Prostaglandin E2 Receptor 4 (EP4) as a Therapeutic Target to Impede Breast Cancer-Associated Angiogenesis and Lymphangiogenesis
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Physiological Roles of Angiogenesis and Lymphangiogenesis
1.2. Molecular Regulators of Angiogenesis and Lymphangiogenesis
1.2.1. Angiogenesis: Molecular Regulators
1.2.2. Lymphangiogenesis: Molecular Regulators
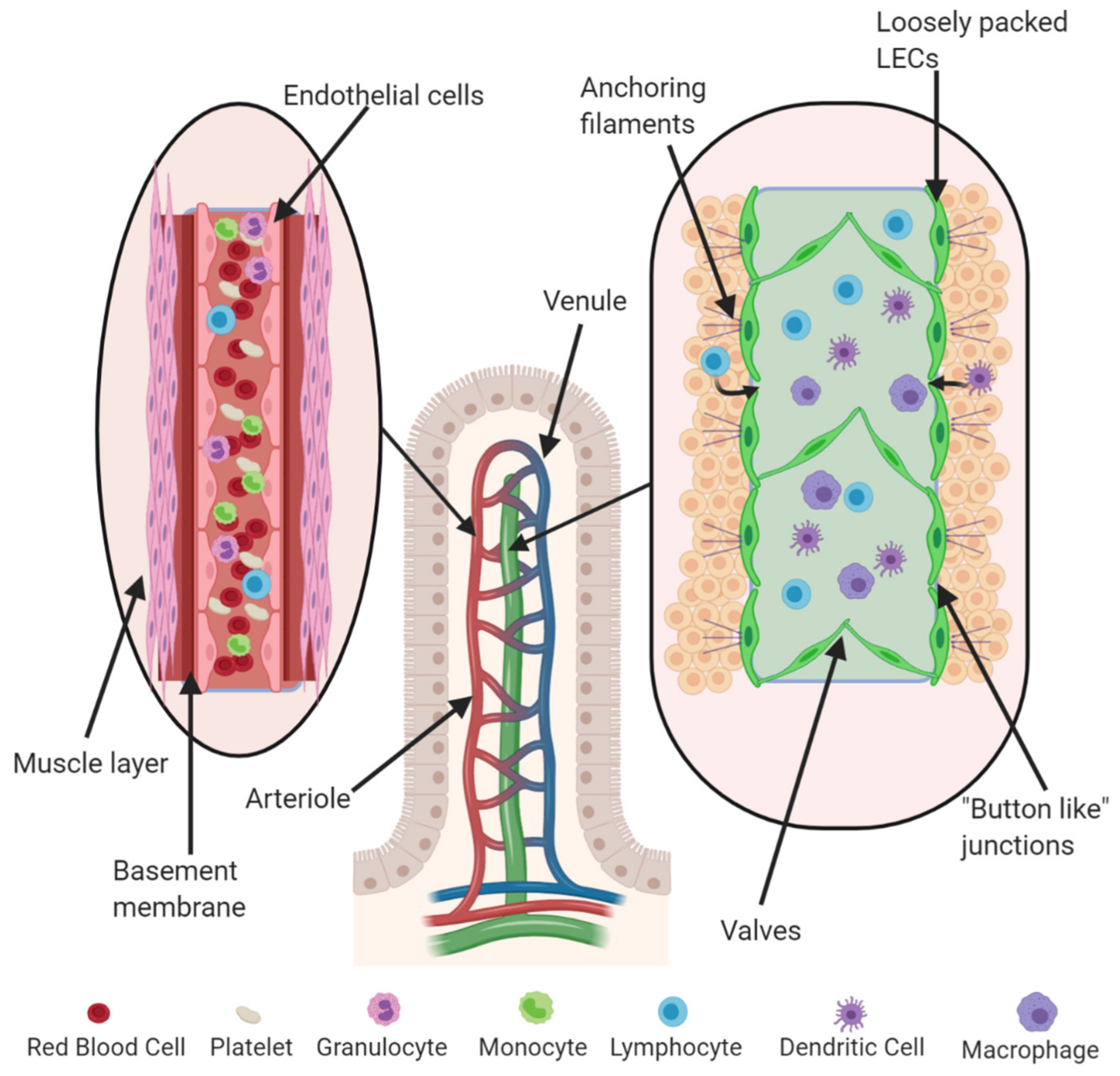
1.3. Experimental Models to Investigate Angiogenesis and Lymphangiogenesis
1.3.1. Angiogenesis Models: In Vitro and In Vivo
1.3.2. Lymphangiogenesis Models: In Vitro and In Vivo
1.4. Roles of COX-2/PGE2 in Angiogenesis and Lymphangiogenesis
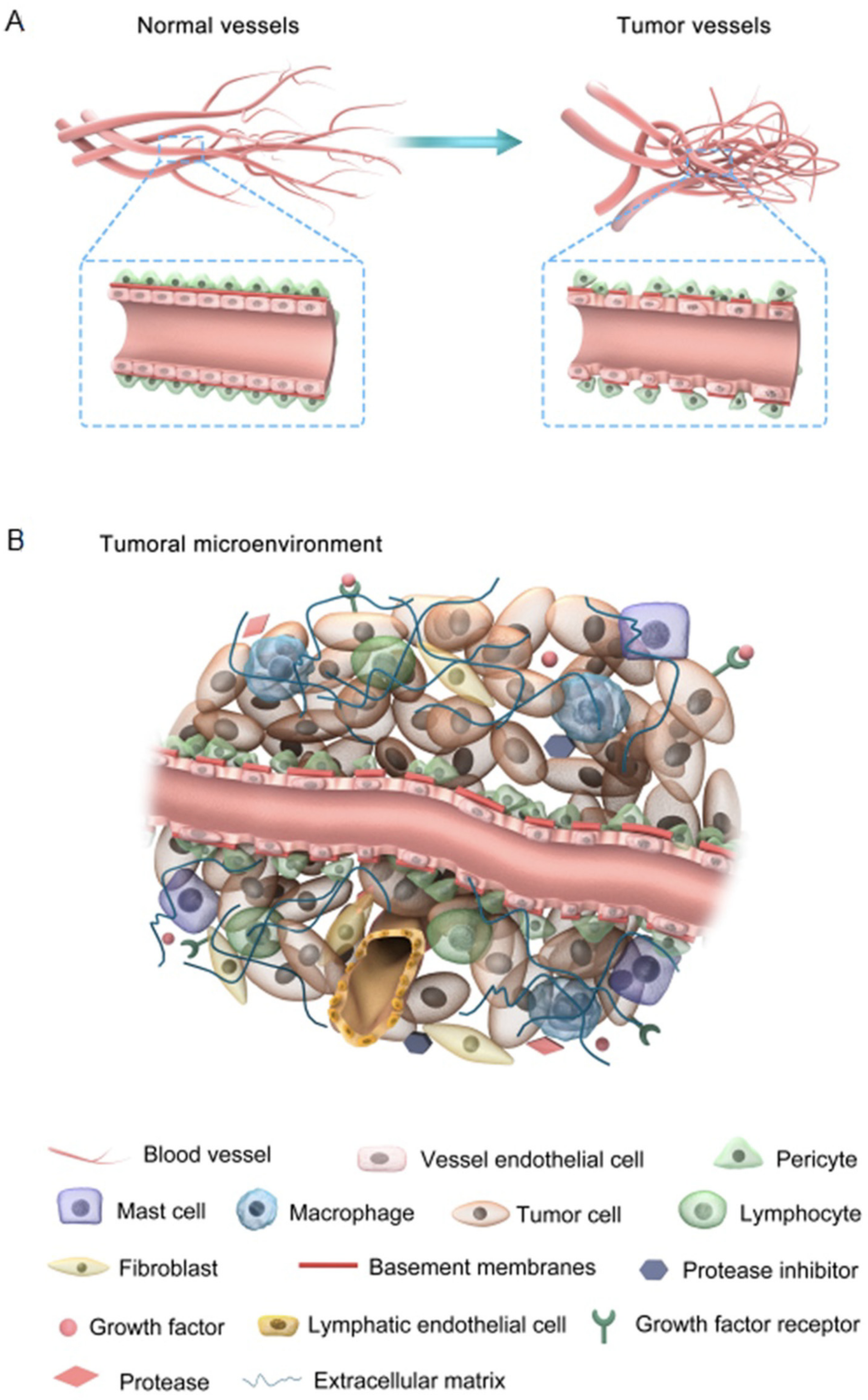
2. Tumor-Associated Angiogenesis and Lymphangiogenesis: Hijacking Inflammatory Mediators
2.1. Tumor-Associated Angiogenesis (TAA)
2.2. Tumor-Associated Lymphangiogenesis (TAL)
2.3. Roles of COX-2-Mediated TAA and TAL in Tumor Cell Survival, Nutrition, and Metastasis
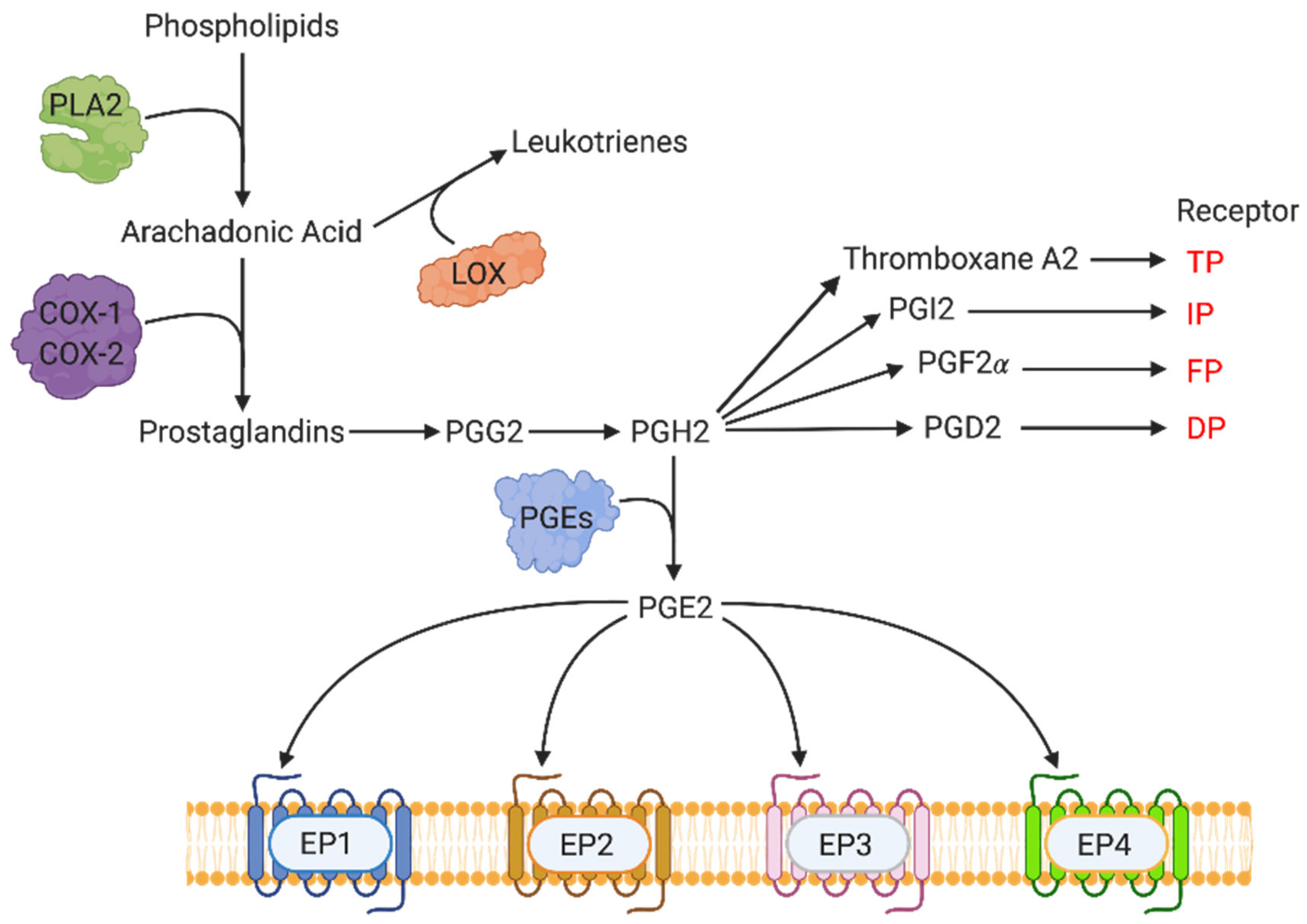
3. COX-2-Mediated Molecular Pathways: Key Events in the Synthesis of Prostanoids
3.1. EP Receptors and Molecular Signaling Pathways
3.2. COX-2/EP Receptors and Breast Cancer
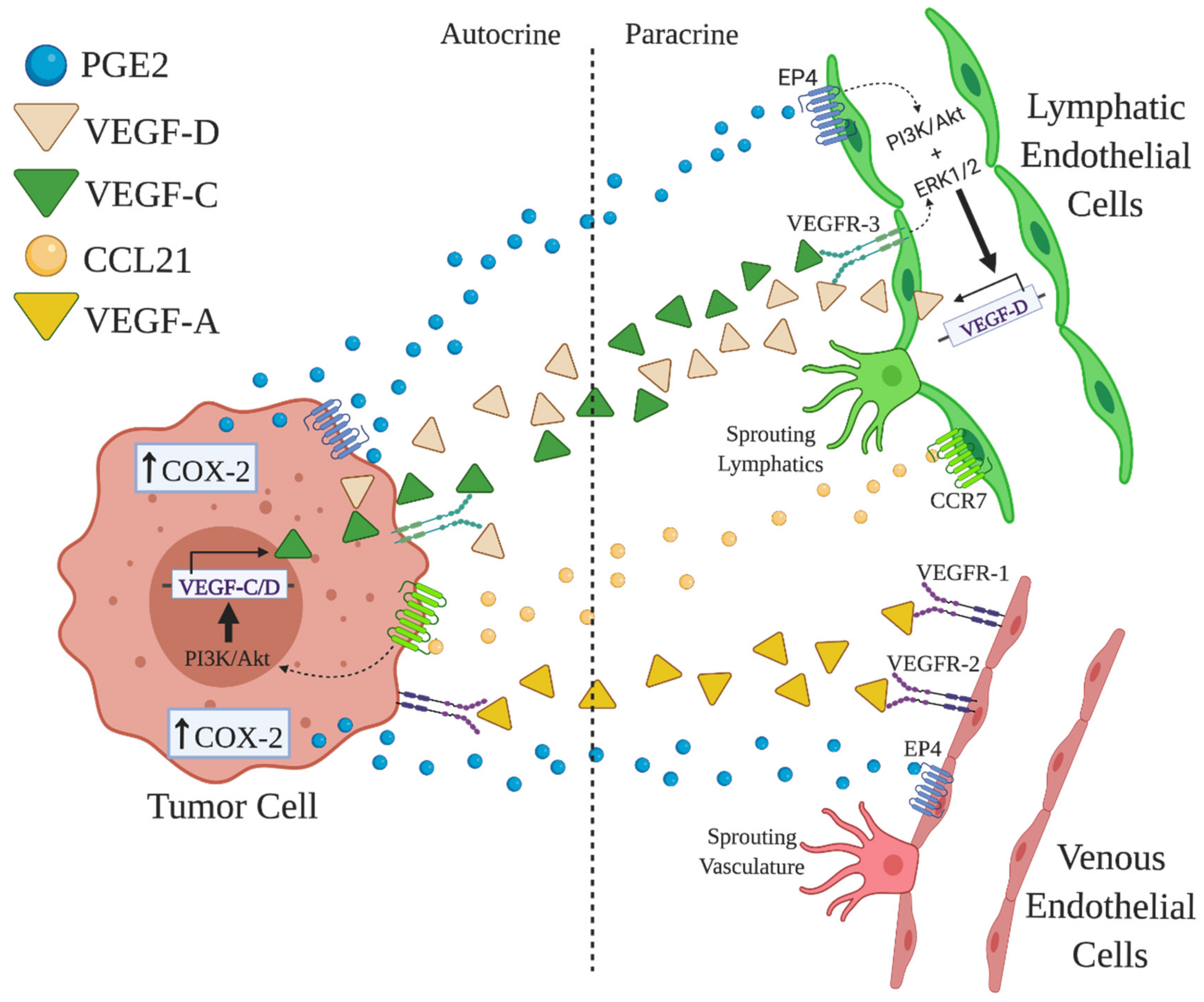
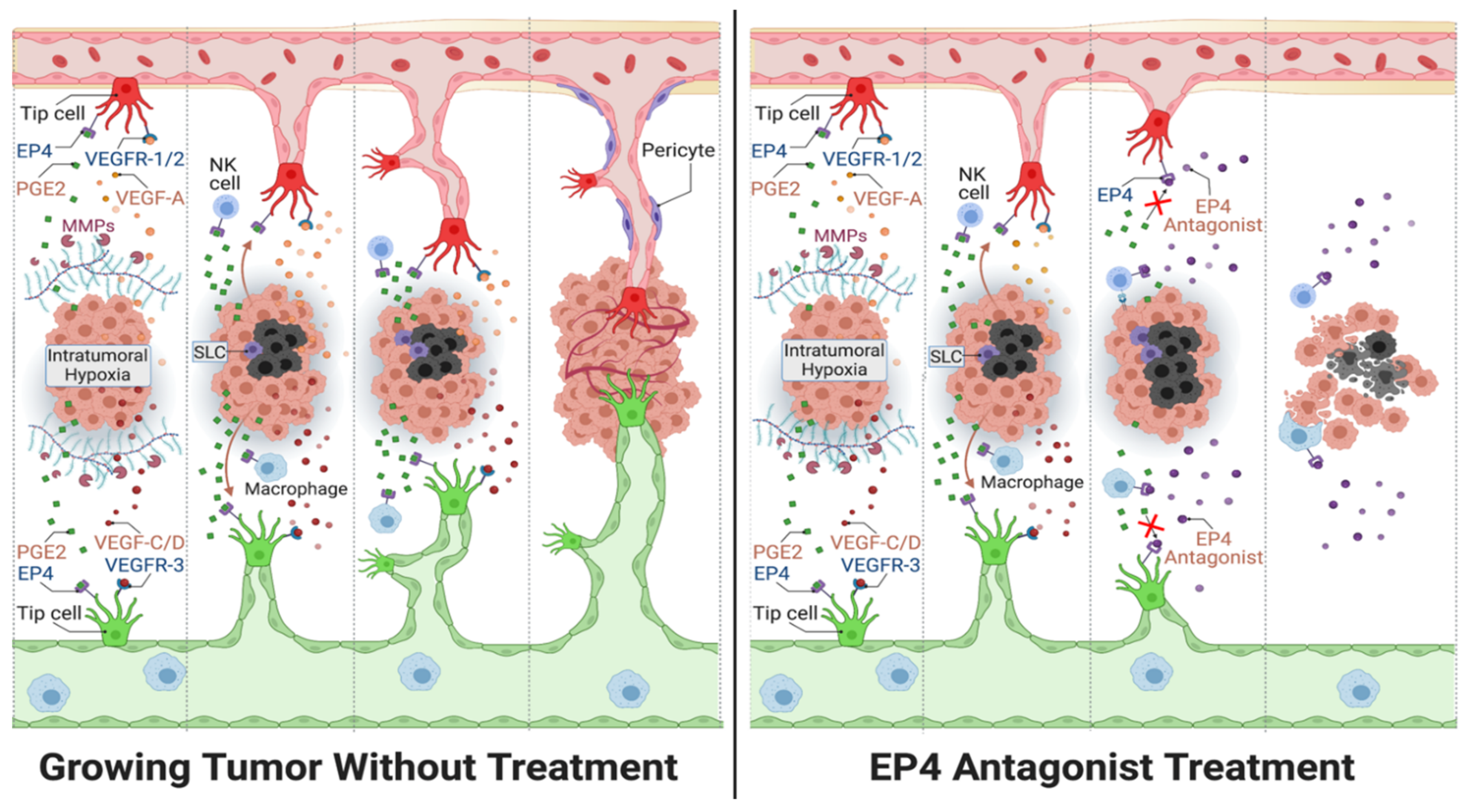
3.3. Roles of COX-2/EP4 in TAA and TAL
3.4. Roles of COX-2/EP4-Induced microRNAs (miRNA)s in TAA and TAL
4. COX-2/EP4 as Therapeutic Targets in Breast Cancer: Experimental Models
4.1. Spontaneous and Syngeneic Murine Breast Cancer Models
4.1.1. Spontaneous Murine Breast Cancer
4.1.2. Syngeneic Murine Breast Cancer Models Using Cell Lines
4.2. Human Breast Cancer Cell Line and Patient-Derived Xenografts in Immune-Deficient Mice
4.3. Patient-Derived Xenografts of Triple-Negative Breast Cancer (TNBC)
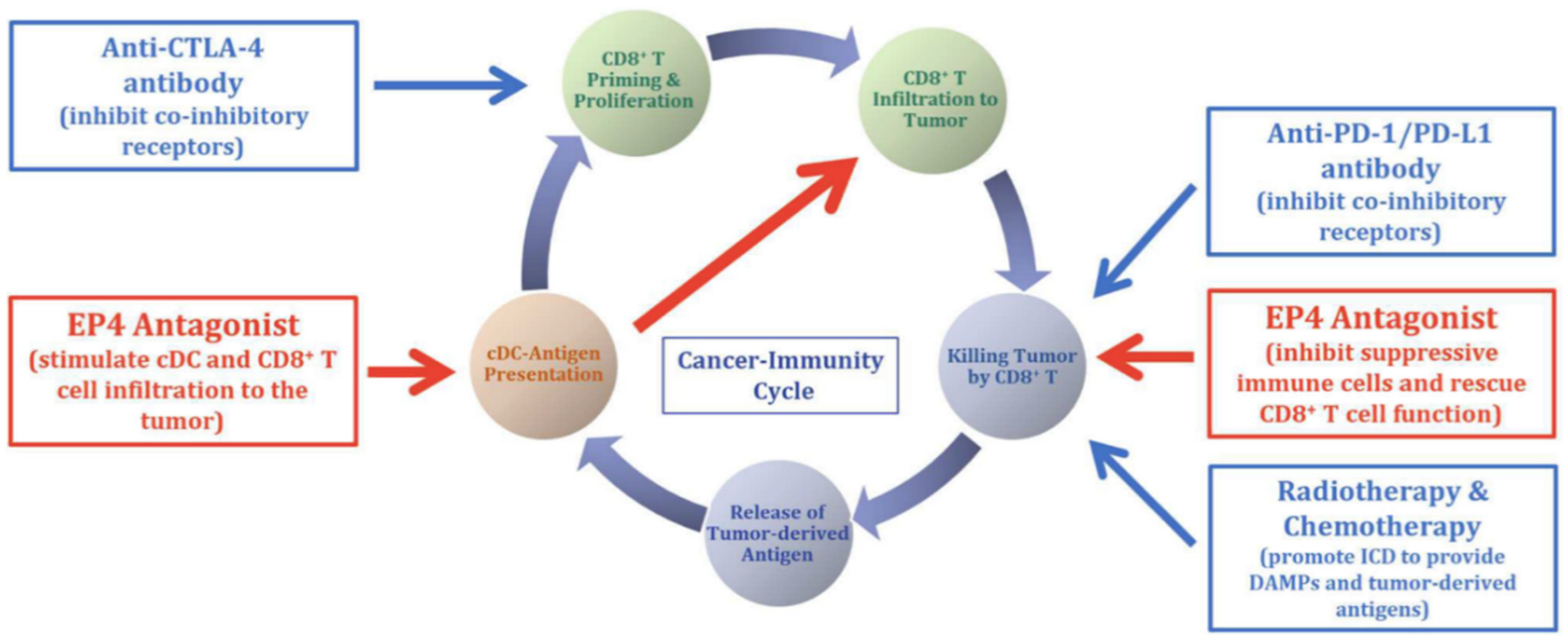
4.4. EP4 Antagonists Used in Combination Therapies
4.5. Currently Ongoing Human Trials with EP4 Antagonist as Single Agent or Combination Therapies
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otrock, Z.; Mahfouz, R.; Makarem, J.; Shamseddine, A. Understanding the Biology of Angiogenesis: Review of the Most Important Molecular Mechanisms. Blood Cells Mol. Dis. 2007, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Jeltsch, M. Hyperplasia of Lymphatic Vessels in VEGF-C Transgenic Mice. Science 1997, 276, 1423–1425. [Google Scholar] [CrossRef]
- Nyberg, P.; Xie, L.; Kalluri, R. Endogenous Inhibitors of Angiogenesis. Cancer Res 2005, 65, 3967–3979. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Kessler, O. The Semaphorins: Versatile Regulators of Tumour Progression and Tumour Angiogenesis. Nat. Rev. Cancer 2008, 8, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Worzfeld, T.; Offermanns, S. Semaphorins and Plexins as Therapeutic Targets. Nat. Rev. Drug Discov. 2014, 13, 603–621. [Google Scholar] [CrossRef]
- Lala, P.K.; Nandi, P. Mechanisms of Trophoblast Migration, Endometrial Angiogenesis in Preeclampsia: The Role of Decorin. Cell Adhes. Migr. 2016, 10, 111–125. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin. Am. J. Pathol. 2012, 181, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular Endothelial Growth Factor Mediates Angiogenic Activity during the Proliferative Phase of Wound Healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar] [PubMed]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal Blood Vessel Development and Lethality in Embryos Lacking a Single VEGF Allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Yeo, K.T.; Berse, B.; Yeo, T.K.; Senger, D.R.; Dvorak, H.F.; van de Water, L. Expression of Vascular Permeability Factor (Vascular Endothelial Growth Factor) by Epidermal Keratinocytes during Wound Healing. J. Exp. Med. 1992, 176, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Pardali, E.; Goumans, M.-J.; ten Dijke, P. Signaling by Members of the TGF-β Family in Vascular Morphogenesis and Disease. Trends Cell Biol. 2010, 20, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Reitmaier, B.; Regenbogen, J.; Slowey, R.M.; Opalenik, S.R.; Wolf, E.; Goppelt, A.; Davidson, J.M. CARP, a Cardiac Ankyrin Repeat Protein, Is Up-Regulated during Wound Healing and Induces Angiogenesis in Experimental Granulation Tissue. Am. J. Pathol. 2005, 166, 303–312. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular Endothelial Growth Factor C Is Required for Sprouting of the First Lymphatic Vessels from Embryonic Veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.E.; Halford, M.M.; Roufail, S.; Williams, R.A.; Hibbs, M.L.; Grail, D.; Kubo, H.; Stacker, S.A.; Achen, M.G. Vascular Endothelial Growth Factor D Is Dispensable for Development of the Lymphatic System. MCB 2005, 25, 2441–2449. [Google Scholar] [CrossRef]
