Ovulatory Follicular Fluid Facilitates the Full Transformation Process for the Development of High-Grade Serous Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. FF Promotes Intraperitoneal Tumorigenesis of HGSC Cells and Transformation of Human Fimbrial Epithelial Cells
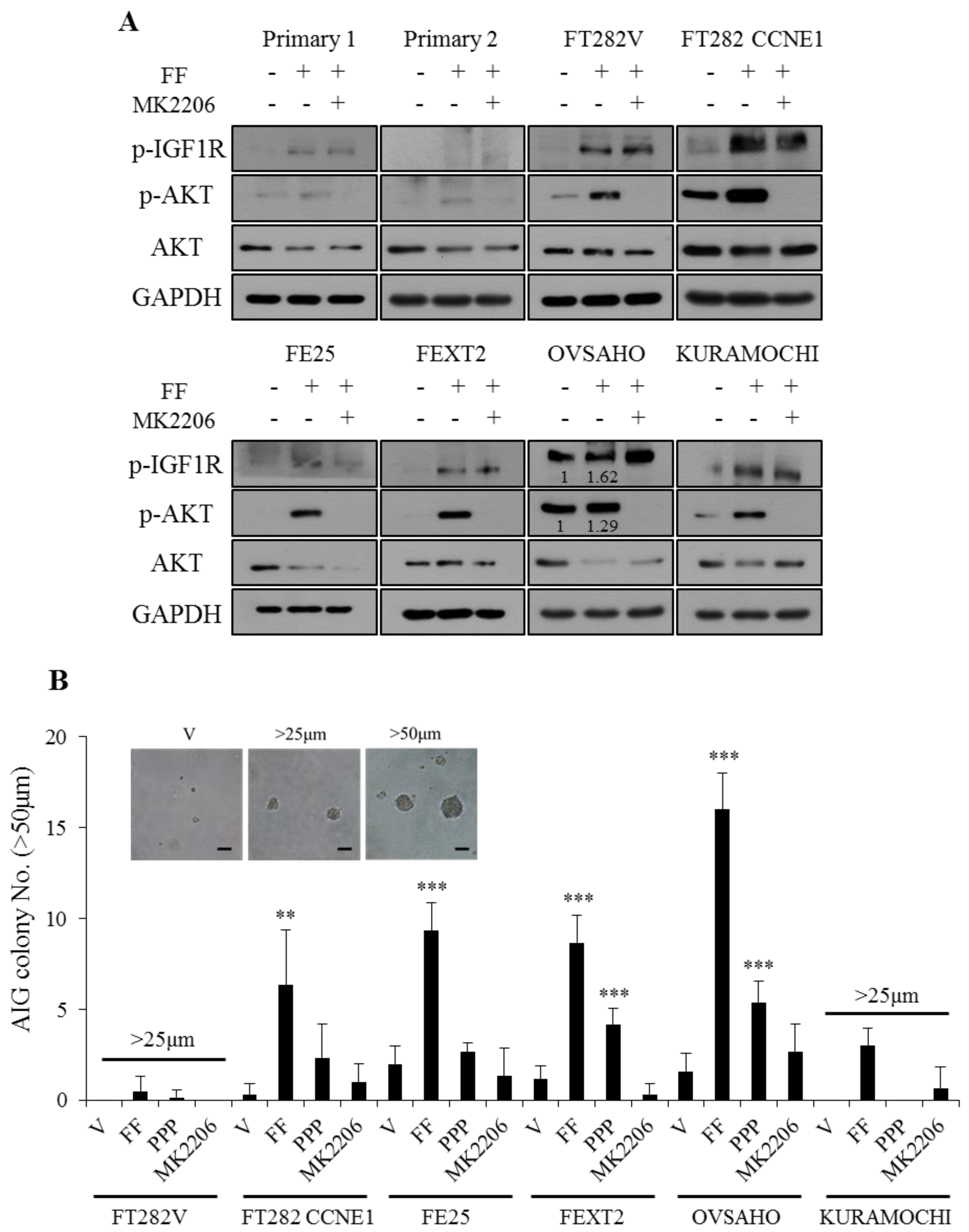
2.2. FF Induces Phosphorylation of IGF-1R/AKT With Subsequent Increase of AIG
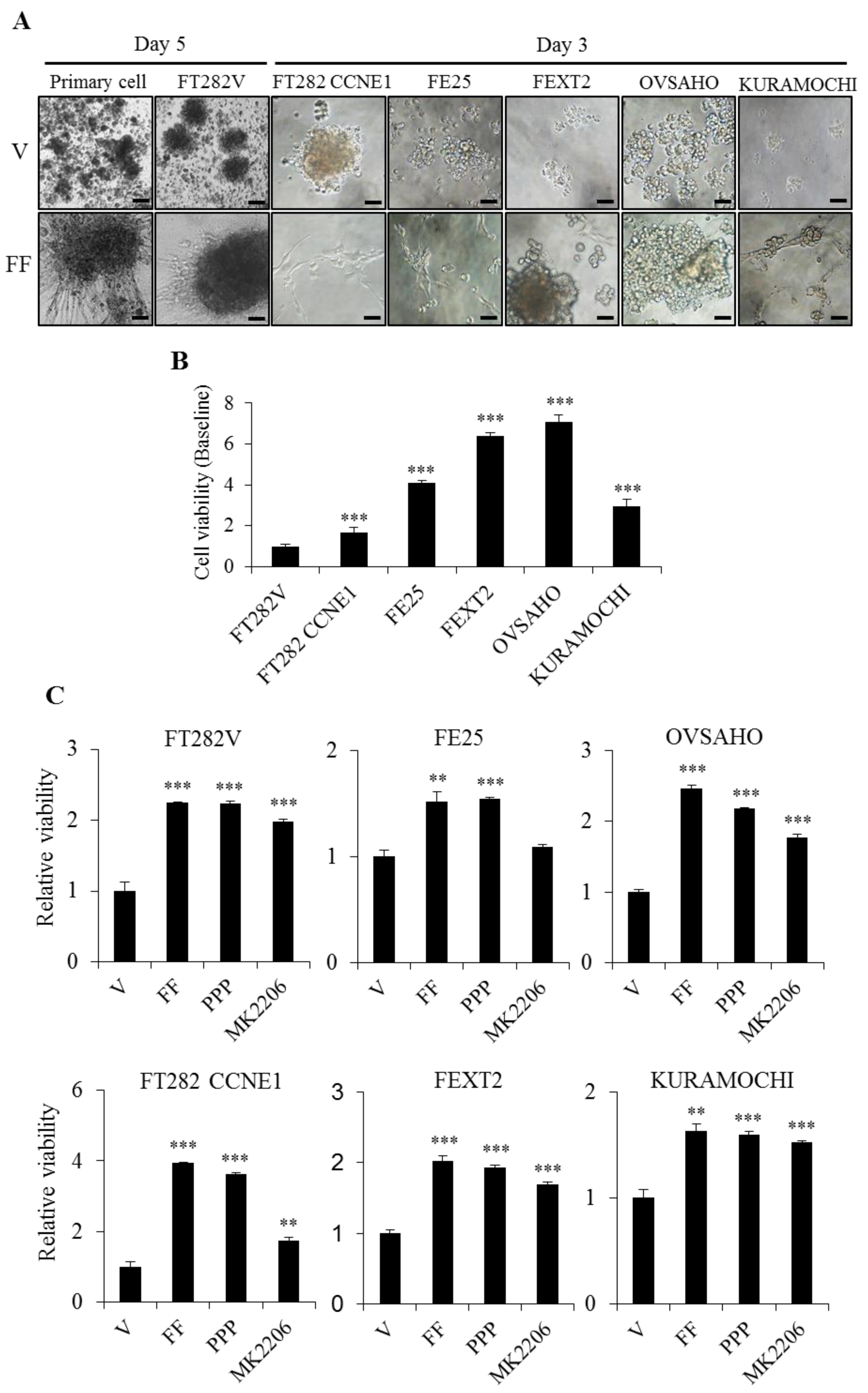
2.3. FF Enhanced Cell Proliferation Through Non-IGF-1R Dependent AKT Signaling
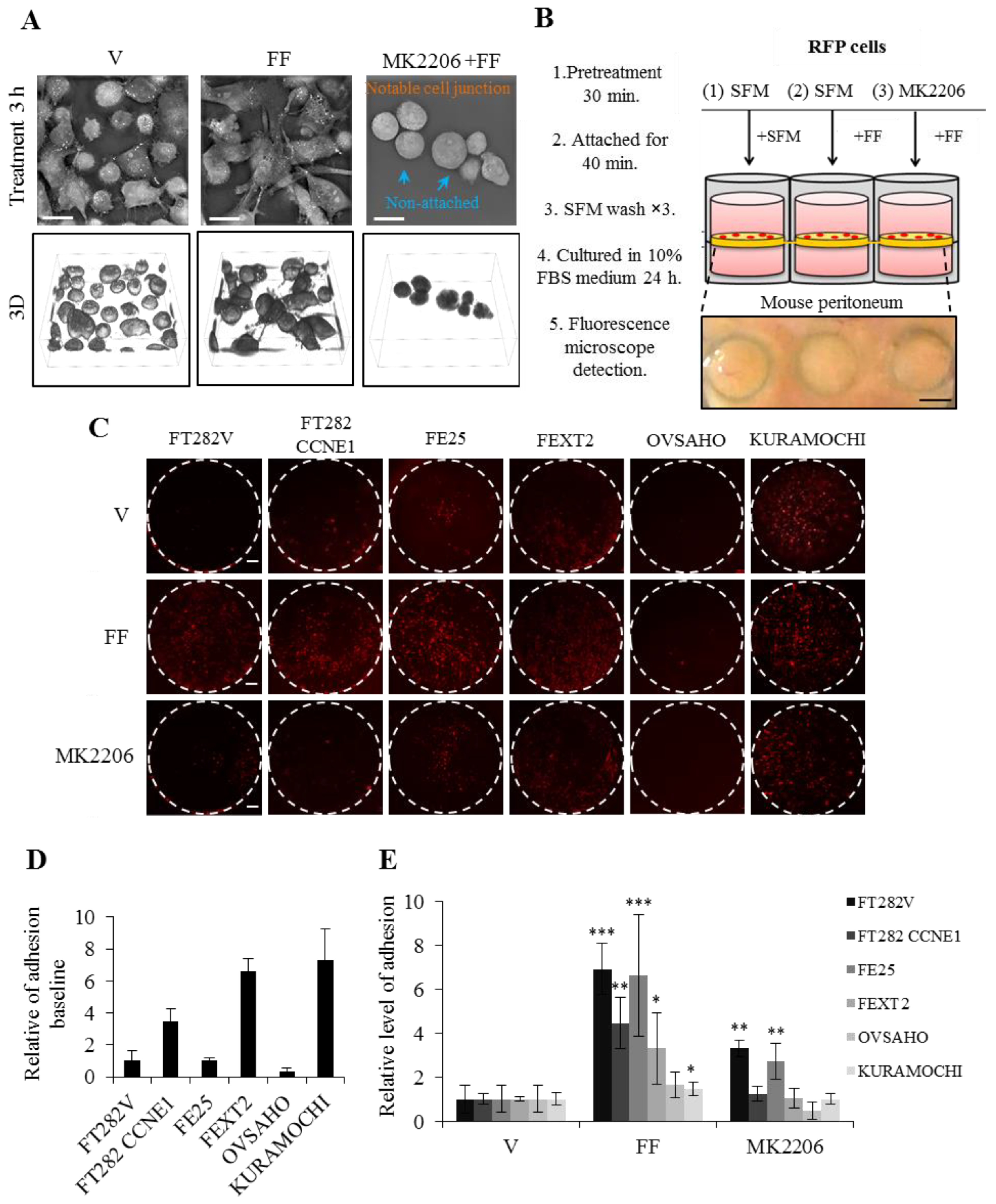
2.4. FF Highly Increases Matrix Attachment, and Enhances Anoikis Resistance, Partly Dependent on AKT
2.5. FF Increases Attached Growth of FE and HGSC Cells on Peritoneum, Partially Dependent on AKT
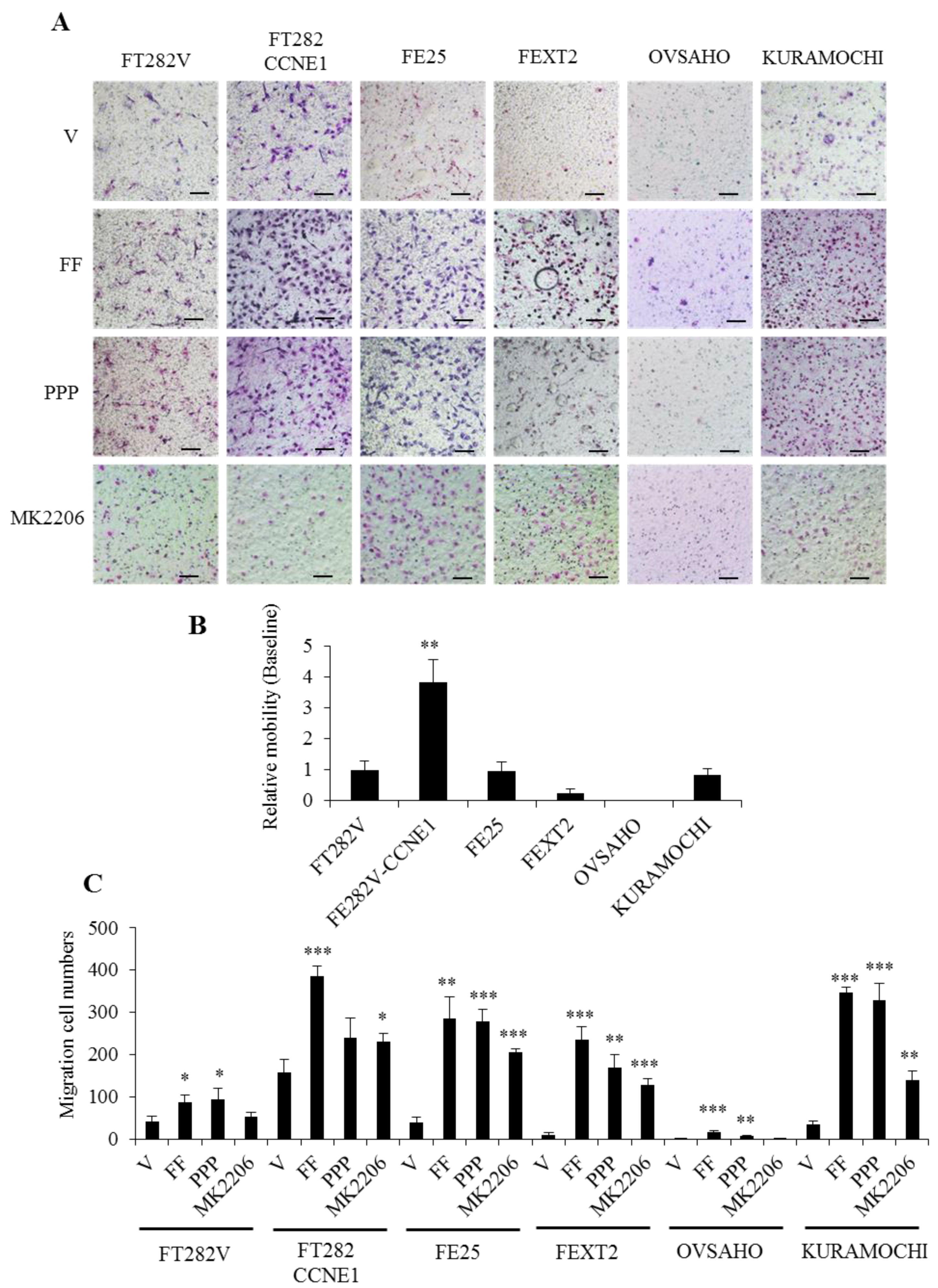
2.6. FF Highly Promotes the Motility of Transforming FE Cells, Partially Mediated by AKT
2.7. FF Promotes the Invasion of Transforming FE Cells, Largely Dependent on AKT
2.8. FF Increase EMT in Transforming FTE Cells Independent of AKT
3. Discussion
4. Material and Methods
4.1. Clinical Specimens
4.2. Human Fallopian Tube Fimbrial Epithelial Cell Lines
Cell Sources
4.3. Xenograft Tumor Model
4.4. Immunohistochemistry Analysis
4.5. Western Blot Analysis
4.6. AIG
4.7. Anoikis Resistance Assay
4.8. Ex Vivo Peritoneal Attachment Growth Assay
4.9. Cell Motility and Invasion Assay
4.10. Epithelial to Mesenchymal Transition (EMT) Assay
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, M.P.; Forman, D.; Bryant, H.; Butler, J.; Rachet, B.; Maringe, C.; Nur, U.; Tracey, E.; Coory, M.; Hatcher, J.; et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef]
- Dao, F.; Schlappe, B.A.; Tseng, J.; Lester, J.; Nick, A.M.; Lutgendorf, S.K.; McMeekin, S.; Coleman, R.L.; Moore, K.N.; Karlan, B.Y.; et al. Characteristics of 10-year survivors of high-grade serous ovarian carcinoma. Gynecol. Oncol. 2016, 141, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Levanon, K.; Crum, C.; Drapkin, R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J. Clin. Oncol. 2008, 26, 5284–5293. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Shih, I.M.; Kurman, R.J. