Remission-Stage Ovarian Cancer Cell Vaccine with Cowpea Mosaic Virus Adjuvant Prevents Tumor Growth
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
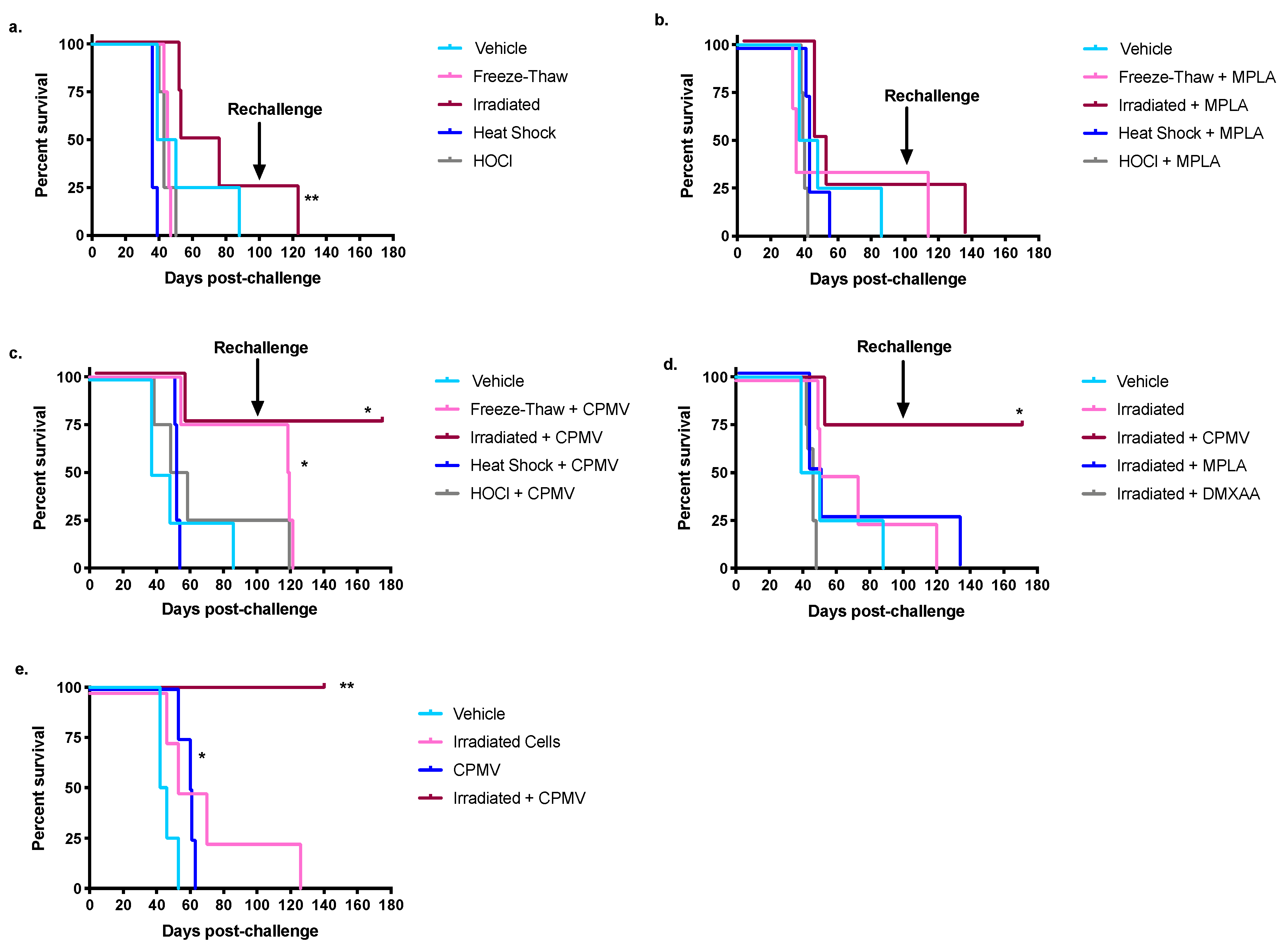
2.1. CPMV Is an Effective Adjuvant When Combined with Irradiated Syngeneic Ovarian Cancer Cells
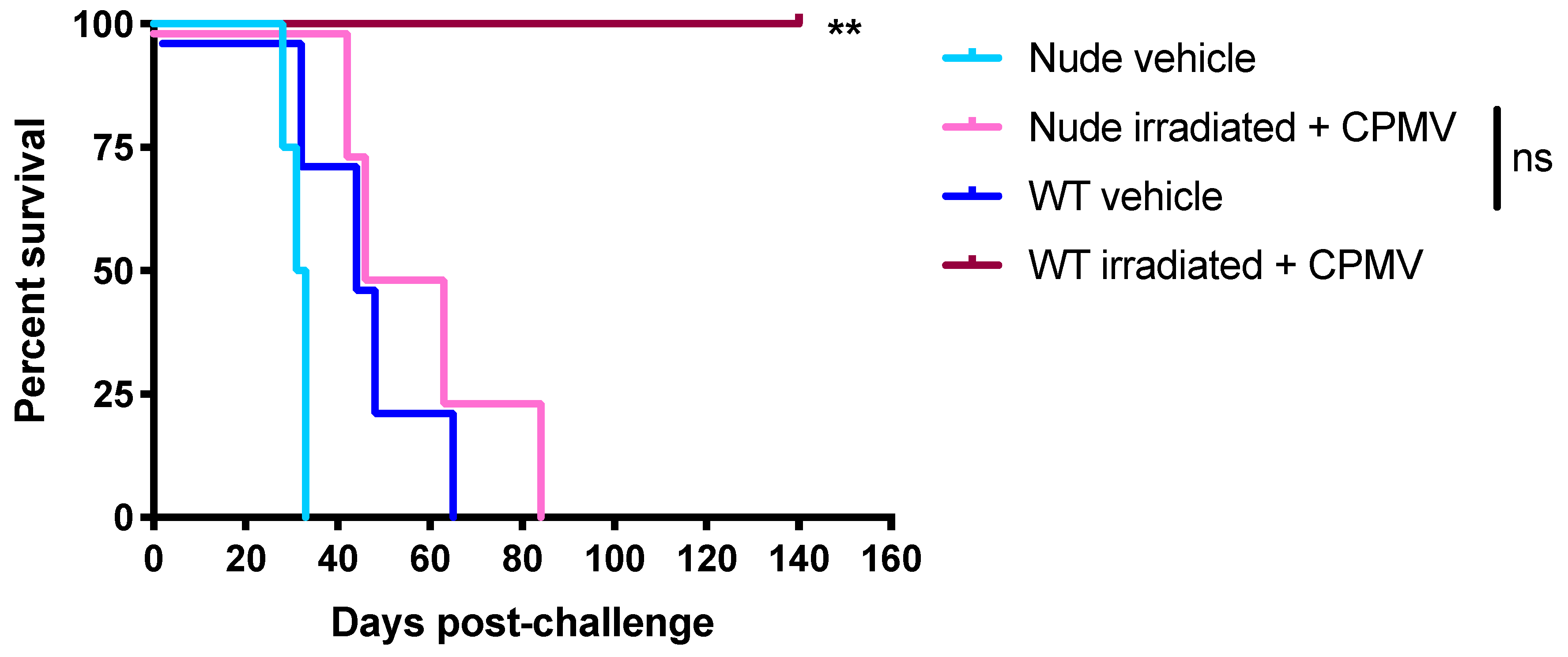
2.2. The Survival Benefit Provided by the Combination of CPMV and Irradiated Cells Is T Cell-Dependent
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tumor Models
4.3. Vaccine Antigen Preparation
4.4. Vaccine Adjuvant Preparation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, C.; Tonini, G.; Pisano, C.; Di Napoli, M.; Cecere, S.C.; Tambaro, R.; Facchini, G.; Pignata, S. Ovarian cancer standard of care: Are there real alternatives? Chin. J. Cancer 2015, 34, 17–27. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Zou, J.P.; Mu, J.; Wijesuriya, R.; Ono, S.; Walunas, T.; Bluestone, J.; Fujiwara, H.; Hamaoka, T. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: The effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997, 57, 4036–4041. [Google Scholar] [PubMed]
- Kudrin, A. Overview of cancer vaccines: Considerations for development. Hum. Vaccines Immunother. 2012, 8, 1335–1353. [Google Scholar] [CrossRef] [PubMed]
- Worzfeld, T.; Pogge von Strandmann, E.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Muller, R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front. Oncol. 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, T.; Wilson, J.; Burke, F.; Kulbe, H.; Li, N.F.; Pluddemann, A.; Charles, K.; Gordon, S.; Balkwill, F.R. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J. Immunol. 2006, 176, 5023–5032. [Google Scholar] [CrossRef]
- Landskron, J.; Helland, O.; Torgersen, K.M.; Aandahl, E.M.; Gjertsen, B.T.; Bjorge, L.; Tasken, K. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol. Immunother. 2015, 64, 337–347. [Google Scholar] [CrossRef]
- Chang, D.K.; Peterson, E.; Sun, J.; Goudie, C.; Drapkin, R.I.; Liu, J.F.; Matulonis, U.; Zhu, Q.; Marasco, W.A. Anti-CCR4 monoclonal antibody enhances antitumor immunity by modulating tumor-infiltrating Tregs in an ovarian cancer xenograft humanized mouse model. Oncoimmunology 2016, 5, e1090075. [Google Scholar] [CrossRef]
- Condamine, T.; Ramachandran, I.; Youn, J.I.; Gabrilovich, D.I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 2015, 66, 97–110. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, F.; Xu, Q.; Han, L.; Xu, J.; Gao, L.; Sun, X.; Li, Y.; Li, Y.; Qian, M.; et al. Revisiting ovarian cancer microenvironment: A friend or a foe? Protein Cell 2018, 9, 674–692. [Google Scholar] [CrossRef]
