Simple Summary
Colorectal cancer (CRC) is one of the most common cancer worldwide. CRC is derived from polyps and many factors, such as Matrix Metalloproteinases (MMPs) can gain the progression of colorectal carcinogenesis. Many investigations have indicated the role of MMPs in CRC development while there is not enough knowledge about the function of MMPs in precancerous conditions. This review summarizes the current information about the role of MMPs in polyps and CRC progression.
Abstract
Colorectal cancer (CRC) is the third and second cancer for incidence and mortality worldwide, respectively, and is becoming prevalent in developing countries. Most CRCs derive from polyps, especially adenomatous polyps, which can gradually transform into CRC. The family of Matrix Metalloproteinases (MMPs) plays a critical role in the initiation and progression of CRC. Prominent MMPs, including MMP-1, MMP-2, MMP-7, MMP-8, MMP-9, MMP-12, MMP-13, MMP-14, and MMP-21, have been detected in CRC patients, and the expression of most of them correlates with a poor prognosis. Moreover, many studies have explored the inhibition of MMPs and targeted therapy for CRC, but there is not enough information about the role of MMPs in polyp malignancy. In this review, we discuss the role of MMPs in colorectal cancer and its pathogenesis
1. Introduction
At approximately 11% of all diagnosed cancer cases, CRC is the third most common cancer and the second most lethal cancer worldwide [1,2]. It is today well known that several factors contribute to the CRC pathogenesis, driving complex genetic and epigenetic processes that, ultimately, transform normal colonic mucosa to cancerous tissue [3]. CRC may initiate from benign polyps with the mucosal origin and can develop into carcinoma. Colorectal polyps, especially adenomas, are proliferative lesions that have been defined as the precursor of CRC. Therefore, the early detection and removal of these polyps can interrupt the progression of the adenoma-carcinoma sequence [4,5].
Many molecular signaling pathways are involved in CRC initiation and progression, such as ERK/MAPK, TGF-β, PI3K/Akt, Src/FAK, and β-catenin pathways. These pathways can promote the hallmarks of cancer such as inflammation, angiogenesis, metastasis, and invasion, also via the activation and overexpression of MMPs [6,7]. Thus, MMPs have been suggested as potential prognostic factors for the malignancy risk of colorectal polyps. MMPs are proteolytic enzymes implicated in the degradation of stromal connective tissues and of the extracellular matrix (ECM), a complex network that plays a key role in sustaining signaling transduction and thus cancer development and progression [8]. As such, MMPs have key roles in tumor initiation, progression, and metastasis and can affect tumor cell behavior by cleaving proapoptotic agents and producing an aggressive phenotype [9]. Because of these roles, MMPs have been detected as biomarkers in CRC progression [10]. A new challenge in CRC treatment is finding an effective pharmacological and therapeutic method for suppression of MMPs and targeted therapy of CRC [11]. This review will deal with the role of MMPs in colorectal carcinogenesis from colorectal polyps to CRC.
2. CRC Pathogenesis and Molecular Classification
Colorectal polyps result from atypical cell proliferation in the colorectal tissue. Based on histological and morphological features, colorectal polyps are divided into neoplastic (adenoma) and non-neoplastic (hyperplastic, hamartomatous, and inflammatory) types [5,12]. Neoplastic polyps, also known as adenomatous polyps, are subclassified by their histological characteristics as tubular, villous, or tubulovillous adenomas. Previous investigations demonstrated that approximately 5–10% of neoplastic polyps are villous adenomas and most of them show dysplasia. Approximately 10–15% of neoplastic polyps show morphological features of both villous and tubular types [13]. Adenomas are not usually transformed to carcinoma, but there is evidence that the adenoma-carcinoma sequence originates from adenomatous polyps [14]. Also, hyperplastic polyps may possess malignancy potential [15]. CRC is caused by the misregulation of some oncogenes such as KRAS and c-MYC and tumor suppressor genes such as P53 and APC, which control cellular signal transduction [16,17,18].
