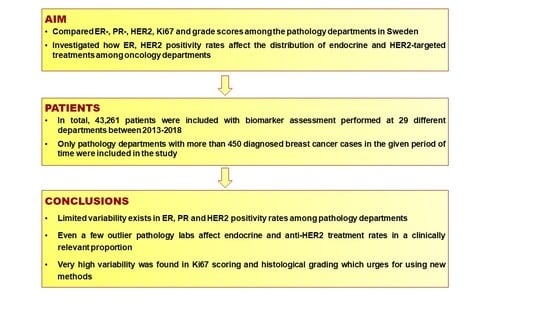
Variability in Breast Cancer Biomarker Assessment and the Effect on Oncological Treatment Decisions: A Nationwide 5-Year Population-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Biomarker Assessment
2.3. Quality Assurance of Pathology Data Reported to the NKBC
2.4. Questionnaire on Analytical Procedures
2.5. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
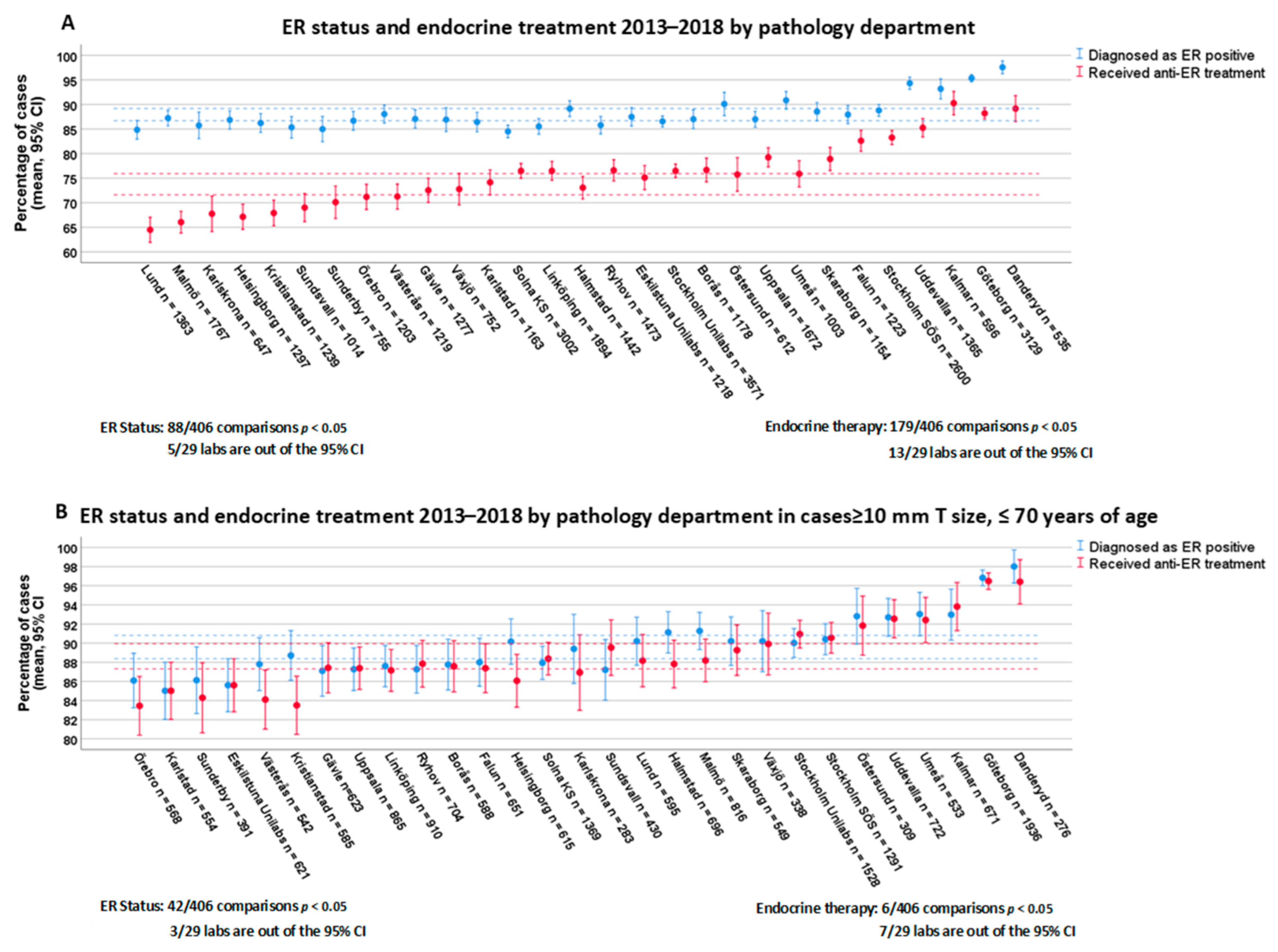
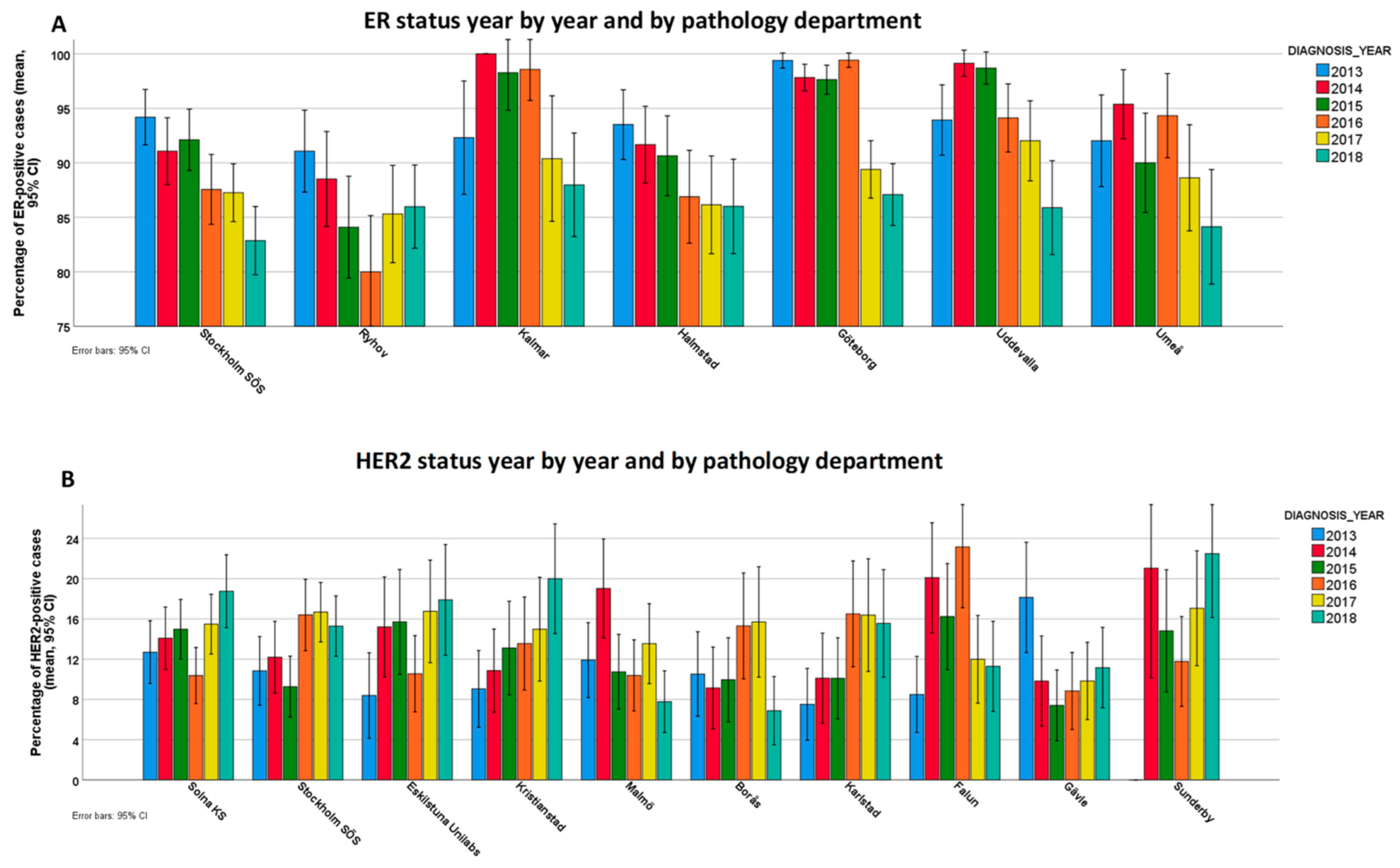
3.2. Variability of ER Positivity Rates and Endocrine Therapy
3.3. Variability of PR Positivity Rates
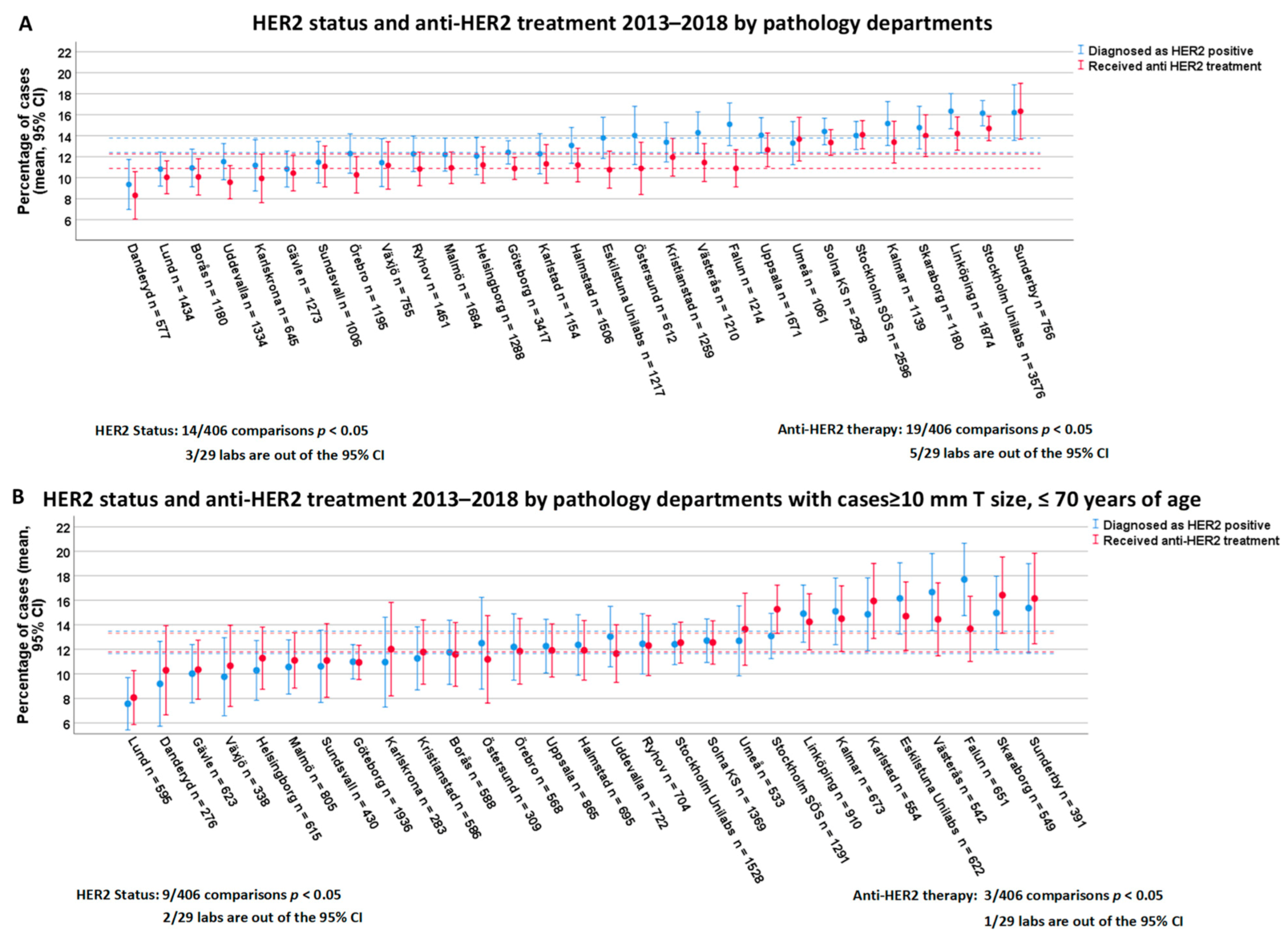
3.4. Variability of HER2 Positivity Rates and Anti-HER2 Treatment
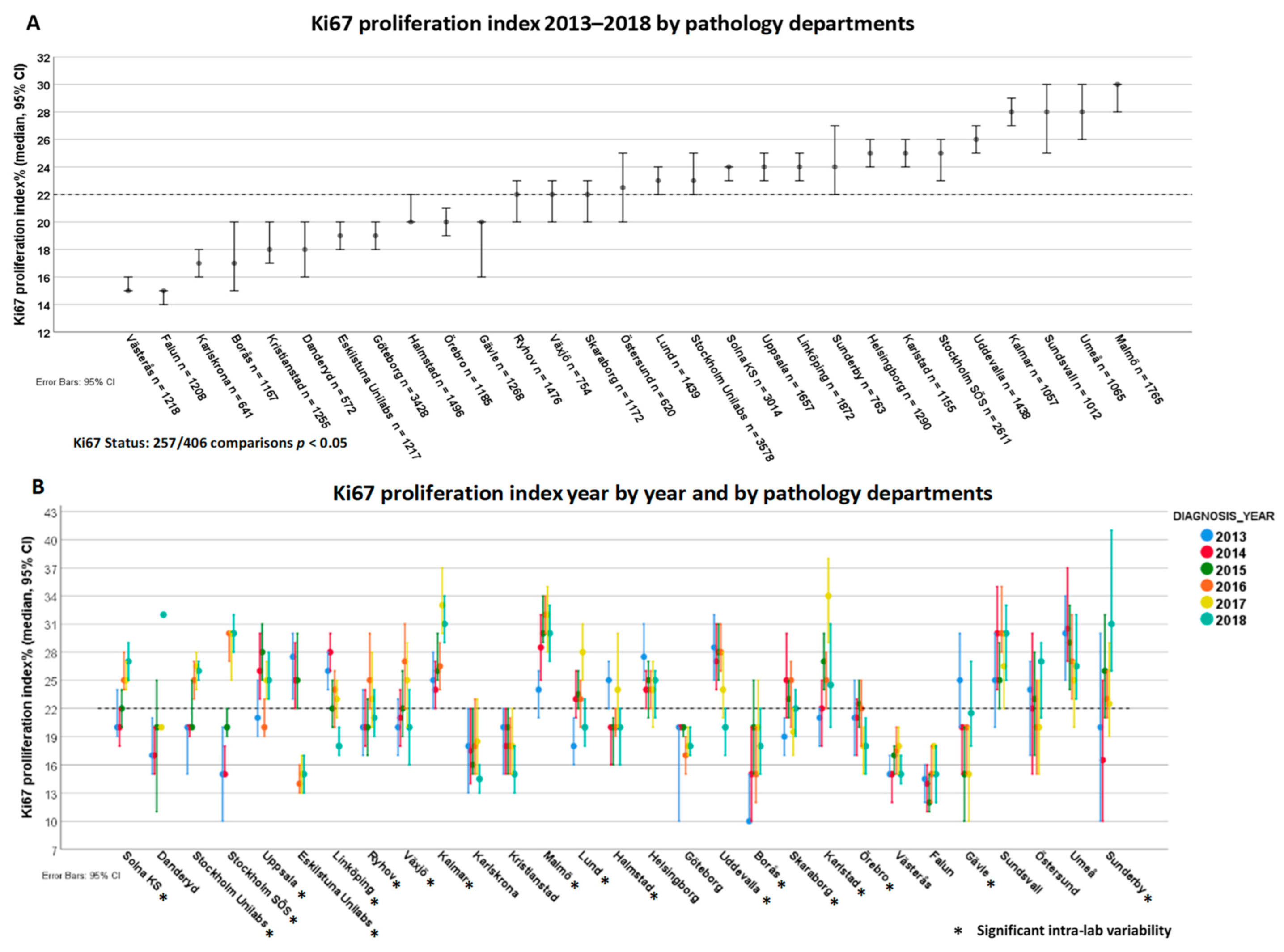
3.5. Variability of Ki67 Proliferation Index
