Osteopathic Treatment and Evaluation in the Clinical Setting of Childhood Hematological Malignancies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Precision-Based Exercise Protocols and OT
2.2. Osteopathic Evaluation
2.3. Evaluation of the Satisfaction of the OT Intervention
2.4. Study Design and Statistical Analysis
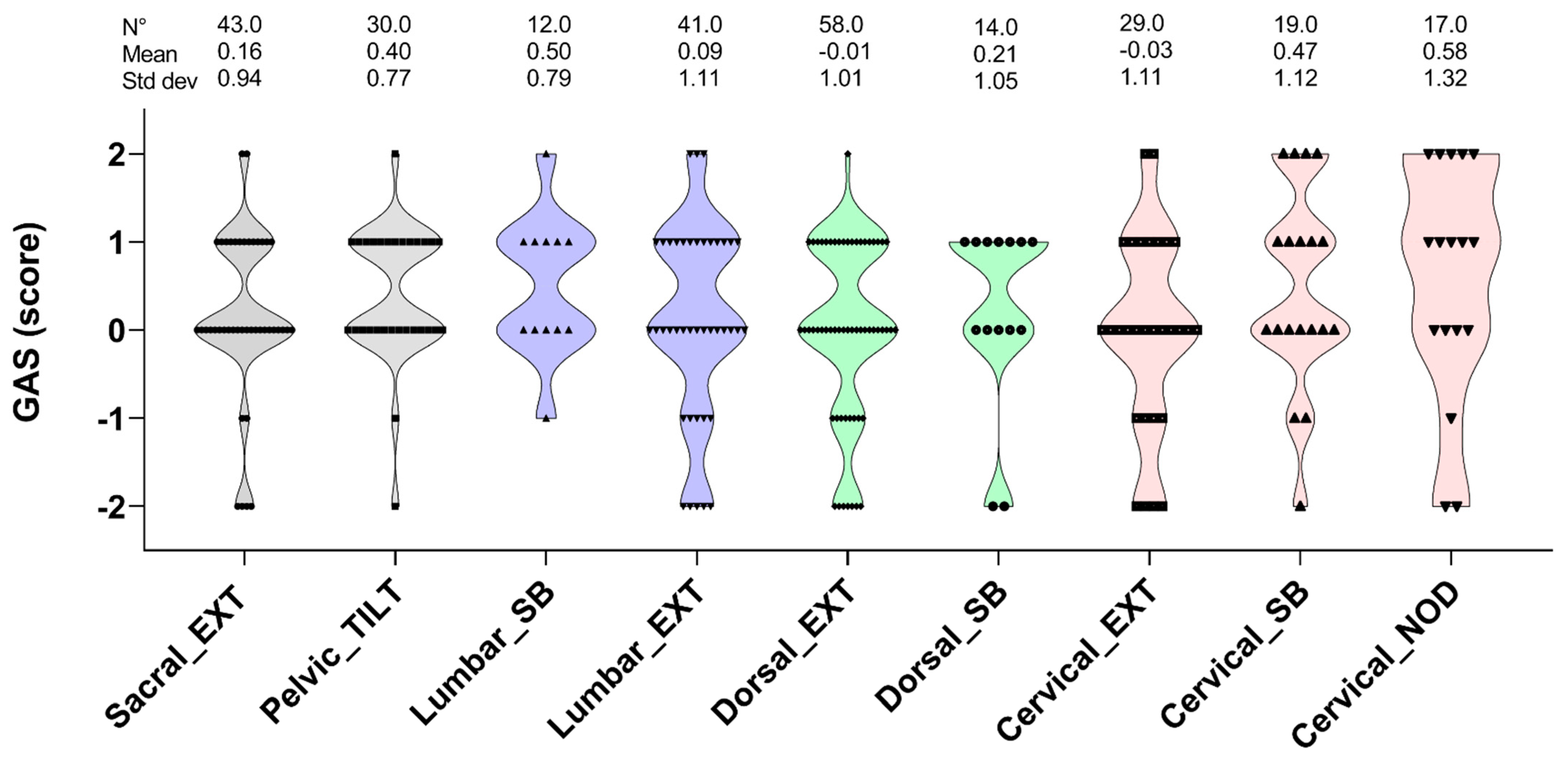
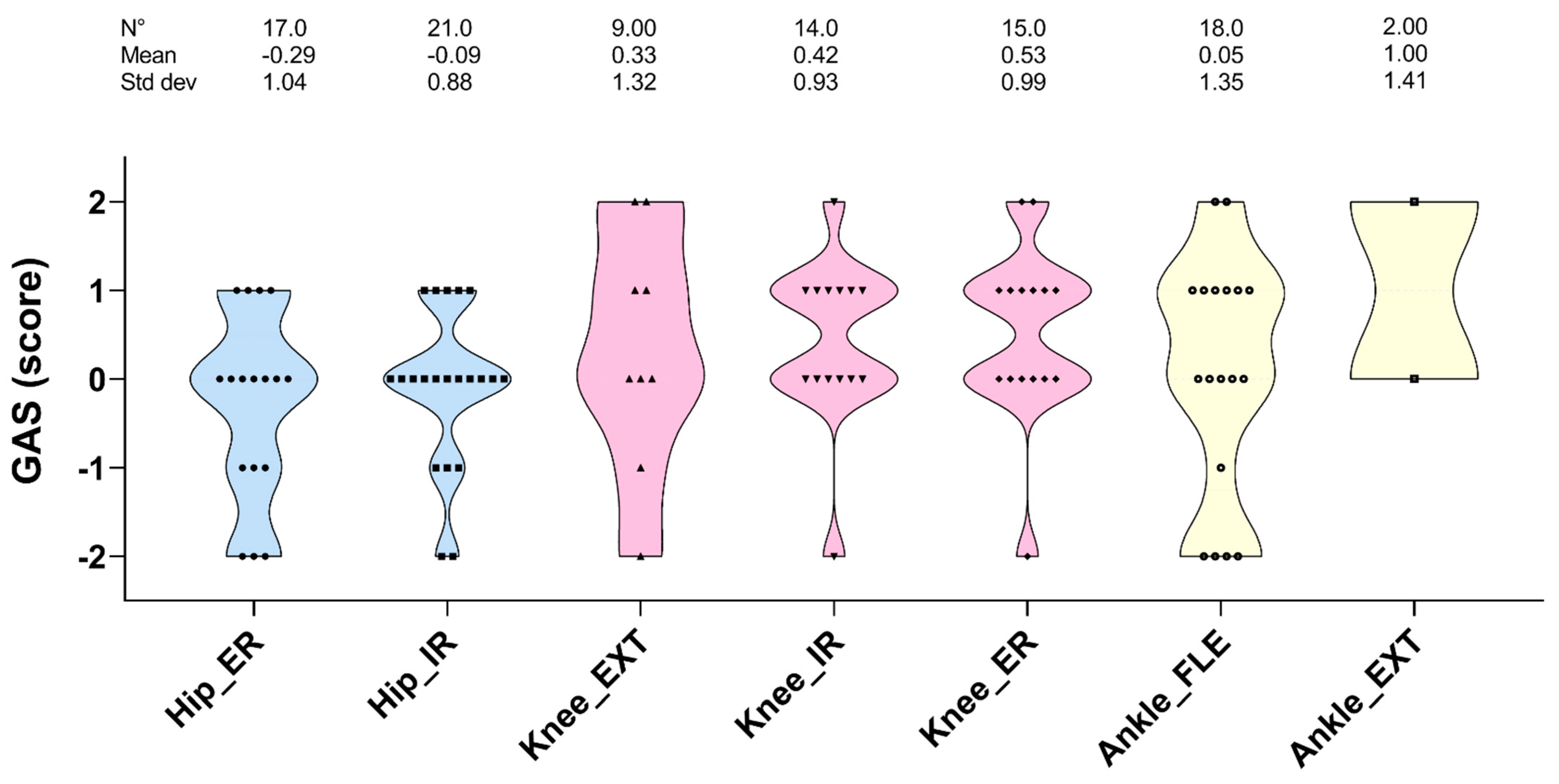
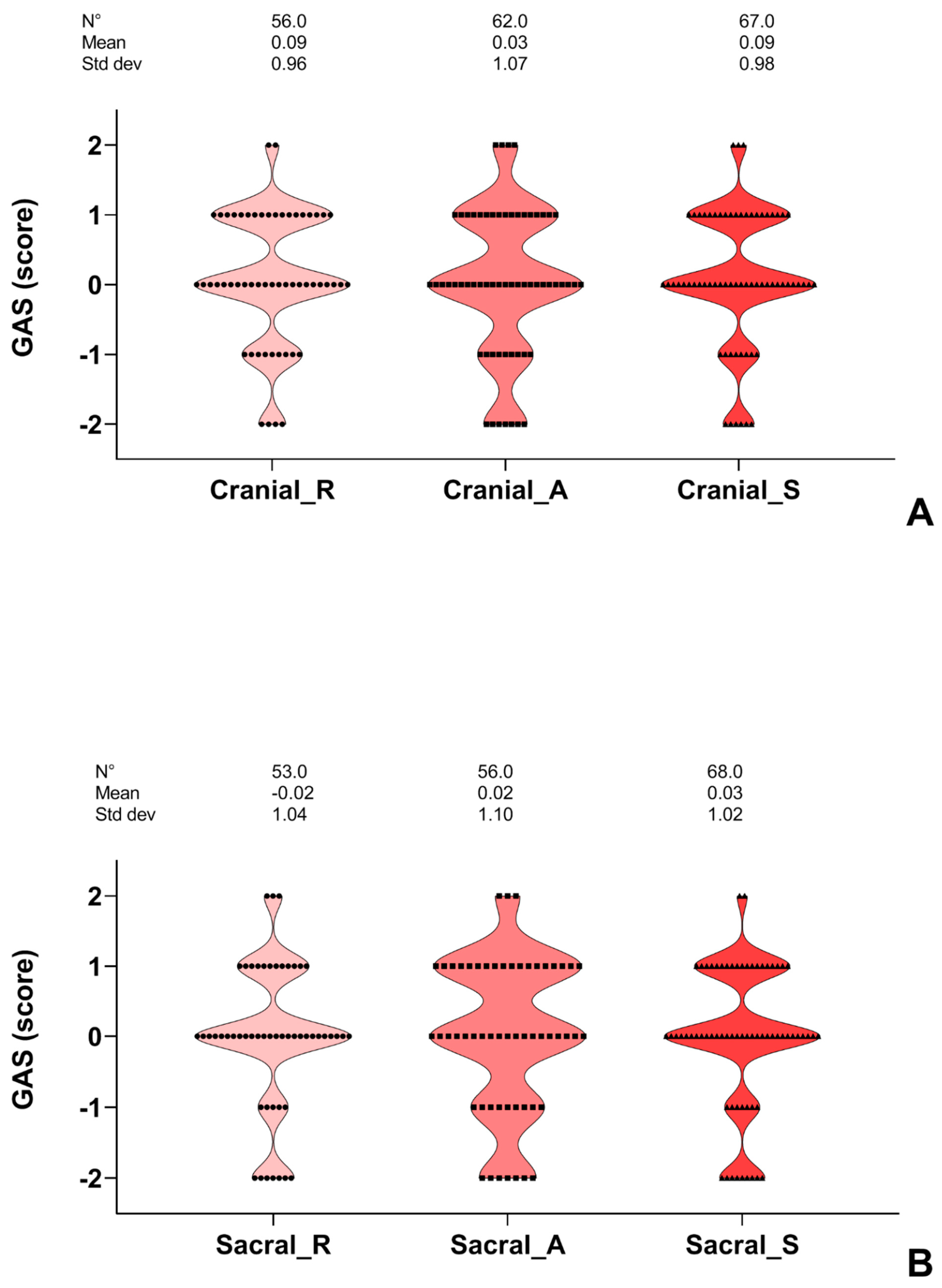
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- AIRTUM Working Group; CCM; AIEOP Working Group. Italian cancer figures, report 2012: Cancer in children and adolescents. Epidemiol. Prev. 2013, 37 (Suppl. 1), 1–225. [Google Scholar]
- Vassal, G.; Schrappe, M.; Pritchard-Jones, K.; Arnold, F.; Basset, L.; Biondi, A.; Bode, G.; Eggert, A.; Hjorthi, L.; Kameric, L.; et al. The SIOPE strategic plan: A European cancer plan for children and adolescents. J. Cancer Policy 2016, 8, 17–32. [Google Scholar] [CrossRef]
- Akyay, A.; Olcay, L.; Sezer, N.; Sonmez, C.A. Muscle strength, motor performance, cardiac and muscle biomarkers in detection of muscle side effects during and after acute lymphoblastic leukemia treatment in children. J Pediatr. Hematol./Oncol. 2014, 36, 594–598. [Google Scholar] [CrossRef]
- Nysom, K.; Holm, K.; Olsen, J.H.; Hertz, H.; Hesse, B. Pulmonary function after treatment for acute lymphoblastic leukaemia in childhood. Br. J. Cancer 1998, 78, 21–27. [Google Scholar] [CrossRef][Green Version]
- Lanfranconi, F.; Pollastri, L.; Ferri, A.; Fraschini, D.; Masera, G.; Miserocchi, G. Near infrared spectroscopy (NIRS) as a new non-invasive tool to detect oxidative skeletal muscle impairment in children survived to acute lymphoblastic leukaemia. PLoS ONE 2014, 9, e99282. [Google Scholar]
- Marchese, V.G.; Connolly, B.H.; Able, C.; Booten, A.; Bowen, P.; Porter, B.; Rai, S.; Hancock, M.; Pui, C.H.; Howard, S.; et al. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescent, and young adults with acute lymphoblastic leukemia. Phys. Ther. 2008, 88, 341–350. [Google Scholar]
- Harila-Saari, A.H.; Huuskonen, U.E.J.; Tolonen, U.; Vainionpää, L.K.; Lanning, B.M. Motor nervous pathway function is impaired after treatment of childhood acute lymphoblastic leukemia: A study with motor evoked potentials. Med. Pediatr. Oncol. 2001, 36, 345–351. [Google Scholar] [CrossRef]
