CXCL10 Is an Agonist of the CC Family Chemokine Scavenger Receptor ACKR2/D6
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Proteins
2.2. Chemokine Processing by Dipeptidyl Peptidase 4
2.3. Chemokine-Induced β-Arrestin Recruitment
2.4. Chemokine Binding
2.5. Chemokine-Induced Receptor Mobilisation to the Plasma Membrane
2.6. Chemokine-Induced Receptor-Arrestin Delivery to Endosomes
2.7. Chemokine Scavenging
2.8. Chemokine Internalization
2.9. Inhibition of Chemokine Uptake by Anti-mACKR2 Antibodies
2.10. Data and Statistical Analysis
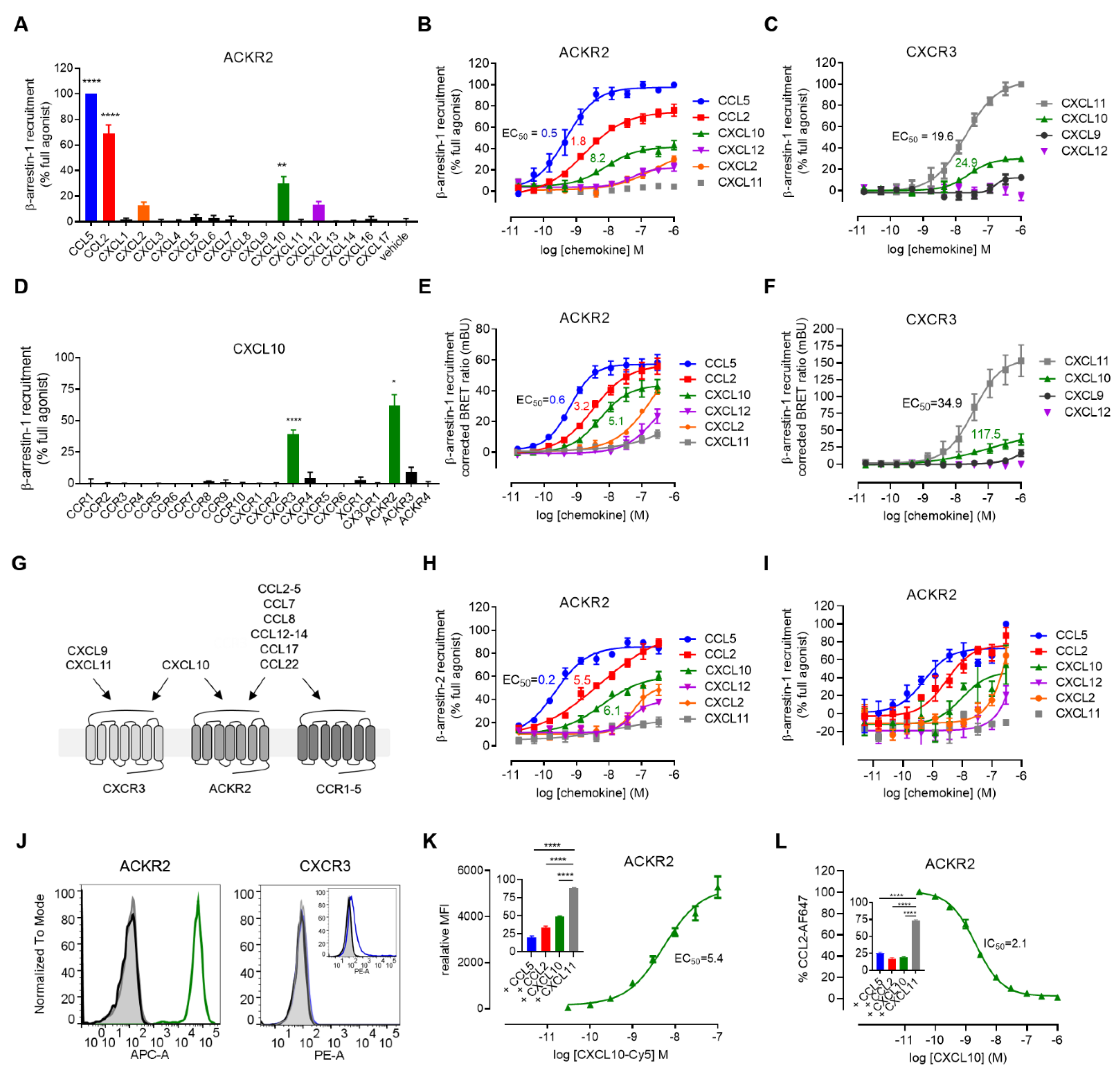
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, G.J.; Locati, M.; Mantovani, A.; Rot, A.; Thelen, M. The biochemistry and biology of the atypical chemokine receptors. Immunol. Lett. 2012, 145, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bachelerie, F.; Graham, G.J.; Locati, M.; Mantovani, A.; Murphy, P.M.; Nibbs, R.; Rot, A.; Sozzani, S.; Thelen, M. New nomenclature for atypical chemokine receptors. Nat. Immunol. 2014, 15, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Blair, E.; Simpson, C.V.; O’Hara, M.; Blackburn, P.E.; Rot, A.; Graham, G.J.; Nibbs, R.J. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol. Biol. Cell 2004, 15, 2492–2508. [Google Scholar] [CrossRef]
- Galliera, E.; Jala, V.R.; Trent, J.O.; Bonecchi, R.; Signorelli, P.; Lefkowitz, R.J.; Mantovani, A.; Locati, M.; Haribabu, B. beta-Arrestin-dependent constitutive internalization of the human chemokine decoy receptor D6. J. Biol. Chem. 2004, 279, 25590–25597. [Google Scholar] [CrossRef] [PubMed]
- Vacchini, A.; Cancellieri, C.; Milanesi, S.; Badanai, S.; Savino, B.; Bifari, F.; Locati, M.; Bonecchi, R.; Borroni, E.M. Control of Cytoskeletal Dynamics by beta-Arrestin1/Myosin Vb Signaling Regulates Endosomal Sorting and Scavenging Activity of the Atypical Chemokine Receptor ACKR2. Vaccines 2020, 8, 542. [Google Scholar] [CrossRef]
- Comerford, I.; Milasta, S.; Morrow, V.; Milligan, G.; Nibbs, R. The chemokine receptor CCX-CKR mediates effective scavenging of CCL19 in vitro. Eur. J. Immunol. 2006, 36, 1904–1916. [Google Scholar] [CrossRef]
- Meyrath, M.; Szpakowska, M.; Zeiner, J.; Massotte, L.; Merz, M.P.; Benkel, T.; Simon, K.; Ohnmacht, J.; Turner, J.D.; Kruger, R.; et al. The atypical chemokine receptor ACKR3/CXCR7 is a broad-spectrum scavenger for opioid peptides. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, C.V.; Morrow, V.; Milasta, S.; Comerford, I.; Milligan, G.; Graham, G.J.; Isaacs, N.W.; Nibbs, R.J. Multiple roles for the C-terminal tail of the chemokine scavenger D6. J. Biol. Chem. 2008, 283, 7972–7982. [Google Scholar] [CrossRef]
- Montpas, N.; St-Onge, G.; Nama, N.; Rhainds, D.; Benredjem, B.; Girard, M.; Hickson, G.; Pons, V.; Heveker, N. Ligand-specific conformational transitions and intracellular transport are required for atypical chemokine receptor 3-mediated chemokine scavenging. J. Biol. Chem. 2018, 293, 893–905. [Google Scholar] [CrossRef]