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology 2013, 62, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Ducie, J.; Dao, F.; Considine, M.; Olvera, N.; Shaw, P.A.; Kurman, R.J.; Shih, I.M.; Soslow, R.A.; Cope, L.; Levine, D.A. Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat. Commun. 2017, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Leeper, K.; Garcia, R.; Swisher, E.; Goff, B.; Greer, B.; Paley, P. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol. Oncol. 2002, 87, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Folkins, A.K.; Jarboe, E.A.; Saleemuddin, A.; Lee, Y.; Callahan, M.J.; Drapkin, R.; Garber, J.E.; Muto, M.G.; Tworoger, S.; Crum, C.P. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol. Oncol. 2008, 109, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am. J. Surg. Pathol. 2007, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Kurman, R.J.; Vang, R.; Sehdev, A.S.; Han, G.; Soslow, R.; Wang, T.L.; Shih, I.M. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma—Evidence supporting the clonal relationship of the two lesions. J. Pathol. 2012, 226, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.C.; Wang, P.; Lin, S.F.; Zhang, M.; Song, Q.; Chu, T.; Wang, B.G.; Kurman, R.J.; Vang, R.; Kinzler, K.; et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J. Pathol. 2019, 248, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.Y.; Fang, C.; Huang, H.S.; Wang, J.; Chu, T.Y. Natural history of ovarian high-grade serous carcinoma from time effects of ovulation inhibition and progesterone clearance of p53-defective lesions. Mod. Pathol. 2020, 33, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Jarboe, E.; Folkins, A.; Nucci, M.R.; Kindelberger, D.; Drapkin, R.; Miron, A.; Lee, Y.; Crum, C.P. Serous carcinogenesis in the fallopian tube: A descriptive classification. Int. J. Gynecol. Pathol. 2008, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bijron, J.G.; Seldenrijk, C.A.; Zweemer, R.P.; Lange, J.G.; Verheijen, R.H.; van Diest, P.J. Fallopian tube intraluminal tumor spread from noninvasive precursor lesions: A novel metastatic route in early pelvic carcinogenesis. Am. J. Surg. Pathol. 2013, 37, 1123–1130. [Google Scholar] [CrossRef]
- Wang, K.H.; Chu, S.C.; Chu, T.Y. Loss of calponin h1 confers anoikis resistance and tumor progression in the development of high-grade serous carcinoma originating from the fallopian tube epithelium. Oncotarget 2017, 8, 61133–61145. [Google Scholar] [CrossRef]
