Radiotherapy–Immunotherapy Combination: How Will We Bridge the Gap Between Pre-Clinical Promise and Effective Clinical Delivery?
Abstract
Simple Summary
Abstract
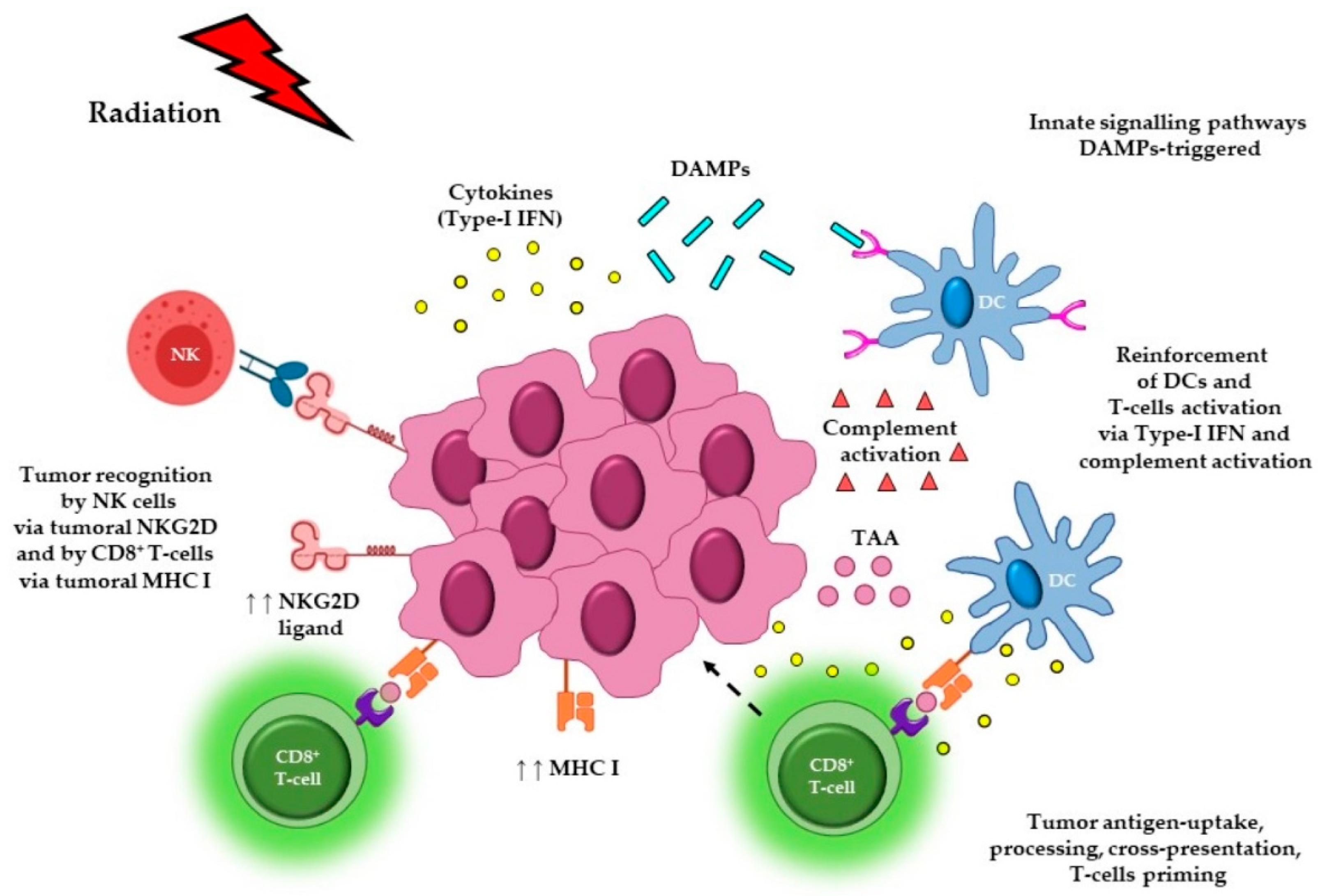
1. Introduction
- What is the optimal radiation dose and fractionation to induce the most effective anti-tumor immune responses?
- What is the optimal tumor volume and RT treatment field to elicit the most effective tumor control?
- Does schedule of RT–IO agents impact on the induction of systemic anti-tumor immunity, and does this vary with the IO agent and tumor type? Moreover, does the route of delivery (intra-tumoral versus intravenous) of the IO agent(s) influence the generation of both local and abscopal responses in combination with RT?