2.1. Molecular Mechanism of CRC
Specific features characterize CRC and its pathogenesis based on genetic, epigenetic, and transcriptomic factors. Three main molecular abnormalities are involved in CRC carcinogenesis:
- Microsatellite instability (MSI): it consists of mutations in DNA mismatch repair (MMR) genes such as MSH2, MLH1, PMS2, MLH3, MSH3, PMSI, and EXO1; MSI is rare in polyps but it is always found in serrated polyps and about 15–20% of all CRC cases are derived from MSI [19,20].
- Chromosomal instability (CIN): this abnormality is identified in 85% of CRC cases and consists of a gain (1q, 7p, 8q, 13q, 2pq) or loss (8q, 15q, 17p, 18p) of chromosomal genes, activation of proto-oncogenes (KRAS, SRC, c-MYC), and inactivation of tumor suppressor genes (P53, APC) [21].
- CpG Islands Methylator Phenotype (CIMP): these regions, located in the gene promoter, could disturb the activation of tumor suppressor genes. CIMP phenotype is represented by hypermethylation of CpG dinucleotides and premalignant serrated polyps are correlated with CIMP [22,23]
2.2. Molecular Classification Based on Transcriptomic Analysis
Based on gene expression profiles, CRC has been classified into subgroups with distinct molecular and clinical features [24].
- Consensus molecular subtype (CMS) classification: CMS classification provides biological insight into metastatic colorectal cancer (mCRC) carcinogenesis and predicts CRC prognosis [25].
- ◦
- CMS1 (14%) indicates MSI, CIMP, and BRAF mutation and immune activation.
- ◦
- CMS2 (37%) shows Wingless-Type MMTR integration site family member (WNT), MYC signaling activation, and epithelial involvement.
- ◦
- CMS3 (13%) demonstrates MSI, CIMP, and KRAS mutations and metabolic involvement.
- ◦
- CMS4 (23%) includes invasion, metastatic situations, and TGF-β signaling co-activation and angiogenesis. Also, epithelial-mesenchymal transition (EMT) is a crucial event in colorectal carcinogenesis and is involved in CMS4 status. EMT can result in advanced-stage CRC, poor patient survival, and worst clinical features [26,27] and CMS4 subgroup shows the most unfavorable prognosis.
- CRC intrinsic subtypes (CRIS): CRIS is a unique classification exclusively based on the cancer cell-specific transcriptome of CRC since the extrinsic factors of the stroma have not been analyzed. It classifies CRC into five novel transcriptional groups that, thus, further clarify biological understanding of CRC heterogeneity.
- ◦
- CRIS-A is enriched for BRAF-mutated MSI tumors and KRAS-mutated MSS tumors that are without targeted therapeutic options.
- ◦
- CRIS-B is related to invasive tumors with poor prognosis and high TGF-ß signaling. CRIS-B is unconnected to the CMS4 mesenchymal subtype, which also indicates aggressive tumors with TGF-ß pathway activation.
- ◦
- CRIS-C is dependent on EGFR signals and is sensitive to anti-EGFR monoclonal antibody treatment.
- ◦
- CRIS-D shows IGF2 overexpression. This occurrence has been involved in desensitization to the EGFR blockade in patients with KRAS wild-type tumors.
- ◦
- CRIS-E indicates KRAS-mutated, Paneth cell-like CIN tumors refractory to anti-EGFR antibody treatment [28].
3. Structure and Function of MMPs
MMPs are a family of zinc-dependent endopeptidases consisting of a propeptide sequence, a catalytic domain, a hinge region, and a hemopexin (PEX) domain [29]. The propeptide domain is highly conserved and can regulate the sequence that interacts with Zn2+. Also, cystine within this area permits the MMPs to be in the active or inactive status [30]. The catalytic domain possesses a conserved zinc-binding motif which, in the active condition, will disconnect from the propeptide domain. Movement between the catalytic and PEX domain is done via hinge regions [29]. According to their structural domains, MMPs have been categorized into collagenase, gelatinase, stromelysin, matrilysin, and membrane-bound MMPs (MT-MMPs) [31,32].
