Targeted Neoadjuvant Therapies in HR+/HER2−Breast Cancers: Challenges for Improving pCR
Abstract
Simple Summary
Abstract
1. Current Status of Systemic Neoadjuvant Therapy
2. CDK4/6 Inhibitors and Endocrine Therapy
3. PI3K Pathway Inhibitors and Endocrine Therapy
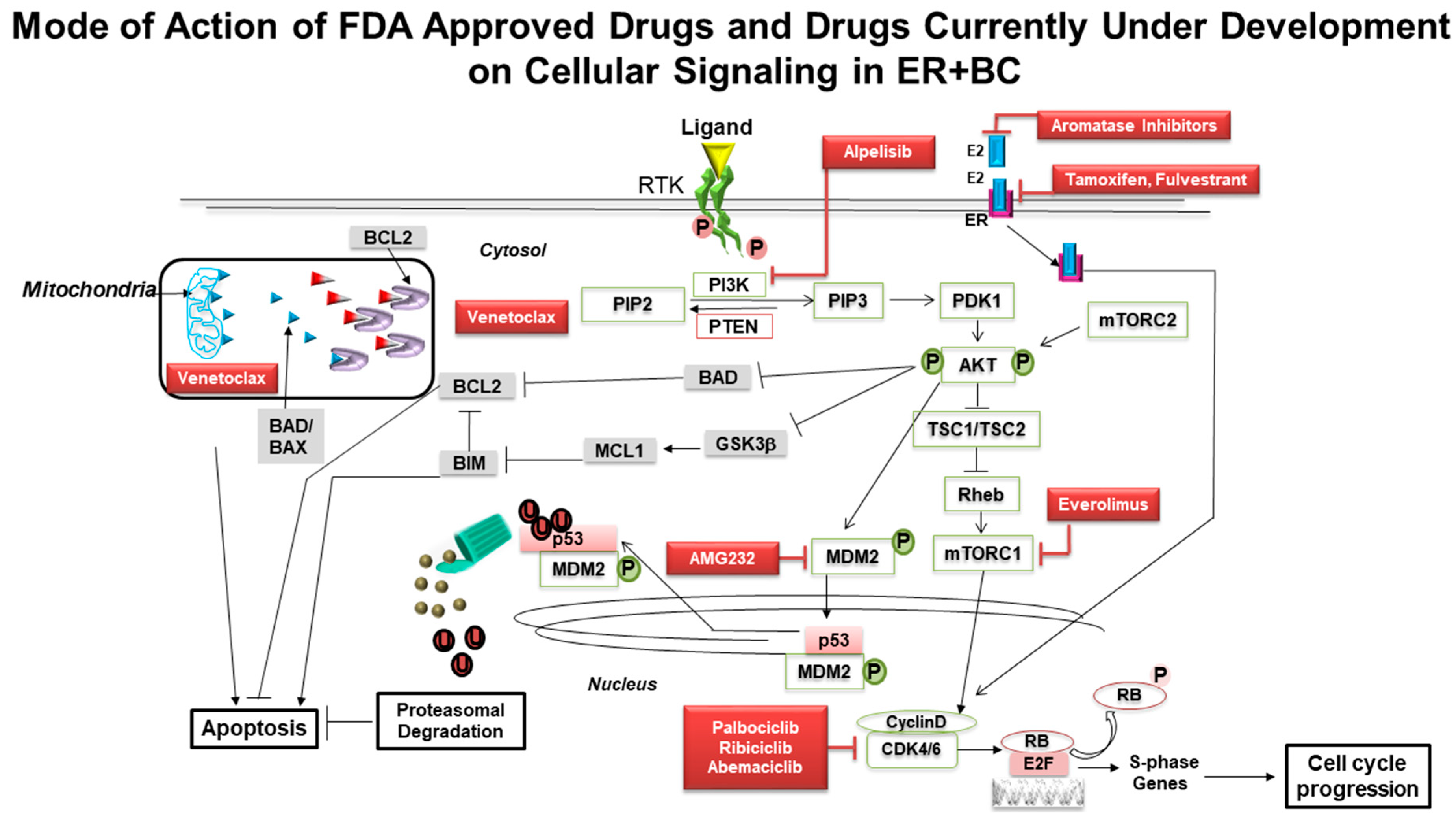
4. Traveling Forward
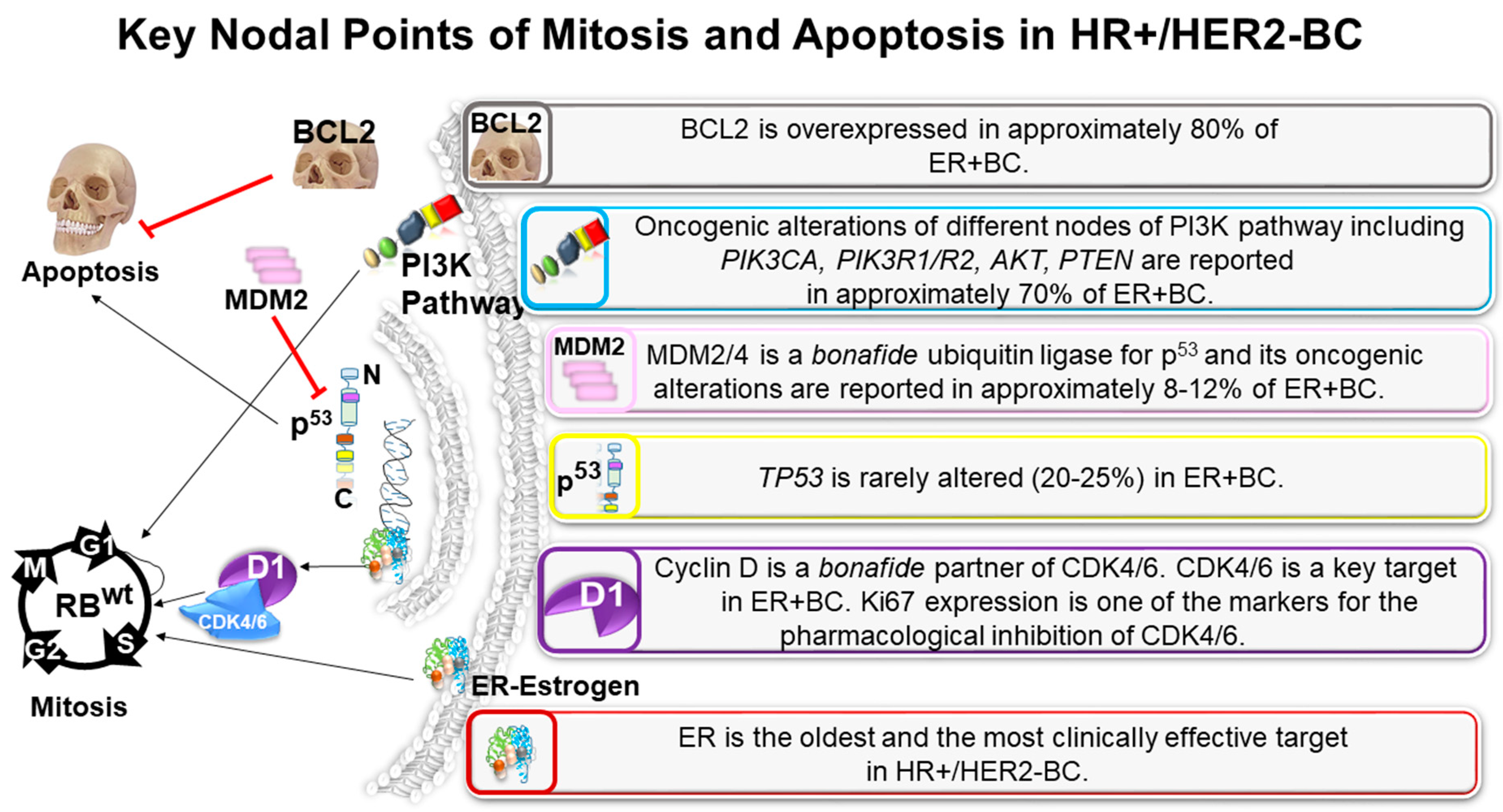
5. A Perfect Apoptosis Plot in HR+/HER2−BC
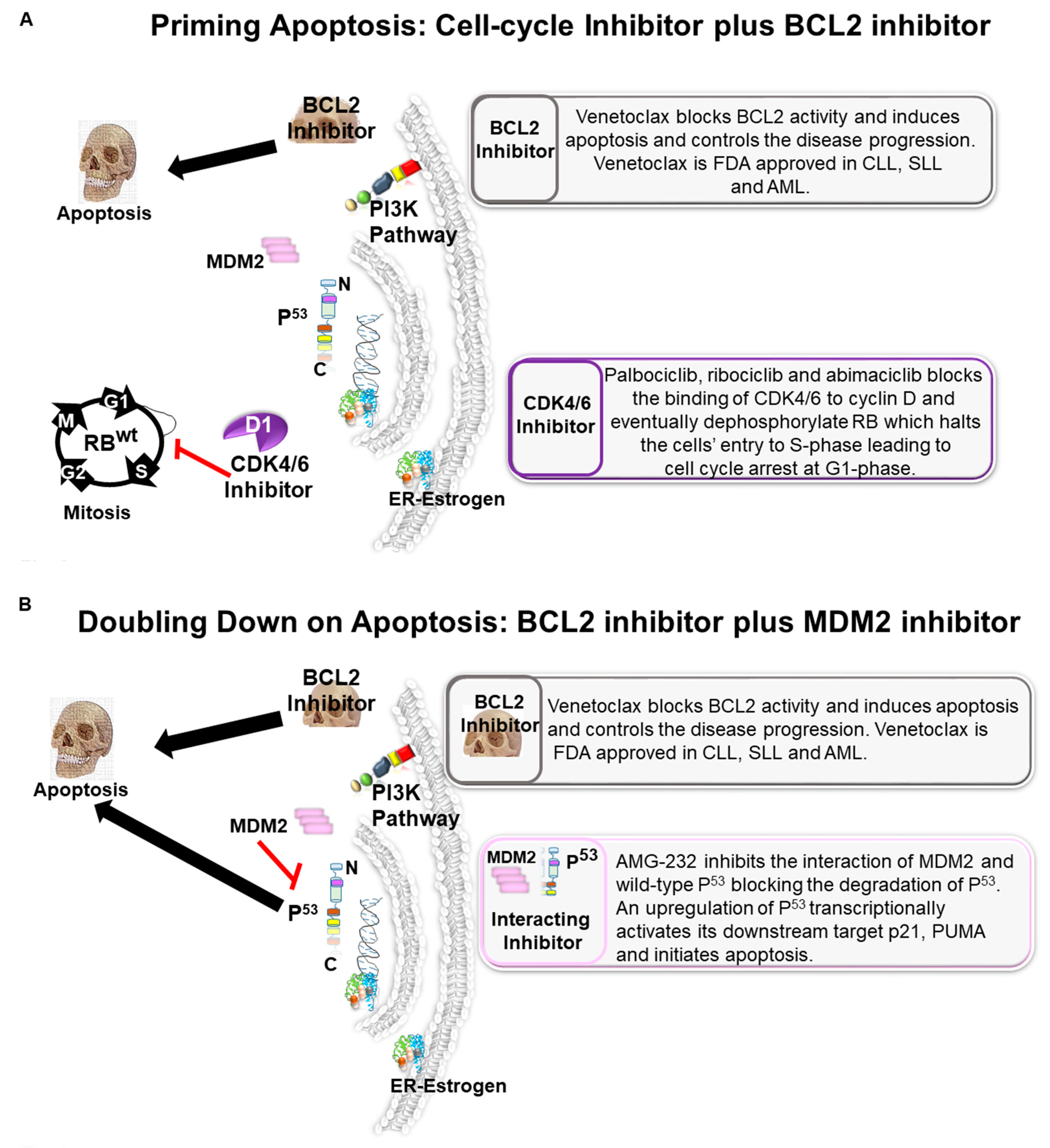
6. Priming Apoptosis: Cell-Cycle Inhibitor Plus BCL2 Inhibitor
7. Doubling Down on Apoptosis: BCL2 Inhibitor Plus MDM2 Inhibitor
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perloff, M.; Lesnick, G.J. Chemotherapy before and after mastectomy in stage III breast cancer. Arch. Surg. 1982, 117, 879–881. [Google Scholar] [CrossRef]
- Schick, P.; Goodstein, J.; Moor, J.; Butler, J.; Senter, K.L. Preoperative chemotherapy followed by mastectomy for locally advanced breast cancer. J. Surg. Oncol. 1983, 22, 278–282. [Google Scholar] [CrossRef]
- Madigan, L.I.; Dinh, P.; Graham, J.D. Neoadjuvant endocrine therapy in locally advanced estrogen or progesterone receptor-positive breast cancer: Determining the optimal endocrine agent and treatment duration in postmenopausal women-a literature review and proposed guidelines. Breast Cancer Res. Bcr 2020, 22, 77. [Google Scholar] [CrossRef]
- Spring, L.M.; Gupta, A.; Reynolds, K.L.; Gadd, M.A.; Ellisen, L.W.; Isakoff, S.J.; Moy, B.; Bardia, A. Neoadjuvant Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1477–1486. [Google Scholar] [CrossRef]
- Alba, E.; Calvo, L.; Albanell, J.; De la Haba, J.R.; Arcusa Lanza, A.; Chacon, J.I.; Sanchez-Rovira, P.; Plazaola, A.; Lopez Garcia-Asenjo, J.A.; Bermejo, B.; et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: Results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 3069–3074. [Google Scholar] [CrossRef]
- Hayashi, N.; Yagata, H.; Tsugawa, K.; Kajiura, Y.; Yoshida, A.; Takei, J.; Yamauchi, H.; Nakamura, S. Response and Prognosis of Docetaxel and Cyclophosphamide as Neoadjuvant Chemotherapy in ER(+) HER2(−) Breast Cancer: A Prospective Phase II Study. Clin. Breast Cancer 2020, 20, 462–468. [Google Scholar] [CrossRef]