- Vlahakis, N.E.; Young, B.A.; Atakilit, A.; Sheppard, D. The Lymphangiogenic Vascular Endothelial Growth Factors VEGF-C and -D Are Ligands for the Integrin A9β1. J. Biol. Chem. 2005, 280, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Wu, J.F.; Ferrando, R.; Lee, J.H.; Wang, Y.L.; Farese, R.V.; Sheppard, D. Fatal Bilateral Chylothorax in Mice Lacking the Integrin A9β1. Mol. Cell. Biol. 2000, 20, 5208–5215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Moyon, D.; Pardanaud, L.; Bréant, C.; Karkkainen, M.J.; Alitalo, K.; Eichmann, A. Abnormal Lymphatic Vessel Development in Neuropilin 2 Mutant Mice. Development 2002, 129, 4797–4806. [Google Scholar]
- Karpanen, T.; Egeblad, M.; Karkkainen, M.J.; Kubo, H.; Ylä-Herttuala, S.; Jäättelä, M.; Alitalo, K. Vascular Endothelial Growth Factor C Promotes Tumor Lymphangiogenesis and Intralymphatic Tumor Growth. Cancer Res. 2001, 61, 1786–1790. [Google Scholar] [PubMed]
- Kubo, H.; Cao, R.; Brakenhielm, E.; Makinen, T.; Cao, Y.; Alitalo, K. Blockade of Vascular Endothelial Growth Factor Receptor-3 Signaling Inhibits Fibroblast Growth Factor-2-Induced Lymphangiogenesis in Mouse Cornea. Proc. Natl. Acad. Sci. USA 2002, 99, 8868–8873. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.K.; Garcia-Cardena, G.; Farnebo, F.; Fannon, M.; Chen, E.J.; Butterfield, C.; Moses, M.A.; Mulligan, R.C.; Folkman, J.; Kaipainen, A. Dose-Dependent Response of FGF-2 for Lymphangiogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 11658–11663. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Björndahl, M.A.; Religa, P.; Clasper, S.; Garvin, S.; Galter, D.; Meister, B.; Ikomi, F.; Tritsaris, K.; Dissing, S.; et al. PDGF-BB Induces Intratumoral Lymphangiogenesis and Promotes Lymphatic Metastasis. Cancer Cell 2004, 6, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Eriksson, A.; Kubo, H.; Alitalo, K.; Cao, Y.; Thyberg, J. Comparative Evaluation of FGF-2–, VEGF-A–, and VEGF-C–Induced Angiogenesis, Lymphangiogenesis, Vascular Fenestrations, and Permeability. Circ. Res. 2004, 94, 664–670. [Google Scholar] [CrossRef]
- Hirakawa, S.; Kodama, S.; Kunstfeld, R.; Kajiya, K.; Brown, L.F.; Detmar, M. VEGF-A Induces Tumor and Sentinel Lymph Node Lymphangiogenesis and Promotes Lymphatic Metastasis. J. Exp. Med. 2005, 201, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, M.A.; Cao, R.; Burton, J.B.; Brakenhielm, E.; Religa, P.; Galter, D.; Wu, L.; Cao, Y. Vascular Endothelial Growth Factor-A Promotes Peritumoral Lymphangiogenesis and Lymphatic Metastasis. Cancer Res. 2005, 65, 9261–9268. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, K.; Hirakawa, S.; Ma, B.; Drinnenberg, I.; Detmar, M. Hepatocyte Growth Factor Promotes Lymphatic Vessel Formation and Function. EMBO J. 2005, 24, 2885–2895. [Google Scholar] [CrossRef]
- Morisada, T.; Oike, Y.; Yamada, Y.; Urano, T.; Akao, M.; Kubota, Y.; Maekawa, H.; Kimura, Y.; Ohmura, M.; Miyamoto, T.; et al. Angiopoietin-1 Promotes LYVE-1-Positive Lymphatic Vessel Formation. Blood 2005, 105, 4649–4656. [Google Scholar] [CrossRef]
- Paduch, R. The Role of Lymphangiogenesis and Angiogenesis in Tumor Metastasis. Cell Oncol. 2016, 39, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Ucuzian, A.A.; Greisler, H.P. In Vitro Models of Angiogenesis. World J. Surg. 2007, 31, 654–663. [Google Scholar] [CrossRef]
- Morin, K.T.; Tranquillo, R.T. In Vitro Models of Angiogenesis and Vasculogenesis in Fibrin Gel. Exp. Cell Res. 2013, 319, 2409–2417. [Google Scholar] [CrossRef]
- Norrby, K. In Vivo Models of Angiogenesis. J. Cell. Mol. Med. 2006, 10, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Staton, C.A.; Reed, M.W.R.; Brown, N.J. A Critical Analysis of Current in Vitro and in Vivo Angiogenesis Assays: Current in Vitro and in Vivo Angiogenesis Assays. Int. J. Exp. Pathol. 2009, 90, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Nault, B.; Ugwuagbo, K.C.; Maiti, S.; Majumder, M. Mir526b and Mir655 Promote Tumour Associated Angiogenesis and Lymphangiogenesis in Breast Cancer. Cancers 2019, 11, 938. [Google Scholar] [CrossRef]
- Roudsari, L.C.; West, J.L. Studying the Influence of Angiogenesis in in Vitro Cancer Model Systems. Adv. Drug Deliv. Rev. 2016, 97, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, A.; Helker, C.S.M.; Herzog, W. Angiogenesis in Zebrafish. Semin. Cell Dev. Biol. 2014, 31, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ugwuagbo, K.C.; Maiti, S.; Omar, A.; Hunter, S.; Nault, B.; Northam, C.; Majumder, M. Prostaglandin E2 Promotes Embryonic Vascular Development and Maturation in Zebrafish. Biol. Open 2019, 8, bio039768. [Google Scholar] [CrossRef] [PubMed]
