T Cell Aging in Patients with Colorectal Cancer—What Do We Know So Far?
Abstract
:Simple Summary
Abstract
1. Colorectal Cancer in Aged Individuals
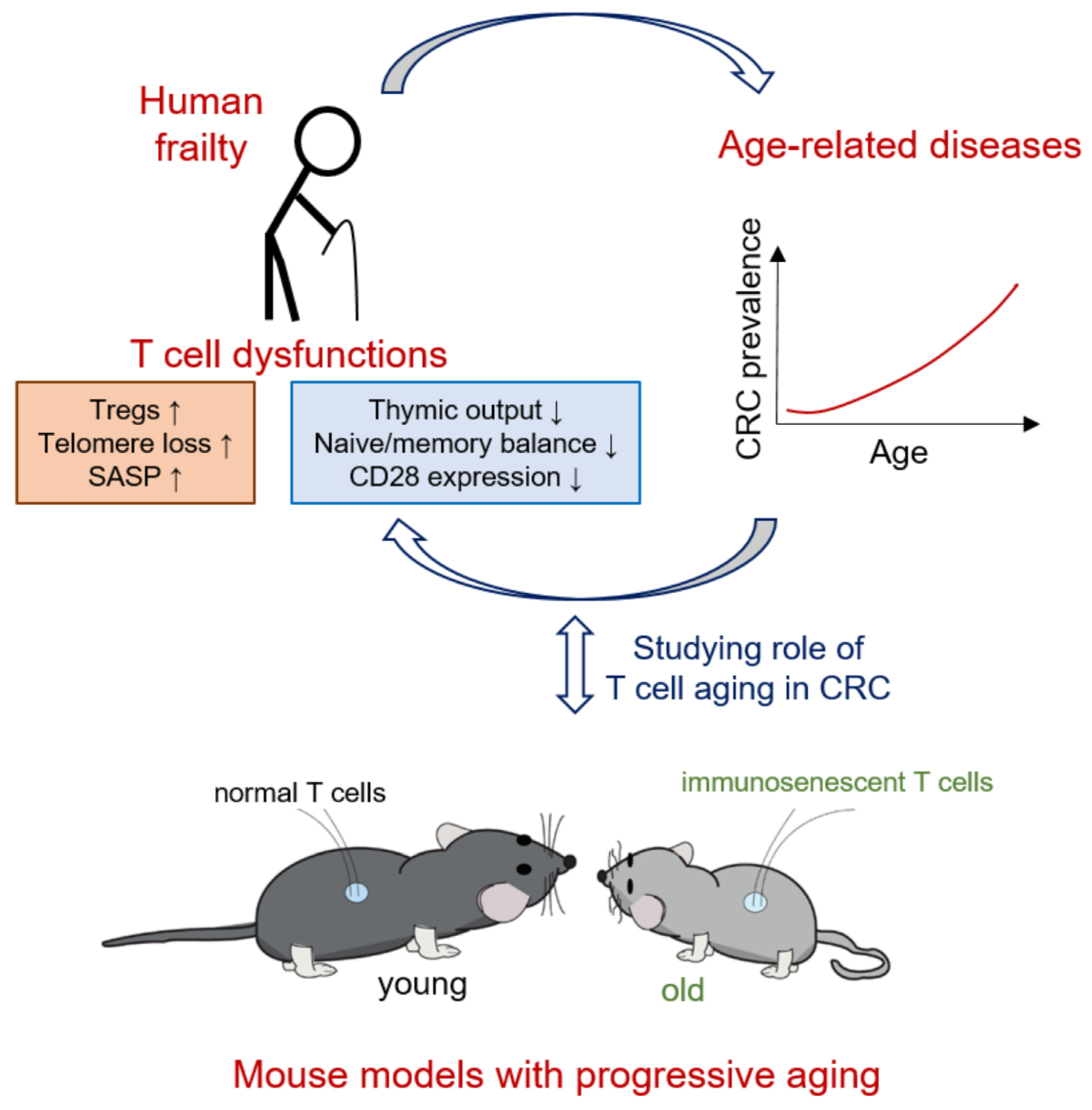
2. Pathophysiology of T Cell Aging
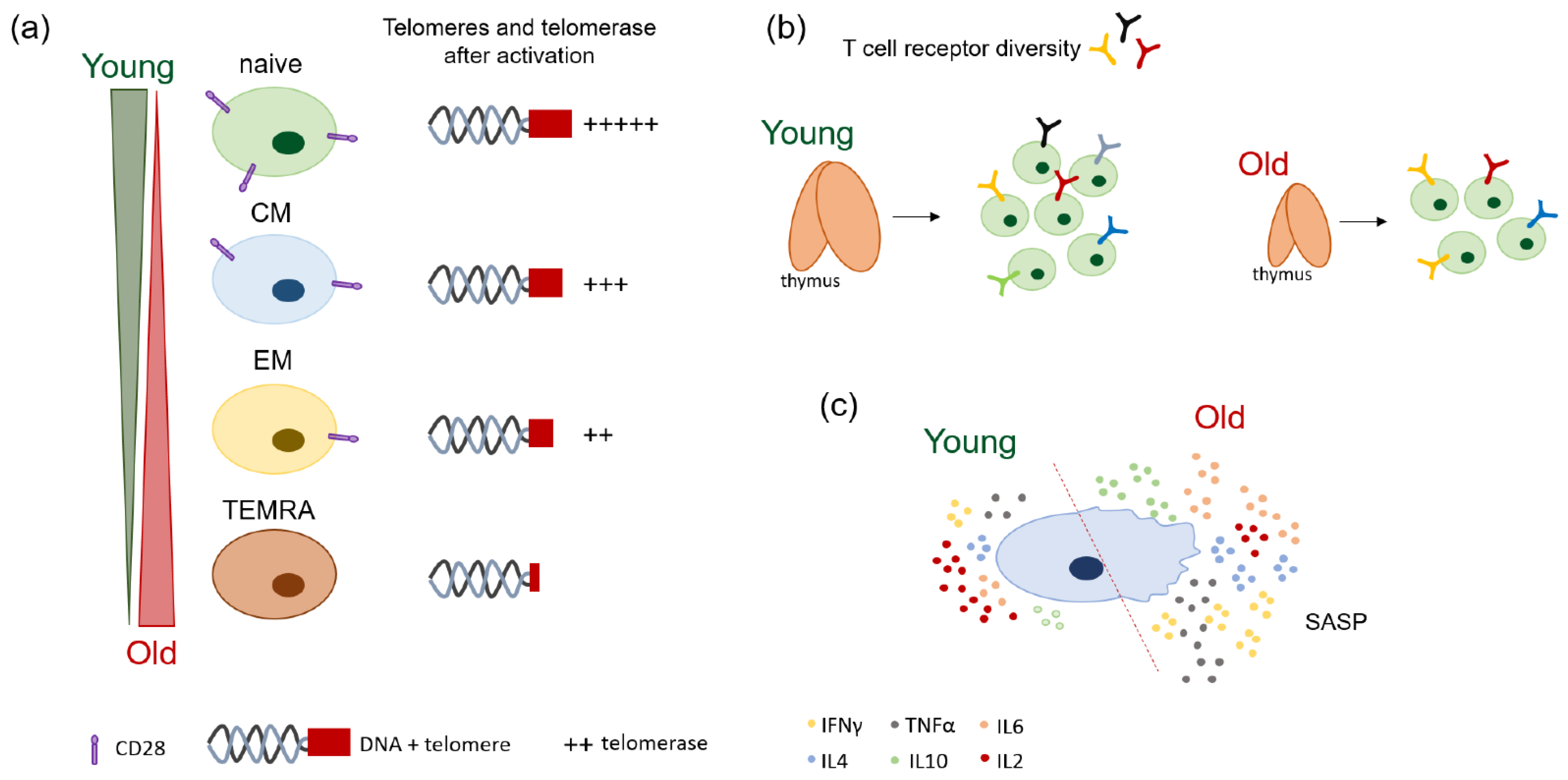
2.1. Age-Related Changes of T Cell Receptor (TCR) Diversity
2.2. Naïve/Memory Shifts and Loss of Co-Stimulatory Molecules with Age
2.3. Loss of Telomeres and Telomerase Activity with Age
2.4. Mechanisms of T Cell Proliferation, Apoptosis, and Cellular Senescence during Aging
2.5. Patterns of Cytokine Production in the Elderly
3. Importance of Adaptive Immune T Cells in CRC
4. Effect of T Cell Aging in Patients with CRC
5. Potential of Using Animal Models to Elucidate the Role of Aged T Cells in CRC
5.1. Old Wildtype Mice
5.2. Mice with Telomere Length Deficiency
5.3. Klotho−/− Mice
5.4. Mice with Deficiencies in DNA Repair Genes
5.5. IL10-Deficient Mice
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 11 October 2021).
- Age and Cancer Risk. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed on 6 December 2021).