- Cox, C.L.; Montgomery, M.; Oeffinger, K.C.; Leisenring, W.; Zeltzer, L.; Whitton, J.; Mertens, C.; Hudson, M.; Robison, L. Promoting physical activity in childhood cancer survivors. Cancer 2009, 115, 642–654. [Google Scholar] [CrossRef] [PubMed]
- YildizKabak, V.; Calders, P.; Duger, T.; Mohammed, J.; van Breda, E. Short and long-term impairments of cardiopulmonary fitness level in previous childhood cancer cases: A systematic review. Support. Care Cancer 2019, 27, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.K.; Kaste, S.C.; Zhu, L.; Pui, C.H.; Jeha, S.; Nathan, P.C. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk. Lymphoma 2015, 56, 1004–1011. [Google Scholar] [PubMed]
- Deisenroth, A.; Sontgerath, R.; Schuster, A.J.; von Busch, C.; Huber, G.; Eckert, K.; Kulozik, A.; Wiskemann, J. Muscle strength and QoL in patients with childhood cancer at early phase of primary treatment. Pediatr. Hematol. Oncol. 2016, 33, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.; van den Bos, C.; Stijnen, T.; Pieters, R. Decrease in Peripheral Muscle Strength and Ankle Dorsiflexion as Long-Term Side Effects of Treatment for Childhood Cancer. Pediatr. Blood Cancer 2008, 50, 833–837. [Google Scholar] [CrossRef] [PubMed]
- San Juan, A.F.; Fleck, S.J.; Chamorro-Vina, C.; Maté-Muñoz, J.L.; Moral, S.; Pérez, M.; Cardona, C.; Del Valle, M.F.; Hernández, M.; Ramírez, M.; et al. Effects of an intrahospital exercise program intervention for children with leukemia. Med. Sci. Sports Exerc. 2007, 39, 13–21. [Google Scholar] [CrossRef]
- Perondi, M.B.; Gualano, B.; Artioli, G.G. Effects of a combined aerobic and strength training program in youth patients with acute lymphoblastic leukemia. J. Sports Sci. Med. 2012, 1, 387–392. [Google Scholar]
- Fiuza-Luces, C.; Padilla, J.R.; Soares-Miranda, L.; Santana-Sosa, E.; Quiroga, J.V.; Santos-Lozano, A.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Lorenzo-González, R.; Verde, Z.; et al. Exercise Intervention in Pediatric Patients with Solid Tumors: The Physical Activity in Pediatric Cancer Trial. Med. Sci. Sports Exerc. 2017, 49, 223–230. [Google Scholar] [CrossRef]