- Daniel, D.; Urs, S.; Guley, K.; Krueger, S.; Draper, D.; Wong, A.; Evens, H.; Higginbottom, C.; Saims, D.; Wise, S.; et al. Abstract 5691: Evaluation of immunomodulatory agents in classically immunologically ’cold’ cancers using syngeneic mouse models of breast and ovarian cancer. Cancer Res. 2018, 78, 5691. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Gray, H.J.; Benigno, B.; Berek, J.; Chang, J.; Mason, J.; Mileshkin, L.; Mitchell, P.; Moradi, M.; Recio, F.O.; Michener, C.M.; et al. Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial. J. Immunother. Cancer 2016, 4, 34. [Google Scholar] [CrossRef]
- Chiang, C.L.; Kandalaft, L.E.; Tanyi, J.; Hagemann, A.R.; Motz, G.T.; Svoronos, N.; Montone, K.; Mantia-Smaldone, G.M.; Smith, L.; Nisenbaum, H.L.; et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: From bench to bedside. Clin. Cancer Res. 2013, 19, 4801–4815. [Google Scholar] [CrossRef]
- Raza, A.; Merhi, M.; Inchakalody, V.P.; Krishnankutty, R.; Relecom, A.; Uddin, S.; Dermime, S. Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy. J. Transl. Med. 2020, 18, 140. [Google Scholar] [CrossRef]
- Sabbatini, P.; Tsuji, T.; Ferran, L.; Ritter, E.; Sedrak, C.; Tuballes, K.; Jungbluth, A.A.; Ritter, G.; Aghajanian, C.; Bell-McGuinn, K.; et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin. Cancer Res. 2012, 18, 6497–6508. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Tono, Y.; Miyahara, Y.; Muraoka, D.; Harada, N.; Kageyama, S.; Sasaki, T.; Hori, Y.; Soga, N.; Uchida, K.; et al. First-in-human phase I clinical trial of the NY-ESO-1 protein cancer vaccine with NOD2 and TLR9 stimulants in patients with NY-ESO-1-expressing refractory solid tumors. Cancer Immunol. Immunother. 2020, 69, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Gao, Q.; Wilson, K.L.; Heyerick, A.; Plebanski, M. A Nanoparticle Based Sp17 Peptide Vaccine Exposes New Immuno-Dominant and Species Cross-reactive B Cell Epitopes. Vaccines 2015, 3, 875–893. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Cao, W.L.; Li, F.Q.; Shi, L.N.; Jia, X. Anti-Sp17 monoclonal antibody with antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity activities against human ovarian cancer cells. Med. Oncol. 2012, 29, 2923–2931. [Google Scholar] [CrossRef]
- Brunette, L.L.; Mhawech-Fauceglia, P.Y.; Ji, L.; Skeate, J.G.; Brand, H.E.; Lawrenson, K.; Walia, S.; Chiriva-Internati, M.; Groshen, S.; Roman, L.D.; et al. Validity and prognostic significance of sperm protein 17 as a tumor biomarker for epithelial ovarian cancer: A retrospective study. BMC Cancer 2018, 18, 970. [Google Scholar] [CrossRef]
- Gulley, J.L.; Arlen, P.M.; Tsang, K.Y.; Yokokawa, J.; Palena, C.; Poole, D.J.; Remondo, C.; Cereda, V.; Jones, J.L.; Pazdur, M.P.; et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin. Cancer Res. 2008, 14, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, X.; Wang, J.; Hui, X.; Zhang, Y.; Cai, Y.; Ren, M.; Guo, M.; Zhao, F.; Dou, J. Ovarian Cancer Stem Cells with High ROR1 Expression Serve as a New Prophylactic Vaccine for Ovarian Cancer. J. Immunol. Res. 2019, 2019, 9394615. [Google Scholar] [CrossRef] [PubMed]
