Precision Nutrition to Activate Thermogenesis as a Complementary Approach to Target Obesity and Associated-Metabolic-Disorders
Simple Summary
Abstract
1. Introduction
2. Methodology of Searching
3. Addressing Obesity through Precision Nutrition: Nutrigenetics and Nutrigenomics
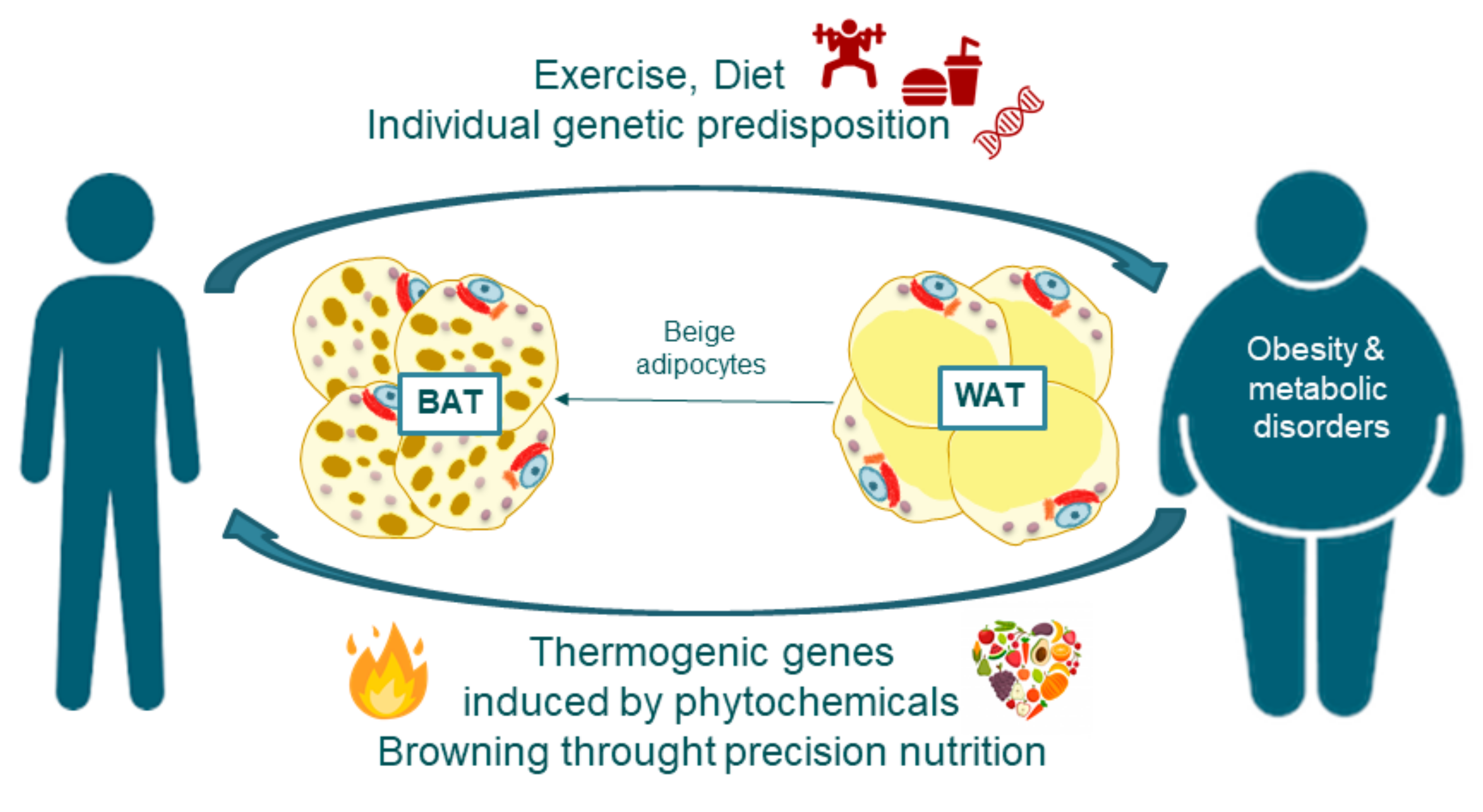
4. Adipose Tissue Features and Fate: The Browning Process
4.1. White and Brown Adipose Tissue Biogenesis
4.2. Adipose Tissue Browning
5. Thermogenesis within Adipose Tissue, Inductors and Their Implications in Obesity Disorders
5.1. Molecules and Processes Implicated in Mitochondrial Thermogenesis
5.2. Adrenergic Nervous System Activation of Thermogenesis Upon Cold Exposure
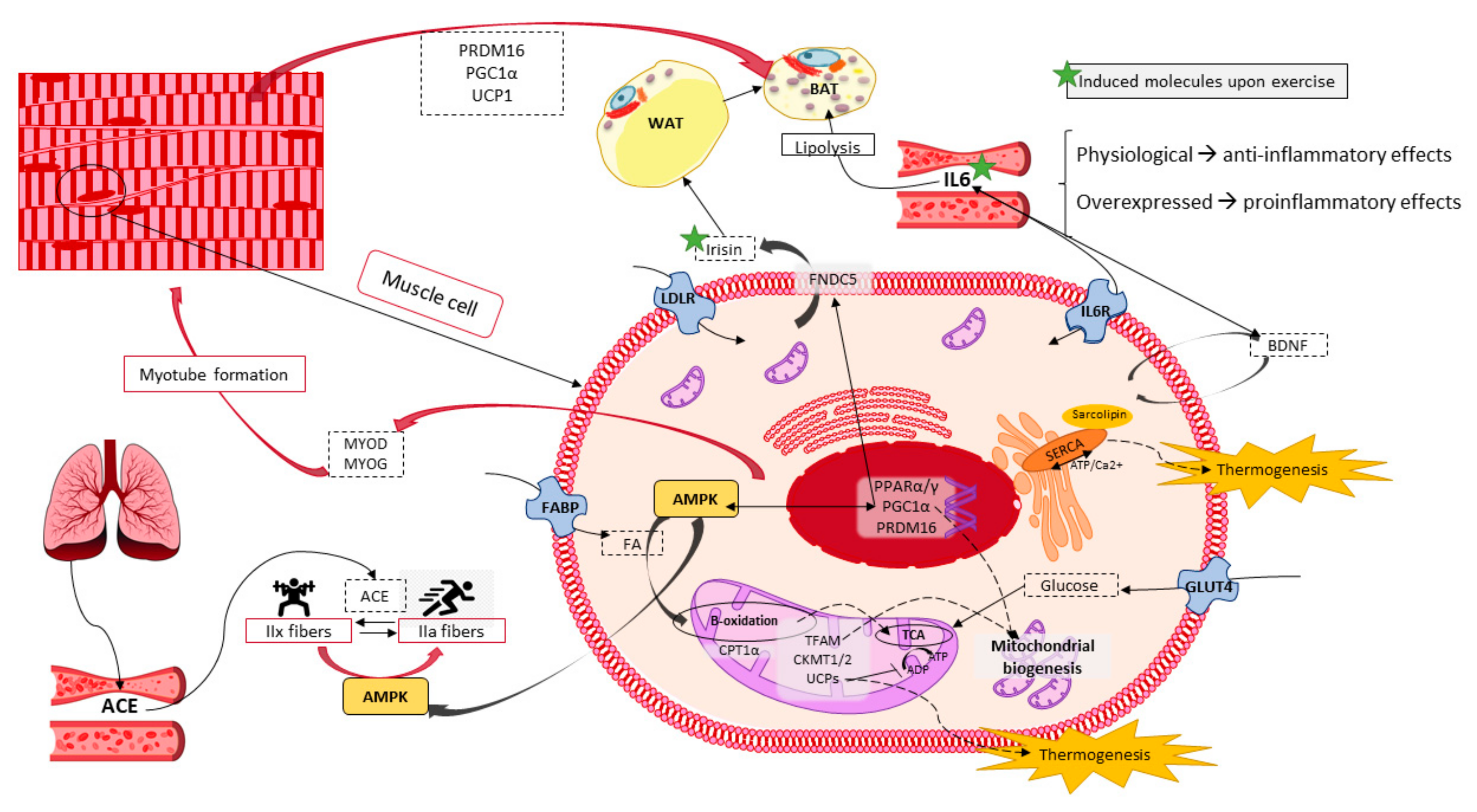
6. Skeletal Muscle Potential in Energy Expenditure and Heat Production
6.1. Skeletal Muscle Features and Functions
6.2. Thermogenesis within Skeletal Muscle
6.3. Exercise Performance as a Molecular Inductor of Thermogenesis and Browning
7. Results, Discussion and Conclusions
7.1. Phytochemicals as Thermogenic and Anti-Adipogenic Agents
7.1.1. Pomegranate
7.1.2. Ginkgo Biloba
7.1.3. Milk Thistle
7.1.4. Soy
7.1.5. Resveratrol
7.2. Relevance of Research on Bioactive Compounds to Augment Energy Expenditure
7.3. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AICAR | 5-aminoimidazole-4-carboxamide ribonucleotide |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| AMPK | Adenosine monophosphate -activated protein kinase |
| FTO | Alpha-Ketoglutarate Dependent Dioxygenase |
| APO-A | Apolipoprotein A |
| AT | Adipose Tissue |
| b3-AR | β-3-adrenergic receptors |
| b3-AR | β-3-adrenergic receptors |
| BDNF | Brain Derived Neurotrophic Factor |
| BAT | Brown Adipose Tissue |
| CVD | Cardiovascular disease |
| CPT1a | Carnitine Palmitoyltransferase 1A |
| C/EBPα | CCAAT-enhancer-binding protein α |
| CIDEA | Cell death-inducing DNA fragmentation factor-α-like effector A |
| DNA | Deoxyribonucleic acid |
| EE | Energy expenditure |
| FABP4 | Fatty Acid Binding Protein 4 |
| FAS | Fatty acid synthase |
| FTO | Fat mass and obesity-associated protein |
| FGF21 | Fibroblast growth factor-21 |
| FNDC5 | Fibronectin Type III Domain Containing 5 |
| GLUT | Glucose transporter |
| HDL | High density lipoprotein |
| HFD | High Fat Diet |
| IL6 | Interleukin 6 |
| IGF2BP2 | Insulin-like growth factor 2 mRNA-binding protein 2 |
| IR | Insulin Receptor |
| IRS | Insulin Receptor Substrate |
| LEP | Leptin |
| LDL | Low density lipoprotein |
| MC4R | Melanocortin 4 receptor |
| Myf5 | Myogenic factor 5 |
| OPA1 | Mitochondrial dynamin like GTPase |
| mtROS | Mitochondrial reactive oxygen species |
| TFAM | Mitochondrial Transcription Factor A |
| TNFa | Necrosis tumoral factor a |
| NE | Norepinephrine |
| GTPase | Nucleotide guanosine triphosphate hydrolase |
| OPA1 | Optic atrophy 1 |
| PPARs | Peroxisome Proliferator-Activated Receptors |
| PGC1α | Peroxisome proliferator-activated receptor gamma—coactivator 1a |
| PINK1 | Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase -induced kinase 1 |
| PRDM16 | PR domain containing 16 |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SNS | Sympathetic Nervous System |
| SNP | Single Nucleotide Polymorphisms |
| SERCA | Sarco/endoplasmic reticulum calcium ATPase |
| Sirt1 | Sirtuin 1 |
| SREBP1c | Sterol regulatory element-binding transcription factor 1 |
| T3 | Triiodothyronine |
| T2DM | Type 2 diabetes mellitus |
| UCPs | Uncoupling proteins |
| VEGF | Vascular endothelial growth factor |
| WAT | White Adipose Tissue |
References
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Pérez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Gálvez, B.G. ‘Adipaging’: Ageing and Obesity Share Biological Hallmarks Related to a Dysfunctional Adipose Tissue: Adipaging. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Nowack, J.; Giroud, S.; Arnold, W.; Ruf, T. Muscle Non-Shivering Thermogenesis and Its Role in the Evolution of Endothermy. Front. Physiol. 2017, 8, 889. [Google Scholar] [CrossRef]
- Bal, N.C.; Periasamy, M. Uncoupling of Sarcoendoplasmic Reticulum Calcium ATPase Pump Activity by Sarcolipin as the Basis for Muscle Non-Shivering Thermogenesis. Phil. Trans. R. Soc. B 2020, 375, 20190135. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in Regulating Fatty Acid β-Oxidation: Potential Underlying Mechanisms and Their Involvement in Obesity and Weight Loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Fang, H.; Guo, F.; Li, F.; Chen, A.; Huang, S. Flavonoids as Inducers of White Adipose Tissue Browning and Thermogenesis: Signalling Pathways and Molecular Triggers. Nutr. Metab. 2019, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, J.; Li, L. Phytochemicals as Potential Candidates to Combat Obesity via Adipose Non-Shivering Thermogenesis. Pharmacol. Res. 2019, 147, 104393. [Google Scholar] [CrossRef]
- Trayhurn, P. Origins and Early Development of the Concept That Brown Adipose Tissue Thermogenesis Is Linked to Energy Balance and Obesity. Biochimie 2017, 134, 62–70. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Non-Shivering Thermogenesis as a Mechanism to Facilitate Sustainable Weight Loss: Non-Shivering Thermogenesis and Weight Loss. Obes. Rev. 2017, 18, 819–831. [Google Scholar] [CrossRef]
- Song, D.; Cheng, L.; Zhang, X.; Wu, Z.; Zheng, X. The Modulatory Effect and the Mechanism of Flavonoids on Obesity. J. Food Biochem. 2019, 43. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Wood dos Santos, T.; Cristina Pereira, Q.; Teixeira, L.; Gambero, A.; Villena, J.A.; Lima Ribeiro, M. Effects of Polyphenols on Thermogenesis and Mitochondrial Biogenesis. IJMS 2018, 19, 2757. [Google Scholar] [CrossRef]
- Kang, H.; Lee, S.; Otieno, D.; Ha, K. Flavonoids, Potential Bioactive Compounds, and Non-Shivering Thermogenesis. Nutrients 2018, 10, 1168. [Google Scholar] [CrossRef]
- Azhar, Y.; Parmar, A.; Miller, C.N.; Samuels, J.S.; Rayalam, S. Phytochemicals as Novel Agents for the Induction of Browning in White Adipose Tissue. Nutr. Metab. (Lond.) 2016, 13, 89. [Google Scholar] [CrossRef]
- Fenech, M.; El-Sohemy, A.; Cahill, L.; Ferguson, L.R.; French, T.-A.C.; Tai, E.S.; Milner, J.; Koh, W.-P.; Xie, L.; Zucker, M.; et al. Nutrigenetics and Nutrigenomics: Viewpoints on the Current Status and Applications in Nutrition Research and Practice. J. Nutr. Nutr. 2011, 4, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Peña-Romero, A.C.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. The Future of Nutrition: Nutrigenomics and Nutrigenetics in Obesity and Cardiovascular Diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 3030–3041. [Google Scholar] [CrossRef]
- Braconi, D.; Bernardini, G.; Millucci, L.; Santucci, A. Foodomics for Human Health: Current Status and Perspectives. Expert Rev. Proteom. 2018, 15, 153–164. [Google Scholar] [CrossRef]
- Bush, C.L.; Blumberg, J.B.; El-Sohemy, A.; Minich, D.M.; Ordovás, J.M.; Reed, D.G.; Behm, V.A.Y. Toward the Definition of Personalized Nutrition: A Proposal by The American Nutrition Association. J. Am. Coll. Nutr. 2020, 39, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R. Nutrigenomics Approaches to Functional Foods. J. Am. Diet. Assoc. 2009, 109, 452–458. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Qu, W. Dietary Apigenin Promotes Lipid Catabolism, Thermogenesis, and Browning in Adipose Tissues of HFD-Fed Mice. Food Chem. Toxicol. 2019, 133, 110780. [Google Scholar] [CrossRef]
- Goni, L.; Cuervo, M.; Milagro, F.I.; Martínez, J.A. Future Perspectives of Personalized Weight Loss Interventions Based on Nutrigenetic, Epigenetic, and Metagenomic Data. J. Nutr. 2015, 146, 905S–912S. [Google Scholar] [CrossRef]
- Xi, B.; AGEN-T2D Consortium; Takeuchi, F.; Chandak, G.R.; Kato, N.; Pan, H.W.; Zhou, D.H.; Pan, H.Y.; Mi, J. Common Polymorphism near the MC4R Gene Is Associated with Type 2 Diabetes: Data from a Meta-Analysis of 123,373 Individuals. Diabetologia 2012, 55, 2660–2666. [Google Scholar] [CrossRef]