2. The Effect of Radiotherapy Dose and Fractionation on Anti-Cancer Immune Responses
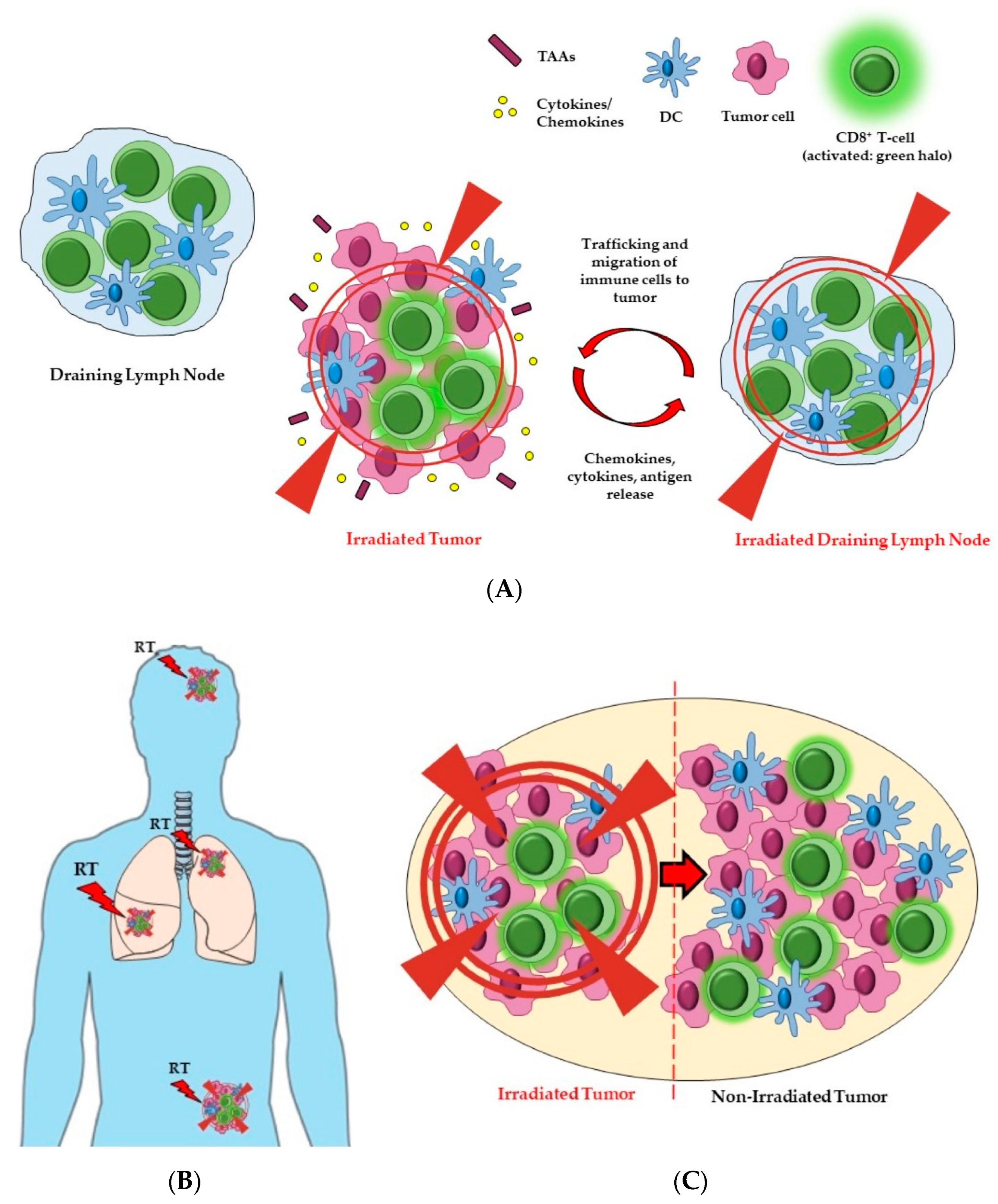
3. The Potential Effects of Radiotherapy Treatment Volumes on Immune Responses and Tumor Control
3.1. Irradiation to Draining Lymph Nodes
3.2. Irradiation to Single or Multiple Tumor Sites (Oligometastases)
3.3. Partial Tumor Irradiation
4. Combining Radiotherapy with Immunomodulatory Agents
5. Scheduling and Site of Delivery of IO Agents in Combination with RT
5.1. Scheduling
5.2. Site and Route of Delivery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| APCs | Antigen Presenting Cells |
| CAFs | Cancer Associated Fibroblasts |
| CTLs | Cytotoxic T Lymphocytes |
| CTLA-4 | Cytotoxic T Lymphocyte-Associated protein-4 |
| CTV | Clinical Target Volume |
| DAMPs | Damage Associated Molecular Patterns |
| DCs | Dendritic Cells |
| DLNs | Draining Lymph Nodes |
| ENI | Elective Nodal Irradiation |
| FDA | Food and Drug Administration |
| GITR | Glucocorticoid-Induced TNFR-Related protein |
| Gy | Gray |
| ICAMs | Intercellular Adhesion Molecules |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| ICD | Immunogenic Cell Death |
| ICI | Immune Check-point Inhibitors |
| IFN | Interferon |
| IMRT | Intensity-Modulated Radiation Therapy |
| IO | Immuno-Oncology |
| irAEs | Immune Related Adverse Events |
| I.T. | Intra Tumoral |
| i.v. | intra-venous |
| LLC | Lewis Lung Carcinoma |
| MDSCs | Myeloid Derived Suppressor Cells |
| MHC I | Major Histocompatibility Complex class I |
| MOSART | Multi-Organ Site Ablative Radiation Therapy |
| NKG2D | Natural Killer Group 2D |
| NKs | Natural Killer Cells |
| NSCLC | Non-Small Cell Lung Cancer |
| PD-1 | Programmed Death-1 |
| PD-L1 | Programmed Death Ligand-1 |
| p.t. | peri-tumoral |
| RT | Radiotherapy |
| SABR | Stereotactic ABlative Radiotherapy |
| s.c. | sub-cutaneous |
| SFRT | Spatially Fractionated RadioTherapy |
| TAAs | Tumor-Associated Antigens |
| TGF-β | Transforming Growth Factor-Beta |
| TIGIT | T cell ImmunoGlobulin and ITIM domain |
| TILs | Tumor Infiltrating Lymphocytes |
| TLRs | Toll-Like Receptors |
| TME | Tumor Microenvironment |
| TNF | Tumor Necrosis Factor |
| Tregs | regulatory T lymphocytes |
References
- Schoenhals, J.E.; Skrepnik, T.; Selek, U.; Cortez, M.A.; Li, A.; Welsh, J.W. Optimizing Radiotherapy with Immunotherapeutic Approaches. In Immunotherapy, 1st ed.; Naing, A., Hajjar, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 53–71. ISBN 978-3-319-53156-4. [Google Scholar]
- Ko, E.C.; Formenti, S.C. Radiation therapy to enhance tumor immunotherapy: A novel application for an established modality. Int. J. Radiat. Biol. 2019, 95, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, C.; Ellsworth, S.G.; Wilks, M.Q.; Keane, F.K.; Loeffler, J.S. Assessing the interactions between radiotherapy and antitumour immunity. Nat. Rev. Clin. Oncol. 2019, 16, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Reynders, K.; Illidge, T.; Siva, S.; Chang, J.Y.; De Ruysscher, D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015, 41, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, 1–21. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; De Nardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Hilmi, M.; Nicolle, R.; Bousquet, C.; Neuzillet, C. Cancer-Associated Fibroblasts: Accomplices in the Tumor Immune Evasion. Cancers (Basel) 2020, 12, 2969. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Tan, Y.; Wei, Q.; Yu, W. Cancer-associated fibroblasts in radiotherapy: Challenges and new opportunities. Cell Commun. Signal. 2019, 17, 47. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Grass, G.D.; Krishna, N.; Kim, S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer 2015, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, J.; Illidge, T.M. The influence of radiation in the context of developing combination immunotherapies in cancer. Ther. Adv. Vaccines Immunother. 2017, 5, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, J.; Cheadle, E.J.; Dovedi, S.J.; Illidge, T.M. Immuno-regulatory antibodies for the treatment of cancer. Expert Opin. Biol. Ther. 2015, 15, 787–801. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Ko, E.C.; Formenti, S.C. Radiotherapy and checkpoint inhibitors: A winning new combination? Ther. Adv. Med. Oncol. 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Mondini, M.; Levy, A.; Meziani, L.; Milliat, F.; Deutsch, E. Radiotherapy–immunotherapy combinations—Perspectives and challenges. Mol. Oncol. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Arnold, K.M.; Flynn, N.J.; Raben, A.; Romak, L.; Yu, Y.; Dicker, A.P.; Mourtada, F.; Sims-Mourtada, J. The Impact of Radiation on the Tumor Microenvironment: Effect of Dose and Fractionation Schedules. Cancer Growth Metastasis 2018, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Filatenkov, A.; Baker, J.; Mueller, A.M.S.; Kenkel, J.; Ahn, G.; Dutt, S.; Zhang, N.; Kohrt, H.; Jensen, K.; Dejbakhsh-jones, S.; et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin. Cancer Res. 2015, 21, 3727–3740. [Google Scholar] [CrossRef] [PubMed]