- Farsinejad, S.; Cattabiani, T.; Muranen, T.; Iwanicki, M. Ovarian Cancer Dissemination-A Cell Biologist’s Perspective. Cancers 2019, 11, 1957. [Google Scholar] [CrossRef]
- Collaborative Group on Epidemiological Studies of Ovarian Cancers; Beral, V.; Doll, R.; Hermon, C.; Peto, R.; Reeves, G. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008, 371, 303–314. [Google Scholar] [CrossRef]
- Holschneider, C.H.; Berek, J.S. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin. Surg. Oncol. 2000, 19, 3–10. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Moorman, P.G.; Lowery, W.J.; Gierisch, J.M.; Coeytaux, R.R.; Urrutia, R.P.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sanders, G.D.; et al. Oral contraceptive pills as primary prevention for ovarian cancer: A systematic review and meta-analysis. Obstet. Gynecol. 2013, 122, 139–147. [Google Scholar] [CrossRef]
- Huang, H.S.; Chu, S.C.; Hsu, C.F.; Chen, P.C.; Ding, D.C.; Chang, M.Y.; Chu, T.Y. Mutagenic, surviving and tumorigenic effects of follicular fluid in the context of p53 loss: Initiation of fimbria carcinogenesis. Carcinogenesis 2015, 36, 1419–1428. [Google Scholar] [CrossRef]
- Huang, H.S.; Hsu, C.F.; Chu, S.C.; Chen, P.C.; Ding, D.C.; Chang, M.Y.; Chu, T.Y. Haemoglobin in pelvic fluid rescues Fallopian tube epithelial cells from reactive oxygen species stress and apoptosis. J. Pathol. 2016, 240, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.F.; Huang, H.S.; Chen, P.C.; Ding, D.C.; Chu, T.Y. IGF-axis confers transformation and regeneration of fallopian tube fimbria epithelium upon ovulation. EBioMedicine 2019, 41, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Karst, A.M.; Jones, P.M.; Vena, N.; Ligon, A.H.; Liu, J.F.; Hirsch, M.S.; Etemadmoghadam, D.; Bowtell, D.D.; Drapkin, R. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer Res. 2014, 74, 1141–1152. [Google Scholar] [CrossRef]