- Chamorro-Viña, C.; Ruiz, J.R.; Santana-Sosa, E.; González Vicent, M.; Madero, L.; Pérez, M.; Fleck, S.J.; Pérez, A.; Ramírez, M.; Lucía, A. Exercise during hematopoietic stem cell transplant hospitalization in children. Med. Sci. Sports Exerc. 2010, 42, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Kabak, V.Y.; Duger, T.; Cetinkaya, D.U. Investigation of the Effects of an Exercise Program on Physical Functions and Activities of Daily Life in Pediatric Hematopoietic Stem Cell Transplantation. Pediatric Blood Cancer 2016, 63, 1643–1648. [Google Scholar] [CrossRef]
- Braam, K.I.; van der Torre, P.; Takken, T.; Veening, M.A.; van Dulmen-den Broeder, E.; Kaspers, G.J.L. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst. Rev. 2016, CD008796. [Google Scholar] [CrossRef]
- Morales, J.S.; Santana-Sosa, E.; Santos-Lozano, A.; Baño-Rodrigo, A.; Valenzuela, P.; Rincón-Castanedo, C.; Fernández-Moreno, D.; González Vicent, M.; Pérez-Somarriba, M.; Madero, L.; et al. Inhospital exercise benefits in childhood cancer: A prospective cohort study. Scand. J. Med. Sci. Sports 2020, 30, 126–134. [Google Scholar] [CrossRef]
- Lanfranconi, F.; Zardo, W.; Moriggi, T.; Villa, E.; Radaelli, G.; Radaelli, S.; Paoletti, F.; Bottes, E.; Miraglia, T.; Pollastri, L.; et al. Precision-basedexerciseas a new therapeutic option for children and adolescents with haematologicalmalignancies. Sci. Rep. 2020, 10, 12892. [Google Scholar] [CrossRef]
- Miladinia, M.; Baraz, S.; Shariati, A.; Malehi, A.S. Effects of Slow-Stroke Back Massage on Symptom Cluster in Adult Patients with Acute Leukemia: Supportive Care in Cancer Nursing. Cancer Nurs. 2017, 40, 31–38. [Google Scholar] [CrossRef]
- World Health Organization. Benchmarks for Training in Traditional/Complementary and Alternative Medicine: Benchmarks for trining in Osteopathy; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44356 (accessed on 12 December 2021).
- Arienti, C.; Bosisio, T.; Ratti, S.; Miglioli, R.; Negrini, S. Osteopathic Manipulative Treatment Effect on Pain Relief and QoL in Oncology Geriatric Patients: A Nonrandomized Controlled Clinical Trial. Integr. Cancer Ther. 2018, 17, 1163–1171. [Google Scholar] [CrossRef]