- Grapin, M.; Richard, C.; Limagne, E.; Boidot, R.; Morgand, V.; Bertaut, A.; Derangere, V.; Laurent, P.; Thibaudin, M.; Fumet, J.D.; et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: A promising new combination. J. Immunother. Cancer 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but Not Single-Dose Radiotherapy Induces an Immune-Mediated Abscopal Effect when Combined with Anti—CTLA-4 Antibody. Clin. Cancer Res. 2009, 15, 5379–5389. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Zhang, X.; Niedermann, G. Abscopal Effects With Hypofractionated Schedules Extending Into the Effector Phase of the Tumor-Specific T-Cell Response. Radiat. Oncol. Biol. 2018, 101, 63–73. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Barbes, B.; Mayorga, L.; Sanchez-Paulete, A.R.; Ponz-Sarvise, M.; Pérez-Gracia, J.L.; Melero, I. Brachytherapy attains abscopal effects when combined with immunostimulatory monoclonal antibodies. Brachytherapy 2017, 16, 1246–1251. [Google Scholar] [CrossRef]
- Deng, L.; Weichselbaum, R.R.; Fu, Y.; Deng, L.; Liang, H.; Burnette, B.; Beckett, M. Irradiation and anti—PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Cheadle, E.J.; Popple, A.L.; Poon, E.; Morrow, M.; Stewart, R.; Yusko, E.C.; Sanders, C.M.; Vignali, M.; Emerson, R.O.; et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin. Cancer Res. 2017, 23, 5514–5527. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-bhalla, G.; Mckenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5469. [Google Scholar] [CrossRef]
- Deloch, L.; Derer, A.; Hartmann, J.; Frey, B.; Fietkau, R.; Gaipl, U.S. Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation. Front. Oncol. 2016, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Stamell, E.F.; Wolchok, J.D.; Gnjatic, S.; Lee, N.Y.; Brownell, I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.A.; Wilhite, T.J.; Balboni, T.A.; Alexander, B.M.; Spektor, A.; Ott, P.A.; Ng, A.K.; Hodi, F.S.; Jonathan, D.; Chandra, R.A.; et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015, 4, e1046028. [Google Scholar] [CrossRef]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non–Small Cell Lung Cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Chachoua, A.; Demaria, S.; Formenti, S.C. Abscopal Responses in Metastatic Non-Small Cell Lung Cancer (NSCLC) Patients Treated on a Phase 2 Study of Combined Radiation Therapy and Ipilimumab: Evidence for the In Situ Vaccination Hypothesis of Radiation. Radiat. Oncol. Biol. 2000, 93, S66–S67. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Cesaire, M.; Le Mauff, B.; Rambeau, A.; Toutirais, O.; Thariat, J. Mechanisms of radiation-induced lymphopenia and therapeutic impact. Bull. Cancer 2020, 107, 813–822. [Google Scholar] [CrossRef]
- Takeshima, T.; Chamoto, K.; Wakita, D.; Ohkuri, T.; Togashi, Y.; Shirato, H.; Kitamura, H.; Nishimura, T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: Its potentiation by combination with TH1 cell therapy. Cancer Res. 2010, 70, 2697–2706. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Ghasemzadeh, A.; Nirschl, T.R.; Theodros, D.; Kochel, C.M.; Francica, B.J.; Muroyama, Y.; Anders, R.A.; Sharabi, A.B.; Velarde, E.; et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin. Cancer Res. 2018, 24, 5058–5071. [Google Scholar] [CrossRef]
- Buchwald, Z.S.; Nasti, T.H.; Lee, J.; Eberhardt, C.S.; Wieland, A.; Im, S.J.; Lawson, D.; Curran, W.; Ahmed, R.; Khan, M.K. Tumor-draining lymph node is important for a robust abscopal effect stimulated by radiotherapy. J. Immunother. Cancer 2020, 8, e000867. [Google Scholar] [CrossRef]
- Lugade, A.A.; Moran, J.P.; Gerber, S.A.; Rose, R.C.; Frelinger, J.G.; Lord, E.M. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. J. Immunol. 2005, 174, 7516–7523. [Google Scholar] [CrossRef]
- Chuong, M.; Chang, E.T.; Choi, E.Y.; Mahmood, J.; Lapidus, R.G.; Davila, E.; Carrier, F. Exploring the Concept of Radiation “Booster Shot” in Combination with an Anti-PD-L1 mAb to Enhance Anti-Tumor Immune Effects in Mouse Pancreas Tumors. J. Clin. Oncol. Res. 2017, 5, 1058. [Google Scholar]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11, 728–734. [Google Scholar]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Luke, J.J.; Lemons, J.M.; Karrison, T.G.; Pitroda, S.P.; Melotek, J.M.; Zha, Y.; Hania, A.; Arina, A.; Khodarev, N.N.; Janisch, L.; et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. Clin. Oncol. 2018, 36, 1611–1618. [Google Scholar] [CrossRef]