- Chiriva-Internati, M.; Yu, Y.; Mirandola, L.; Jenkins, M.R.; Chapman, C.; Cannon, M.; Cobos, E.; Kast, W.M. Cancer testis antigen vaccination affords long-term protection in a murine model of ovarian cancer. PLoS ONE 2010, 5, e10471. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, I.; Lee, K.L.; Chen, K.; Shukla, S.; Steinmetz, N.F. Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus: Cargo-loading and delivery. J. Control. Release 2013, 172, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Albakri, M.M.; Veliz, F.A.; Fiering, S.N.; Steinmetz, N.F.; Sieg, S.F. Endosomal toll-like receptors play a key role in activation of primary human monocytes by cowpea mosaic virus. Immunology 2020, 159, 183–192. [Google Scholar] [CrossRef]
- Lizotte, P.H.; Wen, A.M.; Sheen, M.R.; Fields, J.; Rojanasopondist, P.; Steinmetz, N.F.; Fiering, S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016, 11, 295–303. [Google Scholar] [CrossRef]
- Murray, A.A.; Wang, C.; Fiering, S.; Steinmetz, N.F. In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma. Mol. Pharm. 2018, 15, 3700–3716. [Google Scholar] [CrossRef]
- Kerstetter-Fogle, A.; Shukla, S.; Wang, C.; Beiss, V.; Harris, P.L.R.; Sloan, A.E.; Steinmetz, N.F. Plant Virus-Like Particle In Situ Vaccine for Intracranial Glioma Immunotherapy. Cancers 2019, 11, 515. [Google Scholar] [CrossRef]
- Cai, H.; Wang, C.; Shukla, S.; Steinmetz, N.F. Cowpea Mosaic Virus Immunotherapy Combined with Cyclophosphamide Reduces Breast Cancer Tumor Burden and Inhibits Lung Metastasis. Adv. Sci. (Weinh.) 2019, 6, 1802281. [Google Scholar] [CrossRef]
- Czapar, A.E.; Tiu, B.D.B.; Veliz, F.A.; Pokorski, J.K.; Steinmetz, N.F. Slow-Release Formulation of Cowpea Mosaic Virus for In Situ Vaccine Delivery to Treat Ovarian Cancer. Adv. Sci. (Weinh.) 2018, 5, 1700991. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Wang, C.; Beiss, V.; Steinmetz, N.F. Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer. ACS Nano 2020, 14, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fiering, S.N.; Steinmetz, N.F. Cowpea Mosaic Virus Promotes Anti-Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model. Adv. Ther. 2019, 2. [Google Scholar] [CrossRef]
- Patel, R.; Czapar, A.E.; Fiering, S.; Oleinick, N.L.; Steinmetz, N.F. Radiation Therapy Combined with Cowpea Mosaic Virus Nanoparticle in Situ Vaccination Initiates Immune-Mediated Tumor Regression. ACS Omega 2018, 3, 3702–3707. [Google Scholar] [CrossRef]
- Vandenberk, L.; Garg, A.D.; Verschuere, T.; Koks, C.; Belmans, J.; Beullens, M.; Agostinis, P.; De Vleeschouwer, S.; Van Gool, S.W. Irradiation of necrotic cancer cells, employed for pulsing dendritic cells (DCs), potentiates DC vaccine-induced antitumor immunity against high-grade glioma. Oncoimmunology 2016, 5, e1083669. [Google Scholar] [CrossRef]