- Graff, M.; Scott, R.A.; Justice, A.E.; Young, K.L.; Feitosa, M.F.; Barata, L.; Winkler, T.W.; Chu, A.Y.; Mahajan, A.; Hadley, D.; et al. Genome-Wide Physical Activity Interactions in Adiposity ― A Meta-Analysis of 200,452 Adults. PLoS Genet 2017, 13, e1006528. [Google Scholar] [CrossRef]
- Yu, K.; Li, L.; Zhang, L.; Guo, L.; Wang, C. Association between MC4R Rs17782313 Genotype and Obesity: A Meta-Analysis. Gene 2020, 733, 144372. [Google Scholar] [CrossRef]
- Akbarian, S.-A.; Salehi-Abargouei, A.; Pourmasoumi, M.; Kelishadi, R.; Nikpour, P.; Heidari-Beni, M. Association of Brain-Derived Neurotrophic Factor Gene Polymorphisms with Body Mass Index: A Systematic Review and Meta-Analysis. Adv. Med. Sci. 2018, 63, 43–56. [Google Scholar] [CrossRef]
- Jia, H.; Yu, L.; Jiang, Z.; Ji, Q. Association Between IGF2BP2 Rs4402960 Polymorphism and Risk of Type 2 Diabetes Mellitus: A Meta-Analysis. Arch. Med. Res. 2011, 42, 361–367. [Google Scholar] [CrossRef]
- Qi, Q.; Kilpeläinen, T.O.; Downer, M.K.; Tanaka, T.; Smith, C.E.; Sluijs, I.; Sonestedt, E.; Chu, A.Y.; Renström, F.; Lin, X.; et al. FTO Genetic Variants, Dietary Intake and Body Mass Index: Insights from 177 330 Individuals. Hum. Mol. Genet. 2014, 23, 6961–6972. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Reyes, T.; Astudillo-López, C.C.; Salgado-Goytia, L.; Muñoz-Valle, J.F.; Salgado-Bernabé, A.B.; Guzmán-Guzmán, I.P.; Castro-Alarcón, N.; Moreno-Godínez, M.E.; Parra-Rojas, I. Interaction of Dietary Fat Intake with APOA2, APOA5 and LEPR Polymorphisms and Its Relationship with Obesity and Dyslipidemia in Young Subjects. Lipids Health Dis. 2015, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- De Toro-Martín, J.; Arsenault, B.; Després, J.-P.; Vohl, M.-C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef]
- Silvester, A.J.; Aseer, K.R.; Yun, J.W. Dietary Polyphenols and Their Roles in Fat Browning. J. Nutr. Biochem. 2019, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Ramírez, B.; Unamuno, X.; Portincasa, P.; Gómez-Ambrosi, J.; Frühbeck, G.; Becerril, S. Impact of Adipokines and Myokines on Fat Browning. J. Physiol. Biochem. 2020, 76, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What We Talk About When We Talk About Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E. Adipose Tissue: From Lipid Storage Compartment to Endocrine Organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Wikstrom, J.D.; Mahdaviani, K.; Liesa, M.; Sereda, S.B.; Si, Y.; Las, G.; Twig, G.; Petrovic, N.; Zingaretti, C.; Graham, A.; et al. Hormone-Induced Mitochondrial Fission Is Utilized by Brown Adipocytes as an Amplification Pathway for Energy Expenditure. EMBO J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.L.; Tseng, Y.-H. Brown Fat Fuel Utilization and Thermogenesis. Trends Endocrinol. Metab. 2014, 25, 168–177. [Google Scholar] [CrossRef]
- Tapia, P.; Fernández-Galilea, M.; Robledo, F.; Mardones, P.; Galgani, J.E.; Cortés, V.A. Biology and Pathological Implications of Brown Adipose Tissue: Promises and Caveats for the Control of Obesity and Its Associated Complications: Brown Adipose Tissue in Health and Disease. Biol. Rev. 2018, 93, 1145–1164. [Google Scholar] [CrossRef] [PubMed]
- Poulos, S.P.; Hausman, D.B.; Hausman, G.J. The Development and Endocrine Functions of Adipose Tissue. Mol. Cell. Endocrinol. 2010, 323, 20–34. [Google Scholar] [CrossRef]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The Different Shades of Fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Mottillo, E.P.; Granneman, J.G. Adipose Tissue Plasticity from WAT to BAT and in Between. Biochim. Et Biophys. Acta (Bba) Mol. Basis Dis. 2014, 1842, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. IJMS 2019, 20, 5058. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and Beige Fat: Development, Function and Therapeutic Potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive Thermogenesis in Adipocytes: Is Beige the New Brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef]
- Castro, É.; Silva, T.E.O.; Festuccia, W.T. Critical Review of Beige Adipocyte Thermogenic Activation and Contribution to Whole-Body Energy Expenditure. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct FeatuRes. of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, A.D.; Kooijman, S.; Schilperoort, M.; Rensen, P.C.N.; Boon, M.R. Regulation of Brown Fat by AMP-Activated Protein Kinase. Trends Mol. Med. 2015, 21, 571–579. [Google Scholar] [CrossRef]
- Desjardins, E.M.; Steinberg, G.R. Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for Obesity, Insulin Resistance, and Type 2 Diabetes. Curr. Diab. Rep. 2018, 18, 80. [Google Scholar] [CrossRef]
- Jager, S.; Handschin, C.; St.-Pierre, J.; Spiegelman, B.M. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a Nodal Regulator of Mitochondrial Biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK Regulates Energy Expenditure by Modulating NAD+ Metabolism and SIRT1 Activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. PPARγ: A Nuclear Regulator of Metabolism, Differentiation, and Cell Growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/α-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Hondares, E.; Rosell, M.; Díaz-Delfín, J.; Olmos, Y.; Monsalve, M.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome Proliferator-Activated Receptor α (PPARα) Induces PPARγ Coactivator 1α (PGC-1α) Gene Expression and Contributes to Thermogenic Activation of Brown Fat: INVOLVEMENT OF PRDM16. J. Biol. Chem. 2011, 286, 43112–43122. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 Regulates PGC-1 and Browning of White Adipose Tissues in Adaptive Thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Blondin, D.P.; Haman, F. Shivering and nonshivering thermogenesis in skeletal muscles. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 153–173. ISBN 978-0-444-63912-7. [Google Scholar]
- Contreras, C.; Nogueiras, R.; Diéguez, C.; Medina-Gómez, G.; López, M. Hypothalamus and Thermogenesis: Heating the BAT, Browning the WAT. Mol. Cell. Endocrinol. 2016, 438, 107–115. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Bouillaud, F.; Alves-Guerra, M.-C.; Ricquier, D. UCPs, at the Interface between Bioenergetics and Metabolism. Biochim. Et Biophys. Acta (Bba) Mol. Cell Res. 2016, 1863, 2443–2456. [Google Scholar] [CrossRef]
- Nicholls, D.G. The Hunt for the Molecular Mechanism of Brown Fat Thermogenesis. Biochimie 2017, 134, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lettieri Barbato, D.; Tatulli, G.; Vegliante, R.; Cannata, S.M.; Bernardini, S.; Ciriolo, M.R.; Aquilano, K. Dietary Fat Overload Reprograms Brown Fat Mitochondria. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.-T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 Emission and Cellular Redox State Link Excess Fat Intake to Insulin Resistance in Both Rodents and Humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, A.; Oh, K.J.; Lee, S.C.; Kim, W.K.; Bae, K.H. Lee; Park; Oh; Lee; Kim; Bae The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. IJMS 2019, 20, 4924. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial Fusion and Fission in Cell Life and Death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial Fission and Fusion: A Dynamic Role in Aging and Potential Target for Age-Related Disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef] [PubMed]
- Wrighton, K.H. Mitophagy Turns Beige Adipocytes White. Nat. Rev. Mol. Cell Biol. 2016, 17, 607. [Google Scholar] [CrossRef]
- Altshuler-Keylin, S.; Shinoda, K.; Hasegawa, Y.; Ikeda, K.; Hong, H.; Kang, Q.; Yang, Y.; Perera, R.M.; Debnath, J.; Kajimura, S. Beige Adipocyte Maintenance Is Regulated by Autophagy-Induced Mitochondrial Clearance. Cell Metab. 2016, 24, 402–419. [Google Scholar] [CrossRef]