- Saaber, F.; Schutz, D.; Miess, E.; Abe, P.; Desikan, S.; Ashok Kumar, P.; Balk, S.; Huang, K.; Beaulieu, J.M.; Schulz, S.; et al. ACKR3 Regulation of Neuronal Migration Requires ACKR3 Phosphorylation, but Not beta-Arrestin. Cell Rep. 2019, 26, 1473–1488.e1479. [Google Scholar] [CrossRef] [PubMed]
- Matti, C.; Salnikov, A.; Artinger, M.; D’Agostino, G.; Kindinger, I.; Uguccioni, M.; Thelen, M.; Legler, D.F. ACKR4 Recruits GRK3 Prior to beta-Arrestins but Can Scavenge Chemokines in the Absence of beta-Arrestins. Front. Immunol. 2020, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Nibbs, R.J.; Wylie, S.M.; Pragnell, I.B.; Graham, G.J. Cloning and characterization of a novel murine beta chemokine receptor, D6. Comparison to three other related macrophage inflammatory protein-1alpha receptors, CCR-1, CCR-3, and CCR-5. J. Biol. Chem. 1997, 272, 12495–12504. [Google Scholar] [CrossRef]
- Fra, A.M.; Locati, M.; Otero, K.; Sironi, M.; Signorelli, P.; Massardi, M.L.; Gobbi, M.; Vecchi, A.; Sozzani, S.; Mantovani, A. Cutting edge: Scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J. Immunol. 2003, 170, 2279–2282. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Torre, Y.M.; Galliera, E.; Bonecchi, R.; Bodduluri, H.; Vago, G.; Vecchi, A.; Mantovani, A. Silent chemoattractant receptors: D6 as a decoy and scavenger receptor for inflammatory CC chemokines. Cytokine Growth Factor Rev. 2005, 16, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Bonini, J.A.; Martin, S.K.; Dralyuk, F.; Roe, M.W.; Philipson, L.H.; Steiner, D.F. Cloning, expression, and chromosomal mapping of a novel human CC-chemokine receptor (CCR10) that displays high-affinity binding for MCP-1 and MCP-3. DNA Cell Biol. 1997, 16, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, T.; Cook, D.N.; Nibbs, R.J.; Rot, A.; Nixon, C.; McLean, P.; Alcami, A.; Lira, S.A.; Wiekowski, M.; Graham, G.J. The chemokine receptor D6 limits the inflammatory response in vivo. Nat. Immunol. 2005, 6, 403–411. [Google Scholar] [CrossRef]
- Lee, K.M.; McKimmie, C.S.; Gilchrist, D.S.; Pallas, K.J.; Nibbs, R.J.; Garside, P.; McDonald, V.; Jenkins, C.; Ransohoff, R.; Liu, L.; et al. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood 2011, 118, 6220–6229. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.D.; King, V.; Baldwin, H.; Burden, D.; Thorrat, A.; Holmes, S.; McInnes, I.B.; Nicoll, R.; Shams, K.; Pallas, K.; et al. Elevated expression of the chemokine-scavenging receptor D6 is associated with impaired lesion development in psoriasis. Am. J. Pathol. 2012, 181, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Graham, G.J. Atypical Chemokine Receptors and Their Roles in the Resolution of the Inflammatory Response. Front. Immunol. 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Wilson, G.J.; Pingen, M.; Fukuoka, A.; Hansell, C.A.H.; Bartolini, R.; Medina-Ruiz, L.; Graham, G.J. Placental chemokine compartmentalisation: A novel mammalian molecular control mechanism. PLoS Biol. 2019, 17, e3000287. [Google Scholar] [CrossRef]