- Mazzola, R.; Jereczek-Fossa, B.A.; Franceschini, D.; Tubin, S.; Filippi, A.R.; Tolia, M.; Lancia, A.; Minniti, G.; Corradini, S.; Arcangeli, S.; et al. Oligometastasis and local ablation in the era of systemic targeted and immunotherapy. Radiat. Oncol. 2020, 15, 92. [Google Scholar] [CrossRef]
- Gomez, D.R.; Blumenschein, G.R.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef]
- Barsoumian, H.B.; Ramapriyan, R.; Younes, A.I.; Caetano, M.S.; Menon, H.; Comeaux, N.I.; Cushman, T.R.; Schoenhals, J.E.; Cadena, A.P.; Reilly, T.P.; et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J. Immunother. Cancer 2020, 8, e000537. [Google Scholar] [CrossRef]
- Yan, W.; Khan, M.K.; Wu, X.; Simone, C.B.; Fan, J.; Gressen, E.; Zhang, X.; Limoli, C.L.; Bahig, H.; Tubin, S.; et al. Spatially fractionated radiation therapy: History, present and the future. Clin. Transl. Radiat. Oncol. 2020, 20, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Markovsky, E.; Budhu, S.; Samstein, R.M.; Li, H.; Russell, J.; Zhang, Z.; Drill, E.; Bodden, C.; Chen, Q.; Powell, S.N.; et al. An Antitumor Immune Response Is Evoked by Partial-Volume Single-Dose Radiation in 2 Murine Models. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Lemons, J.M.; Luke, J.J.; Janisch, L.; Hseu, R.; Melotek, J.M.; Chmura, S.J. The ADscopal Effect? Control of Partially Irradiated Versus Completely Irradiated Tumors on a Prospective Trial of Pembrolizumab and SBRT Per NRG-BR001. Int. J. Radiat. Oncol. 2017, 99, S87. [Google Scholar] [CrossRef]
- Barsoumian, H.; Cushman, T.R.; Caetano, M.D.S.; Cadena, A.; Younes, A.; Tang, C.; Simon, G.R.; Cortez, M.A.; Welsh, J.W. Low Dose Radiation Improves Anti-Tumor Responses in a Phase 2 Prospective Trial of Concurrent or Sequential Stereotactic Radiation and Ipilimumab in Patients with Metastatic Lesions. Int. J. Radiat. Oncol. 2018, 102, S26. [Google Scholar] [CrossRef]
- Menon, H.; Chen, D.; Ramapriyan, R.; Verma, V.; Barsoumian, H.B.; Cushman, T.R.; Younes, A.I.; Cortez, M.A.; Erasmus, J.J.; De Groot, P.; et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J. Immunother. Cancer 2019, 7, 237. [Google Scholar] [CrossRef]
- Urwyler, P.; Earnshaw, I.; Bermudez, M.; Perucha, E.; Wu, W.; Ryan, S.; Mcdonald, L.; Karagiannis, S.N.; Taams, L.S.; Powell, N.; et al. Mechanisms of checkpoint inhibition-induced adverse events. Clin. Exp. Immunol. 2020, 200, 141–154. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Garasa, S.; Barbes, B.; Solorzano, J.L.; Perez-Gracia, J.L.; Labiano, S.; Sanmamed, M.F.; Azpilikueta, A.; Bolaños, E.; et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res. 2016, 76, 5994–6005. [Google Scholar] [CrossRef]
- Park, S.S.; Dong, H.; Liu, X.; Harrington, S.M.; Krco, C.J.; Grams, M.P.; Mansfield, A.S.; Furutani, K.M.; Olivier, K.R.; Kwon, E.D. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol. Res. 2015, 3, 610–619. [Google Scholar] [CrossRef]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef]
- Oweida, A.; Lennon, S.; Calame, D.; Korpela, S.; Bhatia, S.; Sharma, J.; Graham, C.; Binder, D.; Serkova, N.; Raben, D.; et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology 2017, 6, e1356153. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Jiang, T.; Xie, H.; Zhu, Z.; Zhou, F.; Zhou, C. Combined Radiotherapy and Anti–PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.; Yin Lim, S.; D’Costa, Z.; Jones, K.; Diana, A.; Sansom, O.J.; Kruger, P.; Liu, S.; McKenna, W.G.; Dushek, O.; et al. PD -L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol. Med. 2017, 9, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, E.W.; Lukyanov, Y.; Kawashima, N.; Alonso-Basanta, M.; Wang, S.-C.; Liu, M.; Jure-Kunkel, M.; Zagzag, D.; Demaria, S.; Formenti, S.C. Radiotherapy Enhances Antitumor Effect of Anti-CD137 Therapy in a Mouse Glioma Model. Radiat. Res. 2010, 173, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Ota, Y.; Murata, M.; Sugaru, E.; Koga-yamakawa, E.; Eguchi, K.; Hirose, Y.; Yamamoto, S.; Honeychurch, J.; et al. Intravenous administration of the selective toll-like receptor 7 agonist DSR-29133 leads to anti-tumor efficacy in murine solid tumor models which can be potentiated by combination with fractionated radiotherapy. Oncotarget 2016, 7, 17035–17046. [Google Scholar] [CrossRef] [PubMed]