- Maggiani, A.; Tremolizzo, L.; Della Valentina, A.; Andrea Della Valentina Mapelli, L.; Sosio, S.; Milano, V.; Bianchi, M.; Badi, F.; Lavazza, C.; Grandini, M.; et al. Osteopathic Manual Treatment for Amyotrophic Lateral sclerosis: A Feasibility Pilot Study. Open Neurol. J. 2016, 10, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Vismara, L.; Cimolin, V.; Menegoni, F.; Zaina, F.; Galli, M.; Negrini, S.; Villa, V.; Capodaglio, P. Osteopathic manipulative treatment in obese patients with cronic low back pain: A pilot study. Man. Ther. 2012, 17, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Gamber, R.G.; Shores, J.H.; Russo, D.P.; Jimenez, C.; Rubin, B.R. Osteopathic manipulative treatment in conjunction with medication relieves pain associated with fibromyalgia syndrome: Results of a randomized clinical pilot project. J. Am. Osteopath. Assoc. 2002, 102, 321–325. [Google Scholar] [PubMed]
- Schwerla, F.; Kaiser, A.K.; Gietz, R.; Kastner, R. Osteopathic treatment of patients with long-term sequelae of whiplash injury: Effect on neck pain disability and QoL. J. Altern. Complement. Med. 2013, 19, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Licciardone, J.C.; Kearns, C.M.; Hodge, L.M.; Bergamini, M.V. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: Results from the osteopathic trial. J. Am. Osteopath. Assoc. 2012, 112, 596–605. [Google Scholar] [CrossRef]
- Cicchitti, L.; Martelli, M.; Cerritelli, F. Chronic inflammatory disease and osteopathy: A systematic review. PLoS ONE 2015, 10, e0121327. [Google Scholar] [CrossRef]
- Krasny-Pacini, A.; Evans, J.; Sohlberg, M.M.; Chevignard, M. Proposed Criteria for Appraising Goal Attainment Scales Used as Outcome Measures in Rehabilitation Research. Arch. Phys. Med. Rehabil. 2016, 97, 157–170. [Google Scholar] [CrossRef]
- Urach, S.; Gaasterland, C.; Posch, M.; Jilma, B.; Roes, K.; Rosenkranz, G.; Van der Lee, J.H.; Ristl, R. Statistical analysis of Goal Attainment Scaling endpoints in randomised trials. Stat. Methods Med. Res. 2019, 28, 1893–1910. [Google Scholar] [CrossRef]
- Haupt, R.; Spinetta, J.J.; Ban, I. Long term survivors of childhood cancer: Cure and care. The ERICE Statement. Eur. J. Cancer 2007, 43, 1778–1780. [Google Scholar] [CrossRef] [PubMed]