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Tsoi, K.K.F.; Hirai, H.W.; Chan, F.C.H.; Griffiths, S.; Sung, J.J.Y. Predicted Increases in Incidence of Colorectal Cancer in Developed and Developing Regions, in Association With Ageing Populations. Clin. Gastroenterol. Hepatol. 2017, 15, 892–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer. 2019, 144, 2992–3000. [Google Scholar] [CrossRef] [Green Version]
- Steele, S.R.; Park, G.E.; Johnson, E.K.; Martin, M.J.; Stojadinovic, A.; Maykel, J.A.; Causey, M.W. The impact of age on colorectal cancer incidence, treatment, and outcomes in an equal-access health care system. Dis. Colon Rectum 2014, 57, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Braendegaard Winther, S.; Baatrup, G.; Pfeiffer, P.; Qvortrup, C.; Academy of Geriatric Cancer, R. Trends in colorectal cancer in the elderly in Denmark, 1980–2012. Acta Oncol. 2016, 55 (Suppl. S1), 29–39. [Google Scholar] [CrossRef] [Green Version]
- Jafari, M.D.; Jafari, F.; Halabi, W.J.; Nguyen, V.Q.; Pigazzi, A.; Carmichael, J.C.; Mills, S.D.; Stamos, M.J. Colorectal Cancer Resections in the Aging US Population: A Trend Toward Decreasing Rates and Improved Outcomes. JAMA Surg. 2014, 149, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, H.; Maeda, K.; Hanai, T.; Sato, H.; Masumori, K.; Koide, Y.; Katsuno, H.; Endo, T.; Shiota, M.; Sugihara, K. Surgical management of colorectal cancer for the aging population-A survey by the Japanese Society for Cancer of Colon and Rectum. Asian J. Surg. 2018, 41, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, U.; Spath, C.; Muller, T.C.; Maak, M.; Janssen, K.P.; Wilhelm, D.; Kleeff, J.; Bader, F.G. Colorectal cancer surgery remains effective with rising patient age. Int. J. Colorectal Dis. 2014, 29, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, O.; Watts, E.; Bull, C.A.; Morris, R.; Acheson, A.; Banerjea, A. Colorectal cancer outcomes in patients aged over 85 years. Ann. R. Coll. Surg. Engl. 2016, 98, 216–221. [Google Scholar] [CrossRef]
- Schlichtemeier, S.; Logaraj, A.; Gill, A.J.; Engel, A. Colorectal cancer resection in the Australian nonagenarian patient. Colorectal Dis. 2017, 19, 243–250. [Google Scholar] [CrossRef]
- Ahiko, Y.; Shida, D.; Horie, T.; Tanabe, T.; Takamizawa, Y.; Sakamoto, R.; Moritani, K.; Tsukamoto, S.; Kanemitsu, Y. Controlling nutritional status (CONUT) score as a preoperative risk assessment index for older patients with colorectal cancer. BMC Cancer 2019, 19, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, R.; Giobbie-Hurder, A.; McCleary, N.J.; Ott, P.; Hodi, F.S.; Rahma, O. Efficacy of PD-1 & PD-L1 inhibitors in older adults: A meta-analysis. J. Immunother Cancer. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Thabane, L.; Papaioannou, A.; Ioannidis, G.; Levine, M.A.; Adachi, J.D. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord. 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Jiang, Q.; McDermott, J.; Han, J.J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell 2018, 17, e12802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strait, J.B.; Lakatta, E.G. Aging-Associated Cardiovascular Changes and Their Relationship to Heart Failure. Heart Fail. Clin. 2012, 8, 143–164. [Google Scholar] [CrossRef] [Green Version]
- Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef]
- Mariuzza, R.A.; Agnihotri, P.; Orban, J. The structural basis of T-cell receptor (TCR) activation: An enduring enigma. J. Biol Chem. 2020, 295, 914–925. [Google Scholar] [CrossRef]
- Sewell, A.K. Why must T cells be cross-reactive? Nat. Rev. Immunol. 2012, 12, 668–677. [Google Scholar] [CrossRef]
- Vanhanen, R.; Heikkila, N.; Aggarwal, K.; Hamm, D.; Tarkkila, H.; Patila, T.; Jokiranta, T.S.; Saramaki, J.; Arstila, T.P. T cell receptor diversity in the human thymus. Mol. Immunol. 2016, 76, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Qi, Q.; Liu, Y.; Cheng, Y.; Glanville, J.; Zhang, D.; Lee, J.Y.; Olshen, R.A.; Weyand, C.M.; Boyd, S.D.; Goronzy, J.J. Diversity and clonal selection in the human T-cell repertoire. Proc. Natl. Acad. Sci. USA 2014, 111, 13139–13144. [Google Scholar] [CrossRef] [Green Version]
- Egorov, E.S.; Kasatskaya, S.A.; Zubov, V.N.; Izraelson, M.; Nakonechnaya, T.O.; Staroverov, D.B.; Angius, A.; Cucca, F.; Mamedov, I.Z.; Rosati, E.; et al. The Changing Landscape of Naive T Cell Receptor Repertoire With Human Aging. Front. Immunol. 2018, 9, 1618. [Google Scholar] [CrossRef] [Green Version]