- Contreras, C.; Nogueiras, R.; Diéguez, C.; Rahmouni, K.; López, M. Traveling from the Hypothalamus to the Adipose Tissue: The Thermogenic Pathway. Redox Biol. 2017, 12, 854–863. [Google Scholar] [CrossRef]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vázquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B Increases Brown Adipose Tissue Thermogenesis through Both Central and Peripheral Actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Luby-Phelps, K.; Spurgin, S.B.; An, Y.A.; Wang, Q.A.; Holland, W.L.; Scherer, P.E. Brown Adipose Tissue Derived VEGF-A Modulates Cold Tolerance and Energy Expenditure. Mol. Metab. 2014, 3, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Collazo, P.; Martínez-Sánchez, N.; Milbank, E.; Contreras, C. Incendiary Leptin. Nutrients 2020, 12, 472. [Google Scholar] [CrossRef]
- Caron, A.; Lee, S.; Elmquist, J.K.; Gautron, L. Leptin and Brain–Adipose Crosstalks. Nat. Rev. Neurosci. 2018, 19, 153–165. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Bottinelli, R.; Reggiani, C. Human Skeletal Muscle Fibres: Molecular and Functional Diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef]
- Westerblad, H.; Bruton, J.D.; Katz, A. Skeletal Muscle: Energy Metabolism, Fiber Types, Fatigue and Adaptability. Exp. Cell Res. 2010, 316, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Justice, J.N.; Thompson, L. Lipotoxicity, Aging, and Muscle Contractility: Does Fiber Type Matter? GeroScience 2019, 41, 297–308. [Google Scholar] [CrossRef]
- Bal, N.C.; Singh, S.; Reis, F.C.G.; Maurya, S.K.; Pani, S.; Rowland, L.A.; Periasamy, M. Both Brown Adipose Tissue and Skeletal Muscle Thermogenesis Processes Are Activated during Mild to Severe Cold Adaptation in Mice. J. Biol. Chem. 2017, 292, 16616–16625. [Google Scholar] [CrossRef]
- Amat, R.; Solanes, G.; Giralt, M.; Villarroya, F. SIRT1 Is Involved in Glucocorticoid-Mediated Control of Uncoupling Protein-3 Gene Transcription. J. Biol. Chem. 2007, 282, 34066–34076. [Google Scholar] [CrossRef]
- Mall, S.; Broadbridge, R.; Harrison, S.L.; Gore, M.G.; Lee, A.G.; East, J.M. The Presence of Sarcolipin Results in Increased Heat Production by Ca 2+ -ATPase. J. Biol. Chem. 2006, 281, 36597–36602. [Google Scholar] [CrossRef]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin Is a Newly Identified Regulator of Muscle-Based Thermogenesis in Mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef]
- Maurya, S.K.; Herrera, J.L.; Sahoo, S.K.; Reis, F.C.G.; Vega, R.B.; Kelly, D.P.; Periasamy, M. Sarcolipin Signaling Promotes Mitochondrial Biogenesis and Oxidative Metabolism in Skeletal Muscle. Cell Rep. 2018, 24, 2919–2931. [Google Scholar] [CrossRef] [PubMed]
- Koltai, E.; Hart, N.; Taylor, A.W.; Goto, S.; Ngo, J.K.; Davies, K.J.A.; Radak, Z. Age-Associated Declines in Mitochondrial Biogenesis and Protein Quality Control Factors Are Minimized by Exercise Training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R127–R134. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Chung, E.; Diffee, G.; Ji, L.L. Exercise Training Attenuates Aging-Associated Mitochondrial Dysfunction in Rat Skeletal Muscle: Role of PGC-1α. Exp. Gerontol. 2013, 48, 1343–1350. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient Control of Glucose Homeostasis through a Complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Iizuka, K.; Machida, T.; Hirafuji, M. Skeletal Muscle Is an Endocrine Organ. J. Pharm. Sci. 2014, 125, 125–131. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Schnyder, S.; Handschin, C. Skeletal Muscle as an Endocrine Organ: PGC-1α, Myokines and Exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Bal, N.C.; Sopariwala, D.H.; Pant, M.; Rowland, L.A.; Shaikh, S.A.; Periasamy, M. Sarcolipin Is a Key Determinant of the Basal Metabolic Rate, and Its Overexpression Enhances Energy Expenditure and Resistance against Diet-Induced Obesity. J. Biol. Chem. 2015, 290, 10840–10849. [Google Scholar] [CrossRef]
- Maurya, S.K.; Periasamy, M. Sarcolipin Is a Novel Regulator of Muscle Metabolism and Obesity. Pharmacol. Res. 2015, 102, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Mercader, J.; Palou, A. A Nutritional Perspective on UCP1-Dependent Thermogenesis. Biochimie 2017, 134, 99–117. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.-X.; Wang, X.; Zhang, L.; Qu, W.; Bao, B.; Liu, C.-A.; Liu, J. Dietary Luteolin Activates Browning and Thermogenesis in Mice through an AMPK/PGC1α Pathway-Mediated Mechanism. Int. J. Obes. 2016, 40, 1841–1849. [Google Scholar] [CrossRef]
- Aziz, S.A.; Wakeling, L.A.; Miwa, S.; Alberdi, G.; Hesketh, J.E.; Ford, D. Metabolic Programming of a Beige Adipocyte Phenotype by Genistein. Mol. Nutr. Food Res. 2017, 61, 1600574. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Dong, L.; Hu, X.; Feng, F.; Chen, F. 6-Gingerol, a Functional Polyphenol of Ginger, Promotes Browning through an AMPK-Dependent Pathway in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2019, 67, 14056–14065. [Google Scholar] [CrossRef]
- Sun, W.; Yu, S.; Han, H.; Yuan, Q.; Chen, J.; Xu, G. Resveratrol Inhibits Human Visceral Preadipocyte Proliferation and Differentiation In Vitro. Lipids 2019, 54, 679–686. [Google Scholar] [CrossRef]
- Grossini, E.; Farruggio, S.; Raina, G.; Mary, D.; Deiro, G.; Gentilli, S. Effects of Genistein on Differentiation and Viability of Human Visceral Adipocytes. Nutrients 2018, 10, 978. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Bindels, L.B.; Geurts, L.; Van Hul, M.; Cani, P.D.; Delzenne, N.M. A Polyphenolic Extract from Green Tea Leaves Activates Fat Browning in High-Fat-Diet-Induced Obese Mice. J. Nutr. Biochem. 2017, 49, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.E.; Rodriguez, A.; Romero-Valdovinos, M.; Mendoza-Lorenzo, P.; Mansour, C.; Ceballos, G.; Villarreal, F.; Ramirez-Sanchez, I. Browning Effects of (-)-Epicatechin on Adipocytes and White Adipose Tissue. Eur. J. Pharmacol. 2017, 811, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, J.-Z.; Shen, J.; Chen, L.; He, T.; Jin, M.; Liu, H. Pentamethylquercetin Induces Adipose Browning and Exerts Beneficial Effects in 3T3-L1 Adipocytes and High-Fat Diet-Fed Mice. Sci. Rep. 2017, 7, 1123. [Google Scholar] [CrossRef]
- Lee, S.G.; Parks, J.S.; Kang, H.W. Quercetin, a Functional Compound of Onion Peel, Remodels White Adipocytes to Brown-like Adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Picó, C.; Teresa Macarulla, M.; Oliver, P.; Miranda, J.; Palou, A.; Portillo, M.P. A Combination of Resveratrol and Quercetin Induces Browning in White Adipose Tissue of Rats Fed an Obesogenic Diet: Polyphenol Combination and Brite Cell Induction. Obesity 2017, 25, 111–121. [Google Scholar] [CrossRef]
- Al-Muammar, M.N.; Khan, F. Obesity: The Preventive Role of the Pomegranate (Punica Granatum). Nutrition 2012, 28, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of Anti-Obesity Effects of the Pomegranate Leaf Extract in High-Fat Diet Induced Obese Mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Cerd, B.; Llorach, R.; Cern, J.J.; Espn, J.C.; Toms-Barbern, F.A. Evaluation of the Bioavailability and Metabolism in the Rat of Punicalagin, an Antioxidant Polyphenol from Pomegranate Juice. Eur. J. Nutr. 2003, 42, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yan, C.; Shi, Y.; Cao, K.; Xu, J.; Wang, X.; Chen, C.; Luo, C.; Li, Y.; Gao, J.; et al. Mitochondrial Dysfunction in Obesity-Associated Nonalcoholic Fatty Liver Disease: The Protective Effects of Pomegranate with Its Active Component Punicalagin. Antioxid. Redox Signal. 2014, 21, 1557–1570. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Pu, W.; Dong, Z.; Sun, L.; Zang, W.; Gao, F.; Zhang, Y.; Feng, Z.; Liu, J. Punicalagin, an Active Component in Pomegranate, Ameliorates Cardiac Mitochondrial Impairment in Obese Rats via AMPK Activation. Sci. Rep. 2015, 5, 14014. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate Juice Consumption for 3 Years by Patients with Carotid Artery Stenosis Reduces Common Carotid Intima-Media Thickness, Blood Pressure and LDL Oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- Mirmiran, P.; Fazeli, M.R.; Asghari, G.; Shafiee, A.; Azizi, F. Effect of Pomegranate Seed Oil on Hyperlipidaemic Subjects: A Double-Blind Placebo-Controlled Clinical Trial. Br. J. Nutr. 2010, 104, 402–406. [Google Scholar] [CrossRef]