- Kendall, F.P.; McCreary, E.K.; Provance, P.G.; Rodgers, M.M.; Romani, W.A. Muscle Testing and Function with Posture and Pain, 5th ed.; Lippincott Williams e Wilkins: Baltimore, MD, USA, 2005; pp. 60–64. [Google Scholar]
- Ferguson, A. A review of the physiology of cranial osteopathy. J. Osteopath. Med. 2003, 6, 74–84. [Google Scholar] [CrossRef]
- Kasparian, H.; Signoret, G.; Kasparian, J. Quantification of Motion Palpation. J. Am. Osteopath. Assoc. 2015, 115, 604. [Google Scholar] [CrossRef][Green Version]
- Bovend’Eerdt, T.; Botell REWade, D.T. Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clin. Rehabil. 2009, 23, 352–361. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Gui, C.; Walsh, J.P.; Lodhia, P.; Suarez-Ahedo, C.; Domb, B.G. Correlation Between Changes in Visual Analog Scale and Patient-Reported Outcome Scores and Patient Satisfaction After Hip Arthroscopic Surgery. Orthop. J. Sports Med. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Gras, M.; Vallard, A.; Brosse, C.; Beneton, A.; Sotton, S.; Guyotat, D.; Fournel, P.; Daguenet, E.; Magné, N.; Morisson, S. Use of Complementary and Alternative Medicines among Cancer Patients: A Single-Center Study. Oncology 2019, 97, 18–25. [Google Scholar] [CrossRef]
- Anderson, J.G.; Taylor, A.G. Use of Complementary Therapies for Cancer Symptom Management: Results of the 2007 National Health Interview Survey. J. Altern. Complement. Med. 2012, 18, 235–241. [Google Scholar] [CrossRef]
- Steel, A.; Tricou, C.; Monsarrat, T.; Ruer, M.; Deslandes, C.; Sisoix, C.; Filbet, M. The perceptions and experiences of osteopathic treatment among cancer patients in palliative care: A qualitative study. Support. Care Cancer 2018, 26, 3627–3633. [Google Scholar] [CrossRef]
- Brisson, P.; Patel, H.; Scorpio, R.; Feins, N. Rotary atlanto-axial subluxation with torticollis following central-venous catheter insertion. Pediatr. Surg. Int. 2000, 16, 421–423. [Google Scholar] [CrossRef]
- Tseng-Tien, H.; Hudson, M.M.; Stokes, D.C.; Krasin, M.J.; Spunt, S.L.; Ness, K.K. Pulmonary Outcomes in Survivors of Childhood Cancer A Systematic Review. Chest 2011, 140, 881–901. [Google Scholar]
- Bossi, G.; Cerveri, I.; Volpini, E.; Corsico, A.; Baio, A.; Corbella, F.; Klersy, C.; Arico, M. Long-term pulmonary sequelae after treatment of childhood Hodgkin’s disease. Ann. Oncol. 1997, 8 (Suppl. 1), S19–S24. [Google Scholar] [CrossRef]