- Naylor, K.; Li, G.; Vallejo, A.N.; Lee, W.W.; Koetz, K.; Bryl, E.; Witkowski, J.; Fulbright, J.; Weyand, C.M.; Goronzy, J.J. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005, 174, 7446–7452. [Google Scholar] [CrossRef]
- Yoshida, K.; Cologne, J.B.; Cordova, K.; Misumi, M.; Yamaoka, M.; Kyoizumi, S.; Hayashi, T.; Robins, H.; Kusunoki, Y. Aging-related changes in human T-cell repertoire over 20 years delineated by deep sequencing of peripheral T-cell receptors. Exp. Gerontol. 2017, 96, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naive to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, C.E.; Cavanagh, M.M.; Jin, J.; Weyand, C.M.; Goronzy, J.J. Functional pathways regulated by microRNA networks in CD8 T-cell aging. Aging Cell 2019, 18, e12879. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.M.; Fox, A.; Harland, K.L.; Russ, B.E.; Li, J.; Nguyen, T.H.O.; Loh, L.; Olshanksy, M.; Naeem, H.; Tsyganov, K.; et al. Age-Related Decline in Primary CD8(+) T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8(+) T Cells. Cell Rep. 2018, 23, 3512–3524. [Google Scholar] [CrossRef]
- Shin, M.S.; Yim, K.; Moon, K.; Park, H.J.; Mohanty, S.; Kim, J.W.; Montgomery, R.R.; Shaw, A.C.; Krishnaswamy, S.; Kang, I. Dissecting alterations in human CD8+ T cells with aging by high-dimensional single cell mass cytometry. Clin. Immunol. 2019, 200, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Le Page, A.; Dupuis, G.; Larbi, A.; Witkowski, J.M.; Fulop, T. Signal transduction changes in CD4(+) and CD8(+) T cell subpopulations with aging. Exp. Gerontol. 2018, 105, 128–139. [Google Scholar] [CrossRef]
- Elyahu, Y.; Hekselman, I.; Eizenberg-Magar, I.; Berner, O.; Strominger, R.; Schiller, M.; Mittal, K.; Nemirovsky, A.; Eremenko, E.; Vital, A.; et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci. Adv. 2019, 5, eaaw8330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, S.K.; Delaney, C.; Toubai, T.; Ghosh, A.; Reddy, P.; Banerjee, R.; Yung, R. Aging is associated with increased regulatory T-cell function. Aging Cell 2014, 13, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.K.; Delaney, C.; Toubai, T.; Ghosh, A.; Reddy, P.; Banerjee, R.; Yung, R. The number of human peripheral blood CD4(+) CD25(high) regulatory T cells increases with age. Clin. Exp. Immunol. 2005, 140, 540–546. [Google Scholar]
- Deng, B.L.; Zhang, W.Q.; Zhu, Y.C.; Li, Y.Y.; Li, D.; Li, B. FOXP3(+) regulatory T cells and age-related diseases. Febs. J. 2021. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.; Zhu, L.J.; Chang, T.T.; Mao, Y.Q.; Li, J. An Association between Immunosenescence and CD4(+)CD25(+) Regulatory T Cells: A Systematic Review. Biomed. Environ. Sci. 2010, 23, 327–332. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, Y.F.; Han, C.; Li, P.; Zhang, H. Colorectal Cancer Progression Is Associated with Accumulation of Th17 Lymphocytes in Tumor Tissues and Increased Serum Levels of Interleukin-6. Tohoku J. Exp. Med. 2014, 233, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Damjanovic, A.; Metter, E.J.; Nguyen, H.; Truong, T.; Najarro, K.; Morris, C.; Longo, D.L.; Zhan, M.; Ferrucci, L.; et al. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clin. Sci. 2015, 128, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Larbi, A.; Fulop, T. From “Truly Naive” to “Exhausted Senescent” T Cells: When Markers Predict Functionality. Cytom Part. A. 2014, 85a, 25–35. [Google Scholar] [CrossRef]
- Leng, Q.B.; Bentwich, Z.; Borkow, G. CTLA-4 upregulation during aging. Mech. Ageing Dev. 2002, 123, 1419–1421. [Google Scholar] [CrossRef]
- Weng, N.P.; Akbar, A.N.; Goronzy, J. CD28(−) T cells: Their role in the age-associated decline of immune function. Trends Immunol. 2009, 30, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.R.; Blackburn, E.H. Telomeres and telomerase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.E.; Tedone, E.; O’Hara, R.; Cornelius, C.; Lai, T.P.; Ludlow, A.; Wright, W.E.; Shay, J.W. The Maintenance of Telomere Length in CD28+ T Cells During T Lymphocyte Stimulation. Sci. Rep. 2017, 7, 6785. [Google Scholar] [CrossRef] [Green Version]
- Patrick, M.S.; Cheng, N.L.; Kim, J.; An, J.; Dong, F.; Yang, Q.; Zou, I.; Weng, N.P. Human T Cell Differentiation Negatively Regulates Telomerase Expression Resulting in Reduced Activation-Induced Proliferation and Survival. Front. Immunol. 2019, 10, 1993. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.C.; Leung, J.M.; Ngan, D.A.; Nashta, N.F.; Guillemi, S.; Harris, M.; Lima, V.D.; Um, S.J.; Li, Y.; Tam, S.; et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: Evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS ONE 2015, 10, e0124426. [Google Scholar] [CrossRef] [Green Version]