- Walker, A.J.; Wedam, S.; Amiri-Kordestani, L.; Bloomquist, E.; Tang, S.; Sridhara, R.; Chen, W.; Palmby, T.R.; Fourie Zirkelbach, J.; Fu, W.; et al. FDA Approval of Palbociclib in Combination with Fulvestrant for the Treatment of Hormone Receptor-Positive, HER2−Negative Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4968–4972. [Google Scholar] [CrossRef]
- Shah, A.; Bloomquist, E.; Tang, S.; Fu, W.; Bi, Y.; Liu, Q.; Yu, J.; Zhao, P.; Palmby, T.R.; Goldberg, K.B.; et al. FDA Approval: Ribociclib for the Treatment of Postmenopausal Women with Hormone Receptor-Positive, HER2−Negative Advanced or Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2999–3004. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Pedersini, R.; Cabiddu, M.; Borgonovo, K.; Parati, M.C.; Ghilardi, M.; Amoroso, V.; Berruti, A.; Barni, S. Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: An adjusted indirect analysis of randomized controlled trials. Breast Cancer Res. Treat. 2019, 174, 597–604. [Google Scholar] [CrossRef]
- Huang, L.; Xu, A.M. Short-term outcomes of neoadjuvant hormonal therapy versus neoadjuvant chemotherapy in breast cancer: Systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anticancer. Ther. 2017, 17, 327–334. [Google Scholar] [CrossRef]
- Yao, L.T.; Wang, M.Z.; Wang, M.S.; Yu, X.T.; Guo, J.Y.; Sun, T.; Li, X.Y.; Xu, Y.Y. Neoadjuvant endocrine therapy: A potential strategy for ER-positive breast cancer. World J. Clin. Cases 2019, 7, 1937–1953. [Google Scholar] [CrossRef]
- Bahrami, N.; Chang, G.; Kanaya, N.; Sauer, T.; Park, D.; Loeng, M.; Gravdehaug, B.; Chen, S.; Geisler, J. Changes in serum estrogenic activity during neoadjuvant therapy with letrozole and exemestane. J. Steroid Biochem. Mol. Biol. 2020, 200, 105641. [Google Scholar] [CrossRef]
- Eiermann, W.; Paepke, S.; Appfelstaedt, J.; Llombart-Cussac, A.; Eremin, J.; Vinholes, J.; Mauriac, L.; Ellis, M.; Lassus, M.; Chaudri-Ross, H.A.; et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001, 12, 1527–1532. [Google Scholar] [CrossRef]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Lin, L.; Snider, J.; Prat, A.; Parker, J.S.; Luo, J.; DeSchryver, K.; Allred, D.C.; et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2342–2349. [Google Scholar]