- Nysom, K.; Holm, K.; Hertz, H.; Hesse, B. Risk factors for reduced pulmonary function after malignant lymphoma in childhood. Med. Pediatr. Oncol. 1998, 30, 240–248. [Google Scholar] [CrossRef]
- Oguz, A.; Tayfun, T.; Citak, E.C.; Karadeniz, C.; Tatlicioglu, T.; Boyunaga, O.; Bora, H. Long-term pulmonary function in survivors of childhood Hodgkin disease and non-Hodgkin lymphoma. Pediatr. Blood Cancer 2007, 49, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.; Smith, S.; Kennel, M.; Schilling, S.; Kalpakjian, C. The Association of Performance Status and Disease Severity in Patients with Chronic Graft-vs-Host Disease. Arch. Phys. Med. Rehabil. 2018, 100, 606–612. [Google Scholar] [CrossRef]
- Porcelli, S.; Marzorati, M.; Lanfranconi, F.; Vago, P.; Pisot, R.; Grassi, B. Role of skeletal muscles impairment and brain oxygenation in limiting oxidative metabolism during exercise after bed rest. J. Appl. Physiol. 2010, 109, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Song, A.H.; Advincula, A.P. Adolescent Chronic Pelvic Pain. J. Pediatr. Adolesc. Gynecol. 2005, 18, 371–377. [Google Scholar] [CrossRef]
- Powell, J. The approach to chronic pelvic pain in the adolescent. Obstet. Gynecol. Clin. N. Am. 2014, 41, 343–355. [Google Scholar] [CrossRef]
- Beulertz, J.; Bloch, W.; Prokop, A.; Rustler, V.; Fitzen, C.; Herich, L.; Streckmann, F.; Baumann, F.T. Limitations in Ankle Dorsiflexion Range of Motion, Gait, and Walking Efficiency in Childhood Cancer Survivors. Cancer Nurs. 2016, 39, 117–124. [Google Scholar] [CrossRef]
- Beulertz, J.; Wurz, A.; Culos-Reed, N.; Chamorro Viña, C.; Bloch, W.; Baumann, F.T. Ankle Dorsiflexion in Childhood Cancer Patients: A Review of the Literature. Cancer Nurs. 2015, 38, 447–457. [Google Scholar] [CrossRef]
- Wright, M.J.; Halton, J.M.; Barr, R.D. Limitation of ankle range of motion in survivors of acute lymphoblastic leukemia: A cross-sectional study. Med. Pediatr. Oncol. 1999, 32, 279–282. [Google Scholar] [CrossRef]
- Mostoufi-Moab, S.; Ward, L.M. Skeletal Morbidity in Children and Adolescents During and Following Cancer Therapy. Horm. Res. Paediatr. 2019, 91, 137–151. [Google Scholar] [CrossRef]