- Adlard, A.L.; Dovedi, S.J.; Telfer, B.A.; Koga-yamakawa, E.; Pollard, C.; Honeychurch, J.; Illidge, T.M.; Murata, M.; Robinson, D.T.; Jewsbury, P.J.; et al. A novel systemically administered Toll-like receptor 7 agonist potentiates the effect of ionizing radiation in murine solid tumor models. Int. J. Cancer 2014, 829, 820–829. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Melis, M.H.M.; Wilkinson, R.W.; Adlard, A.L.; Stratford, I.J.; Honeychurch, J.; Illidge, T.M. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood 2013, 121, 251–259. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Lipowska-Bhalla, G.; Beers, S.A.; Cheadle, E.J.; Mu, L.; Glennie, M.J.; Illidge, T.M.; Honeychurch, J. Antitumor efficacy of radiation plus immunotherapy depends upon dendritic cell activation of effector CD8+ T cells. Cancer Immunol. Res. 2016, 4, 621–630. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Yu, D.; Kandimalla, E.R.; Sun, H.B.; Agrawal, S.; Guha, C. An In Situ Autologous Tumor Vaccination with Combined Radiation Therapy and TLR9 Agonist Therapy. PLoS ONE 2012, 7, e38111. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, H.J.; Ko, H.J.; Yoon, B.I.; Choe, J.; Kim, K.C.; Hahn, T.W.; Han, J.A.; Choi, S.S.; Jung, Y.M.; et al. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget 2017, 8, 24932–24948. [Google Scholar] [CrossRef]
- Patel, M.A.; Kim, J.E.; Theodros, D.; Tam, A.; Velarde, E.; Kochel, C.M.; Francica, B.; Nirschl, T.R.; Ghasemzadeh, A.; Mathios, D.; et al. Agonist anti-GITR monoclonal antibody and stereotactic radiation induce immune-mediated survival advantage in murine intracranial glioma. J. Immunother. Cancer 2016, 4, 28. [Google Scholar] [CrossRef]
- Honeychurch, J.; Glennie, M.J.; Johnson, P.W.M.; Illidge, T.M. Anti-CD40 monoclonal antibody therapy in combination with irradiation results in a CD8 T-cell—Dependent immunity to B-cell lymphoma. Blood 2003, 102, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Yasmin-Karim, S.; Mueller, R.; Viswanathan, A.N.; Ngwa, W. Single Radiotherapy Fraction with Local Anti-CD40 Therapy Generates Effective Abscopal Responses in Mouse Models of Cervical Cancer. Cancers 2020, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Yasmin-Karim, S.; Bruck, P.T.; Moreau, M.; Kunjachan, S.; Chen, G.Z.; Kumar, R.; Grabow, S.; Dougan, S.K.; Ngwa, W. Radiation and Local Anti-CD40 Generate an Effective in situ Vaccine in Preclinical Models of Pancreatic Cancer. Front. Immunol. 2018, 9, 2030. [Google Scholar] [CrossRef] [PubMed]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newel, P.; Bahjat, K.S.; et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef]
- Yokouchi, H.; Yamazaki, K.; Chamoto, K.; Kikuchi, E.; Shinagawa, N.; Oizumi, S.; Hommura, F.; Nishimura, T.; Nishimura, M. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 2008, 99, 361–367. [Google Scholar] [CrossRef]
- Boutros, C.; Chaput-Gras, N.; Lanoy, E.; Larive, A.; Mateus, C.; Routier, E.; Sun, R.; Tao, Y.G.; Massard, C.; Bahleda, R.; et al. Dose escalation phase 1 study of radiotherapy in combination with anti-cytotoxic-T-lymphocyte-associated antigen 4 monoclonal antibody ipilimumab in patients with metastatic melanoma. J. Immunother. Cancer 2020, 8, e000627. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; Van Der Noort, V.; De Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.L.N.; De Langen, A.J.; et al. Effect of Pembrolizumab after Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients with Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef]
- Maity, A.; Mick, R.; Huang, A.C.; George, S.M.; Farwell, M.D.; Lukens, J.N.; Berman, A.T.; Mitchell, T.C.; Bauml, J.; Schuchter, L.M.; et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br. J. Cancer 2018, 119, 1200–1207. [Google Scholar] [CrossRef]
- Frank, M.J.; Reagan, P.M.; Bartlett, N.L.; Gordon, L.I.; Friedberg, J.W.; Czerwinski, D.K.; Long, S.R.; Hoppe, R.T.; Janssen, R.; Candia, A.F.; et al. In situ vaccination with a tlr9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 2018, 8, 1258–1269. [Google Scholar] [CrossRef]
- Brody, J.D.; Ai, W.Z.; Czerwinski, D.K.; Torchia, J.A.; Levy, M.; Advani, R.H.; Kim, Y.H.; Hoppe, R.T.; Knox, S.J.; Shin, L.K.; et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: A phase I/II study. J. Clin. Oncol. 2010, 28, 4324–4332. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Kropp, L.M.; De Los Santos, J.F.; McKee, S.B.; Conry, R.M. Radiotherapy to Control Limited Melanoma Progression Following Ipilimumab. J. Immunother. 2016, 39, 373–378. [Google Scholar] [CrossRef]
- Qian, J.M.; Yu, J.B.; Kluger, H.M.; Chiang, V.L.S. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016, 122, 3051–3058. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Illidge, T.M. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology 2015, 4, e1016709. [Google Scholar] [CrossRef]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Kaira, K.; Hashimoto, K.; Mouri, A.; Miura, Y.; Shiono, A.; Nishihara, F.; Murayama, Y.; Noda, S.; Kato, S.; et al. Radiotherapy is an independent prognostic marker of favorable prognosis in non-small cell lung cancer patients after treatment with the immune checkpoint inhibitor, nivolumab. Thorac. Cancer 2019, 10, 992–1000. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; Van de Vijver, K.K.; De Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Demaria, S.; Romano, E.; Brackstone, M.; Formenti, S.C. Immune induction strategies to enhance responses to PD-1 blockade: Lessons from the TONIC trial. J. Immunother. Cancer 2019, 7, 318. [Google Scholar] [CrossRef]