- Martinez de la Torre, Y.; Locati, M.; Buracchi, C.; Dupor, J.; Cook, D.N.; Bonecchi, R.; Nebuloni, M.; Rukavina, D.; Vago, L.; Vecchi, A.; et al. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur. J. Immunol. 2005, 35, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Teoh, P.J.; Menzies, F.M.; Hansell, C.A.; Clarke, M.; Waddell, C.; Burton, G.J.; Nelson, S.M.; Nibbs, R.J. Atypical chemokine receptor ACKR2 mediates chemokine scavenging by primary human trophoblasts and can regulate fetal growth, placental structure, and neonatal mortality in mice. J. Immunol. 2014, 193, 5218–5228. [Google Scholar] [CrossRef] [PubMed]
- Hansell, C.A.; Schiering, C.; Kinstrie, R.; Ford, L.; Bordon, Y.; McInnes, I.B.; Goodyear, C.S.; Nibbs, R.J. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood 2011, 117, 5413–5424. [Google Scholar] [CrossRef]
- McKimmie, C.S.; Singh, M.D.; Hewit, K.; Lopez-Franco, O.; Le Brocq, M.; Rose-John, S.; Lee, K.M.; Baker, A.H.; Wheat, R.; Blackbourn, D.J.; et al. An analysis of the function and expression of D6 on lymphatic endothelial cells. Blood 2013, 121, 3768–3777. [Google Scholar] [CrossRef] [PubMed]
- Savino, B.; Castor, M.G.; Caronni, N.; Sarukhan, A.; Anselmo, A.; Buracchi, C.; Benvenuti, F.; Pinho, V.; Teixeira, M.M.; Mantovani, A.; et al. Control of murine Ly6C(high) monocyte traffic and immunosuppressive activities by atypical chemokine receptor D6. Blood 2012, 119, 5250–5260. [Google Scholar] [CrossRef]
- Castanheira, F.; Borges, V.; Sonego, F.; Kanashiro, A.; Donate, P.B.; Melo, P.H.; Pallas, K.; Russo, R.C.; Amaral, F.A.; Teixeira, M.M.; et al. The Atypical Chemokine Receptor ACKR2 is Protective Against Sepsis. Shock 2018, 49, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Massara, M.; Bonavita, O.; Savino, B.; Caronni, N.; Mollica Poeta, V.; Sironi, M.; Setten, E.; Recordati, C.; Crisafulli, L.; Ficara, F.; et al. ACKR2 in hematopoietic precursors as a checkpoint of neutrophil release and anti-metastatic activity. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Lee, K.M.; Danuser, R.; Stein, J.V.; Graham, D.; Nibbs, R.J.; Graham, G.J. The chemokine receptors ACKR2 and CCR2 reciprocally regulate lymphatic vessel density. Embo J. 2014, 33, 2564–2580. [Google Scholar] [CrossRef]
- Nibbs, R.J.; Graham, G.J. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 2013, 13, 815–829. [Google Scholar] [CrossRef]