- Kuhn, E.; Wang, T.L.; Doberstein, K.; Bahadirli-Talbott, A.; Ayhan, A.; Sehdev, A.S.; Drapkin, R.; Kurman, R.J.; Shih, I.M. CCNE1 amplification and centrosome number abnormality in serous tubal intraepithelial carcinoma: Further evidence supporting its role as a precursor of ovarian high-grade serous carcinoma. Mod. Pathol. 2016, 29, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.; Park, J.S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Sen, P.; Ganguly, P.; Ganguly, N. Modulation of DNA methylation by human papillomavirus E6 and E7 oncoproteins in cervical cancer. Oncol. Lett. 2018, 15, 11–22. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Papp, E.; Hallberg, D.; Konecny, G.E.; Bruhm, D.C.; Adleff, V.; Noe, M.; Kagiampakis, I.; Palsgrove, D.; Conklin, D.; Kinose, Y.; et al. Integrated Genomic, Epigenomic, and Expression Analyses of Ovarian Cancer Cell Lines. Cell Rep. 2018, 25, 2617–2633. [Google Scholar] [CrossRef]
- Shin, H.Y.; Yang, W.; Lee, E.J.; Han, G.H.; Cho, H.; Chay, D.B.; Kim, J.H. Establishment of five immortalized human ovarian surface epithelial cell lines via SV40 T antigen or HPV E6/E7 expression. PLoS ONE 2018, 13, e0205297. [Google Scholar] [CrossRef]
- Desmeules, P.; Trudel, D.; Turcotte, S.; Sirois, J.; Plante, M.; Gregoire, J.; Renaud, M.C.; Orain, M.; Tetu, B.; Bairati, I. Prognostic significance of TIMP-2, MMP-2, and MMP-9 on high-grade serous ovarian carcinoma using digital image analysis. Hum. Pathol. 2015, 46, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Emori, M.M.; Papp, E.; MacDuffie, E.; Konecny, G.E.; Velculescu, V.E.; Drapkin, R. Beyond genomics: Critical evaluation of cell line utility for ovarian cancer research. Gynecol. Oncol. 2015, 139, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, M.F. Incessant ovulation—A factor in ovarian neoplasia? Lancet 1971, 2, 163. [Google Scholar] [CrossRef]
- Casagrande, J.T.; Louie, E.W.; Pike, M.C.; Roy, S.; Ross, R.K.; Henderson, B.E. ”Incessant ovulation” and ovarian cancer. Lancet 1979, 2, 170–173. [Google Scholar] [CrossRef]
- Lin, S.F.; Gerry, E.; Shih, I.M. Tubal origin of ovarian cancer—The double-edged sword of haemoglobin. J. Pathol. 2017, 242, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chen, P.C.; Chen, C.H.; Hsu, C.F.; Huang, R.L.; Ding, D.C.; Chu, T.Y. Platelet-Derived Growth Factor in the Ovarian Follicle Attracts the Stromal Cells of the Fallopian Tube Fimbriae. PLoS ONE 2016, 11, e0158266. [Google Scholar] [CrossRef]