- Mattano, L.A., Jr.; Devidas, M.; Nachman, J.B.; Sather, N.H.; Hunger, S.P.; Steinherz, P.G.; Gaynon, P.S.; Seibel, N.L. Children’s Oncoogy Group. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: Results from the CCG-1961 randomised cohort trial. Lancet Oncol. 2012, 13, 906–915. [Google Scholar] [CrossRef]
- Kawedia, J.D.; Kaste, S.C.; Pei, D.; Panetta, J.C.; Cai, X.; Cheng, C.; Neale, G.; Howard, S.C.; Evans, W.E.; Pui, C.; et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood 2011, 117, 2340–2347. [Google Scholar] [CrossRef]
- Smith, R.S.; Asher, A. Rehabilitation in Chronic Graft-Versus-Host Disease. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 143–151. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Borrell, S.; Simpson, R.J.; Ramírez, M.; Lucia, A. Physical function and QoL in patients with chronic graft-versus-host-disease: A summary of preclinical and clinical studies and a call for exercise intervention trials in patients. Bone Marrow Transpl. 2016, 51, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Flowers, M.E.D. Recognizing and managing chronic graft-versus-host disease. Hemat. Am. Soc. Hematol. Educ. Program 2008, 2008, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Figueroa, J.; Barrón-Calvillo, E.; García-Parra, C.; Galindo-Delgado, P.; Contreras-Ochoa, C.; Lagunas-Martínez, A.; Campos-Romero, F.H.; Silva-Estrada, J.A.; Limón-Rojas, A.E. Probiotic Supplementation Decreases Chemotherapy-induced Gastrointestinal Side Effects in Patients with Acute Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, N.S.S.; Rings, E.H.H.M.; Tissin, W.J.E. Risk analysis, diagnosis and management of gastrointestinal mucositis in pediatric cancer patients. Crit. Rev. Oncol./Hemat. 2014, 94, 87–97. [Google Scholar] [CrossRef]
- Mazlum, S.; Chaharsoughi, N.T.; Banihashem, A.; Hamidreza, B.V. The effect of massage therapy on chemotherapy-induced nausea and vomiting in pediatric cancer. Iran. J. Nurs. Midwifery Res. 2013, 18, 280–284. [Google Scholar]
- Field, T. Pediatric Massage Therapy Research: A Narrative Review. Children 2019, 6, 78. [Google Scholar] [CrossRef]
- Peryer, G.; Golder, S.; Junqueira, D.; Vohra, S.; Loke, Y.K. Chapter 19: Adverse effects. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 12 December 2021).