- Vroegrijk, I.O.C.M.; van Diepen, J.A.; van den Berg, S.; Westbroek, I.; Keizer, H.; Gambelli, L.; Hontecillas, R.; Bassaganya-Riera, J.; Zondag, G.C.M.; Romijn, J.A.; et al. Pomegranate Seed Oil, a Rich Source of Punicic Acid, Prevents Diet-Induced Obesity and Insulin Resistance in Mice. Food Chem. Toxicol. 2011, 49, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Pang, W.; Zhang, Z.; Zhao, J.; Wang, X.; Liu, Y.; Wang, X.; Feng, Z.; Zhang, Y.; Sun, W.; et al. Pomegranate Extract and Exercise Provide Additive Benefits on Improvement of Immune Function by Inhibiting Inflammation and Oxidative Stress in High-Fat-Diet-Induced Obesity in Rats. J. Nutr. Biochem. 2016, 32, 20–28. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate Supplementation Accelerates Recovery of Muscle Damage and Soreness and Inflammatory Markers after a Weightlifting Training Session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef]
- Ammar, A.; MounaTurki; Trabelsi, K.; Bragazzi, N.L.; Boukhris, O.; Bouaziz, M.; Ayadi, F.; El Abed, K.; Driss, T.; Souissi, N.; et al. Effects of Natural Polyphenol-Rich Pomegranate Juice on the Acute and Delayed Response of Homocysteine and Steroidal Hormones Following Weightlifting Exercises: A Double-Blind, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2020, 17, 15. [Google Scholar] [CrossRef]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate Polyphenols and Urolithin A Inhibit α-Glucosidase, Dipeptidyl Peptidase-4, Lipase, Triglyceride Accumulation and Adipogenesis Related Genes in 3T3-L1 Adipocyte-like Cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Shi, X.C.; Xie, B.C.; Zhu, M.Q.; Chen, Y.; Chu, X.Y.; Cai, G.H.; Liu, M.; Yang, S.Z.; Mitchell, G.A.; et al. Urolithin A Exerts Antiobesity Effects through Enhancing Adipose Tissue Thermogenesis in Mice. PLoS Biol. 2020, 18, e3000688. [Google Scholar] [CrossRef] [PubMed]
- Eisvand, F.; Razavi, B.M.; Hosseinzadeh, H. The Effects of Ginkgo Biloba on Metabolic Syndrome: A Review. Phytother. Res. 2020, 34, 1798–1811. [Google Scholar] [CrossRef]
- Banin, R.M.; Hirata, B.K.S.; Andrade, I.S.; Zemdegs, J.C.S.; Clemente, A.P.G.; Dornellas, A.P.S.; Boldarine, V.T.; Estadella, D.; Albuquerque, K.T.; Oyama, L.M.; et al. Beneficial Effects of Ginkgo Biloba Extract on Insulin Signaling Cascade, Dyslipidemia, and Body Adiposity of Diet-Induced Obese Rats. Braz. J. Med. Biol. Res. 2014, 47, 780–788. [Google Scholar] [CrossRef]
- Hirata, B.K.S.; Banin, R.M.; Dornellas, A.P.S.; de Andrade, I.S.; Zemdegs, J.C.S.; Caperuto, L.C.; Oyama, L.M.; Ribeiro, E.B.; Telles, M.M. Ginkgo Biloba Extract Improves Insulin Signaling and Attenuates Inflammation in Retroperitoneal Adipose Tissue Depot of Obese Rats. Mediat. Inflamm. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hirata, B.K.S.; Cruz, M.M.; de Sá, R.D.C.C.; Farias, T.S.M.; Machado, M.M.F.; Bueno, A.A.; Alonso-Vale, M.I.C.; Telles, M.M. Potential Anti-Obesogenic Effects of Ginkgo Biloba Observed in Epididymal White Adipose Tissue of Obese Rats. Front. Endocrinol. 2019, 10, 284. [Google Scholar] [CrossRef]
- Liu, G.; Grifman, M.; Macdonald, J.; Moller, P.; Wong-Staal, F.; Li, Q.-X. Isoginkgetin Enhances Adiponectin Secretion from Differentiated Adiposarcoma Cells via a Novel Pathway Involving AMP-Activated Protein Kinase. J. Endocrinol. 2007, 194, 569–578. [Google Scholar] [CrossRef]
- Gautam, J.; Kushwaha, P.; Swarnkar, G.; Khedgikar, V.; Nagar, G.K.; Singh, D.; Singh, V.; Jain, M.; Barthwal, M.; Trivedi, R. EGb 761 Promotes Osteoblastogenesis, Lowers Bone Marrow Adipogenesis and Atherosclerotic Plaque Formation. Phytomedicine 2012, 19, 1134–1142. [Google Scholar] [CrossRef]
- Bu, S.; Yuan, C.Y.; Xue, Q.; Chen, Y.; Cao, F. Bilobalide Suppresses Adipogenesis in 3T3-L1 Adipocytes via the AMPK Signaling Pathway. Molecules 2019, 24, 3503. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-L.; Park, J.-G.; Kang, H.J.; Kim, W.; Cho, M.J.; Jang, J.-H.; Kwon, M.-G.; Kim, S.; Lee, S.-H.; Lee, J.; et al. Ginkgetin, a Biflavone from Ginkgo Biloba Leaves, Prevents Adipogenesis through STAT5-Mediated PPARγ and C/EBPα Regulation. Pharmacol. Res. 2019, 139, 325–336. [Google Scholar] [CrossRef]
- Hosoda, S.; Kawazoe, Y.; Shiba, T.; Numazawa, S.; Manabe, A. Anti-Obesity Effect of Ginkgo Vinegar, a Fermented Product of Ginkgo Seed Coat, in Mice Fed a High-Fat Diet and 3T3-L1 Preadipocyte Cells. Nutrients 2020, 12, 230. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Wang, Y.; Zhu, T. Silymarin Improved Diet-Induced Liver Damage and Insulin Resistance by Decreasing Inflammation in Mice. Pharm. Biol. 2016, 54, 2995–3000. [Google Scholar] [CrossRef]
- Tajmohammadi, A.; Razavi, B.M.; Hosseinzadeh, H. Silybum Marianum (Milk Thistle) and Its Main Constituent, Silymarin, as a Potential Therapeutic Plant in Metabolic Syndrome: A Review: Silybum Marianum and Metabolic Syndrome. Phytother. Res. 2018, 32, 1933–1949. [Google Scholar] [CrossRef]
- Barbagallo, I.; Vanella, L.; Cambria, M.T.; Tibullo, D.; Godos, J.; Guarnaccia, L.; Zappalà, A.; Galvano, F.; Li Volti, G. Silibinin Regulates Lipid Metabolism and Differentiation in Functional Human Adipocytes. Front. Pharmacol. 2016, 6. [Google Scholar] [CrossRef]
- Park Silibinin Attenuates Adipogenesis in 3T3-L1 Preadipocytes through a Potential Upregulation of the Insig Pathway. Int. J. Mol. Med. 2009, 23. [CrossRef]
- Suh, H.J.; Cho, S.Y.; Kim, E.Y.; Choi, H.-S. Blockade of Lipid Accumulation by Silibinin in Adipocytes and Zebrafish. Chem. -Biol. Interact. 2015, 227, 53–62. [Google Scholar] [CrossRef]
- Alsaggar, M.; Bdour, S.; Ababneh, Q.; El-Elimat, T.; Qinna, N.; Alzoubi, K.H. Silibinin Attenuates Adipose Tissue Inflammation and Reverses Obesity and Its Complications in Diet-Induced Obesity Model in Mice. BMC Pharm. Toxicol 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Ørgaard, A.; Jensen, L. The Effects of Soy Isoflavones on Obesity. Exp. Biol. Med. (Maywood) 2008, 233, 1066–1080. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Pan, M.-H.; Ho, C.-T. Anti-Obesity Molecular Mechanism of Soy Isoflavones: Weaving the Way to New Therapeutic Routes. Food Funct. 2017, 8, 3831–3846. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Pang, D.; Luo, Q.; Chen, X.; Gao, Q.; Shi, L.; Liu, W.; Zou, Y.; Li, L.; Chen, Z. Soy Isoflavones Regulate Lipid Metabolism through an AKT/MTORC1 Pathway in Diet-Induced Obesity (DIO) Male Rats. Molecules 2016, 21, 586. [Google Scholar] [CrossRef]
- Rasbach, K.A.; Schnellmann, R.G. Isoflavones Promote Mitochondrial Biogenesis. J. Pharm. Exp. 2008, 325, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Liu, S.; Si, H. Antiadipogenic Effects and Mechanisms of Combinations of Genistein, Epigallocatechin-3-Gallate, and/or Resveratrol in Preadipocytes. J. Med. Food 2017, 20, 162–170. [Google Scholar] [CrossRef]
- Seo, S.G.; Yang, H.; Shin, S.H.; Min, S.; Kim, Y.A.; Yu, J.G.; Lee, D.E.; Chung, M.-Y.; Heo, Y.-S.; Kwon, J.Y.; et al. A Metabolite of Daidzein, 6,7,4’-Trihydroxyisoflavone, Suppresses Adipogenesis in 3T3-L1 Preadipocytes via ATP-Competitive Inhibition of PI3K. Mol. Nutr. Food Res. 2013, 57, 1446–1455. [Google Scholar] [CrossRef]
- Kim, M.-H.; Park, J.-S.; Seo, M.-S.; Jung, J.-W.; Lee, Y.-S.; Kang, K.-S. Genistein and Daidzein Repress Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells via Wnt/β-Catenin Signalling or Lipolysis: Anti-Adipogenic Effect of Isoflavones. Cell Prolif. 2010, 43, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Della-Fera, M.A.; Hausman, D.B.; Rayalam, S.; Ambati, S.; Baile, C.A. Genistein Inhibits Differentiation of Primary Human Adipocytes. J. Nutr. Biochem. 2009, 20, 140–148. [Google Scholar] [CrossRef]
- Hall, J.M.; Powell, H.R.; Rajic, L.; Korach, K.S. The Role of Dietary Phytoestrogens and the Nuclear Receptor PPARγ in Adipogenesis: An in Vitro Study. Environ. Health Perspect. 2019, 127, 037007. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, S.; Broderick, T.L.; Al-Nakkash, L. Feeding Obese Diabetic Mice a Genistein Diet Induces Thermogenic and Metabolic Change. J. Med. Food 2018, 21, 332–339. [Google Scholar] [CrossRef]