- King, S.M.; Hilliard, T.S.; Wu, L.Y.; Jaffe, R.C.; Fazleabas, A.T.; Burdette, J.E. The impact of ovulation on fallopian tube epithelial cells: Evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr. Relat. Cancer 2011, 18, 627–642. [Google Scholar] [CrossRef]
- Allen, B.J.; Brown, J.K.; Mountford, M.H.; Tamat, S.R.; Patwardhan, A.; Moore, D.E.; Ichihashi, M.; Mishima, Y.; Kahl, S.B. In vitro and in vivo studies of boron conjugated melanoma affined biochemicals. Strahlenther. Onkol. 1989, 165, 163–165. [Google Scholar]
- Roggiani, F.; Mezzanzanica, D.; Rea, K.; Tomassetti, A. Guidance of Signaling Activations by Cadherins and Integrins in Epithelial Ovarian Cancer Cells. Int. J. Mol. Sci 2016, 17, 1387. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Uruski, P.; Tykarski, A.; Ksiazek, K. The peritoneal “soil” for a cancerous “seed”: A comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell. Mol. Life Sci. 2018, 75, 509–525. [Google Scholar] [CrossRef]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; Karaminejadranjbar, M.; Hu, Z.; et al. An evolving story of the metastatic voyage of ovarian cancer cells: Cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 2019, 38, 2885–2898. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.; Ricardo, S.; Amaral, A.L.; Huang, Y.L.; Nunes, M.; Neves, J.P.; Mendes, N.; Lopez, M.N.; Bartosch, C.; Ferreira, V.; et al. Regulation of invasion and peritoneal dissemination of ovarian cancer by mesothelin manipulation. Oncogenesis 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Zhang, Z.; Li, H.; Cheng, W.; Liu, J. Cancer stem cells, epithelial-mesenchymal transition, and drug resistance in high-grade ovarian serous carcinoma. Hum. Pathol. 2013, 44, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.A.; Testa, J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005, 24, 7455–7464. [Google Scholar] [CrossRef] [PubMed]
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res. 2005, 94, 29–86. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, C.G.; Altuna, S.C.; Habeeb, B.S.; Trapani, D.; Khan, S.Z. The Potential of PI3K/AKT/mTOR Signaling as a Druggable Target for Endometrial and Ovarian Carcinomas. Curr. Drug Targets 2020, 21, 946–961. [Google Scholar] [CrossRef]