- Zucchetti, G.; Rossi, F.; Chamorro Vina, C.; Bertorello, N.; Fagioli, F. Exercise program for children and adolescents with leukemia and lymphoma during treatment: A comprehensive review. Pediatr. Blood Cancer 2018, 65, e26924. [Google Scholar] [CrossRef] [PubMed]
| Had—MAd | LAd | |
|---|---|---|
| Patients (n) | 75 | 29 |
| Age (yrs) | 11.08 ± 4.46 | 10.00 ± 4.30 |
| Age (yrs, range) | 3–22 | 3–18 |
| Sex (% Female) | 54.50 | 46.00 |
| Aged 3 < x < 11 yrs (%) | 50.13 | 50.00 |
| Aged 12 < x < 22 yrs (%) | 49.87 | 50.00 |
| Treatment protocol | ||
| ALL (%) | 59.70 | 62.29 |
| HSCT (%) | 27.78 | 28.95 |
| Treatment protocol | ||
| AML (%) | 10.50 | 9.84 |
| HSCT (%) | 57.79 | 66.60 |
| Treatment protocol | ||
| HL (%) | 13.28 | 9.84 |
| HSCT (%) | 18.83 | 50.00 |
| Treatment protocol | ||
| NHL (%) | 6.18 | 4.91 |
| HSCT (%) | 18.39 | 100.00 |
| Treatment protocol | ||
| Non onco (%) | 10.35 | 13.11 |
| HSCT (%) | 71.60 | 75.00 |
| >then 1 round (%) | 32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, M.; Zardo, W.; Frittoli, C.; Rivolta, C.; Valdata, V.; Bouquin, F.; Passignani, G.; Maggiani, A.; Jankovic, M.; Cossio, A.; et al. Osteopathic Treatment and Evaluation in the Clinical Setting of Childhood Hematological Malignancies. Cancers 2021, 13, 6321. https://doi.org/10.3390/cancers13246321
Barbieri M, Zardo W, Frittoli C, Rivolta C, Valdata V, Bouquin F, Passignani G, Maggiani A, Jankovic M, Cossio A, et al. Osteopathic Treatment and Evaluation in the Clinical Setting of Childhood Hematological Malignancies. Cancers. 2021; 13(24):6321. https://doi.org/10.3390/cancers13246321
Chicago/Turabian StyleBarbieri, Monica, William Zardo, Chiara Frittoli, Clara Rivolta, Valeria Valdata, Federico Bouquin, Greta Passignani, Alberto Maggiani, Momcilo Jankovic, Andrea Cossio, and et al. 2021. "Osteopathic Treatment and Evaluation in the Clinical Setting of Childhood Hematological Malignancies" Cancers 13, no. 24: 6321. https://doi.org/10.3390/cancers13246321
APA StyleBarbieri, M., Zardo, W., Frittoli, C., Rivolta, C., Valdata, V., Bouquin, F., Passignani, G., Maggiani, A., Jankovic, M., Cossio, A., Biondi, A., Balduzzi, A., & Lanfranconi, F. (2021). Osteopathic Treatment and Evaluation in the Clinical Setting of Childhood Hematological Malignancies. Cancers, 13(24), 6321. https://doi.org/10.3390/cancers13246321