- Doh, S.J.; Yamakawa, M.; Santosa, S.M.; Montana, M.; Guo, K.; Sauer, J.R.; Curran, N.; Han, K.-Y.; Yu, C.; Ema, M.; et al. Fluorescent Reporter Transgenic Mice for in Vivo Live Imaging of Angiogenesis and Lymphangiogenesis. Angiogenesis 2018, 21, 677–698. [Google Scholar] [CrossRef]
- Guedez, L.; Rivera, A.M.; Salloum, R.; Miller, M.L.; Diegmueller, J.J.; Bungay, P.M.; Stetler-Stevenson, W.G. Quantitative Assessment of Angiogenic Responses by the Directed in Vivo Angiogenesis Assay. Am. J. Pathol. 2003, 162, 1431–1439. [Google Scholar] [CrossRef]
- Bruyère, F.; Melen-Lamalle, L.; Blacher, S.; Roland, G.; Thiry, M.; Moons, L.; Frankenne, F.; Carmeliet, P.; Alitalo, K.; Libert, C.; et al. Modeling Lymphangiogenesis in a Three-Dimensional Culture System. Nat. Methods 2008, 5, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Xin, X.; Lala, P.K. A Practical and Sensitive Method of Quantitating Lymphangiogenesis in Vivo. Lab. Invest. 2013, 93, 779–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majumder, M.; Tutunea-Fatan, E.; Xin, X.; Rodriguez-Torres, M.; Torres-Garcia, J.; Wiebe, R.; Timoshenko, A.V.; Bhattacharjee, R.N.; Chambers, A.F.; Lala, P.K. Co-Expression of A9β1 Integrin and VEGF-D Confers Lymphatic Metastatic Ability to a Human Breast Cancer Cell Line MDA-MB-468LN. PLoS ONE 2012, 7, e35094. [Google Scholar] [CrossRef] [PubMed]
- Hosono, K.; Isonaka, R.; Kawakami, T.; Narumiya, S.; Majima, M. Signaling of Prostaglandin E Receptors, EP3 and EP4 Facilitates Wound Healing and Lymphangiogenesis with Enhanced Recruitment of M2 Macrophages in Mice. PLoS ONE 2016, 11, e0162532. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Lim, S.; Ji, H.; Zhang, Y.; Yang, Y.; Honek, J.; Hedlund, E.-M.; Cao, Y. Mouse Corneal Lymphangiogenesis Model. Nat. Protoc. 2011, 6, 817–826. [Google Scholar] [CrossRef]
- Koukourakis, M.I. LYVE-1 Immunohistochemical Assessment of Lymphangiogenesis in Endometrial and Lung Cancer. J. Clin. Pathol. 2005, 58, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Mbonye, U.R.; DeLong, C.J.; Wada, M.; Smith, W.L. Regulation of Intracellular Cyclooxygenase Levels by Gene Transcription and Protein Degradation. Prog. Lipid Res. 2007, 46, 108–125. [Google Scholar] [CrossRef]
- Yuan, C.J.; Mandal, A.K.; Zhang, Z.; Mukherjee, A.B. Transcriptional Regulation of Cyclooxygenase-2 Gene Expression: Novel Effects of Nonsteroidal Anti-Inflammatory Drugs. Cancer Res. 2000, 60, 1084–1091. [Google Scholar]
- Teo, S.T.; Yung, Y.C.; Herr, D.R.; Chun, J. Lysophosphatidic Acid in Vascular Development and Disease. IUBMB Life 2009, 61, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Oyesanya, R.A.; Lee, Z.P.; Wu, J.; Chen, J.; Song, Y.; Mukherjee, A.; Dent, P.; Kordula, T.; Zhou, H.; Fang, X. Transcriptional and Post-transcriptional Mechanisms for Lysophosphatidic Acid-induced Cyclooxygenase-2 Expression in Ovarian Cancer Cells. FASEB J. 2008, 22, 2639–2651. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Majumder, M.; Girish, G.V.; Mohindra, V.; Maruyama, T.; Lala, P.K. Targeting COX-2 and EP4 to Control Tumor Growth, Angiogenesis, Lymphangiogenesis and Metastasis to the Lungs and Lymph Nodes in a Breast Cancer Model. Lab. Invest. 2012, 92, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Lala, P.K.; Nandi, P.; Majumder, M. Roles of Prostaglandins in Tumor-Associated Lymphangiogenesis with Special Reference to Breast Cancer. Cancer Metastasis Rev. 2018, 37, 369–384. [Google Scholar] [CrossRef]
- Majumder, M.; Nandi, P.; Omar, A.; Ugwuagbo, K.; Lala, P. EP4 as a Therapeutic Target for Aggressive Human Breast Cancer. IJMS 2018, 19, 1019. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-R.; Hou, M.-F.; Chang, H.-C.; Hung, W.-C. Cyclooxygenase-2 Up-Regulates CCR7 via EP2/EP4 Receptor Signaling Pathways to Enhance Lymphatic Invasion of Breast Cancer Cells. J. Biol. Chem. 2008, 283, 11155–11163. [Google Scholar] [CrossRef]
- Dimberg, A. Chemokines in Angiogenesis. In The Chemokine System in Experimental and Clinical Hematology; Bruserud, O., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 341, pp. 59–80. ISBN 978-3-642-12638-3. [Google Scholar]
- Wang, D.; DuBois, R.N. Eicosanoids and Cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Howe, L.R. Inflammation and Breast Cancer. Cyclooxygenase/Prostaglandin Signaling and Breast Cancer. Breast Cancer Res. 2007, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.M.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 Pathway: Key Roles in the Hallmarks of Cancer and Adaptation to the Tumour Microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef]