- Ribeiro Gomes, J.; Schmerling, R.A.; Haddad, C.K.; Racy, D.J.; Ferrigno, R.; Gil, E.; Zanuncio, P.; Buzaid, A.C. Analysis of the Abscopal Effect With Anti-PD1 Therapy in Patients With Metastatic Solid Tumors. J. Immunother. 2016, 39, 367–372. [Google Scholar] [CrossRef]
- Haymaker, C.L.; Kim, D.W.; Uemura, M.; Vence, L.M.; Phillip, A.; McQuail, N.; Brown, P.D.; Fernandez, I.; Hudgens, C.W.; Creasy, C.; et al. Metastatic melanoma patient had a complete response with clonal expansion after whole brain radiation and PD-1 blockade. Cancer Immunol. Res. 2017, 5, 100–105. [Google Scholar] [CrossRef]
- Bloom, B.C.; Augustyn, A.; Pezzi, T.A.; Menon, H.; Mayo, L.L.; Shah, S.J.; Schwartz, D.L.; Chmura, S.J.; Johnson, F.M.; Welsh, J.W.; et al. Rescue of Immunotherapy-Refractory Metastatic Merkel Cell Carcinoma With Conventionally Fractionated Radiotherapy and Concurrent Pembrolizumab. Front. Oncol. 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, S.; Zhang, X.; Jia, K.; Wang, H.; Zhou, C.; He, Y. OX40 (CD134) and OX40 ligand, important immune checkpoints in cancer. Onco Targets Ther. 2019, 12, 7347–7353. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Tselikas, L.; de Baere, T.; Houot, R. Intratumoral immunotherapy: Using the tumor as the remedy. Ann. Oncol. 2017, 28, xii33–xii43. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Andtbacka, R.; Harrington, K.; Melero, I.; Leidner, R.; de Baere, T.; Robert, C.; Ascierto, P.A.; Haanen, J.; Brody, J.; et al. Starting the fight in the tumor: Expert recommendations for the development of human intratumoral immunotherapy (HIT-IT). Ann. Oncol. 2018, 29, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.F.; Van Der Sluis, T.C.; Ossendorp, F.; Arens, R.; Melief, C.J.M. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin. Cancer Res. 2013, 19, 5381–5389. [Google Scholar] [CrossRef]
- van Hooren, L.; Sandin, L.C.; Moskalev, I.; Ellmark, P.; Dimberg, A.; Black, P.; Tötterman, T.H.; Mangsbo, S.M. Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer. Eur. J. Immunol. 2017, 47, 385–393. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Chu, J.; Brody, J.; Czerwinski, D.K.; Chester, C.; Sadaram, M.; Advani, R.; Kim, Y.H.; Hoppe, R.T.; Knox, S.J.; et al. Dose-Escalated, Intratumoral TLR9 Agonist and Low-Dose Radiation Induce Abscopal Effects in Follicular Lymphoma. Blood 2014, 124, 3092. [Google Scholar] [CrossRef]
- Johnson, M.L.; Braiteh, F.; Grilley-olson, J.E.; Chou, J.; Davda, J.; Forgie, A.; Li, R.; Jacobs, I.; Kazazi, F.; Hu-lieskovan, S. Assessment of Subcutaneous vs Intravenous Administration of Anti–PD-1 Antibody PF-06801591 in Patients With Advanced Solid Tumors: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2019, 5, 999–1007. [Google Scholar] [CrossRef]
- Irenaeus, S.M.M.; Nielsen, D.; Ellmark, P.; Yachnin, J.; Deronic, A.; Nilsson, A.; Norlén, P.; Veitonmäki, N.; Wennersten, C.S.; Ullenhag, G.J. First-in-human study with intratumoral administration of a CD40 agonistic antibody, ADC-1013, in advanced solid malignancies. Int. J. Cancer 2019, 145, 1189–1199. [Google Scholar] [CrossRef]
| a | |||||||
| Targeted Molecule | Category | Cancer Type | Mouse Model | (Immune) Responses Observed | RT Regimen | RT–IO Agent Schedule | |
| CTLA-4 (Cytotoxic T Lymphocyte- Associated protein 4) | Co-inhibitory | Breast cancer [25,46] Colon cancer [25] | TSA [25] 4T1 [46] MCA38 [25] | CD8+ T cell-mediated tumor inhibition (tumor-specific IFN-γ production); abscopal response [25] | 3 × 8 Gy, 5 × 6 Gy [25] | IO agent given concurrently with RT [25] | |
| Survival advantage promoted by CD8+ T cells, with inhibition of lung metastasis development [46] | 1 × 12 Gy, 2 × 12 Gy [46] | IO agent given following (1, 4, 7 days) RT [46] | |||||
| PD-1 (Programmed Death-1) | Co-inhibitory | Breast cancer [31,41,58,59] Colon cancer [31,41,58] Melanoma [31,41,58] | 4T1 [31,58] 4T1-HA [41] CT26 [31] MC38 [58] 4434 [31] B16-OVA [41,58,59] | CD8+ T cells mediated immune response improving local tumor control; CD8+ T cells mediated long term survival and protection against tumor rechallenge; anti-tumor memory response antigen-specific; IFN-γ production by CD8+ T cells mediating PD-L1 upregulation on cancer cells [31] | 5 × 4 Gy (4T1) [31] 5 × 2 Gy (CT26, 4434) [31] | IO agent given concurrently with RT [31] | |
| Antigen-specific T and B cells mediated responses; increased percentage of antigen-experienced T cells and effector memory T cells; upregulation of tumor-associated MHC; enhanced antigen-presentation in DLNs; increased tumoral T cell infiltration; improved local tumor control [41] | 1 × 12 Gy [41] | IO given concurrently with RT [41] | |||||