- Prasad, S.J.; Farrand, K.J.; Matthews, S.A.; Chang, J.H.; McHugh, R.S.; Ronchese, F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J. Immunol. 2005, 174, 90–98. [Google Scholar] [CrossRef]
- Koido, S.; Hara, E.; Homma, S.; Mitsunaga, M.; Takahara, A.; Nagasaki, E.; Kawahara, H.; Watanabe, M.; Toyama, Y.; Yanagisawa, S.; et al. Synergistic induction of antigen-specific CTL by fusions of TLR-stimulated dendritic cells and heat-stressed tumor cells. J. Immunol. 2007, 179, 4874–4883. [Google Scholar] [CrossRef]
- Ying, M.; Zhen, Q.; Liu, S.; Gong, F.; Xie, Y. Treatment of established colon carcinoma-bearing mice by dendritic cells pulsed with lysates of heat-treated tumor cells. Sci. China C Life Sci. 2009, 52, 831–835. [Google Scholar] [CrossRef]
- Ito, A.; Fujioka, M.; Tanaka, K.; Kobayashi, T.; Honda, H. Screening of cytokines to enhance vaccine effects of heat shock protein 70-rich tumor cell lysate. J. Biosci. Bioeng. 2005, 100, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Ledermann, J.A.; Rad, A.N.; Katz, D.R.; Chain, B.M. Hypochlorous acid enhances immunogenicity and uptake of allogeneic ovarian tumor cells by dendritic cells to cross-prime tumor-specific T cells. Cancer Immunol. Immunother. 2006, 55, 1384–1395. [Google Scholar] [CrossRef]
- Chiang, C.L.; Ledermann, J.A.; Aitkens, E.; Benjamin, E.; Katz, D.R.; Chain, B.M. Oxidation of ovarian epithelial cancer cells by hypochlorous acid enhances immunogenicity and stimulates T cells that recognize autologous primary tumor. Clin. Cancer Res. 2008, 14, 4898–4907. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Hagemann, A.R.; Leskowitz, R.; Mick, R.; Garrabrant, T.; Czerniecki, B.J.; Kandalaft, L.E.; Powell, D.J., Jr.; Coukos, G. Day-4 myeloid dendritic cells pulsed with whole tumor lysate are highly immunogenic and elicit potent anti-tumor responses. PLoS ONE 2011, 6, e28732. [Google Scholar] [CrossRef]
- Cluff, C.W. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: Clinical results. Lipid A Cancer Ther. 2009, 667, 111–123. [Google Scholar]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchell, T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef]
- Weiss, J.M.; Guerin, M.V.; Regnier, F.; Renault, G.; Galy-Fauroux, I.; Vimeux, L.; Feuillet, V.; Peranzoni, E.; Thoreau, M.; Trautmann, A.; et al. The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression. Oncoimmunology 2017, 6, e1346765. [Google Scholar] [CrossRef]
- Shi, M.; Chen, X.; Ye, K.; Yao, Y.; Li, Y. Application potential of toll-like receptors in cancer immunotherapy: Systematic review. Medicine (Baltim.) 2016, 95, e3951. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Dinc, G.; Sharma, R.K.; Yolcu, E.S.; Zhao, H.; Shirwan, H. SA-4-1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res. 2014, 74, 6441–6451. [Google Scholar] [CrossRef]