- Tutunea-Fatan, E.; Majumder, M.; Xin, X.; Lala, P.K. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer-Induced Lymphangiogenesis. Mol. Cancer 2015, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Valdés-Rives, S.A.; González-Arenas, A. Autotaxin-Lysophosphatidic Acid: From Inflammation to Cancer Development. Mediat. Inflamm. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Tang, X.; Dewald, J.; Dong, W.-F.; Mackey, J.R.; Hemmings, D.G.; McMullen, T.P.W.; Brindley, D.N. Tumor-Induced Inflammation in Mammary Adipose Tissue Stimulates a Vicious Cycle of Autotaxin Expression and Breast Cancer Progression. FASEB J. 2015, 29, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Perez, C.E.; Nie, W.; Sinnett-Smith, J.; Rozengurt, E.; Yoo, J. TNF-α Potentiates Lysophosphatidic Acid-Induced COX-2 Expression via PKD in Human Colonic Myofibroblasts. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011; 300, G637–G646. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Gervin, E.; Shin, B.; Opperman, R.; Cullen, M.; Feser, R.; Maiti, S.; Majumder, M. Chemically Induced Hypoxia Enhances MiRNA Functions in Breast Cancer. Cancers 2020, 12, 2008. [Google Scholar] [CrossRef]
- Siemann, D.W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for Its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic Therapy in Oncology: Current Status and Future Directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef]
- Abdalla, A.M.E.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current Challenges of Cancer Anti-Angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics 2018, 8, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Rozic, J.G.; Chakraborty, C.; Lala, P.K. Cyclooxygenase Inhibitors Retard Murine Mammary Tumor Progression by Reducing Tumor Cell Migration, Invasiveness and Angiogenesis. Int. J. Cancer 2001, 93, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Leek, R.D.; Lewis, C.E.; Whitehouse, R.; Greenall, M.; Clarkee, J.; Harris, A.L. Association of Macrophage Infiltration with Angiogenesis and Prognosis in Invasive Breast Carcinoma. Cancer Res. 1996, 56, 4625–4629. [Google Scholar]
- Majumder, M.; Xin, X.; Liu, L.; Girish, G.V.; Lala, P.K. Prostaglandin E2 Receptor EP 4 as the Common Target on Cancer Cells and Macrophages to Abolish Angiogenesis, Lymphangiogenesis, Metastasis, and Stem-like Cell Functions. Cancer Sci. 2014, 105, 1142–1151. [Google Scholar] [CrossRef]
- Lin, E.Y.; Li, J.-F.; Gnatovskiy, L.; Deng, Y.; Zhu, L.; Grzesik, D.A.; Qian, H.; Xue, X.-N.; Pollard, J.W. Macrophages Regulate the Angiogenic Switch in a Mouse Model of Breast Cancer. Cancer Res. 2006, 66, 11238–11246. [Google Scholar] [CrossRef] [PubMed]
- Vaahtomeri, K.; Alitalo, K. Lymphatic Vessels in Tumor Dissemination versus Immunotherapy. Cancer Res. 2020, 80, 3463–3465. [Google Scholar] [CrossRef]
- Lyons, T.R.; O’Brien, J.; Borges, V.F.; Conklin, M.W.; Keely, P.J.; Eliceiri, K.W.; Marusyk, A.; Tan, A.-C.; Schedin, P. Postpartum Mammary Gland Involution Drives Progression of Ductal Carcinoma in Situ through Collagen and COX-2. Nat. Med. 2011, 17, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, R.H.; Achen, M.G.; Stacker, S.A. The Evolving Role of Lymphatics in Cancer Metastasis. Curr. Opin. Immunol. 2018, 53, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Nandi, P.; Girish, G.V.; Majumder, M.; Xin, X.; Tutunea-Fatan, E.; Lala, P.K. PGE2 Promotes Breast Cancer-Associated Lymphangiogenesis by Activation of EP4 Receptor on Lymphatic Endothelial Cells. BMC Cancer 2017, 17, 11. [Google Scholar] [CrossRef]
- Majumder, M.; Landman, E.; Liu, L.; Hess, D.; Lala, P.K. COX-2 Elevates Oncogenic MiR-526b in Breast Cancer by EP4 Activation. Mol. Cancer Res. 2015, 13, 1022–1033. [Google Scholar] [CrossRef]
- Karnezis, T.; Shayan, R.; Fox, S.; Achen, M.G.; Stacker, S.A. The Connection between Lymphangiogenic Signalling and Prostaglandin Biology: A Missing Link in the Metastatic Pathway. Oncotarget 2012, 3, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Iwata, C.; Kano, M.R.; Komuro, A.; Oka, M.; Kiyono, K.; Johansson, E.; Morishita, Y.; Yashiro, M.; Hirakawa, K.; Kaminishi, M.; et al. Inhibition of Cyclooxygenase-2 Suppresses Lymph Node Metastasis via Reduction of Lymphangiogenesis. Cancer Res. 2007, 67, 10181–10189. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Boyle, J.O.; Yang, E.K.; Zhang, F.; Sacks, P.G.; Shah, J.P.; Edelstein, D.; Soslow, R.A.; Koki, A.T.; Woerner, B.M.; et al. Cyclooxygenase-2 Expression Is up-Regulated in Squamous Cell Carcinoma of the Head and Neck. Cancer Res. 1999, 59, 991–994. [Google Scholar] [PubMed]
