Metabolic Health Together with a Lipid Genetic Risk Score Predicts Survival of Small Cell Lung Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient’s Selection and Gene Expression Analysis
2.2. Statistical Analysis
3. Results
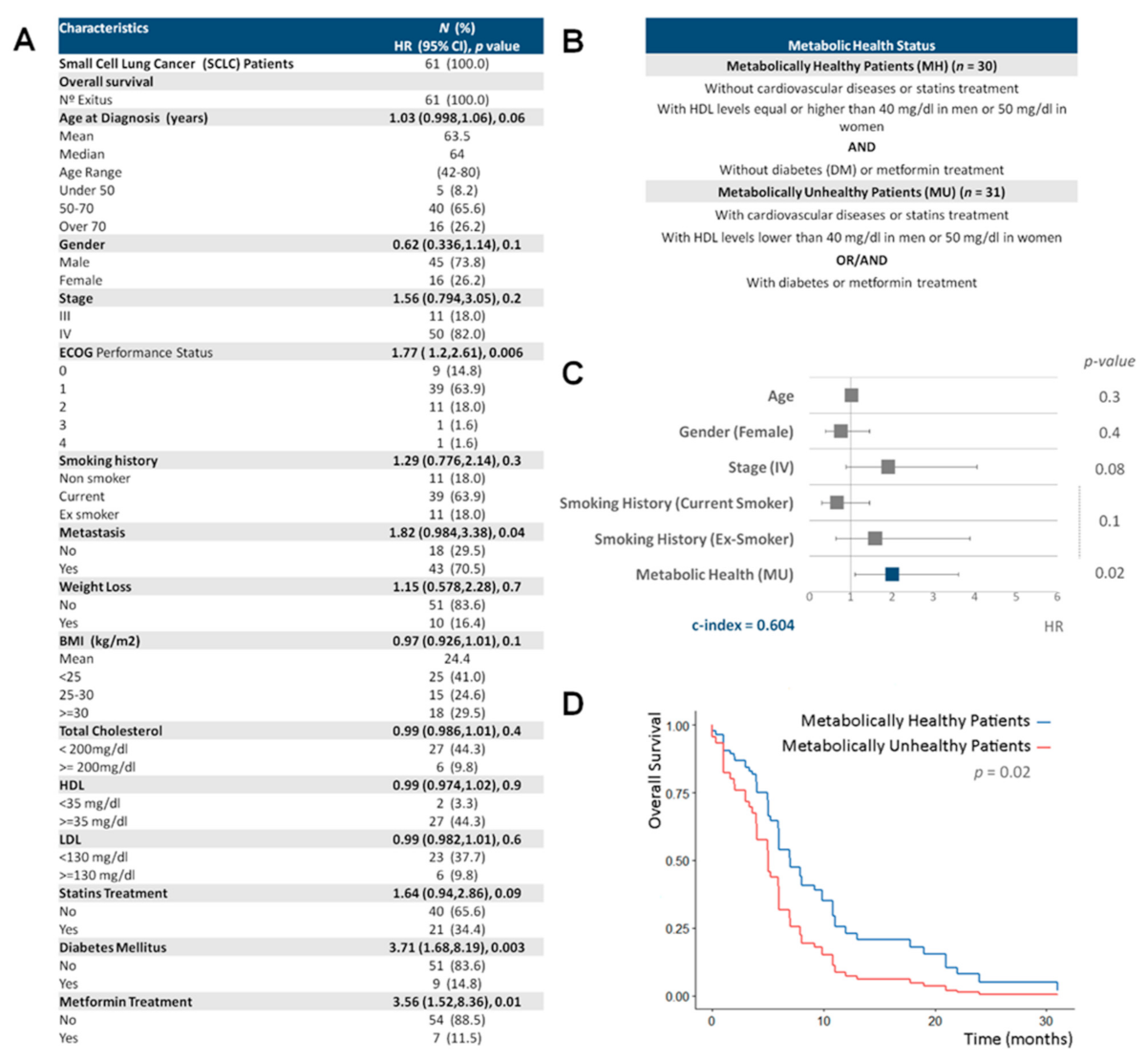
3.1. A Healthy Metabolic Status Contributes to Increase SCLC Survival
3.2. Racemase and Perilipin Expression Levels Are Prognostic Markers of Increased Survival in SCLC Patients
3.3. Metabolic Health, Lipid Metabolism Genetic Risk Score and Smoking Behavior Are the Main Predictors of SCLC Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alpha-Methylacyl-CoA Racemase | (AMACR) |
| BMI | (body mass index) |
| Diabetes mellitus | (DM) |
| ECOG | (Eastern Cooperative Oncology Group) |
| Formalin-fixed, paraffin-embedded | (FFPE) |
| Genetic risk score | (GRS) |
| Hazard ratio | (HR) |
| HDL | (high-density lipoprotein) |
| LDL | (low-density lipoprotein) |
| Metabolic health | (MH) |
| Metabolically healthy | (MH) |
| Metabolically unhealthy | (MU) |
| Overall survival | (OS) |
| Perilipin 1 | (PLIN1) |
| Small cell lung cancer | (SCLC) |
| The World Health Organization | (WHO) |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-Cell Lung Cancer: What We Know, What We Need to Know and the Path Forward. Nat. Rev. Cancer 2017, 17, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid Metabolic Reprogramming in Cancer Cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Vargas, T.; Moreno-Rubio, J.; Herranz, J.; Cejas, P.; Molina, S.; González-Vallinas, M.; Mendiola, M.; Burgos, E.; Aguayo, C.; Custodio, A.B.; et al. ColoLipidGene: Signature of Lipid Metabolism-Related Genes to Predict Prognosis in Stage-II Colon Cancer Patients. Oncotarget 2015, 6, 7348–7363. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; Ramos-Ruiz, R.; Herranz, J.; Martín-Hernández, R.; Vargas, T.; Mendiola, M.; Guerra, L.; Reglero, G.; Feliu, J.; Ramírez de Molina, A. The Transcriptional and Mutational Landscapes of Lipid Metabolism-Related Genes in Colon Cancer. Oncotarget 2018, 9, 5919–5930. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; Merino, M.; Colmenarejo, G.; Moreno-Rubio, J.; Sánchez-Martínez, R.; Quijada-Freire, A.; Gómez de Cedrón, M.; Reglero, G.; Casado, E.; Sereno, M.; et al. Metabolic Enzyme ACSL3 Is a Prognostic Biomarker and Correlates with Anticancer Effectiveness of Statins in Non-Small Cell Lung Cancer. Mol. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; Gómez de Cedrón, M.; Ramírez de Molina, A. Alterations of Lipid Metabolism in Cancer: Implications in Prognosis and Treatment. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Arends, J. Metabolism in Cancer Patients. Anticancer Res. 2010, 30, 1863–1868. [Google Scholar] [PubMed]
