TRPM7 Ion Channel: Oncogenic Roles and Therapeutic Potential in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
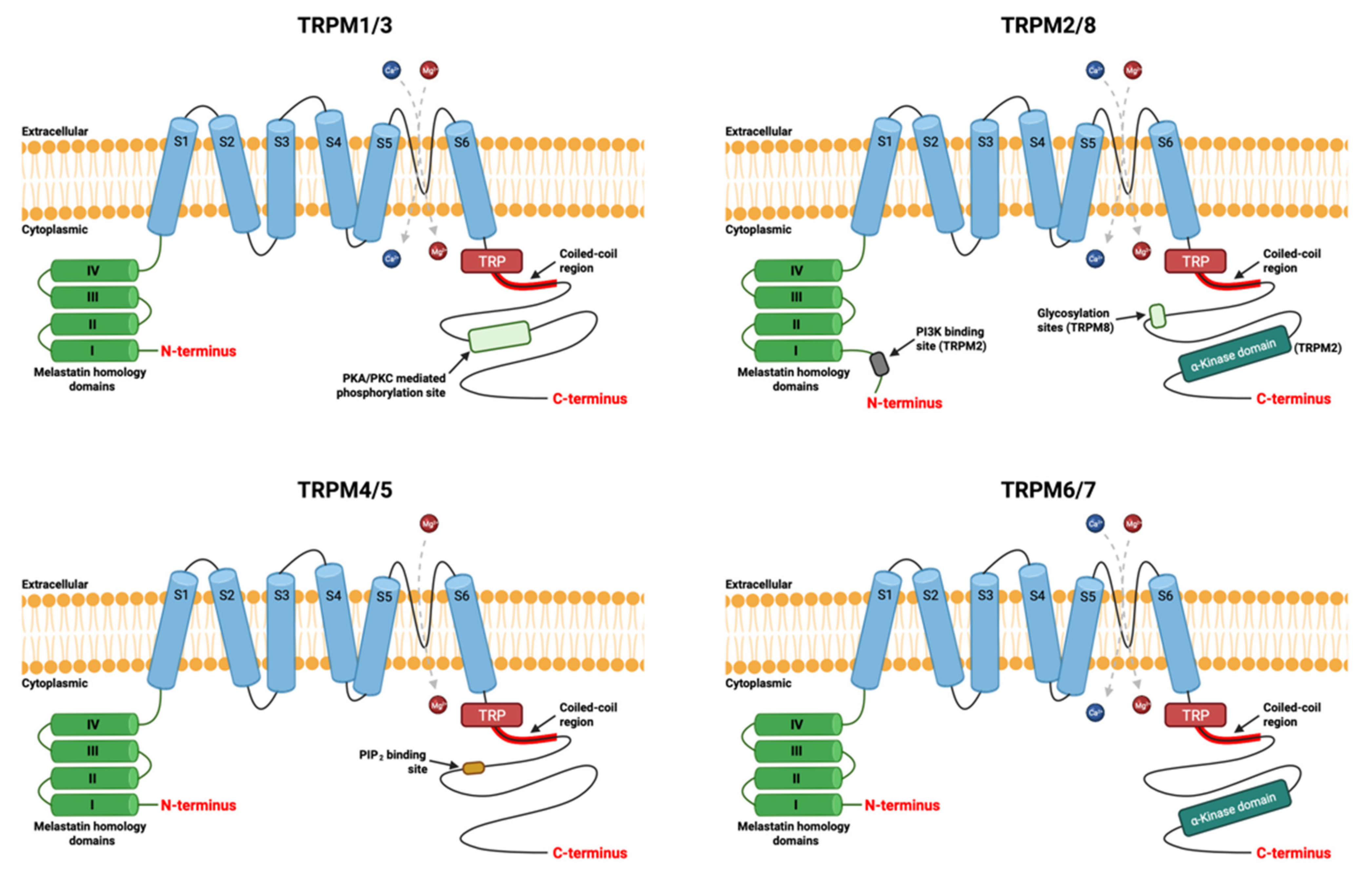
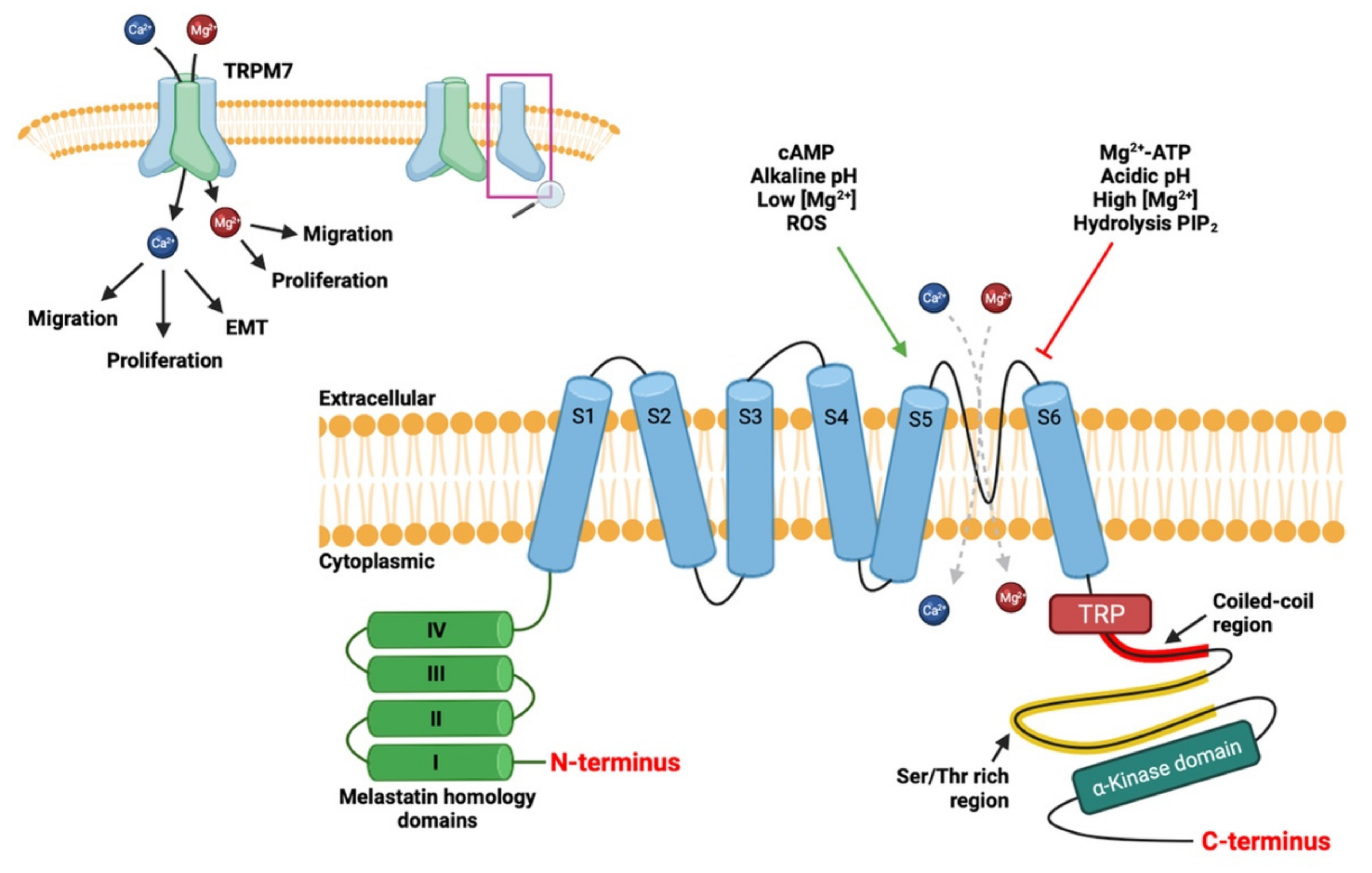
2. TRPM7 Ion Channel
2.1. Structure of Channel
2.2. Physiological Role of TRPM7
3. Role of TRPM7 in Breast Cancer Pathophysiology
3.1. TRPM7 and Proliferation
3.2. TRPM7 and Apoptosis
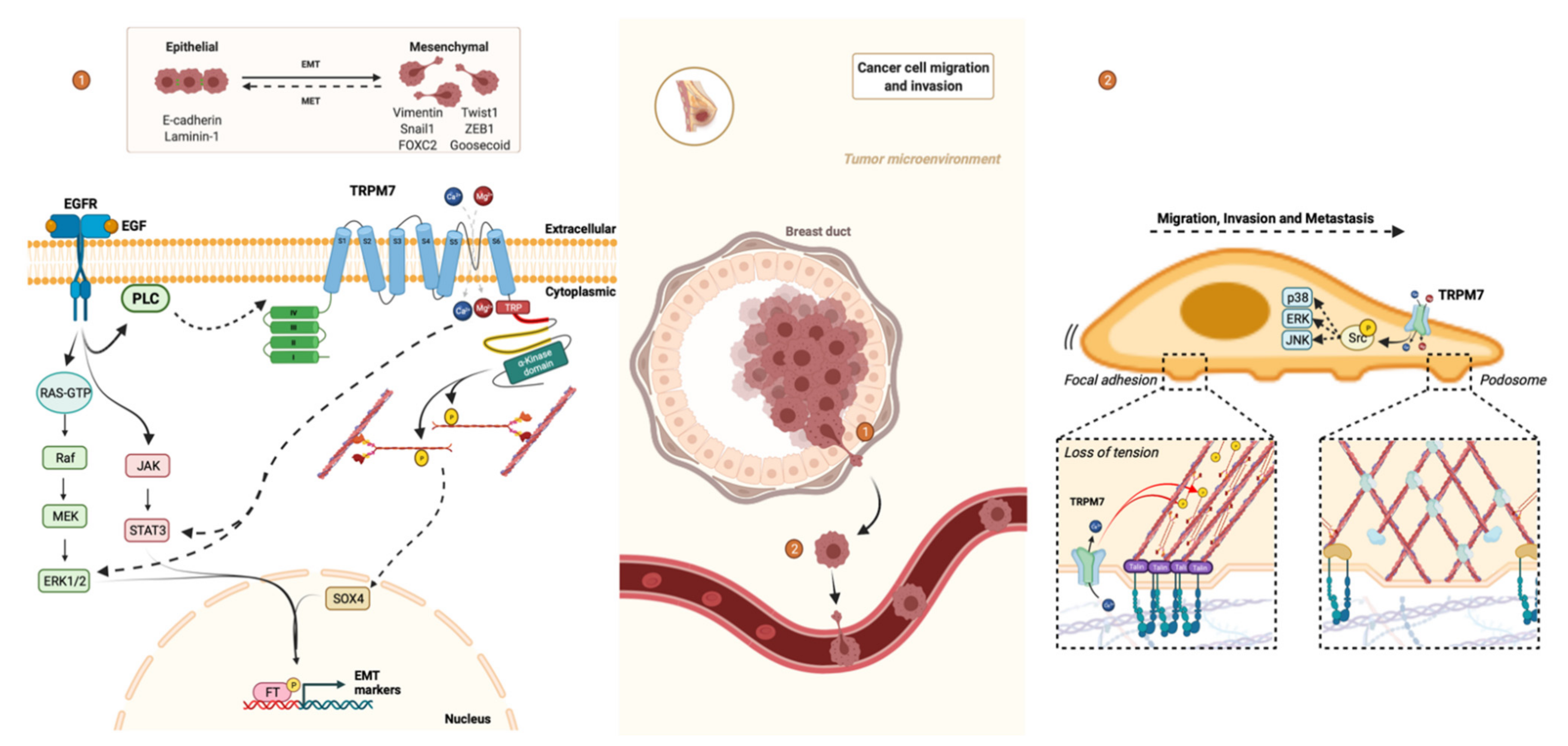
3.3. TRPM7 and Epithelial-Mesenchymal Transition
3.4. TRPM7 and Migration/Invasion
3.5. TRPM7 and Metastasis
3.6. TRPM7 and Microcalcification
4. TRPM7 as Therapeutic Target in Breast Cancer
4.1. Impact on Channel Activity
4.1.1. Lidocaine
4.1.2. Carvacrol
4.1.3. Waixenicin A
4.1.4. Ginsenoside Rd
4.1.5. Sophorae Radix
4.1.6. NS8593
4.2. Impact on Kinase Activity
5. General Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Institut National Du Cancer—Accueil. Available online: https://www.e-cancer.fr/ (accessed on 9 September 2021).