MMPs play a crucial role in the remodeling of the ECM by digestion of ECM components, stimulation of cell surface proteins. Also, they can control the activity of other proteinases, growth factors, chemokines, and cell receptors, and moderate many biological functions [33]. MMPs can regulate cellular growth, migration, survival, and adhesion in biological and pathological statuses (Table 1, Figure 1). Due to the MMP’s key roles, the dysregulation of their expression levels and their activation lead cancerous cells to proliferation, angiogenesis, survival, invasion, malignant transitions, and immune dysregulation [34,35,36]. Also, the tissue inhibitors of metalloproteinase (TIMPs) control the activation of MMPs and have a critical action in precancerous conditions, CRC progression, and metastasis (Table 2, Figure 2) [11,37].

Table 1.
Matrix Metallopeptidases Features in Humans.
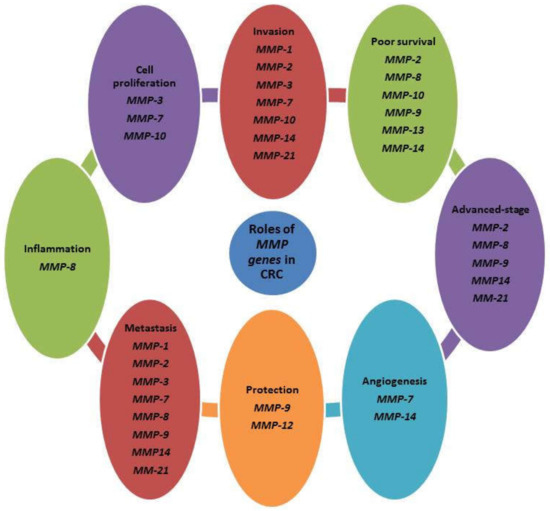
Figure 1.
Summary of the prominent MMP genes in CRC. MMPs play different functions in CRC.

Table 2.
Summary of Investigations about the Roles of MMP Genes and Proteins in Colorectal Polyps and Cancer.
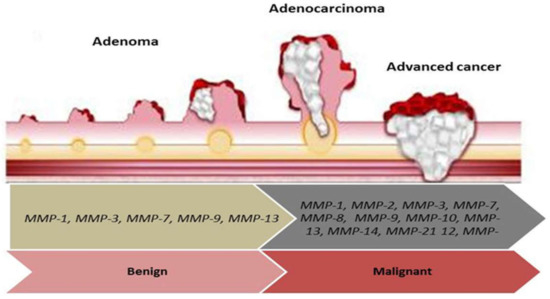
Figure 2.
The diagram indicates the role of MMPs genes in adenoma development, colorectal adenoma-carcinoma sequence, and tumor progression. MMP-1, MMP-3, MMP-7, MMP-9, and MMP-13 are involved in adenoma development. MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-12, MMP-13, MMP-14, and MMP-21 participate in adenoma-carcinoma sequence and tumor progression.
4. The Function of MMPs in Colorectal Polyps and Cancer
4.1. MMP-1, MMP-13, and MMP-8 (Collagenases)
The specific targets for MMP-1 and MMP-13 are in the intestine. MMP-1 can digest type I, II, III, VII, VIII, X collagen, and gelatin. Upregulation of MMP-1 gene was detected in CRC patients compared to normal tissue [6,59]. Eiro et al., found overexpression of MMP-1 gene in serrated, villous, and tubulovillous adenomas (i.e., polyps with high potential for transformation to CRC) [44]. Previous investigations demonstrated the correlation between MMP-1 gene expression and CRC progression: high expression levels of MMP-1 were associated with invasion, advanced stage metastasis, LNM, and shorter overall survival [60,61]. Wang, et al. investigated the role of MMP-1 in the development of CRC. They found that the downregulation of MMP-1 expression inhibited the progression of CRC in vitro and in vivo by suppressing the PI3K/Akt/c-myc signaling pathway and the EMT [6].
