2021. "Are In Vitro Human Blood–Brain–Tumor-Barriers Suitable Replacements for In Vivo Models of Brain Permeability for Novel Therapeutics?" Cancers 13, no. 5: 955. https://doi.org/10.3390/cancers13050955
APA StylePrashanth, A., Donaghy, H., Stoner, S. P., Hudson, A. L., Wheeler, H. R., Diakos, C. I., Howell, V. M., Grau, G. E., & McKelvey, K. J. (2021). Are In Vitro Human Blood–Brain–Tumor-Barriers Suitable Replacements for In Vivo Models of Brain Permeability for Novel Therapeutics? Cancers, 13(5), 955. https://doi.org/10.3390/cancers13050955