- Schonland, S.O.; Lopez, C.; Widmann, T.; Zimmer, J.; Bryl, E.; Goronzy, J.J.; Weyand, C.M. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc. Natl. Acad. Sci. USA 2003, 100, 13471–13476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lustig, A.; Liu, H.B.; Metter, E.J.; An, Y.; Swaby, M.A.; Elango, P.; Ferrucci, L.; Hodes, R.J.; Weng, N.P. Telomere Shortening, Inflammatory Cytokines, and Anti-Cytomegalovirus Antibody Follow Distinct Age-Associated Trajectories in Humans. Front. Immunol. 2017, 8, 1027. [Google Scholar] [CrossRef] [Green Version]
- Tedone, E.; Huang, E.; O’Hara, R.; Batten, K.; Ludlow, A.T.; Lai, T.P.; Arosio, B.; Mari, D.; Wright, W.E.; Shay, J.W. Telomere length and telomerase activity in T cells are biomarkers of high-performing centenarians. Aging Cell 2019, 18, e12859. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Phillips, J.H.; Lanier, L.L. CD28- T lymphocytes. Antigenic and functional properties. J. Immunol. 1993, 150, 1147–1159. [Google Scholar] [PubMed]
- Campisi, J.; di Fagagna, F.D. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Li, M.; Yao, D.; Zeng, X.; Kasakovski, D.; Zhang, Y.; Chen, S.; Zha, X.; Li, Y.; Xu, L. Age related human T cell subset evolution and senescence. Immun. Ageing 2019, 16, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, T.P.; Camous, X.; Nyunt, M.S.Z.; Vasudev, A.; Tan, C.T.Y.; Feng, L.; Fulop, T.; Yap, K.B.; Larbi, A. Markers of T-cell senescence and physical frailty: Insights from Singapore Longitudinal Ageing Studies. NPJ Aging Mech. Dis. 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zamudio, R.I.; Dewald, H.K.; Vasilopoulos, T.; Gittens-Williams, L.; Fitzgerald-Bocarsly, P.; Herbig, U. Senescence-associated beta-galactosidase reveals the abundance of senescent CD8+ T cells in aging humans. Aging Cell 2021, 20, e13344. [Google Scholar] [CrossRef]
- Aggarwal, S.; Gupta, S. Increased apoptosis of T cell subsets in aging humans: Altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J. Immunol. 1998, 160, 1627–1637. [Google Scholar]
- Herndon, F.J.; Hsu, H.C.; Mountz, J.D. Increased apoptosis of CD45RO- T cells with aging. Mech. Ageing Dev. 1997, 94, 123–134. [Google Scholar] [CrossRef]
- Whisler, R.L.; Liu, B.Q.; Chen, M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol. 1996, 169, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Fagiolo, U.; Cossarizza, A.; Scala, E.; Fanales-Belasio, E.; Ortolani, C.; Cozzi, E.; Monti, D.; Franceschi, C.; Paganelli, R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur. J. Immunol. 1993, 23, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Zanni, F.; Vescovini, R.; Biasini, C.; Fagnoni, F.; Zanlari, L.; Telera, A.; Di Pede, P.; Passeri, G.; Pedrazzoni, M.; Passeri, M.; et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8(+) T cells in humans: A contribution to understand the relationship between inflammation and immuno senescence. Exp. Gerontol. 2003, 38, 981–987. [Google Scholar] [CrossRef]
- Saurwein-Teissl, M.; Lung, T.L.; Marx, F.; Gschosser, C.; Asch, E.; Blasko, I.; Parson, W.; Bock, G.; Schonitzer, D.; Trannoy, E.; et al. Lack of antibody production following immunization in old age: Association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 2002, 168, 5893–5899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, K.A.; Kim, H.R.; Kang, I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev. 2009, 130, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Najarro, K.; Nguyen, H.; Chen, G.B.; Xu, M.; Alcorta, S.; Yao, X.; Zukley, L.; Metter, E.J.; Truong, T.; Lin, Y.; et al. Telomere Length as an Indicator of the Robustness of B- and T-Cell Response to Influenza in Older Adults. J. Infect. Dis. 2015, 212, 1261–1269. [Google Scholar] [CrossRef]
- Nguyen, T.H.O.; Sant, S.; Bird, N.L.; Grant, E.J.; Clemens, E.B.; Koutsakos, M.; Valkenburg, S.A.; Gras, S.; Lappas, M.; Jaworowski, A.; et al. Perturbed CD8(+) T cell immunity across universal influenza epitopes in the elderly. J. Leukocyte Biol. 2018, 103, 321–339. [Google Scholar] [CrossRef] [Green Version]