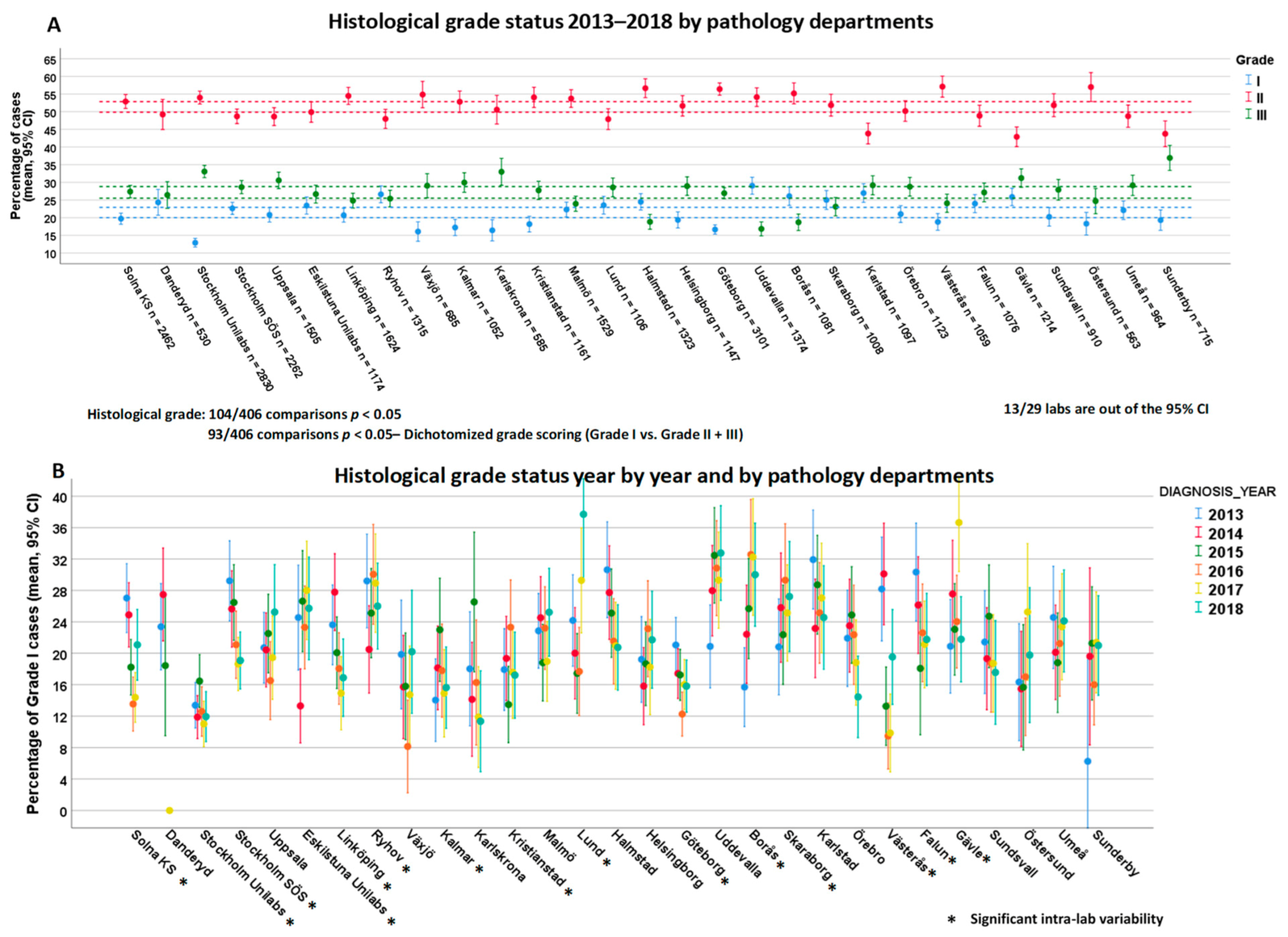
3.6. Variability of Histological Grade
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- McGuire, W.L. Current status of estrogen receptors in human breast cancer. Cancer 1975, 36, 638–644. [Google Scholar] [CrossRef]
- Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; Wang, Y.C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [PubMed]
- Hayes, D.F. Steady progress against HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1336–1338. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.F.; Provencher, L.; et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v8–v30. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, P.L.; Murphy, D.A.; Hammond, M.E.; Allred, D.C.; Valenstein, P.N. Recommendations for