- Conejo-Garcia, J.R.; Benencia, F.; Courreges, M.C.; Kang, E.; Mohamed-Hadley, A.; Buckanovich, R.J.; Holtz, D.O.; Jenkins, A.; Na, H.; Zhang, L.; et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat. Med. 2004, 10, 950–958. [Google Scholar] [CrossRef]
- Chu, C.S.; Boyer, J.; Schullery, D.S.; Gimotty, P.A.; Gamerman, V.; Bender, J.; Levine, B.L.; Coukos, G.; Rubin, S.C.; Morgan, M.A.; et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol. Immunother. 2012, 61, 629–641. [Google Scholar] [CrossRef]
- Wang, C.; Beiss, V.; Steinmetz, N.F. Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Shukla, S.; Wang, C.; Beiss, V.; Cai, H.; Washington, T., 2nd; Murray, A.A.; Gong, X.; Zhao, Z.; Masarapu, H.; Zlotnick, A.; et al. The unique potency of Cowpea mosaic virus (CPMV) in situ cancer vaccine. Biomater. Sci. 2020, 8, 5489–5503. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012, 18, 3281–3292. [Google Scholar] [CrossRef]
- Patel, B.K.; Wang, C.; Lorens, B.; Levine, A.D.; Steinmetz, N.F.; Shukla, S. Cowpea Mosaic Virus (CPMV)-Based Cancer Testis Antigen NY-ESO-1 Vaccine Elicits an Antigen-Specific Cytotoxic T Cell Response. ACS Appl. Bio Mater. 2020, 3, 4179–4187. [Google Scholar] [CrossRef]
- Adams, S.F.; Grimm, A.J.; Chiang, C.L.; Mookerjee, A.; Flies, D.; Jean, S.; McCann, G.A.; Michaux, J.; Pak, H.; Huber, F.; et al. Rapid tumor vaccine using Toll-like receptor-activated ovarian cancer ascites monocytes. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Benencia, F.; Coukos, G. Whole tumor antigen vaccines. Semin. Immunol. 2010, 22, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Coukos, G.; Kandalaft, L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines 2015, 3, 344–372. [Google Scholar] [CrossRef]
- Ophir, E.; Bobisse, S.; Coukos, G.; Harari, A.; Kandalaft, L.E. Personalized approaches to active immunotherapy in cancer. Biochim. Biophys. Acta 2016, 1865, 72–82. [Google Scholar] [CrossRef]
- Chiang, C.L.; Kandalaft, L.E.; Coukos, G. Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines. Int. Rev. Immunol. 2011, 30, 150–182. [Google Scholar] [CrossRef]
- Dranoff, G.; Jaffee, E.; Lazenby, A.; Golumbek, P.; Levitsky, H.; Brose, K.; Jackson, V.; Hamada, H.; Pardoll, D.; Mulligan, R.C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA 1993, 90, 3539–3543. [Google Scholar] [CrossRef]
- Vandenberk, L.; Garg, A.D.; Agostinis, P.; Verschuere, T.; Koks, C.; De Vleeschouwer, S.; Van Gool, S. Irradiation of necrotic tumor cells used to pulse dendritic cells (DCs) potentiates DC vaccine-induced anti-tumor immunity in a mouse model of high-grade glioma. J. Immunother. Cancer 2014, 2. [Google Scholar] [CrossRef][Green Version]