- Kuter, I.; Gee, J.M.; Hegg, R.; Singer, C.F.; Badwe, R.A.; Lowe, E.S.; Emeribe, U.A.; Anderson, E.; Sapunar, F.; Finlay, P.; et al. Dose-dependent change in biomarkers during neoadjuvant endocrine therapy with fulvestrant: Results from NEWEST, a randomized Phase II study. Breast Cancer Res. Treat. 2012, 133, 237–246. [Google Scholar] [CrossRef]
- Lerebours, F.; Rivera, S.; Mouret-Reynier, M.A.; Alran, S.; Venat-Bouvet, L.; Kerbrat, P.; Salmon, R.; Becette, V.; Bourgier, C.; Cherel, P.; et al. Randomized phase 2 neoadjuvant trial evaluating anastrozole and fulvestrant efficacy for postmenopausal, estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer patients: Results of the UNICANCER CARMINA 02 French trial (UCBG 0609). Cancer 2016, 122, 3032–3040. [Google Scholar]
- Carpenter, R.; Doughty, J.C.; Cordiner, C.; Moss, N.; Gandhi, A.; Wilson, C.; Andrews, C.; Ellis, G.; Gui, G.; Skene, A.I. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res. Treat. 2014, 144, 569–576. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Guerrero, A.; Galan, A.; Caranana, V.; Buch, E.; Rodriguez-Lescure, A.; Ruiz, A.; Fuster Diana, C.; Guillem Porta, V. Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2012, 14, 125–131. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J.; Panel members. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Harbeck, N.; Thomssen, C.; Gnant, M. St. Gallen 2013: Brief preliminary summary of the consensus discussion. Breast Care 2013, 8, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hojo, T.; Kinoshita, T.; Imoto, S.; Shimizu, C.; Isaka, H.; Ito, H.; Imi, K.; Wada, N.; Ando, M.; Fujiwara, Y. Use of the neo-adjuvant exemestane in post-menopausal estrogen receptor-positive breast cancer: A randomized phase II trial (PTEX46) to investigate the optimal duration of preoperative endocrine therapy. Breast 2013, 22, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Pariser, A.C.; Sedghi, T.; Soulos, P.R.; Killelea, B.; Gross, C.P.; Mougalian, S.S. Utilization, duration, and outcomes of neoadjuvant endocrine therapy in the United States. Breast Cancer Res. Treat. 