- Tsujii, M.; Kawano, S.; DuBois, R.N. Cyclooxygenase-2 Expression in Human Colon Cancer Cells Increases Metastatic Potential. Proc. Natl. Acad. Sci. USA 1997, 94, 3336–3340. [Google Scholar] [CrossRef] [PubMed]
- Parrett, M.; Harris, R.; Joarder, F.; Ross, M.; Clausen, K.; Robertson, F. Cyclooxygenase-2 Gene Expression in Human Breast Cancer. Int. J. Oncol. 1997. [Google Scholar] [CrossRef]
- Lyons, T.R.; Borges, V.F.; Betts, C.B.; Guo, Q.; Kapoor, P.; Martinson, H.A.; Jindal, S.; Schedin, P. Cyclooxygenase-2–Dependent Lymphangiogenesis Promotes Nodal Metastasis of Postpartum Breast Cancer. J. Clin. Invest. 2014, 124, 3901–3912. [Google Scholar] [CrossRef]
- Tucker, O.N.; Dannenberg, A.J.; Yang, E.K.; Zhang, F.; Teng, L.; Daly, J.M.; Soslow, R.A.; Masferrer, J.L.; Woerner, B.M.; Koki, A.T.; et al. Cyclooxygenase-2 Expression Is up-Regulated in Human Pancreatic Cancer. Cancer Res. 1999, 59, 987–990. [Google Scholar]
- Ristimäki, A.; Sivula, A.; Lundin, J.; Lundin, M.; Salminen, T.; Haglund, C.; Joensuu, H.; Isola, J. Prognostic Significance of Elevated Cyclooxygenase-2 Expression in Breast Cancer. Cancer Res. 2002, 62, 632–635. [Google Scholar]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-Inflammatory Effects of EPA and DHA Are Dependent upon Time and Dose-Response Elements Associated with LPS Stimulation in THP-1-Derived Macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, H.J.; Chang, K.C.; Baek, J.C.; Park, J.K.; Shin, J.K.; Choi, W.J.; Lee, J.H.; Paik, W.Y. DHA and EPA Down-Regulate COX-2 Expression through Suppression of NF-ΚB Activity in LPS-Treated Human Umbilical Vein Endothelial Cells. Korean J. Physiol. Pharmacol. 2009, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Habib, A.; Lubrano, L.; Turco, S.D.; Lazzerini, G.; Bourcier, T.; Weksler, B.B.; De Caterina, R. The Omega-3 Fatty Acid Docosahexaenoate Attenuates Endothelial Cyclooxygenase-2 Induction through Both NADP(H) Oxidase and PKC Inhibition. Proc. Natl. Acad. Sci. USA 2006, 103, 15184–15189. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, G.; Houston, A. Prostaglandin E2 and the EP Receptors in Malignancy: Possible Therapeutic Targets?: PGE 2 Receptors as Targets in Cancer Therapy. Br. J. Pharmacol. 2015, 172, 5239–5250. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E Receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef] [PubMed]
- Markovič, T.; Jakopin, Ž.; Dolenc, M.S.; Mlinarič-Raščan, I. Structural Features of Subtype-Selective EP Receptor Modulators. Drug Discov. Today 2017, 22, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Yatabe, Y.; Achiwa, H.; Muramatsu, H.; Kozaki, K.; Nakamura, S.; Ogawa, M.; Mitsudomi, T.; Sugiura, T.; Takahashi, T. Increased Expression of Cyclooxygenase 2 Occurs Frequently in Human Lung Cancers, Specifically in Adenocarcinomas. Cancer Res. 1998, 58, 3761–3764. [Google Scholar] [PubMed]
- Liu, C.H.; Chang, S.-H.; Narko, K.; Trifan, O.C.; Wu, M.-T.; Smith, E.; Haudenschild, C.; Lane, T.F.; Hla, T. Overexpression of Cyclooxygenase-2 Is Sufficient to Induce Tumorigenesis in Transgenic Mice. J. Biol. Chem. 2001, 276, 18563–18569. [Google Scholar] [CrossRef] [PubMed]
- Chulada, P.C.; Thompson, M.B.; Mahler, J.F.; Doyle, C.M.; Gaul, B.W.; Lee, C.; Tiano, H.F.; Morham, S.G.; Smithies, O.; Langenbach, R. Genetic Disruption of Ptgs-1, as Well as Ptgs-2, Reduces Intestinal Tumorigenesis in Min Mice. Cancer Res. 2000, 60, 4705–4708. [Google Scholar] [PubMed]
- Harris, R.E. COX-2 Blockade in Cancer Prevention and Therapy; Humana Press: Totowa, NJ, USA, 2003; ISBN 978-0-585-43882-5. [Google Scholar]
- Harris, R.E.; Chlebowski, R.T.; Jackson, R.D.; Frid, D.J.; Ascenseo, J.L.; Anderson, G.; Loar, A.; Rodabough, R.J.; White, E.; McTiernan, A.; et al. Breast Cancer and Nonsteroidal Anti-Inflammatory Drugs: Prospective Results from the Women’s Health Initiative. Cancer Res. 2003, 63, 6096–6101. [Google Scholar]
- Gupta, A.K.; Schoen, R.E. Aberrant Crypt Foci: Are They Intermediate Endpoints of Colon Carcinogenesis in Humans? Curr. Opin. Gastroenterol. 2009, 25, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Dannenberg, A.J. COX-2 Inhibitors for the Prevention of Breast Cancer. J. Mammary Gland Biol. Neoplasia 2003, 8, 31–43. [Google Scholar] [CrossRef]
- Sharpe, C.R.; Collet, J.-P.; McNutt, M.; Belzile, E.; Boivin, J.-F.; Hanley, J.A. Nested Case–Control Study of the Effects of Non-Steroidal Anti-Inflammatory Drugs on Breast Cancer Risk and Stage. Br. J. Cancer 2000, 83, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Dinchuk, J.E.; Kargman, S.L.; Oshima, H.; Hancock, B.; Kwong, E.; Trzaskos, J.M.; Evans, J.F.; Taketo, M.M. Suppression of Intestinal Polyposis in ApcΔ716 Knockout Mice by Inhibition of Cyclooxygenase 2 (COX-2). Cell 1996, 87, 803–809. [Google Scholar] [CrossRef]