validating estrogen and progesterone receptor immunohistochemistry assays. Arch. Pathol. Lab. Med. 2010, 134, 930–935. [Google Scholar] [CrossRef]
- Rhodes, A.; Jasani, B.; Balaton, A.J.; Miller, K.D. Immunohistochemical demonstration of oestrogen and progesterone receptors: Correlation of standards achieved on in house tumours with that achieved on external quality assessment material in over 150 laboratories from 26 countries. J. Clin. Pathol. 2000, 53, 292–301. [Google Scholar] [CrossRef]
- Polley, M.Y.; Leung, S.C.; McShane, L.M.; Gao, D.; Hugh, J.C.; Mastropasqua, M.G.; Viale, G.; Zabaglo, L.A.; Penault-Llorca, F.; Bartlett, J.M.; et al. An international Ki67 reproducibility study. J. Natl. Cancer Inst. 2013, 105, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F. Biomarker validation and testing. Mol. Oncol. 2015, 9, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Romond, E.H.; Jeong, J.H.; Rastogi, P.; Swain, S.M.; Geyer, C.E., Jr.; Ewer, M.S.; Rathi, V.; Fehrenbacher, L.; Brufsky, A.; Azar, C.A.; et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2012, 30, 3792–3799. [Google Scholar]
- Russell, S.D.; Blackwell, K.L.; Lawrence, J.; Pippen, J.E., Jr.; Roe, M.T.; Wood, F.; Paton, V.; Holmgren, E.; Mahaffey, K.W. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: A combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J. Clin. Oncol. 2010, 28, 3416–3421. [Google Scholar] [PubMed]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Bilous, M.; Dowsett, M.; Hanna, W.; Isola, J.; Lebeau, A.; Moreno, A.; Penault-Llorca, F.; Ruschoff, J.; Tomasic, G.; van