2019, 178, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mustacchi, G.; Ceccherini, R.; Milani, S.; Pluchinotta, A.; De Matteis, A.; Maiorino, L.; Farris, A.; Scanni, A.; Sasso, F.; Italian Cooperative Group GRETA. Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: Long-term results of the phase III randomized controlled multicenter GRETA trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2003, 14, 414–420. [Google Scholar] [CrossRef]
- Li, J.; Huo, X.; Zhao, F.; Ren, D.; Ahmad, R.; Yuan, X.; Du, F.; Zhao, J. Association of Cyclin-Dependent Kinases 4 and 6 Inhibitors With Survival in Patients With Hormone Receptor-Positive Metastatic Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2020312. [Google Scholar] [CrossRef]
- Ma, C.X.; Gao, F.; Luo, J.; Northfelt, D.W.; Goetz, M.; Forero, A.; Hoog, J.; Naughton, M.; Ademuyiwa, F.; Suresh, R.; et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4055–4065. [Google Scholar] [CrossRef]
- Curigliano, G.; Gomez Pardo, P.; Meric-Bernstam, F.; Conte, P.; Lolkema, M.P.; Beck, J.T.; Bardia, A.; Martínez García, M.; Penault-Llorca, F.; Dhuria, S.; et al. Ribociclib plus letrozole in early breast cancer: A presurgical, window-of-opportunity study. Breast 2016, 28, 191–198. [Google Scholar] [CrossRef]
- Johnston, S.; Puhalla, S.; Wheatley, D.; Ring, A.; Barry, P.; Holcombe, C.; Boileau, J.F.; Provencher, L.; Robidoux, A.; Rimawi, M.; et al. Randomized Phase II Study Evaluating Palbociclib in Addition to Letrozole as Neoadjuvant Therapy in Estrogen Receptor-Positive Early Breast Cancer: PALLET Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 178–189. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Press, M.F.; Chan, D.; Fernandez-Abad, M.; Petru, E.; Rostorfer, R.; Guarneri, V.; Huang, C.S.; Barriga, S.; et al. Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR(+)/HER2(−) Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 566–580. [Google Scholar] [CrossRef]