- Shariati, M.; Meric-Bernstam, F. Targeting AKT for cancer therapy. Expert Opin. Investig. Drugs 2019, 28, 977–988. [Google Scholar] [CrossRef]
- Guadamillas, M.C.; Cerezo, A.; Del Pozo, M.A. Overcoming anoikis—Pathways to anchorage-independent growth in cancer. J. Cell Sci. 2011, 124, 3189–3197. [Google Scholar] [CrossRef]
- Frisch, S.M.; Ruoslahti, E. Integrins and anoikis. Curr. Opin. Cell Biol. 1997, 9, 701–706. [Google Scholar] [CrossRef]
- Sahin, N.; Toylu, A.; Gulekli, B.; Dogan, E.; Kovali, M.; Atabey, N. The levels of hepatocyte growth factor in serum and follicular fluid and the expression of c-Met in granulosa cells in patients with polycystic ovary syndrome. Fertil. Steril. 2013, 99, 264–269.e3. [Google Scholar] [CrossRef]
- Seli, E.; Zeyneloglu, H.B.; Senturk, L.M.; Bahtiyar, O.M.; Olive, D.L.; Arici, A. Basic fibroblast growth factor: Peritoneal and follicular fluid levels and its effect on early embryonic development. Fertil. Steril. 1998, 69, 1145–1148. [Google Scholar] [CrossRef]
- Sun, F.; Feng, M.; Guan, W. Mechanisms of peritoneal dissemination in gastric cancer. Oncol. Lett. 2017, 14, 6991–6998. [Google Scholar] [CrossRef] [PubMed]
- Roque, R.; Costa Sousa, F.; Figueiredo-Dias, M. Epithelial-mesenchymal interconversions in ovarian cancer: The levels and functions of E-cadherin in intraabdominal dissemination. Oncol. Rev. 2020, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Yang-Hartwich, Y.; Gurrea-Soteras, M.; Sumi, N.; Joo, W.D.; Holmberg, J.C.; Craveiro, V.; Alvero, A.B.; Mor, G. Ovulation and extra-ovarian origin of ovarian cancer. Sci. Rep. 2014, 4, 6116. [Google Scholar] [CrossRef] [PubMed]
- Akison, L.K.; Alvino, E.R.; Dunning, K.R.; Robker, R.L.; Russell, D.L. Transient invasive migration in mouse cumulus oocyte complexes induced at ovulation by luteinizing hormone. Biol. Reprod. 2012, 86, 125. [Google Scholar] [CrossRef]
- Honda, T.; Fujiwara, H.; Yoshioka, S.; Yamada, S.; Nakayama, T.; Egawa, M.; Nishioka, Y.; Takahashi, A.; Fujii, S. Laminin and fibronectin concentrations of the follicular fluid correlate with granulosa cell luteinization and oocyte quality. Reprod. Med. Biol. 2004, 3, 43–49. [Google Scholar] [CrossRef][Green Version]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R., Jr.; Ruff, L.E.; Skubitz, A.P. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef]