de Vijver, M. Current perspectives on HER2 testing: A review of national testing guidelines. Mod. Pathol. 2003, 16, 173–182. [Google Scholar] [CrossRef]
- Ellis, I.O.; Bartlett, J.; Dowsett, M.; Humphreys, S.; Jasani, B.; Miller, K.; Pinder, S.E.; Rhodes, A.; Walker, R. Best practice no 176: Updated recommendations for HER2 testing in the UK. J. Clin. Pathol. 2004, 57, 233–237. [Google Scholar] [CrossRef]
- Vyberg, M.; Nielsen, S. Proficiency testing in immunohistochemistry—Experiences from Nordic Immunohistochemical Quality Control (NordiQC). Virchows Arch. 2016, 468, 19–29. [Google Scholar] [CrossRef]
- Choritz, H.; Büsche, G.; Kreipe, H. Quality assessment of HER2 testing by monitoring of positivity rates. Virchows Arch. 2011, 459, 283–289. [Google Scholar] [CrossRef][Green Version]
- Rüschoff, J.; Lebeau, A.; Kreipe, H.; Sinn, P.; Gerharz, C.D.; Koch, W.; Morris, S.; Ammann, J.; Untch, M. Assessing HER2 testing quality in breast cancer: Variables that influence HER2 positivity rate from a large, multicenter, observational study in Germany. Mod. Pathol. 2017, 30, 217–226. [Google Scholar] [CrossRef][Green Version]
- National Quality Registry for Breast Cancer (NKBC). Available online: https://statistik.incanet.se/brostcancer/ (accessed on 26 February 2021).
- Swedish Society of Pathology—Quality and Standardization Committee (KVAST): Breast Cancer Guideline. 2020. Available online: http://www.svfp.se/foreningar/uploads/L15178/kvast/brostpatologi/Brostcancer2020korr.pdf (accessed on 26 February 2021).