- Westmeier, J.; Paniskaki, K.; Karakose, Z.; Werner, T.; Sutter, K.; Dolff, S.; Overbeck, M.; Limmer, A.; Liu, J.; Zheng, X.; et al. Impaired Cytotoxic CD8(+) T Cell Response in Elderly COVID-19 Patients. mBio 2020, 11, e02243-20. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.; Parsons, R.; Botelho, F.; Millar, J.; McNeil, S.; Fulop, T.; McElhaney, J.E.; Andrew, M.K.; Walter, S.D.; Devereaux, P.J.; et al. T-Cell Phenotypes Predictive of Frailty and Mortality in Elderly Nursing Home Residents. J. Am. Geriatr Soc. 2017, 65, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Pages, F.; Berger, A.; Camus, M.; Sanchez-Cabo, F.; Costes, A.; Molidor, R.; Mlecnik, B.; Kirilovsky, A.; Nilsson, M.; Damotte, D.; et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 2005, 353, 2654–2666. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pages, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.O.; Hong, Y.; Etlioglu, H.E.; Cho, Y.B.; Pomella, V.; Van den Bosch, B.; Vanhecke, J.; Verbandt, S.; Hong, H.; Min, J.W.; et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 2020, 52, 594–603. [Google Scholar] [CrossRef]
- Ye, L.L.; Zhang, T.M.; Kang, Z.C.; Guo, G.Q.; Sun, Y.J.; Lin, K.M.; Huang, Q.J.; Shi, X.Y.; Ni, Z.L.; Ding, N.; et al. Tumor-Infiltrating Immune Cells Act as a Marker for Prognosis in Colorectal Cancer. Front. Immunol. 2019, 10, 2368. [Google Scholar] [CrossRef] [Green Version]
- Pages, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredericksen, T.; Mauger, S.; Bindea, G. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 4732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazama, K.; Aoyama, T.; Otake, J.; Shiozawa, M.; Sugano, N.; Sato, S.; Atsumi, Y.; Kano, K.; Murakawa, M.; Maezawa, Y.; et al. Distribution of Regulatory T-Cells and Other Phenotypes of T-Cells in Tumors and Regional Lymph Nodes of Colorectal Cancer Patients. In Vivo 2020, 34, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Krijgsman, D.; de Vries, N.L.; Skovbo, A.; Andersen, M.N.; Swets, M.; Bastiaannet, E.; Vahrmeijer, A.L.; van de Velde, C.J.H.; Heemskerk, M.H.M.; Hokland, M.; et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: The peripheral blood immune cell profile. Cancer Immunol. Immun. 2019, 68, 1011–1024. [Google Scholar] [CrossRef] [Green Version]
- Doulabi, H.; Rastin, M.; Shabahangh, H.; Maddah, G.; Abdollahi, A.; Nosratabadi, R.; Esmaeili, S.A.; Mahmoudi, M. Analysis of Th22, Th17 and CD4(+) cells co-producing IL-17/IL-22 at different stages of human colon cancer. Biomed. Pharmacother. 2018, 103, 1101–1106. [Google Scholar] [CrossRef]
- Yang, R.; Cheng, S.J.; Luo, N.; Gao, R.R.; Yu, K.Z.; Kang, B.X.; Wang, L.; Zhang, Q.M.; Fang, Q.; Zhang, L.; et al. Distinct epigenetic features of tumor-reactive CD8+T cells in colorectal cancer patients revealed by genome-wide DNA methylation analysis. Genome Biol. 2019, 21, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yu, X.; Zheng, L.; Zhang, Y.; Li, Y.; Fang, Q.; Gao, R.; Kang, B.; Zhang, Q.; Huang, J.Y.; et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 2018, 564, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.M.; Murshed, K.; Al-Dhaheri, M.; Khawar, M.; Abu Nada, M.; Elkord, E. Immune Checkpoints in Circulating and Tumor-Infiltrating CD4(+) T Cell Subsets in Colorectal Cancer Patients. Front. Immunol. 2019, 10, 2936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L.; Zhang, L.; Hu, X.; Ren, X.; Zhang, Z. Deep single-cell RNA sequencing data of individual T cells from treatment-naive colorectal cancer patients. Sci. Data 2019, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Falci, C.; Gianesin, K.; Sergi, G.; Giunco, S.; De Ronch, I.; Valpione, S.; Solda, C.; Fiduccia, P.; Lonardi, S.; Zanchetta, M.; et al. Immune senescence and cancer in elderly patients: Results from an exploratory study. Exp. Gerontol. 2013, 48, 1436–1442. [Google Scholar] [CrossRef]
- Giunco, S.; Petrara, M.R.; Bergamo, F.; Del Bianco, P.; Zanchetta, M.; Carmona, F.; Zagonel, V.; De Rossi, A.; Lonardi, S. Immune senescence and immune activation in elderly colorectal cancer patients. Aging 2019, 11, 3864–3875. [Google Scholar] [CrossRef]