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef]
- American Cancer Society|Information and Resources about for Cancer: Breast, Colon, Lung, Prostate, Skin. Available online: https://www.cancer.org (accessed on 12 September 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Tfayli, A.; Temraz, S.; Mrad, R.A.; Shamseddine, A. Breast Cancer in Low- and Middle-Income Countries: An Emerging and Challenging Epidemic. J. Oncol. 2010, 2010, 490631. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Zheng, K.; Tan, J.-X.; Li, F.; Wei, Y.-X.; Yin, X.-D.; Su, X.-L.; Li, H.-Y.; Liu, Q.-L.; Ma, B.-L.; Ou, J.-H.; et al. Relationship between mammographic calcifications and the clinicopathologic characteristics of breast cancer in Western China: A retrospective multi-center study of 7317 female patients. Breast Cancer Res. Treat. 2017, 166, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Nyante, S.J.; Lee, S.S.; Benefield, T.S.; Hoots, T.N.; Henderson, L.M. The association between mammographic calcifications and breast cancer prognostic factors in a population-based registry cohort. Cancer 2016, 123, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Liu, Z.-B.; Xu, L.-H.; Xu, X.-L.; Liu, G.-Y.; Shao, Z.-M. Malignant calcification is an important unfavorable prognostic factor in primary invasive breast cancer. Asia-Pac. J. Clin. Oncol. 2012, 9, 139–145. [Google Scholar] [CrossRef]
- Rauch, G.M.; Hobbs, B.P.; Kuerer, H.M.; Scoggins, M.E.; Benveniste, A.P.; Park, Y.M.; Caudle, A.S.; Ms, P.S.F.; Smith, B.D.; Adrada, B.E.; et al. Microcalcifications in 1657 Patients with Pure Ductal Carcinoma in Situ of the Breast: Correlation with Clinical, Histopathologic, Biologic Features, and Local Recurrence. Ann. Surg. Oncol. 2016, 23, 482–489. [Google Scholar] [CrossRef]
- So, C.L.; Saunus, J.M.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium signalling and breast cancer. Semin. Cell Dev. Biol. 2019, 94, 74–83. [Google Scholar] [CrossRef]
- Li, C.I.; Malone, K.E.; Weiss, N.S.; Boudreau, D.M.; Cushing-Haugen, K.L.; Daling, J.R. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65–79 years. Cancer 2003, 98, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Li, C.I.; Daling, J.R.; Tang, M.-T.C.; Haugen, K.L.; Porter, P.L.; Malone, K.E. Use of Antihypertensive Medications and Breast Cancer Risk Among Women Aged 55 to 74 Years. JAMA Intern. Med. 2013, 173, 1629–1637. [Google Scholar] [CrossRef]
- Largent, J.A.; Bernstein, L.; Horn-Ross, P.L.; Marshall, S.F.; Neuhausen, S.; Reynolds, P.; Ursin, G.; Zell, J.; Ziogas, A.; Anton-Culver, H. Hypertension, antihypertensive medication use, and breast cancer risk in the California Teachers Study cohort. Cancer Causes Control. 2010, 21, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.E.; D’Aloisio, A.A.; Sandler, D.P.; Taylor, J.A. Long-term use of calcium channel blocking drugs and breast cancer risk in a prospective cohort of US and Puerto Rican women. Breast Cancer Res. 2016, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Busby, J.; Mills, K.; Zhang, S.-D.; Liberante, F.; Cardwell, C. Postdiagnostic Calcium Channel Blocker Use and Breast Cancer Mortality. Epidemiology 2018, 29, 407–413. [Google Scholar] [CrossRef]
- Shih, J.-H.; Kao, L.-T.; Chung, C.-H.; Liao, G.-S.; Fann, L.-Y.; Chien, W.-C.; Li, I.-H. Protective Association Between Calcium Channel Blocker Use and Breast Cancer Recurrence in Postsurgical Women: A Population-Based Case-Control Study in Taiwan. J. Clin. Pharmacol. 2020, 60, 785–792. [Google Scholar] [CrossRef]
- Stolarz, A.J.; Lakkad, M.; Klimberg, V.S.; Painter, J.T. Calcium Channel Blockers and Risk of Lymphedema among Breast Cancer Patients: Nested Case–Control Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1809–1815. [Google Scholar] [CrossRef]
- Takada, K.; Kashiwagi, S.; Asano, Y.; Goto, W.; Takahashi, K.; Fujita, H.; Takashima, T.; Tomita, S.; Hirakawa, K.; Ohira, M. Verification of the effects of calcium channel blockers on the immune microenvironment of breast cancer. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Haren, N.; Sevestre, H.; Ouadid-Ahidouch, H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am. J. Physiol. Cell Physiol. 2009, 297, C493–C502. [Google Scholar] [CrossRef] [PubMed]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. High Expression of Transient Receptor Potential Channels in Human Breast Cancer Epithelial Cells and Tissues: Correlation with Pathological Parameters. Cell. Physiol. Biochem. 2011, 28, 813–822. [Google Scholar] [CrossRef]
- Middelbeek, J.; Kuipers, A.J.; Henneman, L.; Visser, D.; Eidhof, I.; Van Horssen, R.; Wieringa, B.; Canisius, S.V.; Zwart, W.; Wessels, L.F.; et al. TRPM7 Is Required for Breast Tumor Cell Metastasis. Cancer Res. 2012, 72, 4250–4261. [Google Scholar] [CrossRef]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Rybarczyk, P.; Sahni, J.; Sevestre, H.; Scharenberg, A.M.; Ouadid-Ahidouch, H. Transient receptor potential melastatin 7 is involved in oestrogen receptor-negative metastatic breast cancer cells migration through its kinase domain. Eur. J. Cancer 2013, 49, 3694–3707. [Google Scholar] [CrossRef]