- Morel, A.; Fernandez, N.; De La Coste, A.; Haddada, H.; Viguier, M.; Polla, B.S.; Antoine, B.; Kahn, A. Gamma-ray irradiation induces B7.1 costimulatory molecule neoexpression in various murine tumor cells. Cancer Immunol. Immunother. 1998, 46, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Herr, W.; Ranieri, E.; Olson, W.; Zarour, H.; Gesualdo, L.; Storkus, W.J. Mature dendritic cells pulsed with freeze-thaw cell lysates define an effective in vitro vaccine designed to elicit EBV-specific CD4(+) and CD8(+) T lymphocyte responses. Blood 2000, 96, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Tirapu, I.; Lewis, A.; Kreutz, M.; McLinden, H.; Diebold, S.S. Freeze-and-thaw-disrupted tumour cells impair the responsiveness of DC to TLR stimulation. Eur. J. Immunol. 2008, 38, 2740–2750. [Google Scholar] [CrossRef]
- Kandalaft, L.E.; Powell, D.J., Jr.; Chiang, C.L.; Tanyi, J.; Kim, S.; Bosch, M.; Montone, K.; Mick, R.; Levine, B.L.; Torigian, D.A.; et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology 2013, 2, e22664. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Webb, J.R.; Milne, K.; Watson, P.; Deleeuw, R.J.; Nelson, B.H. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 2014, 20, 434–444. [Google Scholar] [CrossRef]
- Consortium, O.T.T.A.; Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Toloczko, A.; Hein, A.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef]
- Stanske, M.; Wienert, S.; Castillo-Tong, D.C.; Kreuzinger, C.; Vergote, I.; Lambrechts, S.; Gabra, H.; Gourley, C.; Ganapathi, R.N.; Kolaschinski, I.; et al. Dynamics of the Intratumoral Immune Response during Progression of High-Grade Serous Ovarian Cancer. Neoplasia 2018, 20, 280–288. [Google Scholar] [CrossRef]
- Wick, D.A.; Webb, J.R.; Nielsen, J.S.; Martin, S.D.; Kroeger, D.R.; Milne, K.; Castellarin, M.; Twumasi-Boateng, K.; Watson, P.H.; Holt, R.A.; et al. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin. Cancer Res. 2014, 20, 1125–1134. [Google Scholar] [CrossRef]
- Deniger, D.C.; Pasetto, A.; Prickett, T.D.; Gartner, J.J.; Bharathan, M.; Tran, E.; Robbins, P.F.; Rosenberg, S.A. Mutated Tumor Neoantigens Are Recognized by Tumor Infiltrating Lymphocytes from Metastatic Ovarian Cancer. Cancer Immunother. Cancer Vaccines II 2016, 24, S155. [Google Scholar] [CrossRef]
- Oh, J.; Barve, M.; Matthews, C.M.; Koon, E.C.; Heffernan, T.P.; Fine, B.; Grosen, E.; Bergman, M.K.; Fleming, E.L.; DeMars, L.R.; et al. Phase II study of Vigil(R) DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol. Oncol. 2016, 143, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Peterson, N.; Khalaj, K.; Vitkin, N.; Robinson, A.; Francis, J.A.; Koti, M. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br. J. Cancer 2018, 119, 440–449. [Google Scholar] [CrossRef]