- Di, J.; Liu, M.; Fan, Y.; Gao, P.; Wang, Z.; Jiang, B.; Su, X. Phenotype molding of T cells in colorectal cancer by single-cell analysis. Int. J. Cancer 2020, 146, 2281–2295. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Qu, F.; He, X.; Bao, G.; Liu, X.; Wan, S.; Xing, J. Short leukocyte telomere length predicts poor prognosis and indicates altered immune functions in colorectal cancer patients. Ann. Oncol. 2014, 25, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, J.C.; Penland, L.; Rubinstein, N.D.; Hendrickson, D.G.; Kelley, D.R.; Rosenthal, A.Z. Murine single-cell RNA-seq reveals cell-identity- and tissue-specific trajectories of aging. Genome Res. 2019, 29, 2088–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Gross, D.; Elbaum, P.; Murasko, D.M. Aging affects initiation and continuation of T cell proliferation. Mech. Ageing Dev. 2007, 128, 332–339. [Google Scholar] [CrossRef]
- Oh, J.; Magnuson, A.; Benoist, C.; Pittet, M.J.; Weissleder, R. Age-related tumor growth in mice is related to integrin alpha 4 in CD8(+) T cells. JCI Insight 2018, 3, e122961. [Google Scholar] [CrossRef] [PubMed]
- Ruby, C.E.; Weinberg, A.D. OX40-enhanced tumor rejection and effector T cell differentiation decreases with age. J. Immunol. 2009, 182, 1481–1489. [Google Scholar] [CrossRef] [Green Version]
- Vera, E.; Bernardes de Jesus, B.; Foronda, M.; Flores, J.M.; Blasco, M.A. The rate of increase of short telomeres predicts longevity in mammals. Cell Rep. 2012, 2, 732–737. [Google Scholar] [CrossRef] [Green Version]
- Blasco, M.A.; Lee, H.W.; Hande, M.P.; Samper, E.; Lansdorp, P.M.; DePinho, R.A.; Greider, C.W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 1997, 91, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.J.; Hemann, M.T.; Hathcock, K.S.; Tessarollo, L.; Feigenbaum, L.; Hahn, W.C.; Hodes, R.J. Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo. Mol. Cell Biol. 2004, 24, 7024–7031. [Google Scholar] [CrossRef] [Green Version]
- Raval, A.; Behbehani, G.K.; Nguyen, L.X.T.; Thomas, D.; Kusler, B.; Garbuzov, A.; Ramunas, J.; Holbrook, C.; Park, C.Y.; Blau, H.; et al. Reversibility of Defective Hematopoiesis Caused by Telomere Shortening in Telomerase Knockout Mice. PLoS ONE. 2015, 10, e0131722. [Google Scholar] [CrossRef]
- Blasco, M.A. Immunosenescence phenotypes in the telomerase knockout mouse. Springer Semin Immunopathol. 2002, 24, 75–85. [Google Scholar] [CrossRef]
- Rudolph, K.L.; Chang, S.; Lee, H.W.; Blasco, M.; Gottlieb, G.J.; Greider, C.; DePinho, R.A. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 1999, 96, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Lorente, M.A.; Cano-Martin, A.C.; Blasco, M.A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 2019, 10, 4723. [Google Scholar] [CrossRef] [Green Version]
- Varela, E.; Munoz-Lorente, M.A.; Tejera, A.M.; Ortega, S.; Blasco, M.A. Generation of mice with longer and better preserved telomeres in the absence of genetic manipulations. Nat. Commun. 2016, 7, 11739. [Google Scholar] [CrossRef] [Green Version]
- De Jesus, B.B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; Blasco, M.A. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Jeong, D.J.; Kim, J.; Lee, S.; Park, J.H.; Chang, B.; Jung, S.I.; Yi, L.; Han, Y.; Yang, Y.; et al. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol. Cancer. 2010, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, T.A.; Shahmoon, S.; Zigmond, E.; Etan, T.; Merenbakh-Lamin, K.; Pasmanik-Chor, M.; Har-Zahav, G.; Barshack, I.; Vainer, G.W.; Skalka, N.; et al. Klotho suppresses colorectal cancer through modulation of the unfolded protein response. Oncogene 2019, 38, 794–807. [Google Scholar] [CrossRef]
- Witkowski, J.M.; Soroczynska-Cybula, M.; Bryl, E.; Smolenska, Z.; Jozwik, A. Klotho--a common link in physiological and rheumatoid arthritis-related aging of human CD4+ lymphocytes. J. Immunol. 2007, 178, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Vogel, H.; Holcomb, V.B.; Gu, Y.S.; Hasty, P. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol. Cell Biol. 2007, 27, 8205–8214. [Google Scholar] [CrossRef] [Green Version]
- Mahaney, B.L.; Yu, Y.; Lees-Miller, S.P. The XLF C-Terminal Region Is Required for DNA Binding and Interaction with Ku70/80 In Vitro but Not for Repair of Double-Strand Breaks In Vivo. Environ. Mol. Mutagen. 2011, 52, S44. [Google Scholar]