- Meng, X.; Cai, C.; Wu, J.; Cai, S.; Ye, C.; Chen, H.; Yang, Z.; Zeng, H.; Shen, Q.; Zou, F. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett. 2013, 333, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP channels: An overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Minke, B. TRP channels and Ca2+ signaling. Cell Calcium 2006, 40, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xiao, C.; Liu, H.; Huang, Y.; Dilger, J.P.; Lin, J. Effects of local anesthetics on breast cancer cell viability and migration. BMC Cancer 2018, 18, 666. [Google Scholar] [CrossRef]
- Simon, F.; Varela, D.; Cabello-Verrugio, C. Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell. Signal. 2013, 25, 1614–1624. [Google Scholar] [CrossRef]
- Zholos, A.; Johnson, C.; Burdyga, T.; Melanaphy, D. TRPM Channels in the Vasculature. Transient Receptor Potential Channels 2010, 704, 707–729. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7—Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2007, 1772, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. The TRP Superfamily of Cation Channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef] [PubMed]
- Harteneck, C.; Plant, T.D.; Schultz, G. From worm to man: Three subfamilies of TRP channels. Trends Neurosci. 2000, 23, 159–166. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Fleig, A.; Penner, R. Emerging roles of TRPM channels. Novartis Found. Symp. 2004, 258, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 Provides an Ion Channel Mechanism for Cellular Entry of Trace Metal Ions. J. Gen. Physiol. 2002, 121, 49–60. [Google Scholar] [CrossRef]
- Clark, K.; Middelbeek, J.; Morrice, N.A.; Figdor, C.; Lasonder, E.; Van Leeuwen, F.N. Massive Autophosphorylation of the Ser/Thr-Rich Domain Controls Protein Kinase Activity of TRPM6 and TRPM7. PLoS ONE 2008, 3, e1876. [Google Scholar] [CrossRef]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef]
- Schmitz, C.; Perraud, A.-L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef]
- Runnels, L.; Yue, L.; Clapham, D. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat. Cell Biol. 2002, 4, 329–336. [Google Scholar] [CrossRef]
- Desai, B.N.; Krapivinsky, G.; Navarro, B.; Krapivinsky, L.; Carter, B.C.; Febvay, S.; Delling, M.; Penumaka, A.; Ramsey, I.S.; Manasian, Y.; et al. Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in fas-induced apoptosis. Dev. Cell 2012, 22, 1149–1162. [Google Scholar] [CrossRef]
- Nadler, M.J.S.; Hermosura, M.C.; Inabe, K.; Perraud, A.-L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.-P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef]
- Demeuse, P.; Penner, R.; Fleig, A. TRPM7 Channel Is Regulated by Magnesium Nucleotides via its Kinase Domain. J. Gen. Physiol. 2006, 127, 421–434. [Google Scholar] [CrossRef]
- Chokshi, R.; Matsushita, M.; Kozak, J.A. Detailed examination of Mg2+ and pH sensitivity of human TRPM7 channels. Am. J. Physiol. Cell Physiol. 2012, 302, C1004–C1011. [Google Scholar] [CrossRef]
- Kerschbaum, H.H.; Kozak, J.A.; Cahalan, M.D. Polyvalent Cations as Permeant Probes of MIC and TRPM7 Pores. Biophys. J. 2003, 84, 2293–2305. [Google Scholar] [CrossRef]
- Jiang, J.; Li, M.; Yue, L. Potentiation of TRPM7 Inward Currents by Protons. J. Gen. Physiol. 2005, 126, 137–150. [Google Scholar] [CrossRef]
- Yee, N.S.; Kazi, A.A.; Yee, R.K. Cellular and Developmental Biology of TRPM7 Channel-Kinase: Implicated Roles in Cancer. Cells 2014, 3, 751–777. [Google Scholar] [CrossRef] [PubMed]
- Visser, D.; Middelbeek, J.; van Leeuwen, F.N.; Jalink, K. Function and regulation of the channel-kinase TRPM7 in health and disease. Eur. J. Cell Biol. 2014, 93, 455–465. [Google Scholar] [CrossRef]
- Broertjes, J.; Klarenbeek, J.; Habani, Y.; Langeslag, M.; Jalink, K. TRPM7 residue S1269 mediates cAMP dependence of Ca2+ influx. PLoS ONE 2019, 14, e0209563. [Google Scholar] [CrossRef]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Langeslag, M.; Van Leeuwen, B.; Ran, L.; Ryazanov, A.G.; Figdor, C.; Moolenaar, W.H.; Jalink, K.; van Leeuwen, F.N. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006, 25, 290–301. [Google Scholar] [CrossRef]
- Wei, C.; Wang, X.; Chen, M.; Ouyang, K.; Song, L.-S.; Cheng, H. Calcium flickers steer cell migration. Nat. Cell Biol. 2009, 457, 901–905. [Google Scholar] [CrossRef]
- Tsai, F.-C.; Meyer, T. Ca2+ Pulses Control Local Cycles of Lamellipodia Retraction and Adhesion along the Front of Migrating Cells. Curr. Biol. 2012, 22, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Visser, D.; Langeslag, M.; Kedziora, K.; Klarenbeek, J.; Kamermans, A.; Horgen, F.D.; Fleig, A.; van Leeuwen, F.N.; Jalink, K. TRPM7 triggers Ca2+ sparks and invadosome formation in neuroblastoma cells. Cell Calcium 2013, 54, 404–415. [Google Scholar] [CrossRef]
- Kozak, J.A.; Cahalan, M.D. MIC Channels Are Inhibited by Internal Divalent Cations but Not ATP. Biophys. J. 2003, 84, 922–927. [Google Scholar] [CrossRef]
- Liu, T.; Song, Y.; Chen, H.; Pan, S.; Sun, X. Matrine Inhibits Proliferation and Induces Apoptosis of Pancreatic Cancer Cells In Vitro and In Vivo. Biol. Pharm. Bull. 2010, 33, 1740–1745. [Google Scholar] [CrossRef]
- Elizondo, M.R.; Arduini, B.L.; Paulsen, J.; MacDonald, E.L.; Sabel, J.L.; Henion, P.D.; Cornell, R.; Parichy, D.M. Defective Skeletogenesis with Kidney Stone Formation in Dwarf Zebrafish Mutant for trpm7. Curr. Biol. 2005, 15, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Trappmann, B.; Gautrot, J.; Connelly, J.; Strange, D.G.T.; Li, Y.; Oyen, M.; Stuart, M.A.C.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef]