- Cottu, P.; D’Hondt, V.; Dureau, S.; Lerebours, F.; Desmoulins, I.; Heudel, P.E.; Duhoux, F.P.; Levy, C.; Mouret-Reynier, M.A.; Dalenc, F.; et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 2334–2340. [Google Scholar] [CrossRef]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Munoz, M.; Pare, L.; González Farré, B.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2−negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 33–43. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S., 3rd; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Baselga, J.; Semiglazov, V.; van Dam, P.; Manikhas, A.; Bellet, M.; Mayordomo, J.; Campone, M.; Kubista, E.; Greil, R.; Bianchi, G.; et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 2630–2637. [Google Scholar] [CrossRef]
- Ellis, M.J.; Perou, C.M. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov. 2013, 3, 27–34. [Google Scholar] [CrossRef]
- Andre, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Prat, A.; Egle, D.; Blau, S.; Fidalgo, J.A.P.; Gnant, M.; Fasching, P.A.; Colleoni, M.; Wolff, A.C.; Winer, E.P.; et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2975–2987. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Hlauschek, D.; Oliveira, M.; Zardavas, D.; Jallitsch-Halper, A.; de la Pena, L.; Nuciforo, P.; Ballestrero, A.; Dubsky, P.; Lombard, J.M.; et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2−negative, early-stage breast cancer (LORELEI): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019, 20, 1226–1238. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.; DeMichele, A.; Clark, A.; Zelnak, A.; Yardley, D.; Karuturi, M.S.; Sanft, T.B.; Blau, S.; Hart, L.L.; et al. Triplet therapy (continuous ribociclib, everolimus, exemestane) in HR+/HER2− advanced breast cancer postprogression on a CDK4/6 inhibitor (TRINITI-1): Efficacy, safety, and biomarker results. J. Clin. Onol. 2019, 37 (Suppl. 15), 1016. [Google Scholar] [CrossRef]
- Del Re, M.; Crucitta, S.; Lorenzini, G.; De Angelis, C.; Diodati, L.; Cavallero, D.; Bargagna, I.; Cinacchi, P.; Fratini, B.; Salvadori, B.; et al. PI3K mutations detected in liquid biopsy are associated to reduced sensitivity to CDK4/6 inhibitors in metastatic breast cancer patients. Pharmacol. Res. 2021, 163, 105241. [Google Scholar] [CrossRef]
- Rieder, C.L.; Maiato, H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 2004, 7, 637–651. [Google Scholar] [CrossRef]