- Shams, K.; Wilson, G.J.; Singh, M.; van den Bogaard, E.H.; Le Brocq, M.L.; Holmes, S.; Schalkwijk, J.; Burden, A.D.; McKimmie, C.S.; Graham, G.J. Spread of Psoriasiform Inflammation to Remote Tissues Is Restricted by the Atypical Chemokine Receptor ACKR2. J. Invest. Derm. 2017, 137, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Graham, G.J.; Damodaran, A.; Hu, T.; Lira, S.A.; Sasse, M.; Canasto-Chibuque, C.; Cook, D.N.; Ransohoff, R.M. Cutting edge: The silent chemokine receptor D6 is required for generating T cell responses that mediate experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Pashover-Schallinger, E.; Aswad, M.; Schif-Zuck, S.; Shapiro, H.; Singer, P.; Ariel, A. The atypical chemokine receptor D6 controls macrophage efferocytosis and cytokine secretion during the resolution of inflammation. FASEB J. 2012, 26, 3891–3900. [Google Scholar] [CrossRef] [PubMed]
- Aswad, M.; Assi, S.; Schif-Zuck, S.; Ariel, A. CCL5 Promotes Resolution-Phase Macrophage Reprogramming in Concert with the Atypical Chemokine Receptor D6 and Apoptotic Polymorphonuclear Cells. J. Immunol. 2017, 199, 1393–1404. [Google Scholar] [CrossRef]
- Hansell, C.A.H.; Fraser, A.R.; Hayes, A.J.; Pingen, M.; Burt, C.L.; Lee, K.M.; Medina-Ruiz, L.; Brownlie, D.; Macleod, M.K.L.; Burgoyne, P.; et al. The Atypical Chemokine Receptor Ackr2 Constrains NK Cell Migratory Activity and Promotes Metastasis. J. Immunol. 2018, 201, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, E.; Meyrath, M.; Chevigne, A.; Ostman, A.; Augsten, M.; Szpakowska, M. The diverse and complex roles of atypical chemokine receptors in cancer: From molecular biology to clinical relevance and therapy. Adv. Cancer Res. 2020, 145, 99–138. [Google Scholar]
- Nibbs, R.J.; Gilchrist, D.S.; King, V.; Ferra, A.; Forrow, S.; Hunter, K.D.; Graham, G.J. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J. Clin. Invest. 2007, 117, 1884–1892. [Google Scholar] [CrossRef]
- Noman, M.Z.; Parpal, S.; Van Moer, K.; Xiao, M.; Yu, Y.; Viklund, J.; De Milito, A.; Hasmim, M.; Andersson, M.; Amaravadi, R.K.; et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy. Sci. Adv. 2020, 6, eaax7881. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Maru, S.V.; Holloway, K.A.; Flynn, G.; Lancashire, C.L.; Loughlin, A.J.; Male, D.K.; Romero, I.A. Chemokine production and chemokine receptor expression by human glioma cells: Role of CXCL10 in tumour cell proliferation. J. Neuroimmunol. 2008, 199, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zumwalt, T.J.; Arnold, M.; Goel, A.; Boland, C.R. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration. Oncotarget 2015, 6, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Mikucki, M.E.; Fisher, D.T.; Matsuzaki, J.; Skitzki, J.J.; Gaulin, N.B.; Muhitch, J.B.; Ku, A.W.; Frelinger, J.G.; Odunsi, K.; Gajewski, T.F.; et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.C.; Savino, B.; Mirolo, M.; Buracchi, C.; Germano, G.; Anselmo, A.; Zammataro, L.; Pasqualini, F.; Mantovani, A.; Locati, M.; et al. The atypical chemokine receptor ACKR2 drives pulmonary fibrosis by tuning influx of CCR2(+) and CCR5(+) IFNgamma-producing gammadeltaT cells in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L1010–L1025. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.S.; Schwinn, M.K.; Hall, M.P.; Zimmerman, K.; Otto, P.; Lubben, T.H.; Butler, B.L.; Binkowski, B.F.; Machleidt, T.; Kirkland, T.A.; et al. NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells. ACS Chem. Biol. 2016, 11, 400–408. [Google Scholar] [CrossRef]