- Mammoto, T.; Mammoto, A.; Ingber, D.E. Mechanobiology and Developmental Control. Annu. Rev. Cell Dev. Biol. 2013, 29, 27–61. [Google Scholar] [CrossRef]
- Bellas, E.; Chen, C.S. Forms, forces, and stem cell fate. Curr. Opin. Cell Biol. 2014, 31, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Dufort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef]
- Hanano, T.; Hara, Y.; Shi, J.; Morita, H.; Umebayashi, C.; Mori, E.; Sumimoto, H.; Ito, Y.; Mori, Y.; Inoue, R. Involvement of TRPM7 in Cell Growth as a Spontaneously Activated Ca2+ Entry Pathway in Human Retinoblastoma Cells. J. Pharmacol. Sci. 2004, 95, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.; Iihara, K.; Wei, W.-L.; Xiong, Z.-G.; Arundine, M.; Cerwinski, W.; MacDonald, J.F.; Tymianski, M. A Key Role for TRPM7 Channels in Anoxic Neuronal Death. Cell 2003, 115, 863–877. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Mochida, S.; Krapivinsky, L.; Cibulsky, S.M.; Clapham, D.E. The TRPM7 Ion Channel Functions in Cholinergic Synaptic Vesicles and Affects Transmitter Release. Neuron 2006, 52, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Lim, H.; Yang, D.K.; Jun, J.Y.; Chang, I.Y.; Park, C.; So, I.; Stanfield, P.R.; Kim, K.W. Melastatin-Type Transient Receptor Potential Channel 7 Is Required for Intestinal Pacemaking Activity. Gastroenterology 2005, 129, 1504–1517. [Google Scholar] [CrossRef]
- Chen, L.; Cao, R.; Wang, G.; Yuan, L.; Qian, G.; Guo, Z.; Wu, C.-L.; Wang, X.; Xiao, Y. Downregulation of TRPM7 suppressed migration and invasion by regulating epithelial–mesenchymal transition in prostate cancer cells. Med. Oncol. 2017, 34, 127. [Google Scholar] [CrossRef]
- Chen, J.; Luan, Y.; Yu, R.; Zhang, Z.; Zhang, J.; Wang, W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci. Trends 2014, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Park, E.-J.; Lee, J.H.; Jeon, J.-H.; Kim, S.J.; So, I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008, 99, 2502–2509. [Google Scholar] [CrossRef]
- Du, G.-J.; Li, J.-H.; Liu, W.-J.; Liu, Y.-H.; Zhao, B.; Hou, X.-D.; Qi, X.-X.; Duan, Y.-J.; Li, H.-R.; Li, H. The combination of TRPM8 and TRPA1 expression causes an invasive phenotype in lung cancer. Tumor Biol. 2013, 35, 1251–1261. [Google Scholar] [CrossRef]
- Kim, B.J.; Hong, C. Role of transient receptor potential melastatin type 7 channel in gastric cancer. Integr. Med. Res. 2016, 5, 124–130. [Google Scholar] [CrossRef]
- Lee, E.H.; Chun, S.Y.; Kim, B.; Yoon, B.H.; Lee, J.N.; Kim, B.S.; Yoo, E.S.; Lee, S.; Song, P.H.; Kwon, T.G.; et al. Knockdown of TRPM7 prevents tumor growth, migration, and invasion through the Src, Akt, and JNK pathway in bladder cancer. BMC Urol. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Gao, S.-L.; Kong, C.-Z.; Zhang, Z.; Sheng-Lin, G.; Bi, J.-B.; Liu, X.-K. TRPM7 is overexpressed in bladder cancer and promotes proliferation, migration, invasion and tumor growth. Oncol. Rep. 2017, 38, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Meng, Z.; Liu, T.; Wang, G.; Qian, G.; Cao, T.; Guan, X.; Dan, H.; Xiao, Y.; Wang, X. DecreasedTRPM7inhibits activities and induces apoptosis of bladder cancer cells via ERK1/2 pathway. Oncotarget 2016, 7, 72941–72960. [Google Scholar] [CrossRef]
- Dong, R.; Zhuang, Y.; Wang, Y.; Zhang, Z.; Xu, X.; Mao, Y.; Yu, J. Tumor suppressor miR-192-5p targets TRPM7 and inhibits proliferation and invasion in cervical cancer. Kaohsiung J. Med. Sci. 2021, 37, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Sato-Numata, K.; Okada, Y. TRPM7 is involved in acid-induced necrotic cell death in a manner sensitive to progesterone in human cervical cancer cells. Physiol. Rep. 2019, 7, e14157. [Google Scholar] [CrossRef]
- Liu, L.; Wu, N.; Wang, Y.; Zhang, X.; Xia, B.; Tang, J.; Cai, J.; Zhao, Z.; Liao, Q.; Wang, J. TRPM7 promotes the epithelial–mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. J. Exp. Clin. Cancer Res. 2019, 38, 1–15. [Google Scholar] [CrossRef]
- Pugliese, D.; Armuzzi, A.; Castri, F.; Benvenuto, R.; Mangoni, A.; Guidi, L.; Gasbarrini, A.; Rapaccini, G.L.; Wolf, F.I.; Trapani, V. TRPM7 is overexpressed in human IBD-related and sporadic colorectal cancer and correlates with tumor grade. Dig. Liver Dis. 2020, 52, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Chatterjee, P.K.; Madankumar, S.; Mehdi, S.F.; Xue, X.; Metz, C.N. Magnesium deficiency with high calcium-to-magnesium ratio promotes a metastatic phenotype in the CT26 colon cancer cell line. Magnes. Res. 2020, 33, 68–85. [Google Scholar] [CrossRef]
- Su, F.; Wang, B.-F.; Zhang, T.; Hou, X.-M.; Feng, M.-H. TRPM7 deficiency suppresses cell proliferation, migration, and invasion in human colorectal cancer via regulation of epithelial-mesenchymal transition. Cancer Biomark. 2019, 26, 451–460. [Google Scholar] [CrossRef]
- Mason, M.J.; Schaffner, C.; Floto, R.A.; Teo, Q.A. Constitutive expression of a Mg2+-inhibited K+current and a TRPM7-like current in human erythroleukemia cells. Am. J. Physiol. Cell Physiol. 2012, 302, C853–C867. [Google Scholar] [CrossRef]
- Kim, B.J. Involvement of melastatin type transient receptor potential 7 channels in ginsenoside Rd-induced apoptosis in gastric and breast cancer cells. J. Ginseng Res. 2013, 37, 201–209. [Google Scholar] [CrossRef]