- Dang, Z.C. Dose-Dependent Effects of Soy Phyto-Oestrogen Genistein on Adipocytes: Mechanisms of Action. Obes. Rev. 2009, 10, 342–349. [Google Scholar] [CrossRef]
- Uchitomi, R.; Nakai, S.; Matsuda, R.; Onishi, T.; Miura, S.; Hatazawa, Y.; Kamei, Y. Genistein, Daidzein, and Resveratrols Stimulate PGC-1β-Mediated Gene Expression. Biochem. Biophys. Rep. 2019, 17, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavón, F.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernández-Llebrez, P.; Martínez, A.; Pérez-Valero, V.; et al. Reduction of Body Weight, Liver Steatosis and Expression of Stearoyl-CoA Desaturase 1 by the Isoflavone Daidzein in Diet-Induced Obesity: Pharmacological Effect of Daidzein in Obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ide, T. Effects of Soy Protein and Isoflavone on Hepatic Fatty Acid Synthesis and Oxidation and MRNA Expression of Uncoupling Proteins and Peroxisome Proliferator-Activated Receptor γ in Adipose Tissues of Rats. J. Nutr. Biochem. 2008, 19, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Palacios-González, B.; Zarain-Herzberg, A.; Flores-Galicia, I.; Noriega, L.G.; Alemán-Escondrillas, G.; Zariñan, T.; Ulloa-Aguirre, A.; Torres, N.; Tovar, A.R. Genistein Stimulates Fatty Acid Oxidation in a Leptin Receptor-Independent Manner through the JAK2-Mediated Phosphorylation and Activation of AMPK in Skeletal Muscle. Biochim. Et Biophys. Acta (Bba) Mol. Cell Biol. Lipids 2014, 1841, 132–140. [Google Scholar] [CrossRef]
- Yoshino, M.; Naka, A.; Sakamoto, Y.; Shibasaki, A.; Toh, M.; Tsukamoto, S.; Kondo, K.; Iida, K. Dietary Isoflavone Daidzein Promotes Tfam Expression That Increases Mitochondrial Biogenesis in C2C12 Muscle Cells. J. Nutr. Biochem. 2015, 26, 1193–1199. [Google Scholar] [CrossRef]
- Hirasaka, K.; Maeda, T.; Ikeda, C.; Haruna, M.; Kohno, S.; Abe, T.; Ochi, A.; Mukai, R.; Oarada, M.; Eshima-Kondo, S.; et al. Isoflavones Derived from Soy Beans Prevent MuRF1-Mediated Muscle Atrophy in C2C12 Myotubes through SIRT1 Activation. J. Nutr. Sci. Vitam. 2013, 59, 317–324. [Google Scholar] [CrossRef]
- Zheng, W.; Hemker, M.; Xie, M.; Soukup, S.; Diel, P. Anabolic Activity of a Soy Extract and Three Major Isoflavones in C2C12 Myotubes. Planta Med. 2018, 84, 1022–1029. [Google Scholar] [CrossRef]
- Ogawa, M.; Kitano, T.; Kawata, N.; Sugihira, T.; Kitakaze, T.; Harada, N.; Yamaji, R. Daidzein Down-Regulates Ubiquitin-Specific Protease 19 Expression through Estrogen Receptor β and Increases Skeletal Muscle Mass in Young Female Mice. J. Nutr. Biochem. 2017, 49, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Milton-Laskibar, I.; González, M.; Portillo, M.P. Antiobesity Effects of Resveratrol: Which Tissues Are Involved?: Antiobesity Effects of Resveratrol. Ann. N. Y. Acad. Sci. 2017, 1403, 118–131. [Google Scholar] [CrossRef]
- Milton-Laskíbar, I.; Gómez-Zorita, S.; Arias, N.; Romo-Miguel, N.; González, M.; Fernández-Quintela, A.; Portillo, M.P. Effects of Resveratrol and Its Derivative Pterostilbene on Brown Adipose Tissue Thermogenic Activation and on White Adipose Tissue Browning Process. J. Physiol. Biochem. 2020, 76, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Wu, J.-C.; Ho, C.-T.; Lai, C.-S. Antiobesity Molecular Mechanisms of Action: Resveratrol and Pterostilbene: Antiobesity Molecular Mechanisms of Action. BioFactors 2018, 44, 50–60. [Google Scholar] [CrossRef]
- Christenson, J.; Whitby, S.J.; Mellor, D.; Thomas, J.; McKune, A.; Roach, P.D.; Naumovski, N. The Effects of Resveratrol Supplementation in Overweight and Obese Humans: A Systematic Review of Randomized Trials. Metab. Syndr. Relat. Disord. 2016, 14, 323–333. [Google Scholar] [CrossRef]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an Antioxidant and Pro-Oxidant Agent: Mechanisms and Clinical Implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.; Palou, A.; Bonet, M.L. Resveratrol Enhances Fatty Acid Oxidation Capacity and Reduces Resistin and Retinol-Binding Protein 4 Expression in White Adipocytes. J. Nutr. Biochem. 2011, 22, 828–834. [Google Scholar] [CrossRef]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol Induces Brown-like Adipocyte Formation in White Fat through Activation of AMP-Activated Protein Kinase (AMPK) Α1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Aguirre, L.; Etxeberria, U.; Milagro, F.; Martínez, J.; Portillo, M. Do the Effects of Resveratrol on Thermogenic and Oxidative Capacities in IBAT and Skeletal Muscle Depend on Feeding Conditions? Nutrients 2018, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Koh, Y.-C.; Lee, T.-L.; Wang, B.; Chen, W.-K.; Nagabhushanam, K.; Ho, C.-T. Resveratrol and Oxyresveratrol Activate Thermogenesis via Different Transcriptional Coactivators in High-Fat Diet-Induced Obese Mice. J. Agric. Food Chem. 2019, 67, 13605–13616. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, G.; Rodríguez, V.M.; Miranda, J.; Macarulla, M.T.; Churruca, I.; Portillo, M.P. Thermogenesis Is Involved in the Body-Fat Lowering Effects of Resveratrol in Rats. Food Chem. 2013, 141, 1530–1535. [Google Scholar] [CrossRef]
- Andrade, J.M.O.; Frade, A.C.M.; Guimarães, J.B.; Freitas, K.M.; Lopes, M.T.P.; Guimarães, A.L.S.; de Paula, A.M.B.; Coimbra, C.C.; Santos, S.H.S. Resveratrol Increases Brown Adipose Tissue Thermogenesis Markers by Increasing SIRT1 and Energy Expenditure and Decreasing Fat Accumulation in Adipose Tissue of Mice Fed a Standard Diet. Eur. J. Nutr. 2014, 53, 1503–1510. [Google Scholar] [CrossRef]
- Ku, C.R.; Cho, Y.H.; Hong, Z.-Y.; Lee, H.; Lee, S.J.; Hong, S.; Lee, E.J. The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes. Endocrinol. Metab. 2016, 31, 328. [Google Scholar] [CrossRef] [PubMed]
- Robb, E.L.; Moradi, F.; Maddalena, L.A.; Valente, A.J.F.; Fonseca, J.; Stuart, J.A. Resveratrol Stimulates Mitochondrial Fusion by a Mechanism Requiring Mitofusin-2. Biochem. Biophys. Res. Commun. 2017, 485, 249–254. [Google Scholar] [CrossRef]
- Castrejón-Tellez, V.; Rodríguez-Pérez, J.; Pérez-Torres, I.; Pérez-Hernández, N.; Cruz-Lagunas, A.; Guarner-Lans, V.; Vargas-Alarcón, G.; Rubio-Ruiz, M. The Effect of Resveratrol and Quercetin Treatment on PPAR Mediated Uncoupling Protein (UCP-) 1, 2, and 3 Expression in Visceral White Adipose Tissue from Metabolic Syndrome Rats. IJMS 2016, 17, 1069. [Google Scholar] [CrossRef]
- Aguirre, L.; Milton-Laskibar, I.; Hijona, E.; Bujanda, L.; Rimando, A.M.; Portillo, M.P. Effects of Pterostilbene in Brown Adipose Tissue from Obese Rats. J. Physiol. Biochem. 2016, 73, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol Enhances Brown Adipocyte Formation and Function by Activating AMP-Activated Protein Kinase (AMPK) Α1 in Mice Fed High-Fat Diet. Mol. Nutr. Food Res. 2017, 61, 1600746. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Schweiger, M.; Kotzbeck, P.; Churruca, I.; Simón, E.; Zechner, R.; Portillo, M. del P. Resveratrol Regulates Lipolysis via Adipose Triglyceride Lipase. J. Nutr. Biochem. 2012, 23, 379–384. [Google Scholar] [CrossRef]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.-J.; Nathanielsz, P.W.; Du, M. Resveratrol Supplementation of High-Fat Diet-Fed Pregnant Mice Promotes Brown and Beige Adipocyte Development and Prevents Obesity in Male Offspring: Maternal Resveratrol Promotes Beige Adipogenesis in Offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef]
- Asnani-Kishnani, M.; Rodríguez, A.M.; Serrano, A.; Palou, A.; Bonet, M.L.; Ribot, J. Neonatal Resveratrol and Nicotinamide Riboside Supplementations Sex-Dependently Affect Beige Transcriptional Programming of Preadipocytes in Mouse Adipose Tissue. Front. Physiol. 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Asnani-Kishnani, M.; Rodríguez, A.M.; Palou, A.; Ribot, J.; Bonet, M.L. Programming of the Beige Phenotype in White Adipose Tissue of Adult Mice by Mild Resveratrol and Nicotinamide Riboside Supplementations in Early Postnatal Life. Mol. Nutr. Food Res. 2018, 62, 1800463. [Google Scholar] [CrossRef]