- Minn, A.J.; Boise, L.H.; Thompson, C.B. Expression of Bcl-xL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996, 10, 2621–2631. [Google Scholar] [CrossRef]
- Lok, S.W.; Whittle, J.R.; Vaillant, F.; Teh, C.E.; Lo, L.L.; Policheni, A.N.; Bergin, A.R.T.; Desai, J.; Ftouni, S.; Gandolfo, L.C.; et al. A Phase Ib Dose-Escalation and Expansion Study of the BCL2 Inhibitor Venetoclax Combined with Tamoxifen in ER and BCL2-Positive Metastatic Breast Cancer. Cancer Discov. 2019, 9, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Lucantoni, F.; Dussmann, H.; Llorente-Folch, I.; Prehn, J.H.M. BCL2 and BCL(X)L selective inhibitors decrease mitochondrial ATP production in breast cancer cells and are synthetically lethal when combined with 2-deoxy-D-glucose. Oncotarget 2018, 9, 26046–26063. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Sasso, A.; Abbondanza, C.; Palumbo, G. 17beta-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol. Cell. Biol. 2000, 20, 2890–2901. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Lok, S.W.; Visvader, J.E.; Lindeman, G.J. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene 2016, 35, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Whittle, J.R.; Vaillant, F.; Surgenor, E.; Policheni, A.N.; Giner, G.; Capaldo, B.D.; Chen, H.R.; Liu, H.K.; Dekkers, J.F.; Sachs, N.; et al. Dual Targeting of CDK4/6 and BCL2 Pathways Augments Tumor Response in Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4120–4134. [Google Scholar] [CrossRef]
- Finlay, C.A.; Hinds, P.W.; Levine, A.J. The p53 proto-oncogene can act as a suppressor of transformation. Cell 1989, 57, 1083–1093. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Sdek, P.; Ying, H.; Chang, D.L.; Qiu, W.; Zheng, H.; Touitou, R.; Allday, M.J.; Xiao, Z.X. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol. Cell 2005, 20, 699–708. [Google Scholar] [CrossRef]
- Riley, T.; Sontag, E.; Chen, P.; Levine, A. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 2008, 9, 402–412. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- De, P.; Carlson, J.H.; Leyland-Jones, B.; Williams, C.; Dey, N. Triple Fluorescence staining to Evaluate Mechanism-based Apoptosis following Chemotherapeutic and Targeted Anti-cancer Drugs in Live Tumor Cells. Sci. Rep. 2018, 8, 13192. [Google Scholar] [CrossRef]