- Chen, X.; Brewer, M.A.; Zou, C.; Campagnola, P.J. Adhesion and migration of ovarian cancer cells on crosslinked laminin fibers nanofabricated by multiphoton excited photochemistry. Integr. Biol. 2009, 1, 469–476. [Google Scholar] [CrossRef]
- Yousif, N.G. Fibronectin promotes migration and invasion of ovarian cancer cells through up-regulation of FAK-PI3K/Akt pathway. Cell Biol. Int. 2014, 38, 85–91. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]


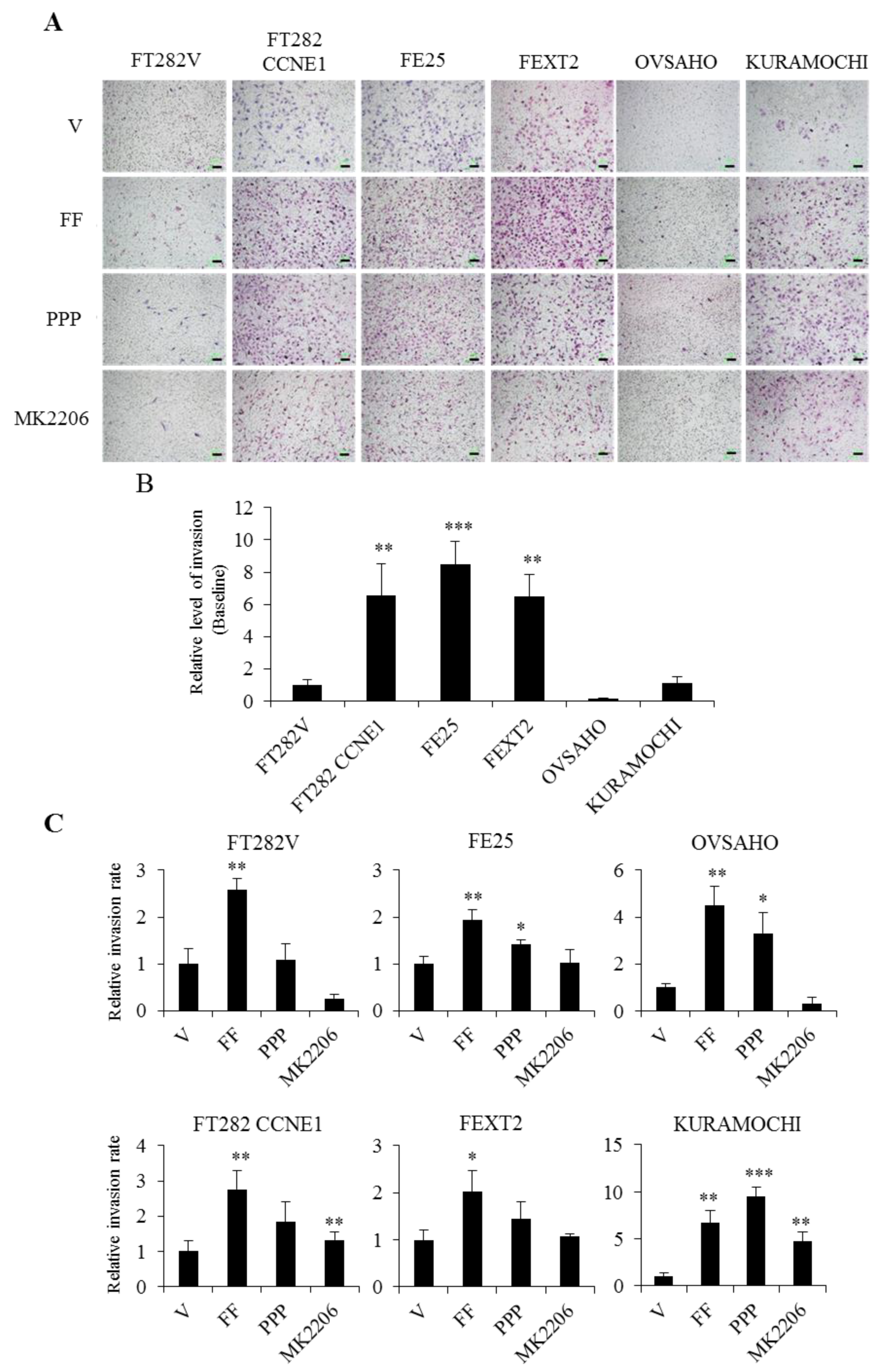
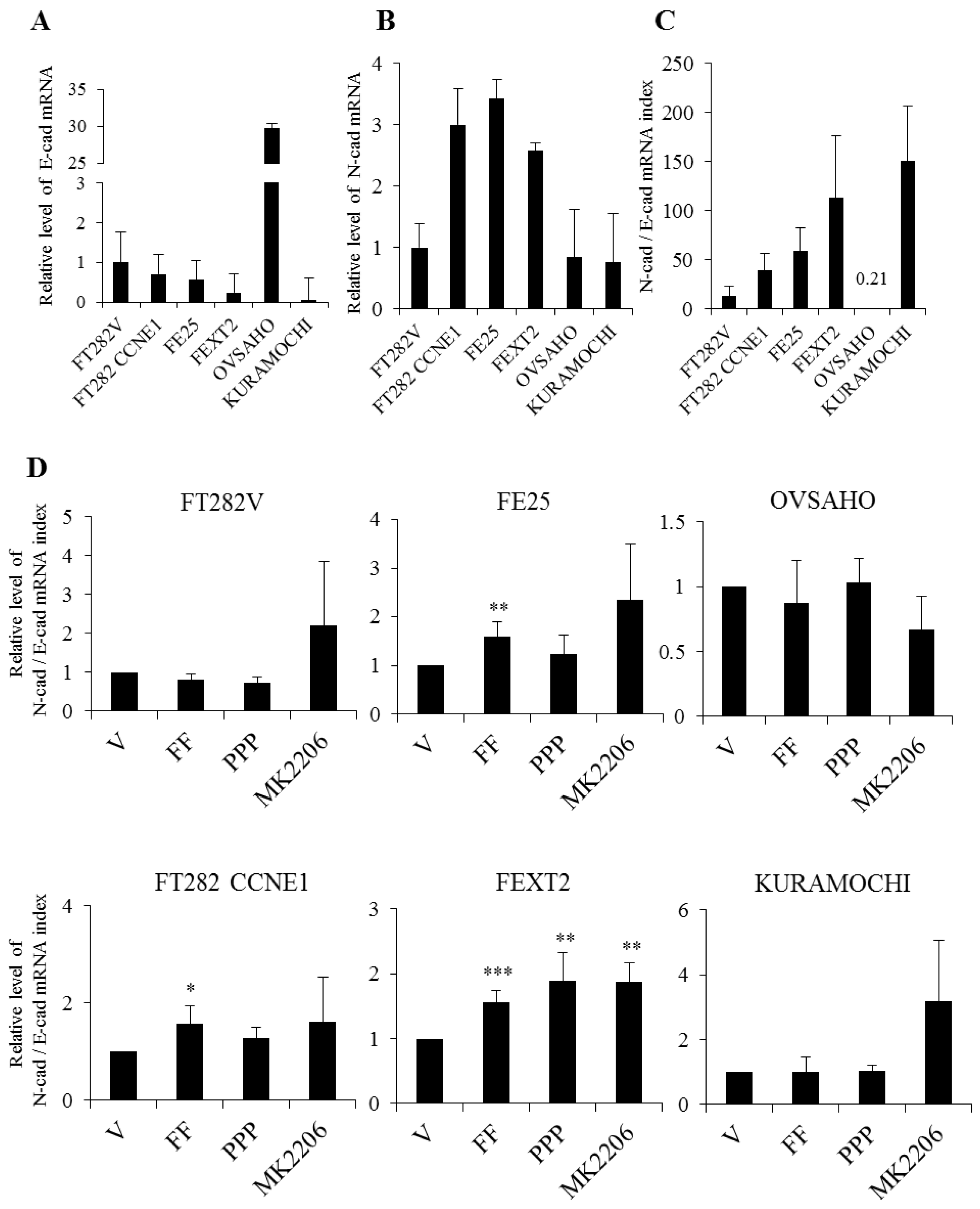
| Cell Line | Origin | Genetic Alterations | Mimic | AIG Colony (>50 μm)/AVG. | Tumorigenesis in NSG Mice |
|---|---|---|---|---|---|
| FT282V [30] | Normal fimbrial epithelium | hTERT + TP53 p.R175H | p53 signature | 0 | 0 |
| FT282-CCNE [30] | FT282 | hTERT + TP53 p.R157H + CCNE1 | STIC | 0.33 | 2/8 (25%) |
| FE25 (p110) | Normal fimbrial epithelium | hTERT + HPV E6/E7 (p53/Rb loss) | STIC | 2 | 0 |
| FEXT2 | ip xenotumor from FE25 + FF | hTERT + HPV E6/E7 (p53/Rb loss) | Perit. STIC | 1.2 | 5/5 (100%) |
| OVSAHO | Abdominal metastasis of HGSC | TP53 p.R342* (Nonsense), Rb-null, NF1 mut; CCNE, FGFR4 amp, BRCA2 homodel; MAP2K4 hetloss. [28,29] | HGSC | 1.6 | 4/4 (100%) |
| KURAMOCHI | Ascites of HGSC | TP53 p.D281Y, BRCA2 mutation; NF1 homodel; KRAS, MYC, FGFR1, CCNE, ARID1A amp. [28,29] | HGSC (BRCAness) | 0 | 5/5 (100%) |
| Cell Line | Mimic | Phenotype Summary * | Growth and Transformation | Peritoneal Metastasis | ||||
|---|---|---|---|---|---|---|---|---|
| AIG ** | Proliferation | EMT (N/E-CAD) | Peritoneal Attachment | Migration | Invasion | |||
| FT282V | p53 signature | E | 0 | 0.3 | 13 | 72 | 41 | 25 |
| FT282-CCNE1 | STIC | M | 0.3 | 1.9 | 40 | 251 | 159 | 162 |
| FE25 | STIC | EM | 2.0 | 1.7 | 59 | 74 | 40 | 210 |
| FEXT2 | Perit. STIC | M | 1.2 | 0.9 | 114 | 474 | 10 | 159 |
| OVSAHO | HGSC | E | 1.6 | 0.3 | 0.21 | 24 | 0.5 | 3 |
| KURAMOCHI | HGSC | M | 0 | 1.2 | 151 | 526 | 35 | 27 |
| Cell Line | AIG ** | Proliferation ** | Anoikis Resistance ** | Peritoneal Attachment ** | Migration ** | Invasion ** | EMT (N-Cad/E-Cad) ** | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +FF | +PPP | +MK | +FF | +PPP | +MK | +FF | +PPP | +MK | +FF | +PPP | +MK | +FF | +PPP | +MK | +FF | +PPP | +MK | +FF | +PPP | +MK | |