MMP-13, another member of the collagenase category, could degenerate type III collagen. According to the strength of the association between pathologic stage and immunoreactivity scoring (IRS) of MMP-13, in high-grade adenomas and CRC, MMP-13 was observed with a moderate and strong staining intensity, respectively [46]. This result indicated that MMP-13 could help to predict metastatic behavior and prognosis of early-stage cancerous and precancerous colorectal adenoma [46,62]. The study of the association between grade dysplasia and MMP-13 expression in 137 biopsies from patients with cancerous and non-cancerous colorectal adenomas showed that the high expression level of MMP-13 IRS could be helpful to predict metastatic state, prognosis, and recrudescence at an early stage of cancerous and precancerous colorectal adenoma. Moreover, the upregulation of MMP-13 IRS from low to high-grade adenoma was considered an early predictive cancer biomarker [46]. Meanwhile, several studies confirmed that upregulation of MMP-13 was related to advanced CRC and liver metastasis [62,63,64]. Also, the expression of MMP-13 on the primary tumor cell surface is increased in inflammatory bowel disease. The expression of MMP-13 is closely related to the progression, early relapse, and high mortality of CRC [63,65].
Another member of collagenase enzymes is MMP-8 which is frequently expressed by neutrophils. MMP-8 cleaves many substrates, such as type I, II, and III collagen. This MMP is mainly considered to play a protective role against cancer. However, more recent findings also suggest an oncogenic function of MMP-8 gene [66,67].
Sirnio et al., found that enhanced-serum MMP-8 level in CRC patients was significantly related to advanced-stage CRC, distant metastasis, lack of MMR, and poor survival. Thus,, they evidenced that MMP-8 is correlated with inflammation and CRC progression [68].
4.2. MMP-2 and MMP-9 (Gelatinase)
MMP-2 and MMP-9, known as gelatinases, can digest type IV collagen and gelatin [69]. Murname et al. showed that MMP-2 protein activity in adenomas with high-grade dysplasia (HGD) was different from adenomas with low-grade dysplasia (LGD). They suggested that the active MMP-2 gene could predict CRC malignancy risk in patients with adenomatous polyps [70]. Some studies also indicated high expression levels of MMP-9 protein in adenomas with HGD compared to adenomas with LGD and normal tissue. As such, researchers speculated that upregulation of MMP-9 is a primary event in the CRC adenoma-carcinoma sequence [41,71]. High expression levels of MMP-2 protein in CRC tumors compared to normal mucosa have also been reported [41,72]. In addition, a statistically significant relationship between upregulation of MMP-2 gene with advanced-stage CRC or CRC progression has been observed [41,73,74,75]. On this basis, MMP-2 has been suggested as a potential biomarker to detect CRC progression and predict patient survival. Furthermore, overexpression of the MMP-2 gene was associated with metastasis of lymph nodes and a decrease of cell adhesion in tumors [73].
Finally, also the upregulation of MMP-9 gene was associated with the advanced stage of CRC and suggested as a biomarker predictive of poor overall survival [41,76]. Chen et al. indicated that the overexpression of MMP-9 gene promoted CRC metastasis through the MKK-3/p38/NF-κB pro-oncogenic pathway. Furthermore, they suggested MMP-9 gene as a potential molecular target for targeted therapy to treat metastatic CRC patients [76].
On the contrary, some investigations reported that MMP-9 gene has a protective role in CRC by stimulating Notch activation resulting in the activation of p21WAF1/Cip1 leading to the suppression of β-catenin [77,78]. In a recent study, although in colitis-associated colon cancer, Walter et al. confirmed this observation by revealing that MMP-9 protein expression was associated with reduced ROS levels, decreased DNA damage, and stimulated mismatch repair pathway [79].
In an interesting study, Wei et al., by analyzing microbiota in tumors obtained by patients with different prognoses, found that the expression of some inflammatory genes, including MMP-9, was associated with the abundance of specific bacteria. High levels of MMP-9 expression were significantly correlated with the high abundance of B. fragilis and F. nucleatum whereas a high level of F. prausnitzii was associated with downregulation of MMP-9 [80].