- Puebla-Osorio, N.; Kim, J.; Ojeda, S.; Zhang, H.; Tavana, O.; Li, S.; Wang, Y.; Ma, Q.; Schluns, K.S.; Zhu, C. A novel Ku70 function in colorectal homeostasis separate from nonhomologous end joining. Oncogene 2014, 33, 2748–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcwhir, J.; Selfridge, J.; Harrison, D.J.; Squires, S.; Melton, D.W. Mice with DNA-Repair Gene (Ercc-1) Deficiency Have Elevated Levels of P53, Liver Nuclear Abnormalities and Die before Weaning. Nat. Genet. 1993, 5, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Weeda, G.; Donker, I.; de Wit, J.; Morreau, H.; Janssens, R.; Vissers, C.J.; Nigg, A.; van Steeg, H.; Bootsma, D.; Hoeijmakers, J.H. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr. Biol. 1997, 7, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.A.; McGowan, S.J.; et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.G.; Williams, C.B. Generation and function of induced regulatory T cells. Front. Immunol. 2013, 4, 152. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, R.; Lohler, J.; Rennick, D.; Rajewsky, K.; Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Berg, D.J.; Davidson, N.; Kuhn, R.; Muller, W.; Menon, S.; Holland, G.; Thompson-Snipes, L.; Leach, M.W.; Rennick, D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Investig. 1996, 98, 1010–1020. [Google Scholar] [CrossRef]
- Strong, M.A.; Vidal-Cardenas, S.L.; Karim, B.; Yu, H.M.; Guo, N.; Greider, C.W. Phenotypes in mTERT(+/−) and mTERT(−/−) Mice Are Due to Short Telomeres, Not Telomere-Independent Functions of Telomerase Reverse Transcriptase. Mol. Cell Biol. 2011, 31, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Min, Y.K.; Jang, J.; Park, H.; Lee, S.; Lee, C.H. Single-cell RNA sequencing reveals distinct cellular factors for response to immunotherapy targeting CD73 and PD-1 in colorectal cancer. J. Immunother. Cancer 2021, 9, e002503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qi, Q.; Pan, Y.; Zhou, Q.; Wu, Y.; Zhuang, J.; Xu, J.; Pan, M.; Han, S. Single-Cell Analysis Reveals Characterization of Infiltrating T Cells in Moderately Differentiated Colorectal Cancer. Front. Immunol. 2020, 11, 620196. [Google Scholar] [CrossRef] [PubMed]
| T Cell Subtype | Role in CRC | Reference |
|---|---|---|
| CD3+ T cells | High numbers correlated with overall better patient survival | [67,70] |
| Bystander CD8+ T cells | Show no chronic antigen exposure, no impact | [76,77] |
| Tumor reactive CD8+ T cells | Highly cytotoxic; frequencies vary amongst patients | [76] |
| Exhausted and TEMRA CD8+ T cells | Often tumor-reactive cells, have high potential as a target in immunotherapy due to high PD-1 expression | [78] |
| Th1 cells | Cytotoxic; high numbers correlated with overall better patient survival | [67,70,71] |
| Th2 cells | No specific impact | [72] |
| Th17 | Increased numbers in later CRC stages are detrimental | [75] |
| Tregs | Increased numbers correlate with worse outcome, can become highly suppressive, Tregs show increased exhaustion marker expression | [72,79,80] |
| Tfh cells | High numbers correlate with better outcome | [71] |
| Th1-like Tfh cells | Newly described, highly cytotoxic | [80,81] |
| Th9, Th22 | Tumor promoting effect | [75] |
| Mouse Type | Age When Phenotype Is Observed | T Cell Phenotypes | Reference |
|---|---|---|---|
| Wildtype | >20 months old | Decreased proliferation Memory phenotype Increased Treg numbers | [31,87] |
| TERC−/− | after multiple crossings | Loss of proliferative potential Development of lymphomas | [91,95] |
| TERT−/− | after multiple crossings | No data | [113] |
| Mice with hyper-long telomeres | any age | No data | [96,97] |
| Klotho−/− | 9 weeks old | Thymus involution Correlated to T cell specific diseases in elderly (RA) | [99,103] |
| Ku70−/− | Normal T cell development Early lymphoma development | [105] | |
| Ku80−/− | T cells arrested at early developmental stage Early lymphoma development | [105] | |
| Vav1Cre+/− × ERCC−/fl | 6 months old | Memory T cell accumulation Senescence markers | [109] |
| IL10−/− | as early as week 3 | Increased CD4+ CD8+ T cells in the gut Increased IFNγ Th1 cells Colitis development | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoma, O.-M.; Neurath, M.F.; Waldner, M.J. T Cell Aging in Patients with Colorectal Cancer—What Do We Know So Far? Cancers 2021, 13, 6227. https://doi.org/10.3390/cancers13246227
Thoma O-M, Neurath MF, Waldner MJ. T Cell Aging in Patients with Colorectal Cancer—What Do We Know So Far? Cancers. 2021; 13(24):6227. https://doi.org/10.3390/cancers13246227
Chicago/Turabian StyleThoma, Oana-Maria, Markus F. Neurath, and Maximilian J. Waldner. 2021. "T Cell Aging in Patients with Colorectal Cancer—What Do We Know So Far?" Cancers 13, no. 24: 6227. https://doi.org/10.3390/cancers13246227
APA StyleThoma, O.-M., Neurath, M. F., & Waldner, M. J. (2021). T Cell Aging in Patients with Colorectal Cancer—What Do We Know So Far? Cancers, 13(24), 6227. https://doi.org/10.3390/cancers13246227