- Kim, B.-J. Involvement of Transient Receptor Potential Melastatin 7 Channels in Sophorae Radix-induced Apoptosis in Cancer Cells—Sophorae Radix and TRPM7. J. Pharmacopunct. 2012, 15, 31–38. [Google Scholar] [CrossRef]
- Kim, B.J.; Nah, S.-Y.; Jeon, J.-H.; So, I.; Kim, S.J. Transient Receptor Potential Melastatin 7 Channels are Involved in Ginsenoside Rg3-Induced Apoptosis in Gastric Cancer Cells. Basic Clin. Pharmacol. Toxicol. 2011, 109, 233–239. [Google Scholar] [CrossRef]
- Wong, R.; Gong, H.; Alanazi, R.; Bondoc, A.; Luck, A.; Sabha, N.; Horgen, F.D.; Fleig, A.; Rutka, J.T.; Feng, Z.-P.; et al. Inhibition of TRPM7 with waixenicin A reduces glioblastoma cellular functions. Cell Calcium 2020, 92, 102307. [Google Scholar] [CrossRef]
- Thuringer, D.; Chanteloup, G.; Winckler, P.; Garrido, C. The vesicular transfer of CLIC1 from glioblastoma to microvascular endothelial cells requires TRPM7. Oncotarget 2018, 9, 33302–33311. [Google Scholar] [CrossRef]
- Alptekin, M.; Eroglu, S.; Tutar, E.; Şencan, S.; Geyik, M.A.; Ulasli, M.; Demiryurek, A.T.; Camci, C. Gene expressions of TRP channels in glioblastoma multiforme and relation with survival. Tumor Biol. 2015, 36, 9209–9213. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.-D.; Li, M.-H.; Shen, J.-F.; Liu, M.-L.; Li, X.-B.; Sun, H.-W.; Branigan, D.; Zeng, Z.; Si, H.-F.; Li, J.; et al. Suppression of TRPM7 inhibits proliferation, migration, and invasion of malignant human glioma cells. CNS Neurosci. Ther. 2014, 21, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Inoue, K.; Leng, T.; Guo, S.; Xiong, Z.-G. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell. Signal. 2014, 26, 2773–2781. [Google Scholar] [CrossRef]
- Qiao, W.; Lan, X.; Ma, H.; Chan, J.; Lui, V.; Yeung, K.; Kwong, D.; Hu, Z.; Tsoi, J.; Matinlinna, J.; et al. Effects of Salivary Mg on Head and Neck Carcinoma via TRPM7. J. Dent. Res. 2019, 98, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, M.-H.; Inoue, K.; Chu, X.-P.; Seeds, J.; Xiong, Z.-G. Transient Receptor Potential Melastatin 7–like Current in Human Head and Neck Carcinoma Cells: Role in Cell Proliferation. Cancer Res. 2007, 67, 10929–10938. [Google Scholar] [CrossRef]
- Takahashi, K.; Umebayashi, C.; Numata, T.; Honda, A.; Ichikawa, J.; Hu, Y.; Yamaura, K.; Inoue, R. TRPM7-mediated spontaneous Ca2+ entry regulates the proliferation and differentiation of human leukemia cell line K562. Physiol. Rep. 2018, 6, e13796. [Google Scholar] [CrossRef]
- Zierler, S.; Yao, G.; Zhang, Z.; Kuo, W.C.; Pörzgen, P.; Penner, R.; Horgen, F.D.; Fleig, A. Waixenicin A Inhibits Cell Proliferation through Magnesium-dependent Block of Transient Receptor Potential Melastatin 7 (TRPM7) Channels. J. Biol. Chem. 2011, 286, 39328–39335. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Rodboon, N.; Samart, P.; Vinayanuwattikun, C.; Klamkhlai, S.; Chanvorachote, P.; Rojanasakul, Y.; Issaragrisil, S. A novel TRPM7/O-GlcNAc axis mediates tumour cell motility and metastasis by stabilising c-Myc and caveolin-1 in lung carcinoma. Br. J. Cancer 2020, 123, 1289–1301. [Google Scholar] [CrossRef]
- Gao, H.; Chen, X.; Du, X.; Guan, B.; Liu, Y.; Zhang, H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium 2011, 50, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Rybarczyk, P.; Bretaudeau, C.; Vanlaeys, A.; Cousin, R.; Brassart-Pasco, S.; Chatelain, D.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Brassart, B.; et al. TRPM7/RPSA Complex Regulates Pancreatic Cancer Cell Migration. Front. Cell Dev. Biol. 2020, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Edwards, M.J.; Ahmad, S.A. Intracellular Ion Channels in Pancreas Cancer. Cell. Physiol. Biochem. 2019, 53, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, P.; Gautier, M.; Hague, F.; Dhennin-Duthille, I.; Chatelain, D.; Kerr-Conte, J.; Pattou, F.; Regimbeau, J.-M.; Sevestre, H.; Ouadid-Ahidouch, H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer 2012, 131, E851–E861. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, J.-Y.; Lu, M.-H.; Shi, Z.; Na, N.; Di, J.-M. Carvacrol Alleviates Prostate Cancer Cell Proliferation, Migration, and Invasion through Regulation of PI3K/Akt and MAPK Signaling Pathways. Oxidative Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Lin, C.-M.; Ma, J.-M.; Zhang, L.; Hao, Z.-Y.; Zhou, J.; Zhou, Z.-Y.; Shi, H.-Q.; Zhang, Y.-F.; Shao, E.-M.; Liang, C.-Z. Inhibition of Transient Receptor Potential Melastain 7 Enhances Apoptosis Induced by TRAIL in PC-3 cells. Asian Pac. J. Cancer Prev. 2015, 16, 4469–4475. [Google Scholar] [CrossRef]
- Sun, Y.; Selvaraj, S.; Varma, A.; Derry, S.; Sahmoun, A.E.; Singh, B.B. Increase in Serum Ca2+/Mg2+ Ratio Promotes Proliferation of Prostate Cancer Cells by Activating TRPM7 Channels. J. Biol. Chem. 2013, 288, 255–263. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Q.-J.; Zhang, Y.; Zhou, H.; Luo, C.-H.; Tang, J.; Wang, Y.; Tang, Y.; Zhao, M.; Zhao, X.-H.; et al. TRPM7 is required for ovarian cancer cell growth, migration and invasion. Biochem. Biophys. Res. Commun. 2014, 454, 547–553. [Google Scholar] [CrossRef]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of somatic mutation in human cancer genomes. Nat. Cell Biol. 2007, 446, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dilger, J.P.; Lin, J. The Role of Transient Receptor Potential Melastatin 7 (TRPM7) in Cell Viability: A Potential Target to Suppress Breast Cancer Cell Cycle. Cancers 2020, 12, 131. [Google Scholar] [CrossRef]