4.3. MMP-3, MMP-10 (Stromelysin)
Another member of MMPs family is MMP-3, or stromelysin-1, which degrades collagen (types II, III, IV, IX, and X), proteoglycans, fibronectin, laminin, and elastin in ECM.
Sipos et al., found a positive association between MMP-3 protein expression and the adenoma–dysplasia–carcinoma sequence. In particular, they reported that high-grade dysplastic sessile adenomatous-stage and early-stage CRC conditions can be differentiated based on the stroma expression of MMP3 [81]. Meaningful positive associations between the protein expression level of MMP-3, invasion, lymph node metastasis, histological type of CRC, and poorly differentiated tumor were reported by Islekel et al. [82]. MMP-3 can activate other MMPs, such as MMP-1, MMP-7, and MMP-9, to promote the progression of tumor initiation [83,84].
MMP-10 also belongs to the stromelysin family. It can digest collagen types II, III, IV, IX, X, proteoglycans, fibronectin, laminin, and elastin. Also, MMP-10 enhances cell growth and invasion in CRC, and its upregulation was found to be associated with poor survival [49,85].
4.4. MMP-7 (Matrilysin)
MMP-7, or matrilysin, digests fibronectin, laminin, type I collagen, and gelatin. It can provide the right condition for vascularization via cleavage of ECM [86]. A major ratio of MMP-7 expression in tumor cells has been reported. Qasim et al., found MMP-7 protein overexpression in villous adenomatous polyps compared to other types of polyps and demonstrated that MMP-7 protein overexpression is an initial event in CRC carcinogenesis that could lead adenomas to CRC [53]. In our laboratory, we observed high expression levels of MMP-7 and VEGF-A mRNA in adenomatous polyps compared to normal tissue. We found that the expression levels of MMP-7 and VEGF-A genes were higher in villous adenoma than in other types of adenomas. Thus, we concluded that the MMP-7 gene overexpression has a critical role in colorectal adenoma angiogenesis and could be a primary event in the adenoma-carcinoma sequence [45].
MMP-7 gene can enhance tumor growth and metastasis [87]. Also, MMP-7 activates other MMPs, such as proMMP9 and proMMP2 [88] In addition. MMP-7 exerts a wide spectrum of activities not only as an enzyme but also as a signaling molecule. In fact, it has been shown that MMP-7 trans-activates EGFR by releasing the heparin-binding epidermal growth factor (HB-EGF) in CRC cells, with consequent cell proliferation and apoptosis regulation [89,90].
4.5. MMP-12 (Metalloelastase)
MMP-12, or metalloelastase, can digest different substrates. Several studies considered MMP-12 gene as an anti-metastatic agent [91,92]. Also, it could inhibit angiogenesis by downregulation of VEGF and enhancement of the endogenous angiogenesis inhibitor angiostatin. Overall, the role of MMP-12 in tumor suppression and increase in overall survival has been widely recognized [93,94,95].
Importantly, Klupp et al., found higher levels of MMP-12 protein expression in sera of CRC patients compared with those of healthy individuals. Also, they suggested an association between MMP-12 protein expression levels and CRC advanced disease and vascular invasion. Furthermore, a significant correlation between the upregulation of MMP-12 expression and poor survival was shown [49].
4.6. MMP-21 (XMMP)
MMP-21 (XMMP) can degenerate aggrecan (cartilage-specific proteoglycan core protein) in the internal region of ECM [96]. Overexpression of MMP-21 protein in CRC compared with normal tissue was shown in many studies [97,98]. Furthermore, significant associations between MMP-21 protein expression and CRC tumor invasion, lymph node metastasis, and distant metastasis were found [97,99]. Wu et al., showed that MMP-21 not only affected CRC progression but also was an independent prognostic biomarker in patients with stage II and stage III CRC cancer. Taken together, these facts led them to conclude that MMP-21 could be used for targeted therapy in CRC [97]. Huang et al., demonstrated that the upregulation of MMP-21 protein was related to shorter overall survival in patients with CRC [98].