- Andrade, J.M.O.; Barcala-Jorge, A.S.; Batista-Jorge, G.C.; Paraíso, A.F.; de Freitas, K.M.; de Farias Lelis, D.; Guimarães, A.L.S.; de Paula, A.M.B.; Santos, S.H.S. Effect of Resveratrol on Expression of Genes Involved Thermogenesis in Mice and Humans. Biomed. Pharmacother. 2019, 112, 108634. [Google Scholar] [CrossRef]
- Abedi-Taleb, E.; Vahabi, Z.; Sekhavati-Moghadam, E.; Khedmat, L.; Jazayeri, S.; Saboor-Yaraghi, A.A. Upregulation of FNDC5 Gene Expression in C2C12 Cells after Single and Combined Treatments of Resveratrol and ATRA. Lipids Health Dis. 2019, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Rayalam, S.; Yang, J.-Y.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol Induces Apoptosis and Inhibits Adipogenesis in 3T3-L1 Adipocytes: RESVERATROL EFFECTS ON 3T3-L1 ADIPOCYTES. Phytother. Res. 2008, 22, 1367–1371. [Google Scholar] [CrossRef]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Miguéliz, I.; Romo-Hualde, A.; López-Yoldi, M.; Martínez, J.; Vizmanos, J.; Milagro, F.; González-Navarro, C. Phenolic Compounds Inhibit 3T3-L1 Adipogenesis Depending on the Stage of Differentiation and Their Binding Affinity to PPARγ. Molecules 2019, 24, 1045. [Google Scholar] [CrossRef]
- Li, S.; Bouzar, C.; Cottet-Rousselle, C.; Zagotta, I.; Lamarche, F.; Wabitsch, M.; Tokarska-Schlattner, M.; Fischer-Posovszky, P.; Schlattner, U.; Rousseau, D. Resveratrol Inhibits Lipogenesis of 3T3-L1 and SGBS Cells by Inhibition of Insulin Signaling and Mitochondrial Mass Increase. Biochim. Et Biophys. Acta (Bba) Bioenerg. 2016, 1857, 643–652. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.; Jin, Q.; You, W.; Cheng, H.; Liu, Y.; Song, E.; Liu, G.; Tan, X.; Zhang, X.; et al. Resveratrol Induces Apoptosis and Inhibits Adipogenesis by Stimulating the SIRT1-AMPKα-FOXO1 Signalling Pathway in Bovine Intramuscular Adipocytes. Mol. Cell Biochem. 2018, 439, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol Exerts Anti-Obesity Effects via Mechanisms Involving down-Regulation of Adipogenic and Inflammatory Processes in Mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef]
- Mendes, K.L.; de Pinho, L.; Andrade, J.M.O.; Paraíso, A.F.; Lula, J.F.; Macedo, S.M.; Feltenberger, J.D.; Guimarães, A.L.S.; de Paula, A.M.B.; Santos, S.H.S. Distinct Metabolic Effects of Resveratrol on Lipogenesis Markers in Mice Adipose Tissue Treated with High-Polyunsaturated Fat and High-Protein Diets. Life Sci. 2016, 153, 66–73. [Google Scholar] [CrossRef]
- De Almeida Pinheiro, T.; de Almeida Pinheiro, T.; Feltenberger, J.D.; Andrade, J.M.O.; Neves Ferreira, E.C.; De Farias Lelis, D.; Guimaraes, A.L.S.; de Paula, A.M.B.; Caldeira, A.P.; Sousa Santos, S.H. Effects of Resveratrol and ACE Inhibitor Enalapril on Glucose and Lipid Profiles in Mice. PPL 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol Induces Mitochondrial Biogenesis in Endothelial Cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef]
- De Ligt, M.; Bruls, Y.M.H.; Hansen, J.; Habets, M.-F.; Havekes, B.; Nascimento, E.B.M.; Moonen-Kornips, E.; Schaart, G.; Schrauwen-Hinderling, V.B.; van Marken Lichtenbelt, W.; et al. Resveratrol Improves Ex Vivo Mitochondrial Function but Does Not Affect Insulin Sensitivity or Brown Adipose Tissue in First Degree Relatives of Patients with Type 2 Diabetes. Mol. Metab. 2018, 12, 39–47. [Google Scholar] [CrossRef]
- Wu, R.-E.; Huang, W.-C.; Liao, C.-C.; Chang, Y.-K.; Kan, N.-W.; Huang, C.-C. Resveratrol Protects against Physical Fatigue and Improves Exercise Performance in Mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Jones, K.E.; Sidhu, R.S.; Haykowsky, M.; Czubryt, M.P.; Gordon, T.; Dyck, J.R.B. Improvements in Skeletal Muscle Strength and Cardiac Function Induced by Resveratrol during Exercise Training Contribute to Enhanced Exercise Performance in Rats: Resveratrol Enhances Exercise Performance. J. Physiol. 2012, 590, 2783–2799. [Google Scholar] [CrossRef]
- Kan, N.-W.; Lee, M.-C.; Tung, Y.-T.; Chiu, C.-C.; Huang, C.-C.; Huang, W.-C. The Synergistic Effects of Resveratrol Combined with Resistant Training on Exercise Performance and Physiological Adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. Ser. A 2017, 72, 1595–1606. [Google Scholar] [CrossRef]
- Ringholm, S.; Olesen, J.; Pedersen, J.T.; Brandt, C.T.; Halling, J.F.; Hellsten, Y.; Prats, C.; Pilegaard, H. Effect of Lifelong Resveratrol Supplementation and Exercise Training on Skeletal Muscle Oxidative Capacity in Aging Mice; Impact of PGC-1α. Exp. Gerontol. 2013, 48, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, A.; Sindhu, G.; Shyni, G.; Preetha Rani, M.; Nisha, V.; Raghu, K. Bilobalide Abates Inflammation, Insulin Resistance and Secretion of Angiogenic Factors Induced by Hypoxia in 3T3-L1 Adipocytes by Controlling NF-ΚB and JNK Activation. Int. Immunopharmacol. 2017, 42, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Zaher, A.O.; Farghaly, H.S.M.; El-Refaiy, A.E.M.; Abd-Eldayem, A.M. Protective Effect of the Standardized Extract of Ginkgo Biloba (EGb761) against Hypertension with Hypercholesterolemia-Induced Renal Injury in Rats: Insights in the Underlying Mechanisms. Biomed. Pharmacother. 2017, 95, 944–955. [Google Scholar] [CrossRef]
- Sayin, F.; Buyukbas, S.; Basarali, M.; Alp, H.; Toy, H.; Ugurcu, V. Effects of Silybum Marianum Extract on High-Fat Diet Induced Metabolic Disorders in Rats. Pol. J. Food Nutr. Sci. 2016, 66, 43–49. [Google Scholar] [CrossRef]
- Poruba, M.; Kazdová, L.; Oliyarnyk, O.; Malinská, H.; Matusková, Z.; Tozzi di Angelo, I.; Skop, V.; Vecera, R. Improvement Bioavailability of Silymarin Ameliorates Severe Dyslipidemia Associated with Metabolic Syndrome. Xenobiotica 2015, 45, 751–756. [Google Scholar] [CrossRef]
- Yao, J.; Zhi, M.; Minhu, C. Effect of Silybin on High-Fat-Induced Fatty Liver in Rats. Braz. J. Med. Biol. Res. 2011, 44, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhi, M.; Gao, X.; Hu, P.; Li, C.; Yang, X. Effect and the Probable Mechanisms of Silibinin in Regulating Insulin Resistance in the Liver of Rats with Non-Alcoholic Fatty Liver. Braz. J. Med. Biol. Res. 2013, 46, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Komatsu, Y.; Murayama, A.; Steffensen, K.R.; Nakagawa, Y.; Nakajima, Y.; Suzuki, M.; Oie, S.; Parini, P.; Vedin, L.-L.; et al. Estrogen Receptor Ligands Ameliorate Fatty Liver through a Nonclassical Estrogen Receptor/Liver X Receptor Pathway in Mice: Hepatology, Vol. XX, No. X, 2013 Han et Al. Hepatology 2014, 59, 1791–1802. [Google Scholar] [CrossRef]
- Heim, M.; Frank, O.; Kampmann, G.; Sochocky, N.; Pennimpede, T.; Fuchs, P.; Hunziker, W.; Weber, P.; Martin, I.; Bendik, I. The Phytoestrogen Genistein Enhances Osteogenesis and Represses Adipogenic Differentiation of Human Primary Bone Marrow Stromal Cells. Endocrinology 2004, 145, 848–859. [Google Scholar] [CrossRef]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Genistein Affects Lipogenesis and Lipolysis in Isolated Rat Adipocytes. J. Steroid Biochem. Mol. Biol. 2000, 75, 265–271. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Park, I.-J.; Shin, J.-I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and Capsaicin Inhibit Adipocyte Differentiation Process via Activating AMP-Activated Protein Kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef]
- Kim, H.-K.; Nelson-Dooley, C.; Della-Fera, M.A.; Yang, J.-Y.; Zhang, W.; Duan, J.; Hartzell, D.L.; Hamrick, M.W.; Baile, C.A. Genistein Decreases Food Intake, Body Weight, and Fat Pad Weight and Causes Adipose Tissue Apoptosis in Ovariectomized Female Mice. J. Nutr. 2006, 136, 409–414. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Vinciguerra, M.; Gjinovci, A.; Kuhne, F.; Klein, M.; Cederroth, M.; Caille, D.; Suter, M.; Neumann, D.; James, R.W.; et al. Dietary Phytoestrogens Activate AMP-Activated Protein Kinase With Improvement in Lipid and Glucose Metabolism. Diabetes 2008, 57, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Torre-Villalvazo, I.; Tovar, A.R.; Ramos-Barragán, V.E.; Cerbón-Cervantes, M.A.; Torres, N. Soy Protein Ameliorates Metabolic Abnormalities in Liver and Adipose Tissue of Rats Fed a High Fat Diet. J. Nutr. 2008, 138, 462–468. [Google Scholar] [CrossRef]