- Petros, A.M.; Gunasekera, A.; Xu, N.; Olejniczak, E.T.; Fesik, S.W. Defining the p53 DNA-binding domain/Bcl-x(L)-binding interface using NMR. FEBS Lett. 2004, 559, 171–174. [Google Scholar] [CrossRef]
- Sot, B.; Freund, S.M.; Fersht, A.R. Comparative biophysical characterization of p53 with the pro-apoptotic BAK and the anti-apoptotic BCL-xL. J. Biol. Chem. 2007, 282, 29193–29200. [Google Scholar] [CrossRef]
- Leu, J.I.; Dumont, P.; Hafey, M.; Murphy, M.E.; George, D.L. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 2004, 6, 443–450. [Google Scholar] [CrossRef]
- Pietsch, E.C.; Perchiniak, E.; Canutescu, A.A.; Wang, G.; Dunbrack, R.L.; Murphy, M.E. Oligomerization of BAK by p53 utilizes conserved residues of the p53 DNA binding domain. J. Biol. Chem. 2008, 283, 21294–21304. [Google Scholar] [CrossRef]
- Rozeboom, B.; Dey, N.; De, P. ER+ metastatic breast cancer: Past, present, and a prescription for an apoptosis-targeted future. Am. J. Cancer Res. 2019, 9, 2821–2831. [Google Scholar]
- Lu, J.; McEachern, D.; Li, S.; Ellis, M.J.; Wang, S. Reactivation of p53 by MDM2 Inhibitor MI-77301 for the Treatment of Endocrine-Resistant Breast Cancer. Mol. Cancer Ther. 2016, 15, 2887–2893. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, N.; Aske, J.; De, P. Targeted Neoadjuvant Therapies in HR+/HER2−Breast Cancers: Challenges for Improving pCR. Cancers 2021, 13, 458. https://doi.org/10.3390/cancers13030458
Dey N, Aske J, De P. Targeted Neoadjuvant Therapies in HR+/HER2−Breast Cancers: Challenges for Improving pCR. Cancers. 2021; 13(3):458. https://doi.org/10.3390/cancers13030458
Chicago/Turabian StyleDey, Nandini, Jennifer Aske, and Pradip De. 2021. "Targeted Neoadjuvant Therapies in HR+/HER2−Breast Cancers: Challenges for Improving pCR" Cancers 13, no. 3: 458. https://doi.org/10.3390/cancers13030458
APA StyleDey, N., Aske, J., & De, P. (2021). Targeted Neoadjuvant Therapies in HR+/HER2−Breast Cancers: Challenges for Improving pCR. Cancers, 13(3), 458. https://doi.org/10.3390/cancers13030458