4.7. MMP-14 (MT1-MMP)
MMP-14, called MT1-MMP, acts on matrix substrates, such as collagens I, II, III, and gelatin. The MMP-14 gene plays a crucial role in many biological and pathological conditions and activation of proMMP2 [92,100]. The role of MMP-14 in angiogenesis and cancer invasion has been identified by previous investigations [101,102,103]. Cui et al., observed statistically significant associations between the overexpression of MMP-14 gene in CRC compared to normal mucosa. Their analysis indicated that high expression levels of MMP-14 were associated with advanced-stage CRC, lymph node metastasis, and poor overall survival. They concluded that the MMP-14 gene is an oncogene and may represent a potential prognostic biomarker in CRC [104].
Yang et al., showed in an in vivo CRC model that the STAT3 phosphorylation activity and the overexpression of MMP14 protein were enhanced by the overexpression of Hes1 gene. Also, they suggested that Hes1 promoted the invasion of colorectal cancerous cells via the STAT3-MMP14 pathway [103]. It was reported that the overexpression of MMP-14 protein was associated with Prox1 gene. When Prox1 gene was deleted, MMP14 protein was increased, and the mice showed slow-growing, matrix-rich, chemotherapy-resistance, and cancerous cells with malignant stromal features, including activation of fibroblasts, blood vessels dysfunction, and lack of cytotoxic T cells [105].
5. The Effects of Polymorphisms of MMP Genes on Colorectal Carcinogenesis
Single-nucleotide polymorphisms (SNPs) are a common genetic variation involving a single base pair in DNA. SNPs are mostly located in the gene promoter region and may have an impact on gene and protein expression levels. The effects of MMP polymorphisms have been observed in many cancers such as CRC and hepatocellular carcinoma [106,107].
In a Japanese population, the MMP-1 1G/2G polymorphism was detected and associated with the development of CRC [108]. In the Iranian population, Kouhkan et al., demonstrated that MMP-1 2G/2G genotype polymorphism was correlated with invasion risk of CRC, especially in smoker men [109]. In the Netherlands, MMP-2-1306C>T SNP was detected in CRC patients, and the T/T genotype was found to be associated with poor overall survival whereas C/C and C/T genotypes showed better outcomes. No difference in overall survival was instead observed among patients with different genotypes of the MMP-9-1562C>T SNP [110]. Also, in a cohort study of Taiwanese CRC patients, Ting et al. indicated that patients carrying the A/A genotype of the MMP-2-1575G>A SNP had a higher risk to develop distant metastasis compared with patients carrying the T/T genotype [111]. In a Polish population with CRC, individuals with the G/G variant genotype of MMP-7-181A>G SNP had a higher risk of lymph node involvement and advanced tumor infiltration than patients carrying the A/A genotype [112]. A Chinese study showed that the MMP-9 R279Q SNP relative to the R/R genotype was correlated with a higher risk of CRC compared with the QQ genotype. Also, the allele frequency of the MMP-1 16071G/2G and MMP-7 181 A/G polymorphisms were not associated with CRC [113]. In a Korean population, the homozygous MMP-9-1562C/C genotype was significantly more frequent in CRC cases than in the control group [114]. In Sweden, researchers found that the A/A genotype of MMP-12-82A>G increased the risk of disseminated malignancy in CRC patients while the A/A genotype of MMP-13-82A>G was not correlated to invasion [115].
Lièvre et al., investigated MMP-3, MMP-7, and MMP-1 genes promoter polymorphisms in 295 patients with large adenomas and 302 patients with small adenomas. The analysis revealed a significant association between MMP-3-1612 ins/del A, MMP-1-1607 ins/del G polymorphism, and small adenomas; also, adenomas were associated with the combined genotype 2G/2G-6A/6A. However, no significant association between MMP-7 polymorphism and the development of adenomas was found. The authors suggested that only the study MMP-3 and MMP-1 gene promoter polymorphisms had potential roles in the development of adenomas from normal colon epithelial cells or in the earliest steps of CRC [57].
Tai et al., showed that MMP-8 rs11225395 related to the risk of CRC and worst outcomes in a subpopulation of the Han Chinese population. On this basis, they suggested MMP-8 rs11225395 polymorphism as a potential biomarker predictive of CRC susceptibility [116].