- Yee, N.S.; Zhou, W.; Liang, I.-C. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis. Model. Mech. 2011, 4, 240–254. [Google Scholar] [CrossRef]
- Yee, N.S. Role of TRPM7 in Cancer: Potential as Molecular Biomarker and Therapeutic Target. Pharmaceuticals 2017, 10, 39. [Google Scholar] [CrossRef]
- Wang, H.; Li, B.; Asha, K.; Pangilinan, R.L.; Thuraisamy, A.; Chopra, H.; Rokudai, S.; Yu, Y.; Prives, C.L.; Zhu, Y. The ion channel TRPM7 regulates zinc-depletion-induced MDMX degradation. J. Biol. Chem. 2021, 297. [Google Scholar] [CrossRef]
- Song, C.; Choi, S.; Oh, K.; Sim, T. Suppression of TRPM7 enhances TRAIL-induced apoptosis in triple-negative breast cancer cells. J. Cell. Physiol. 2020, 235, 10037–10050. [Google Scholar] [CrossRef] [PubMed]
- Day, T.W.; Huang, S.; Safa, A.R. c-FLIP knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and caspase-9-dependent apoptosis in breast cancer cells. Biochem. Pharmacol. 2008, 76, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Azimi, I.; Faville, R.A.; Peters, A.A.; Jalink, K.; Putney, J.W.; Goodhill, G.J.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Induction of epithelial–mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene 2014, 33, 2307–2316. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Nieto, M.A. The Ins and Outs of the Epithelial to Mesenchymal Transition in Health and Disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 347–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Q.; Lei, Y.; Yao, M.; Li, L.; Gao, X.; Feng, J.; Zhang, Y.; Gao, H.; Liu, D.-X.; et al. SOX4 Induces Epithelial–Mesenchymal Transition and Contributes to Breast Cancer Progression. Cancer Res. 2012, 72, 4597–4608. [Google Scholar] [CrossRef]
- Kuipers, A.J.; Middelbeek, J.; van Leeuwen, F.N. Mechanoregulation of cytoskeletal dynamics by TRP channels. Eur. J. Cell Biol. 2012, 91, 834–846. [Google Scholar] [CrossRef]
- Vrenken, K.S.; Jalink, K.; van Leeuwen, F.N.; Middelbeek, J. Beyond ion-conduction: Channel-dependent and -independent roles of TRP channels during development and tissue homeostasis. Biochim. Biophys. Acta (BBA)—Bioenerg. 2016, 1863, 1436–1446. [Google Scholar] [CrossRef]
- Kuipers, A.J.; Middelbeek, J.; Vrenken, K.; Pérez-González, C.; Poelmans, G.; Klarenbeek, J.; Jalink, K.; Trepat, X.; van Leeuwen, F.N. TRPM7 controls mesenchymal features of breast cancer cells by tensional regulation of SOX4. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 2409–2419. [Google Scholar] [CrossRef]
- De La Roche, M.A.; Smith, J.L.; Betapudi, V.; Egelhoff, T.T.; Côté, G.P. Signaling pathways regulating Dictyostelium myosin II. J. Muscle Res. Cell Motil. 2002, 23, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Wennerberg, K. Rho and Rac Take Center Stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Egelhoff, T.T.; Lee, R.J.; Spudich, J.A. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell 1993, 75, 363–371. [Google Scholar] [CrossRef]
- Nakasawa, T.; Takahashi, M.; Matsuzawa, F.; Aikawa, S.; Togashi, Y.; Saitoh, T.; Yamagishi, A.; Yazawa, M. Critical Regions for Assembly of Vertebrate Nonmuscle Myosin II. Biochemistry 2004, 44, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Bae, Y.; Jun, J.; Lee, H.; Kim, N.D.; Lee, K.-B.; Hur, W.; Park, J.-Y.; Sim, T. Identification of TG100-115 as a new and potent TRPM7 kinase inhibitor, which suppresses breast cancer cell migration and invasion. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 947–957. [Google Scholar] [CrossRef]
- Weigelt, B.; Peterse, J.L.; Van ’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Eddy, R.J.; Condeelis, J. The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer 2007, 7, 429–440. [Google Scholar] [CrossRef]
- Kao, K.-J.; Chang, K.-M.; Hsu, H.-C.; Huang, A.T. Correlation of microarray-based breast cancer molecular subtypes and clinical outcomes: Implications for treatment optimization. BMC Cancer 2011, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Haverty, P.M.; Fridlyand, J.; Li, L.; Getz, G.; Beroukhim, R.; Lohr, S.; Wu, T.D.; Cavet, G.; Zhang, Z.; Chant, J. High-resolution genomic and expression analyses of copy number alterations in breast tumors. Genes Chromosom. Cancer 2008, 47, 530–542. [Google Scholar] [CrossRef]
- Gülsün, M.; Demirkazık, F.B.; Arıyürek, M. Evaluation of breast microcalcifications according to breast imaging reporting and data system criteria and Le Gal’s classification. Eur. J. Radiol. 2003, 47, 227–231. [Google Scholar] [CrossRef]
- Tse, G.M.; Tan, P.-H.; Cheung, H.S.; Chu, W.C.W.; Lam, W.W.M. Intermediate to highly suspicious calcification in breast lesions: A radio-pathologic correlation. Breast Cancer Res. Treat. 2007, 110, 1–7. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Davies, J.D.; Reynolds, J.L.; McNair, R.; Jones, G.T.; Sidibe, A.; Schurgers, L.J.; Skepper, J.N.; Proudfoot, D.; Mayr, M.; et al. Calcium Regulates Key Components of Vascular Smooth Muscle Cell–Derived Matrix Vesicles to Enhance Mineralization. Circ. Res. 2011, 109, e1–e12. [Google Scholar] [CrossRef]