- Szpakowska, M.; Meyrath, M.; Reynders, N.; Counson, M.; Hanson, J.; Steyaert, J.; Chevigne, A. Mutational analysis of the extracellular disulphide bridges of the atypical chemokine receptor ACKR3/CXCR7 uncovers multiple binding and activation modes for its chemokine and endogenous non-chemokine agonists. Biochem. Pharm. 2018, 153, 299–309. [Google Scholar] [CrossRef]
- Szpakowska, M.; Nevins, A.M.; Meyrath, M.; Rhainds, D.; D’Huys, T.; Guite-Vinet, F.; Dupuis, N.; Gauthier, P.A.; Counson, M.; Kleist, A.; et al. Different contributions of chemokine N-terminal features attest to a different ligand binding mode and a bias towards activation of ACKR3/CXCR7 compared with CXCR4 and CXCR3. Br. J. Pharm. 2018, 175, 1419–1438. [Google Scholar] [CrossRef]
- Namkung, Y.; Le Gouill, C.; Lukashova, V.; Kobayashi, H.; Hogue, M.; Khoury, E.; Song, M.; Bouvier, M.; Laporte, S.A. Monitoring G protein-coupled receptor and beta-arrestin trafficking in live cells using enhanced bystander BRET. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Schink, K.O.; Raiborg, C.; Stenmark, H. Phosphatidylinositol 3-phosphate, a lipid that regulates membrane dynamics, protein sorting and cell signalling. Bioessays 2013, 35, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Nibbs, R.J.; Wylie, S.M.; Yang, J.; Landau, N.R.; Graham, G.J. Cloning and characterization of a novel promiscuous human beta-chemokine receptor D6. J. Biol. Chem. 1997, 272, 32078–32083. [Google Scholar] [CrossRef] [PubMed]
- Meyrath, M.; Reynders, N.; Uchanski, T.; Chevigne, A.; Szpakowska, M. Systematic reassessment of chemokine-receptor pairings confirms CCL20 but not CXCL13 and extends the spectrum of ACKR4 agonists to CCL22. J. Leukoc. Biol. 2020, 109, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Matti, C.; D’Uonnolo, G.; Artinger, M.; Melgrati, S.; Salnikov, A.; Thelen, S.; Purvanov, V.; Strobel, T.D.; Spannagel, L.; Thelen, M.; et al. CCL20 is a novel ligand for the scavenging atypical chemokine receptor 4. J. Leukoc. Biol. 2020, 107, 1137–1154. [Google Scholar] [CrossRef]
- Lux, M.; Blaut, A.; Eltrich, N.; Bideak, A.; Muller, M.B.; Hoppe, J.M.; Grone, H.J.; Locati, M.; Vielhauer, V. The Atypical Chemokine Receptor 2 Limits Progressive Fibrosis after Acute Ischemic Kidney Injury. Am. J. Pathol. 2019, 189, 231–247. [Google Scholar] [CrossRef]
- Bideak, A.; Blaut, A.; Hoppe, J.M.; Muller, M.B.; Federico, G.; Eltrich, N.; Grone, H.J.; Locati, M.; Vielhauer, V. The atypical chemokine receptor 2 limits renal inflammation and fibrosis in murine progressive immune complex glomerulonephritis. Kidney Int. 2018, 93, 826–841. [Google Scholar] [CrossRef]