- Parhar, R.S.; Lala, P.K. Changes in the Host Natural Killer Cell Population in Mice during Tumor Development. Cell. Immunol. 1985, 93, 265–279. [Google Scholar] [CrossRef]
- Lala, P.K.; Parhar, R.S.; Singh, P. Indomethacin Therapy Abrogates the Prostaglandin-Mediated Suppression of Natural Killer Activity in Tumor-Bearing Mice and Prevents Tumor Metastasis. Cell. Immunol. 1986, 99, 108–118. [Google Scholar] [CrossRef]
- Lala, P.K.; Al-Mutter, N.; Orucevic, A. Effects of Chronic Indomethacin Therapy on the Development and Progression of Spontaneous Mammary Tumors in C3H/HEJ Mice. Int. J. Cancer 1997, 73, 371–380. [Google Scholar] [CrossRef]
- Kundu, N.; Ma, X.; Holt, D.; Goloubeva, O.; Ostrand-Rosenberg, S.; Fulton, A.M. Antagonism of the Prostaglandin E Receptor EP4 Inhibits Metastasis and Enhances NK Function. Breast Cancer Res. Treat. 2009, 117, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, A.V.; Xu, G.; Chakrabarti, S.; Lala, P.K.; Chakraborty, C. Role of Prostaglandin E2 Receptors in Migration of Murine and Human Breast Cancer Cells. Exp. Cell Res. 2003, 289, 265–274. [Google Scholar] [CrossRef]
- Timoshenko, A.V.; Lala, P.K.; Chakraborty, C. PGE2-Mediated Upregulation of INOS in Murine Breast Cancer Cells through the Activation of EP4 Receptors. Int. J. Cancer 2004, 108, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Stevens, J.; Hilton, M.B.; Seaman, S.; Conrads, T.P.; Veenstra, T.D.; Logsdon, D.; Morris, H.; Swing, D.A.; Patel, N.L.; et al. COX-2 Inhibition Potentiates Antiangiogenic Cancer Therapy and Prevents Metastasis in Preclinical Models. Sci. Transl. Med. 2014, 6, 242ra84. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, A.V.; Chakraborty, C.; Wagner, G.F.; Lala, P.K. COX-2-Mediated Stimulation of the Lymphangiogenic Factor VEGF-C in Human Breast Cancer. Br. J. Cancer 2006, 94, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Hiken, J.F.; McDonald, J.I.; Decker, K.F.; Sanchez, C.; Hoog, J.; VanderKraats, N.D.; Jung, K.L.; Akinhanmi, M.; Rois, L.E.; Ellis, M.J.; et al. Epigenetic Activation of the Prostaglandin Receptor EP4 Promotes Resistance to Endocrine Therapy for Breast Cancer. Oncogene 2017, 36, 2319–2327. [Google Scholar] [CrossRef]
- Holt, D.; Ma, X.; Kundu, N.; Fulton, A. Prostaglandin E2 (PGE2) Suppresses Natural Killer Cell Function Primarily through the PGE2 Receptor EP4. Cancer Immunol. Immunother. 2011, 60, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Holt, D.; Kundu, N.; Reader, J.; Goloubeva, O.; Take, Y.; Fulton, A.M. A Prostaglandin E (PGE) Receptor EP4 Antagonist Protects Natural Killer Cells from PGE 2 -Mediated Immunosuppression and Inhibits Breast Cancer Metastasis. OncoImmunology 2013, 2, e22647. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Sugata, Y.; Fujiwara, T.; Matsumoto, R.; Nishibori, M.; Shimizu, K.; Maeda, M.; Kimura, Y.; Kariya, S.; Hattori, H.; et al. E Prostanoid 2 (EP2)/EP4-Mediated Suppression of Antigen-Specific Human T-Cell Responses by Prostaglandin E2. Immunology 2006, 118, 343–352. [Google Scholar] [CrossRef]
- Albu, D.I.; Wang, Z.; Wu, J.; Huang, K.; Li, W.; Liu, D.; Kuznetsov, G.; Chen, Q.; Bao, X.; Woodall-Jappe, M. Abstract 275: ER-886046, an Antagonist of PGE2 Receptor Type-4, Induces an Effective Antitumor Immune Response in Mice by Attenuating Intratumoral MDSCs and TAMs. In Proceedings of the AACR 106th Annual Meeting 2015, Philadelphia, PA, USA, 18–22 August 2015; p. 275. [Google Scholar]
- Kundu, N.; Ma, X.; Kochel, T.; Goloubeva, O.; Staats, P.; Thompson, K.; Martin, S.; Reader, J.; Take, Y.; Collin, P.; et al. Prostaglandin E Receptor EP4 Is a Therapeutic Target in Breast Cancer Cells with Stem-like Properties. Breast Cancer Res. Treat. 2014, 143, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Wicha, M.S.; Liu, S.; Dontu, G. Cancer Stem Cells: An Old Idea—A Paradigm Shift. Cancer Res. 2006, 66, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, B.B. Tumor-Initiating and -Propagating Cells: Cells That We Would to Identify and Control. Neoplasia 2010, 12, 506–515. [Google Scholar] [CrossRef]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.-F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic Resistance of Tumorigenic Breast Cancer Cells to Chemotherapy. JNCI J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer Stem Cells: Current Status and Evolving Complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, G.A. Coxibs and Cardiovascular Disease. N. Engl. J. Med. 2004, 351, 1709–1711. [Google Scholar] [CrossRef]