- Aguirre-Portolés, C.; Fernández, L.P.; Ramírez de Molina, A. Precision Nutrition for Targeting Lipid Metabolism in Colorectal Cancer. Nutrients 2017, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Lausen, B.; Schumacher, M. Maximally Selected Rank Statistics. Biometrics 1992, 48, 73–85. [Google Scholar] [CrossRef]
- Brown, B.W.; Hollander, M.; Korwar, R. Nonparametric Tests of Independence for Censored Data with Application to Heart Transplant Studies; Department of Statistics: Tallahassee, FL, USA, 1973. [Google Scholar]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.; Schulze, A. Lipid Metabolism at the Nexus of Diet and Tumor Microenvironment. Trends Cancer 2019, 5, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Shilo, K.; Dracheva, T.; Mani, H.; Fukuoka, J.; Sesterhenn, I.A.; Chu, W.-S.; Shih, J.H.; Jen, J.; Travis, W.D.; Franks, T.J. Alpha-Methylacyl CoA Racemase in Pulmonary Adenocarcinoma, Squamous Cell Carcinoma, and Neuroendocrine Tumors: Expression and Survival Analysis. Arch. Pathol. Lab. Med. 2007, 131, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Sztalryd, C.; Brasaemle, D.L. The Perilipin Family of Lipid Droplet Proteins: Gatekeepers of Intracellular Lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Shao, H.; Zheng, X. Obesity Paradox in Lung Cancer Prognosis: Evolving Biological Insights and Clinical Implications. J. Thorac. Oncol. 2017, 12, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, M.; Zhou, L.; Zhang, Y.; Liu, W.; Qin, W.; He, R.; Lu, Y.; Wang, Y.; Chen, X.-Z.; et al. Prognostic Significance of PLIN1 Expression in Human Breast Cancer. Oncotarget 2016, 7, 54488–54502. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, L.P.; Merino, M.; Colmenarejo, G.; Moreno Rubio, J.; González Pessolani, T.; Reglero, G.; Casado, E.; Sereno, M.; Ramírez de Molina, A. Metabolic Health Together with a Lipid Genetic Risk Score Predicts Survival of Small Cell Lung Cancer Patients. Cancers 2021, 13, 1112. https://doi.org/10.3390/cancers13051112
Fernández LP, Merino M, Colmenarejo G, Moreno Rubio J, González Pessolani T, Reglero G, Casado E, Sereno M, Ramírez de Molina A. Metabolic Health Together with a Lipid Genetic Risk Score Predicts Survival of Small Cell Lung Cancer Patients. Cancers. 2021; 13(5):1112. https://doi.org/10.3390/cancers13051112
Chicago/Turabian StyleFernández, Lara P., María Merino, Gonzalo Colmenarejo, Juan Moreno Rubio, Tais González Pessolani, Guillermo Reglero, Enrique Casado, María Sereno, and Ana Ramírez de Molina. 2021. "Metabolic Health Together with a Lipid Genetic Risk Score Predicts Survival of Small Cell Lung Cancer Patients" Cancers 13, no. 5: 1112. https://doi.org/10.3390/cancers13051112
APA StyleFernández, L. P., Merino, M., Colmenarejo, G., Moreno Rubio, J., González Pessolani, T., Reglero, G., Casado, E., Sereno, M., & Ramírez de Molina, A. (2021). Metabolic Health Together with a Lipid Genetic Risk Score Predicts Survival of Small Cell Lung Cancer Patients. Cancers, 13(5), 1112. https://doi.org/10.3390/cancers13051112