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Parise, C.A.; Caggiano, V. breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J. Cancer Epidemiol. 2014, 2014, 469251. [Google Scholar] [CrossRef] [PubMed]
- van Dooijeweert, C.; Deckers, I.A.G.; Baas, I.O.; van der Wall, E.; van Diest, P.J. Hormone- and HER2-receptor assessment in 33,046 breast cancer patients: A nationwide comparison of positivity rates between pathology laboratories in the Netherlands. Breast Cancer Res. Treat. 2019, 175, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.A.; Horten, B.C.; Da Silva, M.M. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin. Breast Cancer. 2004, 5, 63–69. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- van Dooijeweert, C.; van Diest, P.J.; Willems, S.M.; Kuijpers, C.; van der Wall, E.; Overbeek, L.I.H.; Deckers, I.A.G. Significant inter- and intra-laboratory variation in grading of invasive breast cancer: A nationwide study of 33,043 patients in the Netherlands. Int. J. Cancer. 2020, 146, 769–780. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Henson, D.E.; Chen, D.; Rajamarthandan, S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: A study of 161 708 cases of breast cancer from the SEER Program. Arch. Pathol. Lab. Med. 2014, 138, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Haglund, M.; Bendahl, P.O.; Hatschek, T.; Kolaric, A.; Kovács, A.; Olsson, A.; Olsson, H.; Strand, C.; Fernö, M. Reproducibility of human epidermal growth factor receptor 2 analysis in primary breast cancer: A national survey performed at pathology departments in Sweden. Acta Oncol. 2009, 48, 860–866. [Google Scholar] [CrossRef]
- Ekholm, M.; Grabau, D.; Bendahl, P.O.; Bergh, J.; Elmberger, G.; Olsson, H.; Russo, L.; Viale, G.; Fernö, M. Highly reproducible results of breast cancer biomarkers when analysed in accordance with national guidelines—A Swedish survey with central re-assessment. Acta Oncol. 2015, 54, 1040–1048. [Google Scholar] [CrossRef]
- Stuart-Harris, R.; Caldas, C.; Pinder, S.E.; Pharoah, P. Proliferation markers and survival in early breast cancer: A systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 2008, 17, 323–334. [Google Scholar] [CrossRef]
- Criscitiello, C.; Disalvatore, D.; De Laurentiis, M.; Gelao, L.; Fumagalli, L.; Locatelli, M.; Bagnardi, V.; Rotmensz, N.; Esposito, A.; Minchella, I.; et al. High Ki-67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast cancer. Breast 2014, 23, 69–75. [Google Scholar] [CrossRef]
- Meyer, J.S.; Alvarez, C.; Milikowski, C.; Olson, N.; Russo, I.; Russo, J.; Glass, A.; Zehnbauer, B.A.; Lister, K.; Parwaresch, R. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: Reproducibility of grade and advantages of proliferation index. Mod. Pathol. 2005, 18, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Frierson, H.F., Jr.; Wolber, R.A.; Berean, K.W.; Franquemont, D.W.; Gaffey, M.J.; Boyd, J.C.; Wilbur, D.C. Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. Am. J. Clin. Pathol. 1995, 103, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Swedish National Breast Cancer Care Program. Available online: https://kunskapsbanken.cancercentrum.se/diagnoser/brostcancer/vardprogram/kategorisering-av-tumoren/ (accessed on 26 February 2021).
- Polley, M.Y.; Leung, S.C.; Gao, D.; Mastropasqua, M.G.; Zabaglo, L.A.; Bartlett, J.M.; McShane, L.M.; Enos, R.A.; Badve, S.S.; Bane, A.L.; et al. An international study to increase concordance in Ki67 scoring. Mod. Pathol. 2015, 28, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Rimm, D.L.; Leung, S.C.Y.; McShane, L.M.; Bai, Y.; Bane, A.L.; Bartlett, J.M.S.; Bayani, J.; Chang, M.C.; Dean, M.; Denkert, C.; et al. An international multicenter study to evaluate reproducibility of automated scoring for assessment of Ki67 in breast cancer. Mod. Pathol. 2019, 32, 59–69. [Google Scholar] [CrossRef]
- Stalhammar, G.; Fuentes Martinez, N.; Lippert, M.; Tobin, N.P.; Molholm, I.; Kis, L.; Rosin, G.; Rantalainen, M.; Pedersen, L.; Bergh, J.; et al. Digital image analysis outperforms manual biomarker assessment in breast cancer. Mod. Pathol. 2016, 29, 318–329. [Google Scholar] [CrossRef]