| CD8+ T cells mediated effective immune response; essential role of DCs for cross-presentation/priming in tumor rejection; reduced total content of effector T cells, MDSCs and Tregs in both irradiated and non-irradiated sites; increased T cells intracellular expression of IFN-γ; increased expression of CD137 and PD-1 on TILs; abscopal response CD8+ T-cells mediated [58] | 3 × 8 Gy [58] | IO given concurrently with RT [58] | |||||
| Renal cell cancer [59] | RENCA [59] | Immune response tumor-specific CD8+ T-cells mediated; abscopal response [59] | 1 × 15Gy [59] | IO given concurrently with RT [59] | |||
| Glioblastoma multiforme [60] | GL261-Luciferase [60] | Increased infiltration of CTLs; Tregs decrease; long term-survival [60] | 1 × 10Gy [60] | IO given concurrently with RT [60] | |||
| PD-L1 (Programmed Death-Ligand 1) | Co-inhibitory | Breast cancer [29,31] Colon cancer [29,31] | TUBO [29] 4T1 [31] CT26 [31] MC38 [29] | CD8+ T cell-mediated Long-term immunity; reduced local MDSCs accumulation CTL-mediated, via cytotoxic TNF-α production; PD-L1 tumoral upregulation; abscopal response [29] | 1 × 12 Gy (TUBO) [29] 1 × 20 Gy (MC38) [29] | IO given concurrently with RT [29] | |
| Melanoma [31] | 4434 [31] | CD8+ T cells mediated immune response improving local tumor control; CD8+ T cell-mediated long-term survival and protection against tumor rechallenge; anti-tumor memory response antigen-specific; IFN-γ production by CD8+ T cells mediating PD-L1 upregulation on cancer cells [31] | 5 × 2 Gy (4434) [31] | IO agent given concurrently with RT [31] | |||
| Head and neck squamous cell carcinoma [61] | B4B8 [61] LY2 [61] | Increased T cell infiltration; PD-L1 tumoral upregulation; enhanced tumor control; improved mice survival [61] | 1 × 10 Gy [61] | IO given before (3 days), concurrently and after (2x/week until end of experiment) RT [61] | |||
| NSCLC [62] | LLC [62] | Enhanced anti-tumor immunity mediated by infiltrated CD8+ T cells; reduced MDSCs accumulation and Tregs infiltration; increased expression of PD-L1 [62] | 3 × 2 Gy [62] | IO given concurrently with RT [62] | |||
| Pancreatic ductal adenocarcinoma [63] | KPC [63] Pan02 [63] | Enhanced infiltration of CD8+ T cells; increased CD8:Tregs ratio; reduced myeloid cells infiltration; improved tumor response [63] | 1 × 12 Gy, 5 × 3 Gy, 1 × 20 Gy [63] | IO given concurrently with RT [63] | |||
| TIGIT (T cell ImmunoGlobulin and ITIM domain) | Co-inhibitory | Colon cancer [24] Melanoma [24] | CT26 [24] B16-F10 [24] | Complete anti-tumor response [24] | 3 × 8 Gy [24] | IO given concurrently with RT [24] | |
| 4-1BB (or CD137) | Co-stimulatory | Breast cancer [58] | 4T1 [58] | See above [58] | See above [58] | See above [58] | |
| Colorectal cancer [58] | MC38 [58] | ||||||
| Melanoma [58] | B16-OVA [58] | ||||||
| Glioma [64] | GL261 [64] | Anti-tumor immunity mediated by increased TILs infiltration and tumor-specific IFN-γ production; complete tumor eradication; long-term survival [64] | 2 × 4 Gy [64] | IO given following (1 day) RT [64] | |||
| TLRs (Toll-Like Receptors) | Activator | Colorectal cancer [65,66] | CT26 [65,66] | CD8+ T cells mediated curative immune response; immune-memory tumor-specific [65] | 5 × 2 Gy [65] | IO given before (1 day) or concurrently with RT [65] | |
| Fibrosarcoma [66] | KHT [66] | CD8+ T cells mediated immunity; reduction in metastatic burden; improved survival of mice; complete tumor resolution [66] | 1 × 15 Gy (KHT) [66] 5 × 2 Gy (CT26) [66] | IO given before (1 h) RT [66] | |||
| Lymphoma [67,68] | A20 [67] EG7 [67,68] | Expansion of antigen-specific CD8+ T cells; development of tumor-specific memory immune response; long-term tumor clearance; improved survival [67] | 1 × 10 Gy (EG7) [67] 5 × 2 Gy (EL4, A20) [67] | IO given concurrently and after (1x/week for up to 5 weeks) RT [67] | |||
| EL4 [67,68] | Long-term immune protection DCs mediated [68] | 1 × 10 Gy (EL4) [68] | IO given concurrently and after (1x/week for up to 5 weeks) RT [68] | ||||
| Lung cancer [69] | 3LL [69] | Specific systemic anti-tumor humoral response; augmented tumoral infiltration of NKDCs, reduced pulmonary metastasis; tumor growth inhibition; improved survival [69] | 1 × 20 Gy [69] | IO given concurrently and after (2x/week for 3 weeks) RT [69] | |||
| Melanoma [70] | B16-F1 [70] B16-F10 [70] | Enhanced systemic anti-tumor immunity via increased number of tumoral CD8+ T cells, reduced number of Tregs and MDSCs; reduced number of lung metastatic nodules; increased population of T cells expressing IFN-γ and TNF-α; decreased tumor growth [70] | 1 × 2 Gy [70] | IO given before (6 h) and after (1 day) RT [70] | |||
| Osteosarcoma [65] | LM8 [65] | See above [65] | See above [65] | See above [65] | |||