- Lephart, E.D.; Porter, J.P.; Lund, T.D.; Bu, L.; Setchell, K.D.; Ramoz, G.; Crowley, W.R. Dietary Isoflavones Alter Regulatory Behaviors, Metabolic Hormones and Neuroendocrine Function in Long-Evans Male Rats. Nutr. Metab. (Lond) 2004, 1, 16. [Google Scholar] [CrossRef]
- Christie, D.R.; Grant, J.; Darnell, B.E.; Chapman, V.R.; Gastaldelli, A.; Sites, C.K. Metabolic Effects of Soy Supplementation in Postmenopausal Caucasian and African American Women: A Randomized, Placebo-Controlled Trial. Am. J. Obstet. Gynecol. 2010, 203, 153.e1–153.e9. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Lin, Y.-J.; Ho, C.-T.; Yen, G.-C. Inhibitory Effects of Garcinol and Pterostilbene on Cell Proliferation and Adipogenesis in 3T3-L1 Cells. Food Funct. 2012, 3, 49–57. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Kim, K.-J.; Koh, E.-J.; Choi, J.; Lee, B.-Y. Anti-Adipogenesis Mechanism of Pterostilbene through the Activation of Heme Oxygenase-1 in 3T3-L1 Cells. Phytomedicine 2017, 33, 7–13. [Google Scholar] [CrossRef]
- Gomez-Zorita, S.; Belles, C.; Briot, A.; Fernández-Quintela, A.; Portillo, M.P.; Carpéné, C. Pterostilbene Inhibits Lipogenic Activity Similar to Resveratrol or Caffeine but Differently Modulates Lipolysis in Adipocytes: Pterostilbene Direct Effects on Human Adipocytes. Phytother. Res. 2017, 31, 1273–1282. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, K.-Y.; Peng, K.-Y.; Day, Y.-J.; Hung, L.-M. Resveratrol Exerts Anti-Obesity Effects in High-Fat Diet Obese Mice and Displays Differential Dosage Effects on Cytotoxicity, Differentiation, and Lipolysis in 3T3-L1 Cells. Endocr. J. 2016, 63, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Portolés, C.; Fernández, L.; Ramírez de Molina, A. Precision Nutrition for Targeting Lipid Metabolism in Colorectal Cancer. Nutrients 2017, 9, 1076. [Google Scholar] [CrossRef]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The Effects of 30 Days Resveratrol Supplementation on Adipose Tissue Morphology and Gene Expression Patterns in Obese Men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F.Y. Effects of Resveratrol in Patients with Type 2 Diabetes Mellitus on Skeletal Muscle SIRT1 Expression and Energy Expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef] [PubMed]



| Ref. | Extract | Treatment | Protocol | Effects |
|---|---|---|---|---|
| [117] | Pomegranate extract | Urolithin A | in vitro (3T3L1) | ↑ adipogenesis, TG, lipase, PPARG, GLUT4, FABP4, adiponectin |
| [107] | Punicalagin | in vivo (HFD mice 400–800 mg/kg/d 5 wk) | ↓ Ch, TG, glc, BW, appetite | |
| [110] | Punicalagin | in vivo (HFD mice 150 mg/kg/d) | ↑ AMPK, PGC1a pathway | |
| [109] | Punicalagin | in vivo (HFD mice 150 mg/kg/d) | ↓ oxidative stress, inflammation markers (IL1, 4, 6, TNFa), hyperlipidemia, hepatic lipid deposition ↑ FAO, PGC1a | |
| [113] | Seed oil | in vivo (HFD mice 1% diet, 12 wk) | ↑ Insulin sensitivity ↓ BW, WAT mass | |
| [114] | Pomegranate extract + exercise training | in vivo (HDF rat 150 mg/kg/d 8 wk, training 60’ 3 times/wk) | ↑ immune function (CD4+) ↓ apoptosis in PBMC, inflammation, oxidative stress | |
| [118] | Urolithin A | in vivo (mice 30 mg/kg/d 10 wk) | ↑ Browning and thermogenesis (T3), improves glucose and insulin homeostasis. ↓ BW | |
| [115] | Pomegranate juice | Prospective cross open cohort-controlled study (athletes 1.5 L/d 2 days with power training) | ↓ muscle damage markers, fatigue, recuperation time | |
| [123] | Ginkgo Biloba | Isoginkgetin | in vitro (3T3L1) | ↑ AMPK, adiponectin pathways, no effects in PPARG nor adipogenesis |
| [125] | Bilobalide | in vitro (3T3L1) | Antiadipogenic effects ↓ differentiation, TG accumulation, ↑ AMPK, CPT1a, HSL, lipolysis | |
| [127] | Vinegar from seed coat | in vitro (3T3L1) | ↓ Lipid accumulation, adipogenesis and differentiation (Cebpd, PPARG) | |
| [191] | Bilobalide | in vitro (3T3L1) | ↓ NFkb ↑ Adiponectin | |
| [124] | Ginkgo Biloba extract | in vitro (primary mice adipocytes and osteoblasts) and in vivo (hamster HCD/HFD 30 d 250 mg/kg/d) | Antiadipogenic effects ↓ PPARG, Ch. ↑ Apoptosis via ROS in WAT | |
| [126] | Ginkgetin | in vitro (3T3L1) and in vivo (mice HFD 5–10 mg/kg/d) | Antiadipogenic. ↓ differentiation, STAT5, PPARG, Cebpa ↑ hypertrophy AT in mice | |
| [121] | Ginkgo Biloba extract | in vivo (rat 2 months HFD + 14 days 500 mg/kg/d) | ↓ Intake, BW, NFkb, TNFa IR ↑ IL10, Akt-P | |
| [120] | Ginkgo Biloba extract | in vivo (rat 2 months HFD + 14 days 500 mg/kg/d) | ↓ Intake, IR ↑ Akt-P, IRS1 | |
| [192] | Ginkgo Biloba extract | in vivo (hypertensive rats 3 wk 100 mg/kg/d) | ↓ BP, Nitrite level ↑ eNOS mRNA, iNOS prot, TNFa, IL6, IL1, GSH | |
| [131] | Milk Thistle Silymarin | Silibinin | in vitro (3T3L1) | ↓ PPARG, FABP4, FASN, SREBP1c, Cebpa en WAT, terminal differentiation, lipogenesis in mature adipocytes |
| [130] | in vitro (mesenquimal stem human adipocytes) | ↓ PPARG, FABP4, FASN, SREBP1c, Cebpa en WAT ↑ SIRT1, PGC1a, UCP1 | ||
| [132] | in vitro (3T3L1) and in zebra fish | ↓ Lipid accumulation (TG, FA), adipogenesis and differentiation (Cebpd, PPARG, FABP4), adipocyte size, ↑ AMPK | ||
| [193] | in vivo (rat 49–77 d HFD 200 mg/kg/d) | ↓ BMI, IR, TG, LDL ↑ Leptin sensitivity | ||
| [128] | in vivo (mice HFD 18 d 30–60 mg/kg/d) | ↓ Lipid accumulation, IR, BP, BW, inflammation Improve glucose metabolism | ||
| [194] | in vivo (rat 4 wk 1% Silymarin in diet) | ↑ HDL, ABC transporter, CytP450 ↓ TG, Ch in serum | ||
| [133] | in vivo (obese mice, 8 wk HFD + 8 wk 50 mg/kg/d intraperitoneal) | ↓ AT inflammation, hypertrophia, BW, IR, restore lipid and glucose homeostasis | ||
| [195] | in vivo (rar 42 d 26 mg/kg/d) | ↑ Serum lipid profile, SOD, GSH, Adiponectin, FAO ↓ IR, Resistin, Oxidative stress, FA synthesis | ||
| [196] | in vivo (rat HFD, 6 wk 0.5 mg/kg/d) | ↓ IR, visceral fat, gluconeogenesis, TG ↑ Lipolysis | ||
| [197] | Soy | Mix of Soy Isoflavones | in vitro | Antiadipogenesis, ↓ SREBP1c |
| [198] | Genistein | in vitro (primary human adipocytes) | ↓ Cebpa, PPARG, LPL, Lipid droplet size ↑ TGFb1 | |
| [141] | Genistein | in vitro (primary human adipocytes) | ↓ Adipogenesis and differentiation (PPARG, Cebpa, FABP4, FASN, SREBP1c) | |
| [140] | Genistein and Daidzein | in vitro (human derived mesenquimal stem cells) | ↓ adipocyte differentiation (PPARG, Cebpa, SREBP1c, GLUT4) | |
| [148] | Genistein | in vitro (3T3L1) and in vivo (mice 0.2% Genistein in diet 58 d) | ↑ FAO, browning induction (FNDC5) mitochondrial function in mice muscle (AMPK, PGC1a, PPARG) ↑ thermogenesis (UCP1, TMEM16), mitochondrial number and respiration rate in adipocytes 3T3L1 | |
| [199] | Genistein | in vitro (primary epididimal rat adipocytes) | ↑ Lipolysis, cAMP via AMPK activation ↓ TG | |
| [200] | Genistein | in vitro (3T3L1) | ↑ AMPK, apoptosis in mature adipocytes ↓ adipogenesis | |
| [137] | Mix of Soy Isoflavones | in vitro (primary adipocytes) | ↑ mitochondrial biogenesis (SIRT1-PGC1a pathway), ATP synthase b | |
| [138] | Soy Isoflavones + Green Tea + Resveratrol | in vitro (3T3L1) | ↓ adipogenesis and differentation (PPARG, Cebpa, FABP4 and perilipin) | |
| [139] | Daidzein | in vitro (3T3L1) | ↓ Adipogenesis (PPARG, Cebpa), lipid accumulation, PI3K-Akt pathway | |
| [97] | Genistein | in vitro (3T3L1) | ↑ thermogenesis in BAT (UCP1, SIRT1, PGC1a, proton leak and oxygen consumption) ↓ Lipid accumulation in WAT (FASN, FABP4, HSL, resistin) | |
| [149] | Daidzein | in vitro (C2C12) | ↑ mitochondrial biogenesis (PGC1a, TFAM, SIRT1 dependent), COX1 | |
| [150] | Mix of Soy Isoflavones | In vitro (C2C12) | ↑ SIRT1, AMPK activation ↓ myotube atrophy | |
| [151] | Mix of Soy Isoflavones | in vitro (C2C12) | ↑ myotube diameter, MHC protein, IGF1 and IGF1R | |