- Acs, B.; Pelekanou, V.; Bai, Y.; Martinez-Morilla, S.; Toki, M.; Leung, S.C.Y.; Nielsen, T.O.; Rimm, D.L. Ki67 reproducibility using digital image analysis: An inter-platform and inter-operator study. Lab. Investig. 2019, 99, 107–117. [Google Scholar] [CrossRef]
- Sotiriou, C.; Wirapati, P.; Loi, S.; Harris, A.; Fox, S.; Smeds, J.; Nordgren, H.; Farmer, P.; Praz, V.; Haibe-Kains, B.; et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J. Natl. Cancer Inst. 2006, 98, 262–272. [Google Scholar] [CrossRef]
- Wang, M.; Klevebring, D.; Lindberg, J.; Czene, K.; Grönberg, H.; Rantalainen, M. Determining breast cancer histological grade from RNA-sequencing data. Breast Cancer Res. 2016, 18, 48. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in breast cancer: Updated recommendations from the international Ki67 in breast cancer working group. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef] [PubMed]
- Arnedos, M.; Nerurkar, A.; Osin, P.; A’Hern, R.; Smith, I.E.; Dowsett, M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann. Oncol. 2009, 20, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.J.; Smit, V.T.; Hooijer, G.K.; Van de Vijver, M.J.; Mesker, W.E.; Tollenaar, R.A.; Nortier, J.W.; Kroep, J.R. Reliability of core needle biopsy for determining ER and HER2 status in breast cancer. Ann. Oncol. 2013, 24, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Rönnlund, C.; de Boniface, J.; Hartman, J. Re-testing of predictive biomarkers on surgical breast cancer specimens is clinically relevant. Breast Cancer Res. Treat. 2019, 174, 795–805. [Google Scholar] [CrossRef] [PubMed]
| Variable | Category | Statistics | Results | |
|---|---|---|---|---|
| Patients | Total n, % | 43,959 | 100 | |
| Valid n, % | 43,261 | 98.4 | ||
| Age (years) | Mean ± SD | 63.5 | ± 13.3 | |
| Missing data | % | 0.1 | - | |
| Tumor size (mm) | Median, IQR | 15 | 13 | |
| Missing data | % | 1.1 | - | |
| Lymph node status | Node-negative | %, CI | 72.0 | 71.0–73.0 |
| Node-positive | %, CI | 28.0 | 27.0–29.0 | |
| Missing data | % | 2.3 | - | |
| Histological grade (n = 38,076) | Grade I | %, CI | 21.4 | 20.0–22.9 |
| Grade II | %, CI | 51.4 | 49.9–52.9 | |
| Grade III | %, CI | 27.2 | 25.5–28.8 | |
| Missing data | % | 1.3 | - | |
| ER | Positive | %, CI | 87.9 | 86.7–89.2 |
| Negative | %, CI | 12.1 | 10.8–13.2 | |
| Missing data | % | 4.4 | ||
| PR | Positive | %, CI | 72.8 | 70.9–74.8 |
| Negative | %, CI | 27.2 | 25.2–29.1 | |
| Missing data | % | 5.7 | - | |
| HER2 | Positive | %, CI | 13.1 | 12.4–13.8 |
| Negative | %, CI | 86.9 | 86.2–87.6 | |
| Missing data | % | 2.3 | - | |
| Ki67 | Median, IQR | 22.0 | 26 | |
| Missing data | % | 2.0 | - | |
| Endocrine treatment | Received | %, CI | 73.8 | 71.6-75.9 |
| Not received | %, CI | 26.2 | 24.1–28.4 | |
| Missing data | % | 0.7 | - | |
| HER2-targeted therapy | Received | %, CI | 11.6 | 10.9–12.3 |
| Not received | %, CI | 88.4 | 87.7–89.1 | |
| Missing data | % | 0.7 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acs, B.; Fredriksson, I.; Rönnlund, C.; Hagerling, C.; Ehinger, A.; Kovács, A.; Røge, R.; Bergh, J.; Hartman, J. Variability in Breast Cancer Biomarker Assessment and the Effect on Oncological Treatment Decisions: A Nationwide 5-Year Population-Based Study. Cancers 2021, 13, 1166. https://doi.org/10.3390/cancers13051166
Acs B, Fredriksson I, Rönnlund C, Hagerling C, Ehinger A, Kovács A, Røge R, Bergh J, Hartman J. Variability in Breast Cancer Biomarker Assessment and the Effect on Oncological Treatment Decisions: A Nationwide 5-Year Population-Based Study. Cancers. 2021; 13(5):1166. https://doi.org/10.3390/cancers13051166
Chicago/Turabian StyleAcs, Balazs, Irma Fredriksson, Caroline Rönnlund, Catharina Hagerling, Anna Ehinger, Anikó Kovács, Rasmus Røge, Jonas Bergh, and Johan Hartman. 2021. "Variability in Breast Cancer Biomarker Assessment and the Effect on Oncological Treatment Decisions: A Nationwide 5-Year Population-Based Study" Cancers 13, no. 5: 1166. https://doi.org/10.3390/cancers13051166
APA StyleAcs, B., Fredriksson, I., Rönnlund, C., Hagerling, C., Ehinger, A., Kovács, A., Røge, R., Bergh, J., & Hartman, J. (2021). Variability in Breast Cancer Biomarker Assessment and the Effect on Oncological Treatment Decisions: A Nationwide 5-Year Population-Based Study. Cancers, 13(5), 1166. https://doi.org/10.3390/cancers13051166