- Duraiswamy, J.; Freeman, G.J.; Coukos, G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013, 73, 6900–6912. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Kashiwagi, S.; Reeves, P.; Nezivar, J.; Yang, Y.; Arrifin, N.H.; Nguyen, M.; Jean-Mary, G.; Tong, X.; Uppal, P.; et al. A novel mycobacterial Hsp70-containing fusion protein targeting mesothelin augments antitumor immunity and prolongs survival in murine models of ovarian cancer and mesothelioma. J. Hematol. Oncol. 2014, 7, 15. [Google Scholar] [CrossRef]
- Huang, R.Y.; Francois, A.; McGray, A.R.; Miliotto, A.; Odunsi, K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2017, 6, e1249561. [Google Scholar] [CrossRef]
- Barber, A.; Zhang, T.; Sentman, C.L. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J. Immunol. 2008, 180, 72–78. [Google Scholar] [CrossRef]
- Spear, P.; Barber, A.; Sentman, C.L. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology 2013, 2, e23564. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Watson, B.; Boardman, C.; Laufer, D.; Piercy, S.; Tustin, N.; Olaleye, D.; Cnaan, A.; Starr, S.E. Humoral and cell-mediated immune responses in healthy children after one or two doses of varicella vaccine. Clin. Infect. Dis. 1995, 20, 316–319. [Google Scholar] [CrossRef]
- Jing, W.; McAllister, D.; Vonderhaar, E.P.; Palen, K.; Riese, M.J.; Gershan, J.; Johnson, B.D.; Dwinell, M.B. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J. Immunother. Cancer 2019, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.A.; Sheen, M.R.; Veliz, F.A.; Fiering, S.N.; Steinmetz, N.F. In Situ Vaccination of Tumors Using Plant Viral Nanoparticles. Methods Mol. Biol. 2019, 2000, 111–124. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stump, C.T.; Ho, G.; Mao, C.; Veliz, F.A.; Beiss, V.; Fields, J.; Steinmetz, N.F.; Fiering, S. Remission-Stage Ovarian Cancer Cell Vaccine with Cowpea Mosaic Virus Adjuvant Prevents Tumor Growth. Cancers 2021, 13, 627. https://doi.org/10.3390/cancers13040627
Stump CT, Ho G, Mao C, Veliz FA, Beiss V, Fields J, Steinmetz NF, Fiering S. Remission-Stage Ovarian Cancer Cell Vaccine with Cowpea Mosaic Virus Adjuvant Prevents Tumor Growth. Cancers. 2021; 13(4):627. https://doi.org/10.3390/cancers13040627
Chicago/Turabian StyleStump, Courtney T., Gregory Ho, Chenkai Mao, Frank A. Veliz, Veronique Beiss, Jennifer Fields, Nicole F. Steinmetz, and Steven Fiering. 2021. "Remission-Stage Ovarian Cancer Cell Vaccine with Cowpea Mosaic Virus Adjuvant Prevents Tumor Growth" Cancers 13, no. 4: 627. https://doi.org/10.3390/cancers13040627
APA StyleStump, C. T., Ho, G., Mao, C., Veliz, F. A., Beiss, V., Fields, J., Steinmetz, N. F., & Fiering, S. (2021). Remission-Stage Ovarian Cancer Cell Vaccine with Cowpea Mosaic Virus Adjuvant Prevents Tumor Growth. Cancers, 13(4), 627. https://doi.org/10.3390/cancers13040627