- Sahmoun, A.E.; Mandavilli, S.; Singh, B.B. Serum calcium levels, TRPM7, TRPC1, microcalcifications, and breast cancer using breast imaging reporting and data system scores. Breast Cancer Targets Ther. 2012, 5, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Grady, S.; Morgan, M.P. Deposition of calcium in an in vitro model of human breast tumour calcification reveals functional role for ALP activity, altered expression of osteogenic genes and dysregulation of the TRPM7 ion channel. Sci. Rep. 2019, 9, 542. [Google Scholar] [CrossRef]
- Sonou, T.; Ohya, M.; Yashiro, M.; Masumoto, A.; Nakashima, Y.; Ito, T.; Mima, T.; Negi, S.; Kimura-Suda, H.; Shigematsu, T. Magnesium prevents phosphate-induced vascular calcification via TRPM7 and Pit-1 in an aortic tissue culture model. Hypertens. Res. 2017, 40, 562–567. [Google Scholar] [CrossRef]
- Louvet, L.; Büchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol. Dial. Transplant. 2013, 28, 869–878. [Google Scholar] [CrossRef]
- Jhan, J.-R.; Andrechek, E.R. Triple-negative breast cancer and the potential for targeted therapy. Pharmacogenomics 2017, 18, 1595–1609. [Google Scholar] [CrossRef]
- Diana, A.; Franzese, E.; Centonze, S.; Carlino, F.; Della Corte, C.M.; Ventriglia, J.; Petrillo, A.; De Vita, F.; Alfano, R.; Ciardiello, F.; et al. Triple-Negative Breast Cancers: Systematic Review of the Literature on Molecular and Clinical Features with a Focus on Treatment with Innovative Drugs. Curr. Oncol. Rep. 2018, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dilger, J.P.; Lin, J. Effects of local anesthetics on cancer cells. Pharmacol. Ther. 2020, 212, 107558. [Google Scholar] [CrossRef]
- Liu, H.; Dilger, J.P.; Lin, J. Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as A Target for Some Breast Cancer Cell Lines. Cancers 2021, 13, 234. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Wu, Y.; Zhang, Y. Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation. Life Sci. 2021, 266, 118894. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Nam, J.H.; Kwon, Y.K.; So, I.; Kim, S.J. The Role of Waixenicin A as Transient Receptor Potential Melastatin 7 Blocker. Basic Clin. Pharmacol. Toxicol. 2013, 112, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Schnitzler, M.M.Y.; Meißner, M.; Schäfer, S.; Abstiens, K.; Hofmann, T.; Gudermann, T. Natural and synthetic modulators of SK (Kca2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br. J. Pharmacol. 2012, 166, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Royds, J.; Khan, A.H.; Buggy, D. An Update on Existing Ongoing Prospective Trials Evaluating the Effect of Anesthetic and Analgesic Techniques During Primary Cancer Surgery on Cancer Recurrence or Metastasis. Int. Anesthesiol. Clin. 2016, 54, e76–e83. [Google Scholar] [CrossRef]
- Leng, T.-D.; Lin, J.; Sun, H.-W.; Zeng, Z.; O’Bryant, Z.; Inoue, K.; Xiong, Z.-G. Local Anesthetic Lidocaine Inhibits TRPM7 Current and TRPM7-Mediated Zinc Toxicity. CNS Neurosci. Ther. 2014, 21, 32–39. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2014, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Parajuli, S.P.; Yeum, C.H.; Park, J.S.; Jeong, H.S.; So, I.; Kim, K.W.; Jun, J.Y.; Choi, S. Effects of Ginseng Total Saponins on Pacemaker Currents of Interstitial Cells of Cajal from the Small Intestine of Mice. Biol. Pharm. Bull. 2007, 30, 2037–2042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.; Lee, M.R.; Lee, G.S.; An, W.G.; Cho, S.I. Effect of Sophora flavescens Aiton extract on degranulation of mast cells and contact dermatitis induced by dinitrofluorobenzene in mice. J. Ethnopharmacol. 2012, 142, 253–258. [Google Scholar] [CrossRef] [PubMed]
| Involved Cancer | Expression | Oncogenic Roles | Ref. |
|---|---|---|---|
| Bladder |
|
| [72,73,74] |
| Breast |
|
| Not reported |
| Cervical |
|
| [75,76,77] |
| Colorectal |
|
| [78,79,80] |
| Erythroleukemia |
|
| [81] |
| Gastric |
|
| [69,82,83,84] |
| Glioblastoma |
|
| [85,86,87,88,89] |
| Head and Neck carcinoma |
|
| [90,91] |
| Leukemia |
|
| [92,93] |
| Lung |
|
| [94,95] |
| Melanoma |
|
| [96] |
| Neuroblastoma |
|
| [55] |
| Pancreatic Adenocarcinoma |
|
| [97,98,99] |
| Prostate |
|
| [67,100,101,102] |
| Ovarian |
|
| [77,103,104] |
| Retinoblastoma |
|
| [63] |
| Impacted Activity | Chemical Modulator | Mechanism of Action | Ref. |
|---|---|---|---|
| Channel Activity | Lidocaine |
| [136,137] |
| Channel Activity | Carvacrol |
| [138] |
| Channel Activity | Waixenicin A |
| [93,139] |
| Channel Activity | Ginsenoside Rd |
| [82] |
| Channel Activity | Sophorae Radix |
| [83] |
| Channel Activity | NS8593 |
| [109,111,140] |
| Kinase Activity | TG100-115 |
| [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordier, C.; Prevarskaya, N.; Lehen’kyi, V. TRPM7 Ion Channel: Oncogenic Roles and Therapeutic Potential in Breast Cancer. Cancers 2021, 13, 6322. https://doi.org/10.3390/cancers13246322
Cordier C, Prevarskaya N, Lehen’kyi V. TRPM7 Ion Channel: Oncogenic Roles and Therapeutic Potential in Breast Cancer. Cancers. 2021; 13(24):6322. https://doi.org/10.3390/cancers13246322
Chicago/Turabian StyleCordier, Clément, Natalia Prevarskaya, and V’yacheslav Lehen’kyi. 2021. "TRPM7 Ion Channel: Oncogenic Roles and Therapeutic Potential in Breast Cancer" Cancers 13, no. 24: 6322. https://doi.org/10.3390/cancers13246322
APA StyleCordier, C., Prevarskaya, N., & Lehen’kyi, V. (2021). TRPM7 Ion Channel: Oncogenic Roles and Therapeutic Potential in Breast Cancer. Cancers, 13(24), 6322. https://doi.org/10.3390/cancers13246322