| FT282V | 0.5 (>25μm) | 0.17 (>25μm) | 0 | ▴▴ | ▴ | ▴ | ▴▴▴▴ | ▴▴▴▴ | ▴▴▴ | 6.9× | N.D. | 3.31× | 2.2× | 2.3× | 1.3× | 2.6× | 1.1× | 0.3× | ✧ | ✧ | ✧ |
| FT282-CCNE1 | 19.2× | 7.1× | 3.0× | ▴▴ | ▴▴ | ▴ | ▴▴▴▴ | ▴▴▴▴ | ▴▴▴ | 4.5× | N.D. | 1.23× | 2.4× | 1.5× | 1.4× | 2.8× | 1.8× | 1.3× | ▴▴▴ | ✧ | ✧ |
| FE25 | 4.7× | 1.3× | 0.7× | ▴ | ▴▴ | ▴ | ▴▴▴ | ▴▴▴ | ▴ | 6.6× | N.D. | 2.72× | 2.0× | 7.0× | 5.2× | 1.9× | 1.4× | 1.0× | ▴▴▴ | ✧ | ✧ |
| FEXT2 | 7.4× | 3.6× | 0.3× | ▴▴ | ▴▴ | ▴ | ▴▴▴▴ | ▴▴▴ | ▴▴▴ | 3.3× | N.D. | 1.03× | 24.0× | 17× | 13× | 2.0× | 1.3× | 1.1× | ▴▴▴ | ▴▴▴ | ▴▴▴ |
| OVSAHO | 10.0× | 3.4× | 1.7× | ▴▴▴ | ▴▴ | ▴ | ▴▴▴▴ | ▴▴▴▴ | ▴▴▴ | 1.6× | N.D. | 0.47× | 33.4× | 11.6× | 0.0× | 4.5× | 3.3× | 0.3× | ✧ | ✧ | ✧ |
| KURAMOCHI | 3.0 (>25μm) | 0 | 0.7 (>25μm) | ▴▴▴ | ▴▴▴ | ▴▴ | ▴▴▴ | ▴▴▴ | ▴▴▴ | 1.5× | N.D. | 1.00× | 9.9× | 9.4× | 4.0× | 6.7× | 9.5× | 4.8× | ✧ | ✧ | ✧ |
| Role of IGF/AKT in FF | IGF and AKT dependent | IGF independent AKT largely dependent | IGF independentAKT partially dependent | IGF independent AKT partially dependent | IGF and AKT partially dependent | IGF independent AKT largely dependent | Nil | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-F.; Chen, P.-C.; Seenan, V.; Ding, D.-C.; Chu, T.-Y. Ovulatory Follicular Fluid Facilitates the Full Transformation Process for the Development of High-Grade Serous Carcinoma. Cancers 2021, 13, 468. https://doi.org/10.3390/cancers13030468
Hsu C-F, Chen P-C, Seenan V, Ding D-C, Chu T-Y. Ovulatory Follicular Fluid Facilitates the Full Transformation Process for the Development of High-Grade Serous Carcinoma. Cancers. 2021; 13(3):468. https://doi.org/10.3390/cancers13030468
Chicago/Turabian StyleHsu, Che-Fang, Pao-Chu Chen, Vaishnavi Seenan, Dah-Ching Ding, and Tang-Yuan Chu. 2021. "Ovulatory Follicular Fluid Facilitates the Full Transformation Process for the Development of High-Grade Serous Carcinoma" Cancers 13, no. 3: 468. https://doi.org/10.3390/cancers13030468
APA StyleHsu, C.-F., Chen, P.-C., Seenan, V., Ding, D.-C., & Chu, T.-Y. (2021). Ovulatory Follicular Fluid Facilitates the Full Transformation Process for the Development of High-Grade Serous Carcinoma. Cancers, 13(3), 468. https://doi.org/10.3390/cancers13030468