- Sjoberg, E.; Meyrath, M.; Milde, L.; Herrera, M.; Lovrot, J.; Hagerstrand, D.; Frings, O.; Bartish, M.; Rolny, C.; Sonnhammer, E.; et al. A Novel ACKR2-Dependent Role of Fibroblast-Derived CXCL14 in Epithelial-to-Mesenchymal Transition and Metastasis of Breast Cancer. Clin. Cancer Res. 2019, 25, 3702–3717. [Google Scholar] [CrossRef]
- Chevigne, A.; Fievez, V.; Szpakowska, M.; Fischer, A.; Counson, M.; Plesseria, J.M.; Schmit, J.C.; Deroo, S. Neutralising properties of peptides derived from CXCR4 extracellular loops towards CXCL12 binding and HIV-1 infection. Biochim. Biophys. Acta 2014, 1843, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.K.; Murphy, P.M. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996, 271, 20545–20550. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Delgado, M.B.; Jones, S.A.; Dewald, B.; Clark-Lewis, I.; Baggiolini, M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur. J. Immunol. 1998, 28, 164–170. [Google Scholar] [CrossRef]
- Scholten, D.J.; Canals, M.; Wijtmans, M.; de Munnik, S.; Nguyen, P.; Verzijl, D.; de Esch, I.J.; Vischer, H.F.; Smit, M.J.; Leurs, R. Pharmacological characterization of a small-molecule agonist for the chemokine receptor CXCR3. Br. J. Pharm. 2012, 166, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Berchiche, Y.A.; Sakmar, T.P. CXC Chemokine Receptor 3 Alternative Splice Variants Selectively Activate Different Signaling Pathways. Mol. Pharm. 2016, 90, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Borroni, E.M.; Anselmo, A.; Doni, A.; Savino, B.; Mirolo, M.; Fabbri, M.; Jala, V.R.; Haribabu, B.; Mantovani, A.; et al. Regulation of D6 chemokine scavenging activity by ligand- and Rab11-dependent surface up-regulation. Blood 2008, 112, 493–503. [Google Scholar] [CrossRef][Green Version]
- Savino, B.; Borroni, E.M.; Torres, N.M.; Proost, P.; Struyf, S.; Mortier, A.; Mantovani, A.; Locati, M.; Bonecchi, R. Recognition versus adaptive up-regulation and degradation of CC chemokines by the chemokine decoy receptor D6 are determined by their N-terminal sequence. J. Biol. Chem. 2009, 284, 26207–26215. [Google Scholar] [CrossRef]
- Mortier, A.; Gouwy, M.; Van Damme, J.; Proost, P.; Struyf, S. CD26/dipeptidylpeptidase IV-chemokine interactions: Double-edged regulation of inflammation and tumor biology. J. Leukoc. Biol. 2016, 99, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Schutyser, E.; Menten, P.; Struyf, S.; Wuyts, A.; Opdenakker, G.; Detheux, M.; Parmentier, M.; Durinx, C.; Lambeir, A.M.; et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 2001, 98, 3554–3561. [Google Scholar] [CrossRef]
- Bonecchi, R.; Locati, M.; Galliera, E.; Vulcano, M.; Sironi, M.; Fra, A.M.; Gobbi, M.; Vecchi, A.; Sozzani, S.; Haribabu, B.; et al. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J. Immunol. 2004, 172, 4972–4976. [Google Scholar] [CrossRef]