- Graham, D.J. COX-2 Inhibitors, Other NSAIDs, and Cardiovascular Risk: The Seduction of Common Sense. JAMA 2006, 296, 1653. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H.; Xu, W.; Regan, J.W. Prostaglandin E 2 Induced Functional Expression of Early Growth Response Factor-1 by EP 4, but Not EP 2, Prostanoid Receptors via the Phosphatidylinositol 3-Kinase and Extracellular Signal-Regulated Kinases. J. Biol. Chem. 2003, 278, 12151–12156. [Google Scholar] [CrossRef]
- Xiao, C.-Y.; Hara, A.; Yuhki, K.; Fujino, T.; Ma, H.; Okada, Y.; Takahata, O.; Yamada, T.; Murata, T.; Narumiya, S.; et al. Roles of Prostaglandin I 2 and Thromboxane A 2 in Cardiac Ischemia-Reperfusion Injury: A Study Using Mice Lacking Their Respective Receptors. Circulation 2001, 104, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Meyer-Kirchrath, J.; Kaber, G.; Jacoby, C.; Flögel, U.; Schrader, J.; Rüther, U.; Schrör, K.; Hohlfeld, T. Cardiospecific Overexpression of the Prostaglandin EP 3 Receptor Attenuates Ischemia-Induced Myocardial Injury. Circulation 2005, 112, 400–406. [Google Scholar] [CrossRef][Green Version]
- Thiemermann, C.; Zacharowski, K. Selective Activation of E-Type Prostanoid3-Receptors Reduces Myocardial Infarct Size. Pharmacol. Ther. 2000, 87, 61–67. [Google Scholar] [CrossRef]
- Mediratta, K.; El-Sahli, S.; D’Costa, V.; Wang, L. Current Progresses and Challenges of Immunotherapy in Triple-Negative Breast Cancer. Cancers 2020, 12, 3529. [Google Scholar] [CrossRef] [PubMed]
- Mosalpuria, K.; Hall, C.; Krishnamurthy, S.; Lodhi, A.; Hallman, D.M.; Baraniuk, M.S.; Bhattacharyya, A.; Lucci, A. Cyclooxygenase-2 Expression in Non-Metastatic Triple-Negative Breast Cancer Patients. Mol. Clin. Oncol. 2014, 2, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Kochel, T.J.; Goloubeva, O.G.; Fulton, A.M. Upregulation of Cyclooxygenase-2/Prostaglandin E2 (COX-2/PGE2) Pathway Member Multiple Drug Resistance-Associated Protein 4 (MRP4) and Downregulation of Prostaglandin Transporter (PGT) and 15-Prostaglandin Dehydrogenase (15-PGDH) in Triple-Negative Breast Cancer. Breast Cancer 2016, 10. [Google Scholar] [CrossRef]
- Williams, C.S.; Tsujii, M.; Reese, J.; Dey, S.K.; DuBois, R.N. Host Cyclooxygenase-2 Modulates Carcinoma Growth. J. Clin. Invest. 2000, 105, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.N.; Timoshenko, A.V.; Cai, J.; Lala, P.K. Relationship between Cyclooxygenase-2 and Human Epidermal Growth Factor Receptor 2 in Vascular Endothelial Growth Factor C up-Regulation and Lymphangiogenesis in Human Breast Cancer. Cancer Sci. 2010, 101, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Dunn, L.; Liu, L.; Hasan, A.; Vincent, K.; Brackstone, M.; Hess, D.; Lala, P.K. COX-2 Induces Oncogenic Micro RNA MiR655 in Human Breast Cancer. Sci. Rep. 2018, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, J.; Majumder, M.; Amiri, M.; Hasan, A.; Hess, D.; Lala, P.K. Tumor Suppressor Role of Cytoplasmic Polyadenylation Element Binding Protein 2 (CPEB2) in Human Mammary Epithelial Cells. BMC Cancer 2019, 19, 561. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Huang, Y.-S. CPEB2-EEF2 Interaction Impedes HIF-1α RNA Translation: Translation Control at Elongation. EMBO J. 2012, 31, 959–971. [Google Scholar] [CrossRef]
- Li, N.-N.; Meng, X.-S.; Bao, Y.-R.; Wang, S.; Li, T.-J. Evidence for the Involvement of COX-2/VEGF and PTEN/Pl3K/AKT Pathway the Mechanism of Oroxin B Treated Liver Cancer. Pharmacogn. Mag. 2018, 14, 207. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Hirota, K.; Semenza, G.L. Regulation of Angiogenesis by Hypoxia-Inducible Factor 1. Crit. Rev. Oncol./Hematol. 2006, 59, 15–26. [Google Scholar] [CrossRef]
- Howe, L.R.; Chang, S.-H.; Tolle, K.C.; Dillon, R.; Young, L.J.T.; Cardiff, R.D.; Newman, R.A.; Yang, P.; Thaler, H.T.; Muller, W.J.; et al. HER2/Neu-Induced Mammary Tumorigenesis and Angiogenesis Are Reduced in Cyclooxygenase-2 Knockout Mice. Cancer Res. 2005, 65, 10113–10119. [Google Scholar] [CrossRef]
- Kundu, N.; Fulton, A.M. Selective Cyclooxygenase (COX)-1 or COX-2 Inhibitors Control Metastatic Disease in a Murine Model of Breast Cancer. Cancer Res. 2002, 62, 2343–2346. [Google Scholar]
- Ma, X.; Kundu, N.; Rifat, S.; Walser, T.; Fulton, A.M. Prostaglandin E Receptor EP4 Antagonism Inhibits Breast Cancer Metastasis. Cancer Res. 2006, 66, 2923–2927. [Google Scholar] [CrossRef] [PubMed]
- Albu, D.I.; Wang, Z.; Huang, K.-C.; Wu, J.; Twine, N.; Leacu, S.; Ingersoll, C.; Parent, L.; Lee, W.; Liu, D.; et al. EP4 Antagonism by E7046 Diminishes Myeloid Immunosuppression and Synergizes with Treg-Reducing IL-2-Diphtheria Toxin Fusion Protein in Restoring Anti-Tumor Immunity. OncoImmunology 2017, 6, e1338239. [Google Scholar] [CrossRef] [PubMed]