| Renal cancer [65] | RENCA [65] | ||||||
| GITR (Glucocorticoid- Induced TNFR-Related protein) | Co-stimulatory | Glioblastoma [71] | GL261-luciferase [71] | T cells mediated immune response; higher intra-tumoral level of CD4+ T cells vs. Tregs; elevated IFN-γ and IL-2 production by CD4+ T cells; elevated IFN-γ and TNF-α production by CD8+ T cells; increased mRNA expression of M1 markers and decreased expression of M2 markers in tumor-infiltrated mononuclear cells; improved survival with a certain degree of cure rate [71] | 1 × 10 Gy [71] | IO given concurrently and after (3 days) RT [71] | |
| CD40 (Cluster of Differentiation 40) | Co-stimulatory | B-cell lymphoma [72] | A31 [72] πBCL1 [72] | CD8+ T cells mediated anti-tumor immune response; long-term mice survival [72] | 1 to 8 Gy (A31) [72] 1 × 5 Gy (πBCL1) [72] | IO given after (4 h) RT [72] | |
| Cervical cancer [73] | TC-1 [73] | Tumor regression; long-term survival; development of immune-memory; abscopal response [73] | 1 × 6 Gy [73] | IO given after (within 3 h) RT [73] | |||
| Pancreatic cancer [74] | KPC cells [74] Panc02 [74] | CD8+ T cells immune-memory; augmented T cells priming; long-term survival; abscopal effect [74] | 1 × 5 Gy (Panc02) [74] 1 × 10 Gy (KPC) [74] | IO given after (within 3 h) RT [74] | |||
| OX40 (or CD134) | Co-stimulatory | Colorectal cancer [75] | CT26 [75] | Long-term tumor immunity; increased survival [75] | 1 × 20 Gy [75] 1 × 20 Gy [76] | IO given after (1day) RT [75] | |
| Lung cancer [76] | LLC-OVA [76] | CD8+ T cells mediated anti-tumor immunity; immune-memory; durable tumor immunity and prolonged survival [76] | IO given concurrently and after (4, 7, 10 days) RT [76] | ||||
| b | |||||||
| Targeted Molecule | Category | Cancer Type | Clinical Trial (RegIstration Number and Type) | Trial Phase, Patients Enrolled (n) | (Immune) Responses Observed | RT regimen | RT–IO Agent Schedule |
| CTLA-4 (Cytotoxic T Lymphocyte- Associated protein 4) | Co-inhibitory | Melanoma [77] | NCT01557114; Interventional | Phase 1; n: 19 | Increased CD8+ T cells associated with PFS; partial and complete responses observed [77] | 9 Gy [77] | IO given before (week 1), concurrently (week 4) and after (weeks 7 and 10) RT [77] |
| NSCLC [38] | NCT02221739; Interventional | Phase 1–2; n: 39 | Increased level of serum IFN-β; changes in T cell clones; systemic anti-tumor response T cell-mediated [38] | 5 × 6 Gy, 3 × 9.5 Gy [38] | IO given concurrently and after RT (on day 22, 43, 64 of the treatment regimen) [38] | ||
| PD-1/PD-L1 (Programmed Death/Ligand-1) | Co-inhibitory | NSCLC [78] | NCT02492568; Interventional | Phase 2; n: 92 | Increased ORR, disease control rate, median PFS and OS [78] | 3 × 8 Gy [78] | IO given after (within 7 days) RT [78] |
| Metastatic cancers (NSCLC, melanoma, breast cancer, pancreatic cancer, etc.) [79] | NCT02303990; Interventional | Phase 1; n: 24 | Ki67 increase in PD-1 expressing CD8+ T cell; complete response and prolonged stable disease observed [79] | 3 × 8 Gy [79] 1 × 17 Gy [79] | IO given before (1 week) and concurrently with RT [79] | ||
| TLRs (Toll-Like Receptors) | Activator | B cell lymphoma [80] | NCT02266147; Interventional | Phase 2; n: 29 | Increased effector CD8+ and CD4+ T cells in the TME; decreased T-follicular helper and Tregs cells in the TME; tumor reduction at the treated sites; abscopal response; partial and complete responses observed [80] | 2 × 2 Gy [80] | IO given concurrently and after (1×/week for 5 weeks) RT [80] |
| B cell lymphoma [81] | NCT00185965; Interventional | Phase 1–2; n: 15 | Tumor-reactive memory CD8+ T cells; partial and complete responses observed [81] | 2 × 2 Gy [81] | IO given immediately before and after RT (and weekly for 8 additional consecutive weeks) [81] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, E.; Honeychurch, J.; Illidge, T.M. Radiotherapy–Immunotherapy Combination: How Will We Bridge the Gap Between Pre-Clinical Promise and Effective Clinical Delivery? Cancers 2021, 13, 457. https://doi.org/10.3390/cancers13030457
Romano E, Honeychurch J, Illidge TM. Radiotherapy–Immunotherapy Combination: How Will We Bridge the Gap Between Pre-Clinical Promise and Effective Clinical Delivery? Cancers. 2021; 13(3):457. https://doi.org/10.3390/cancers13030457
Chicago/Turabian StyleRomano, Erminia, Jamie Honeychurch, and Timothy M. Illidge. 2021. "Radiotherapy–Immunotherapy Combination: How Will We Bridge the Gap Between Pre-Clinical Promise and Effective Clinical Delivery?" Cancers 13, no. 3: 457. https://doi.org/10.3390/cancers13030457
APA StyleRomano, E., Honeychurch, J., & Illidge, T. M. (2021). Radiotherapy–Immunotherapy Combination: How Will We Bridge the Gap Between Pre-Clinical Promise and Effective Clinical Delivery? Cancers, 13(3), 457. https://doi.org/10.3390/cancers13030457