| [201] | Genistein | in vivo (mice 0–1500 mg/kg/d 3 wk) | ↑ fat tissue apoptosis ↓ food intake, BW, parametrial and inguinal fat | |
| [136] | Mix of Soy Isoflavones | in vivo (rat HFD 8 wk HFD + 4 wk HFD + 50–400 mg/kg/d) | ↓ BW, lipogenesis, adipogenesis ↑ FAO, lipolysis, Akt-P, mTOR inhibition | |
| [202] | Genistein and Daidzein | in vivo (mice 3 wk 286 ppm geistein + 198 ppm Daidzein) | ↓ BW, WAT mass, serum leptin, insulin, TG in muscle and liver ↑ AMPK, ACC, FAO, mitochondrial biogenesis (PGC1a, TFAM) in muscle and fat | |
| [203] | Soy protein | in vivo (rat HFD 30% Soy protein 180 d) | ↑ UCP1, WAT lipolysis, Leptin sensitivity in hypotalamous, adipocyte perilipin ↓ SREBP1 and adipocyte size in WAT | |
| [204] | Mix of Soy Isoflavones | in vivo (rat 10–600 mg/kg) | ↑ thermogenesis (UCP1, T3 in BAT) ↓ leptin and insulin in serum | |
| [143] | Genistein | in vivo (obese mice 600 mg/kg/d 5 wk) | ↑ body temperature, T3 in serum ↓ hypercorticosteronism | |
| [146] | Daidzein | in vivo (obese rat 50 mg/kg/d 14 d) | ↓ BW, fat in the liver, SCD ↑ FAO and UCP1 in BAT | |
| [147] | Isoflavones and Soy protein | in vivo (rat 0–4 g/kg/d) | ↑ thermogenesis and browning (UCP1,2, 3, PPARa) ↓ WAT adipogenesis (PPARG) | |
| [205] | Isoflavones and Soy protein | Randomized placebo controlled trial (postmenopausal 160 mg/d Isoflavones + 20 g/d Soy protein 3 months) | ↓ abdominal and subcutaneous fat, IL6 No effect in leptin/adiponectin | |
| [176] | Grape Resveratrol | Resveratrol | in vitro (3T3L1) | ↓ Adipogenesis (↓ adipogenesis (PPARG, Cebpa, SREBP1c, FASN) ↑ SIRT1, AMPK activation, apoptosis, TNFa and lipolysis |
| [165] | Resveratrol | in vitro (C2C12 myoblast, PC3 cancer cells, mouse embryonic fibroblast) | ↑ mitofusin 2 expression and respiration rates | |
| [206] | Pterostilbene | in vitro (3T3L1) | ↑ adiponectin. ↓ cell proliferation and differentiation (PPARG, Cebpa, FASN and resistin) | |
| [207] | Pterostilbene | in vitro (3T3L1) | ↑ oxygenase I ↓ Differentiation (PPARG, Cebpa, FABP4) | |
| [208] | Pterostilbene | in vitro (3T3L1) | ↓ Lipogenesis and lipogenic insulin effect | |
| [99] | Resveratrol | in vitro (3T3L1) | ↓ adipogenesis and differentiation (PPARG, Cebpa, SREBP1c, FASN, FABP4) dose dependent | |
| [178] | Resveratrol | in vitro (3T3L1, SGBS) | ↑ mitochondrial biogenesis and mass (AMPK, ATAD3) ↓ lipogenesis | |
| [179] | Resveratrol | in vitro (bovine intramuscular adipocytes) | ↑ SIRT1, AMPK, FOXO1 pathways, HSL ↓ Adipogenesis (FASN, PPARG) | |
| [170] | Resveratrol | in vitro (3T3L1, SGBS) | ↑ FA release, ATGL via AMPK activation | |
| [209] | Resveratrol | in vitro (3T3L1) and in vivo (mice HFD 1–30 mg/kg/d 10 wk) | ↓ lipid deposition in WAT and liver, BW, differentiation capacity (PPARG and perilipin) | |
| [158] | Resveratrol | in vitro (3T3L1) | ↑ mtDNA, oxydative capacity (CPT1a) and thermogenesis (UCP1) ↓ Lipogenesis and resistin | |
| [183] | Resveratrol | in vivo (HFD 15 wk 400 mg/kg/d) | ↑ EE, thermogenesis (UCP1), mtDNA, mitochondrial biogenesis (PGC1a, PPARA) and oxygen consumption in muscle fibers | |
| [162] | Resveratrol | in vivo (rat HFD 30 mg/kg/d) | ↑ SIRT1, COX2, PGC1a and UCP1 protein | |
| [168] | Resveratrol | in vivo (mice HFD + 0.04–0.4% Resveratrol 8 month) | ↑ mitochondrial biogenesis and function (PGC1a, NRF2, UCP1, ATP5a1, TFAM, SIRT1, AMPK activation, and maximal respiration rate) | |
| [163] | Resveratrol | in vivo (mice 8 wk 4 g/kg) | ↑ thermogenesis and mitochondrial function (UCP1, SIRT1, BMP7) | |
| [169] | Resveratrol | in vivo (mice HFD 4 wk 0.1% Resveratrol) | ↑ iBAT mass, thermogenesis and browning (UCP1, AMPK, PRDM16) | |
| [160] | Resveratrol | in vivo (rat ND 30 mg/kg/d 6 wk) | ↑ thermogenesis and mitochondrial function (UCP1, SIRT3, ↓ PGC1a acetylation) | |
| [159] | Resveratrol | in vivo (mice HFD 0.1% Resveratrol) | ↑ thermogenesis, browning and mitochondrial function in iWAT (UCP1, PRDM16, Cidea, PGC1a, AMPK, oxygen consumption and FAO) | |
| [161] | Resveratrol | in vivo (mice HFD 0.5% Resveratrol) | ↑ thermogenesis and mitochondrial function (UCP1, PRDM16, PPARA and adiponectin expression, SIRT1 and PGC1a activation) | |
| [164] | Resveratrol | in vivo (mice HFD/ND + 10 mg/kg/d) | ↑ mitochondrial activity and mass in BAT, extrogen receptor a | |
| [166] | Resveratrol + quercetin | in vivo (rat 4wk high glucose in water + 10–50 mg/kg/d) | ↑ PPARG, UCP2 in WAT, MUFAs and PUFAs | |
| [210] | Pterostilbene | in vivo (rat 15–30 mg/kg/d) | ↑ browning and thermogenesis (UCP1, PPARA, NRF) and oxidative capacity (CPT1a) | |
| [171] | Resveratrol | in vivo (mice HFD 0.2% Resveratrol during pregnancy and lactation/breeding 11 wk) | ↑ EE, BAT function, browning and thermogenesis after weaning (UCP1, PRDM16, Cidea, PGC1a, SIRT1, AMPK) ↓ IR, TG, WAT mass, blood glucose | |
| [172] | Resveratrol | in vivo (postnatal mice 2–20 d 2 mg/kg/d) | ↑ thermogenesis in BAT only in males (UCP1, PGC1a, TMTM26, SLC27a1, CPT1b | |
| [174] | Resveratrol | in vivo (mice 400 mg/kg/d 8 wk) and preclinical (n = 20, 50 mg/d) | ↑ Browning and thermogenesis (UCP1, PRDM16, PGC1a SIRT1 dependent and FNDC5 in subcutaneous AT) | |
| [180] | Resveratrol | in vivo (mice HFD 0.4% Resveratrol 10 wk) | ↓ Adipogenesis (FASN, leptin, PPARG, Cebpa, SREBP1c, FABP4), inflammation (TNFa, IL6, INFa and b), TG, BW, Ch, blood glucose | |
| [181] | Resveratrol | in vivo (mice HFD and HPD, 4 g/kg/d 60 d) | ↓ adipogenesis and lipogenesis (PPARG, Cebpa, SREBP1c, FASN), BW, Ch, AT mass, ACC ↑ HDL | |
| [186] | Resveratrol | in vivo (mice 0–125 mg/kg/d 21 d + swimming training) | ↑ muscle aerobic capacity ↓ muscle fatigue, CK, ammonia, lactate in serum | |
| [187] | Resveratrol | in vivo (rat 4 g/kg/d 12 wk + physical training) | ↑ Force isometric contraction, FAO, physical performance, mitochondrial number and function (oxydative metabolism), cardiac function (FAO) | |
| [188] | Resveratrol | in vivo (mice 25 mg/kg/d 4 wk + climbing exercise) | ↓ muscle fatigue index ↑ muscle glycogen content, insulin sensitivity, muscle hypertrophy | |
| [211] | Resveratrol | Randomized doubleblind crossover trial (11 obese men 30 d 150 mg/d) | ↑ Lipolysis ↓ adipocyte size | |
| [185] | Resveratrol | Randomized, placebo controlled, cross-over trial. (13 relatives to T2DM patients 150 mg/kg/d 30 d) | ↑ SIRT1, PGC1a pathways in skeletal muscle ex vivo No changes in BAT | |
| [212] | Resveratrol | Part of a randomized, double-blind, parallel group trial (10 men T2DM 12 wk 2 g/d) | No changes in BMI, AT mass ↑ resting EE, SIRT1, AMPK expression in muscle | |
| [189]. | Resveratrol | Randomized blind placebo-controlled trial (30 elderly subjects, 500 mg/d 12 wk + regular exercise) | ↑ mitochondrial density, knee extensor muscle peak torque ↓ muscle fatigue index |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reguero, M.; Gómez de Cedrón, M.; Wagner, S.; Reglero, G.; Quintela, J.C.; Ramírez de Molina, A. Precision Nutrition to Activate Thermogenesis as a Complementary Approach to Target Obesity and Associated-Metabolic-Disorders. Cancers 2021, 13, 866. https://doi.org/10.3390/cancers13040866
Reguero M, Gómez de Cedrón M, Wagner S, Reglero G, Quintela JC, Ramírez de Molina A. Precision Nutrition to Activate Thermogenesis as a Complementary Approach to Target Obesity and Associated-Metabolic-Disorders. Cancers. 2021; 13(4):866. https://doi.org/10.3390/cancers13040866
Chicago/Turabian StyleReguero, Marina, Marta Gómez de Cedrón, Sonia Wagner, Guillermo Reglero, José Carlos Quintela, and Ana Ramírez de Molina. 2021. "Precision Nutrition to Activate Thermogenesis as a Complementary Approach to Target Obesity and Associated-Metabolic-Disorders" Cancers 13, no. 4: 866. https://doi.org/10.3390/cancers13040866
APA StyleReguero, M., Gómez de Cedrón, M., Wagner, S., Reglero, G., Quintela, J. C., & Ramírez de Molina, A. (2021). Precision Nutrition to Activate Thermogenesis as a Complementary Approach to Target Obesity and Associated-Metabolic-Disorders. Cancers, 13(4), 866. https://doi.org/10.3390/cancers13040866