- Lala, P.K.; Elkashab, M.; Kerbel, R.S.; Parhar, R.S. Cure of Human Melanoma Lung Metastases in Nude Mice with Chronic Indomethacin Therapy Combined with Multiple Rounds of IL-2: Characteristics of Killer Cells Generated in Situ. Int. Immunol. 1990, 2, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Berry, J.A.; Shoher, A.; Ayers, G.D.; Wei, C.; Lucci, A. COX-2 Involvement in Breast Cancer Metastasis to Bone. Oncogene 2007, 26, 3789–3796. [Google Scholar] [CrossRef]
- Majumder, M.; Xin, X.; Liu, L.; Tutunea-Fatan, E.; Rodriguez-Torres, M.; Vincent, K.; Postovit, L.-M.; Hess, D.; Lala, P.K. COX-2 Induces Breast Cancer Stem Cells via EP4/PI3K/AKT/NOTCH/WNT Axis: Targeting EP4 to Abrogate Breast Cancer Stem Cells. Stem Cells 2016, 34, 2290–2305. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Zhou, Y.; McIntosh, B.E.; Norman, I.G.; Lou, H.E.; Biermann, M.; Sullivan, J.A.; Kamp, T.J.; Thomson, J.A.; Anagnostopoulos, P.V.; et al. A Humanized Mouse Model Generated Using Surplus Neonatal Tissue. Stem Cell Rep. 2018, 10, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Tonomura, N.; Shimizu, A.; Wang, S.; Yang, Y.-G. Reconstitution of a Functional Human Immune System in Immunodeficient Mice through Combined Human Fetal Thymus/Liver and CD34+ Cell Transplantation. Blood 2006, 108, 487–492. [Google Scholar] [CrossRef]
- Dobrolecki, L.E.; Airhart, S.D.; Alferez, D.G.; Aparicio, S.; Behbod, F.; Bentires-Alj, M.; Brisken, C.; Bult, C.J.; Cai, S.; Clarke, R.B.; et al. Patient-Derived Xenograft (PDX) Models in Basic and Translational Breast Cancer Research. Cancer Metastasis Rev. 2016, 35, 547–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yao, L.-C.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.-X.; Shi, W.; Ma, A.-H.; De Vere White, R.W.; Airhart, S.; et al. Humanized Mice in Studying Efficacy and Mechanisms of PD-1-Targeted Cancer Immunotherapy. FASEB J. 2018, 32, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Albu, D.; Huang, K.-C.; Wu, J.; Twine, N.; Nomoto, K.; Woodall-Jappe, M. Combination of EP4 Antagonist and Checkpoint Inhibitors Promotes Anti-Tumor Effector T Cells in Preclinical Tumor Models. J. Immunother. Cancer 2015, 3, P350. [Google Scholar] [CrossRef]
- Planes-Laine, G.; Rochigneux, P.; Bertucci, F.; Chrétien, A.S.; Viens, P.; Sabatier, R.; Gonçalves, A. PD-1/PD-L1 Targeting in Breast Cancer: The First Clinical Evidences Are Emerging. A Literature Review. Cancers 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Viale, G.; Curigliano, G. Recent Advances in Triple Negative Breast Cancer: The Immunotherapy Era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Ching, M.M.; Reader, J.; Fulton, A.M. Eicosanoids in Cancer: Prostaglandin E2 Receptor 4 in Cancer Therapeutics and Immunotherapy. Front. Pharmacol. 2020, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Take, Y.; Koizumi, S.; Nagahisa, A. Prostaglandin E Receptor 4 Antagonist in Cancer Immunotherapy: Mechanisms of Action. Front. Immunol. 2020, 11, 324. [Google Scholar] [CrossRef]
- Okumura, Y.; Yamagishi, T.; Nukui, S.; Nakao, K. Discovery of AAT-008, a Novel, Potent, and Selective Prostaglandin EP4 Receptor Antagonist. Bioorganic Med. Chem. Lett. 2017, 27, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Parikh, A.; Shapiro, G.I.; Varga, A.; Naing, A.; Meric-Bernstam, F.; Ataman, Ö.; Reyderman, L.; Binder, T.A.; Ren, M.; et al. First-in-Human Phase I Study of Immunomodulatory E7046, an Antagonist of PGE 2 -Receptor E-Type 4 (EP4), in Patients with Advanced Cancers. J. Immunother. Cancer 2020, 8, e000222. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Paz Linares, G.A.; Opperman, R.M.; Majumder, M.; Lala, P.K. Prostaglandin E2 Receptor 4 (EP4) as a Therapeutic Target to Impede Breast Cancer-Associated Angiogenesis and Lymphangiogenesis. Cancers 2021, 13, 942. https://doi.org/10.3390/cancers13050942
De Paz Linares GA, Opperman RM, Majumder M, Lala PK. Prostaglandin E2 Receptor 4 (EP4) as a Therapeutic Target to Impede Breast Cancer-Associated Angiogenesis and Lymphangiogenesis. Cancers. 2021; 13(5):942. https://doi.org/10.3390/cancers13050942
Chicago/Turabian StyleDe Paz Linares, Guillermo Antonio, Reid Morgan Opperman, Mousumi Majumder, and Peeyush K. Lala. 2021. "Prostaglandin E2 Receptor 4 (EP4) as a Therapeutic Target to Impede Breast Cancer-Associated Angiogenesis and Lymphangiogenesis" Cancers 13, no. 5: 942. https://doi.org/10.3390/cancers13050942
APA StyleDe Paz Linares, G. A., Opperman, R. M., Majumder, M., & Lala, P. K. (2021). Prostaglandin E2 Receptor 4 (EP4) as a Therapeutic Target to Impede Breast Cancer-Associated Angiogenesis and Lymphangiogenesis. Cancers, 13(5), 942. https://doi.org/10.3390/cancers13050942