- Kleist, A.B.; Getschman, A.E.; Ziarek, J.J.; Nevins, A.M.; Gauthier, P.A.; Chevigne, A.; Szpakowska, M.; Volkman, B.F. New paradigms in chemokine receptor signal transduction: Moving beyond the two-site model. Biochem. Pharm. 2016, 114, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Reynders, N.; Abboud, D.; Baragli, A.; Noman, M.Z.; Rogister, B.; Niclou, S.P.; Heveker, N.; Janji, B.; Hanson, J.; Szpakowska, M.; et al. The Distinct Roles of CXCR3 Variants and Their Ligands in the Tumor Microenvironment. Cells 2019, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Karin, N.; Razon, H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine 2018, 109, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Soejima, K.; Rollins, B.J. A functional IFN-gamma-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J. Immunol. 2001, 167, 6576–6582. [Google Scholar] [CrossRef] [PubMed]
- Neote, K.; Darbonne, W.; Ogez, J.; Horuk, R.; Schall, T.J. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J. Biol. Chem. 1993, 268, 122247–122249. [Google Scholar] [CrossRef]
- Szpakowska, M.; Dupuis, N.; Baragli, A.; Counson, M.; Hanson, J.; Piette, J.; Chevigne, A. Human herpesvirus 8-encoded chemokine vCCL2/vMIP-II is an agonist of the atypical chemokine receptor ACKR3/CXCR7. Biochem. Pharm. 2016, 114, 14–21. [Google Scholar] [CrossRef]
- Gosling, J.; Dairaghi, D.J.; Wang, Y.; Hanley, M.; Talbot, D.; Miao, Z.; Schall, T.J. Cutting edge: Identification of a novel chemokine receptor that binds dendritic cell- and T cell-active chemokines including ELC, SLC, and TECK. J. Immunol. 2000, 164, 2851–2856. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevigné, A.; Janji, B.; Meyrath, M.; Reynders, N.; D’Uonnolo, G.; Uchański, T.; Xiao, M.; Berchem, G.; Ollert, M.; Kwon, Y.-J.; et al. CXCL10 Is an Agonist of the CC Family Chemokine Scavenger Receptor ACKR2/D6. Cancers 2021, 13, 1054. https://doi.org/10.3390/cancers13051054
Chevigné A, Janji B, Meyrath M, Reynders N, D’Uonnolo G, Uchański T, Xiao M, Berchem G, Ollert M, Kwon Y-J, et al. CXCL10 Is an Agonist of the CC Family Chemokine Scavenger Receptor ACKR2/D6. Cancers. 2021; 13(5):1054. https://doi.org/10.3390/cancers13051054
Chicago/Turabian StyleChevigné, Andy, Bassam Janji, Max Meyrath, Nathan Reynders, Giulia D’Uonnolo, Tomasz Uchański, Malina Xiao, Guy Berchem, Markus Ollert, Yong-Jun Kwon, and et al. 2021. "CXCL10 Is an Agonist of the CC Family Chemokine Scavenger Receptor ACKR2/D6" Cancers 13, no. 5: 1054. https://doi.org/10.3390/cancers13051054
APA StyleChevigné, A., Janji, B., Meyrath, M., Reynders, N., D’Uonnolo, G., Uchański, T., Xiao, M., Berchem, G., Ollert, M., Kwon, Y.-J., Noman, M. Z., & Szpakowska, M. (2021). CXCL10 Is an Agonist of the CC Family Chemokine Scavenger Receptor ACKR2/D6. Cancers, 13(5), 1054. https://doi.org/10.3390/cancers13051054