6. Targeting MMPs in CRC Treatment
6.1. Pharmacological Inhibition
Several pharmacological inhibitors of MMPs (MMPIs) have been studied and tested in phase I-III clinical trials, but to date, none of these drugs has been approved for the treatment of cancer, including CRC. Overall, the late stages of the clinical experimentation failed because of the substantial toxicity and weak selectivity of MMPIs [117]. Mainly, candidate MMPIs are represented by small molecules, peptides, and antibodies [118]. Currently, only one broad-spectrum MMPI has been approved by FDA but it has not indication in cancer (i.e., the small molecule periostat) [117,119]. Other MMPIs, such as the small molecule prinomastat, selective for MMP-1, MMP-2, and MMP-9 [120,121,122,123] and the GA-5745/andecaliximab, a selective anti-body against MMP-9, have reached the phase III [124,125]. However, none of these trials includes CRC.
6.2. Inhibition of MMPs by TIMPs
Since MMPs are naturally inhibited by TIMPs, these proteins have also been widely investigated mainly to exploit their ability to discover potential strategies for MMP inhibition [126]. The TIMP family consists of four members of proteins (TIMP1-4) that form a 1:1 complex with MMPs. Dysregulation of this complex due to the increased expression of MMPs or a decreased control by TIMPs has been observed in several diseases, including cancer. TIMPs control the activity of MMPs via binding to them (Figure 3) [126,127,128].
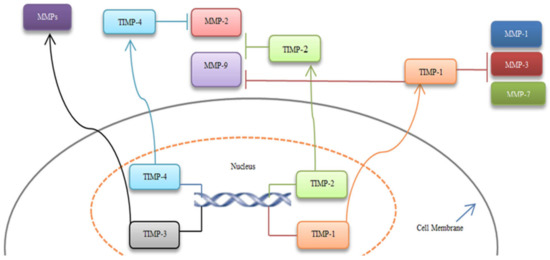
Figure 3.
MMPs inhibition by TIMPs. TIMP-1 inhibits MMP-1, 3, 7, 9. TIMP-2 can suppress MMP-2 and 9, and TIMP-4 blocks MMP-2. These inhibitions result in the primary tumor transitioning to advanced CRC. Moreover, TIMP-3 has a protective effect on CRC cases and could bind to several MMPs [126,127,128].
TIMP-1 inhibits MMP-1, 3, 7, 9 and affects angiogenesis [37,129]. Previous investigations considered a dual activity for the TIMP-1 gene: in particular, TIMP-1 was associated with tumor growth at the early stages of colon cancer, and decreased activity of TIMP-1 could lead to tumor invasion [130,131].
TIMP-2 can suppress MMP-2, MMP-9, and microvascularization [129,132]. Also, downregulation of TIMP-2 is related to invasive CRC [133]. Wang et al., reported that downregulation of TIMP-2 in CRC tumor tissues was meaningfully correlated with the depth of invasion, lymph node metastasis, tumor stage, and poor survival [134].
TIMP-3 is known as a tumor suppressor gene and inhibits several MMPs. TIMP-3 downregulation is associated with advanced CRC [135]. Lin et al., represented that, adenovirus-mediated TIMP-3 transduction in CT26 colon cancer cell line suppressed cell growth and stimulated apoptosis. Also, TIMP-3 transduction inhibited migration and invasion. In vivo data indicated that TIMP-3 prevented in vivo tumor growth and liver metastasis [136].
TIMP-4 protein suppresses MMP-2, and one study showed that overexpression of TIMP-4 increased the survival rate of rectal cancer [128].
Currently, no drug mimicking the TIMP activity has been obtained as well as no gene therapeutic approach able to modulate the activity of TIMPs is available.
6.3. MMPs Regulation by microRNA
MicroRNAs, a class of small, endogenous RNAs of 21–25 nucleotides in length, control gene and protein regulation via binding and digesting target mRNA (Table 3). Suppression of MMPs by microRNAs is a suggested way for CRC treatment. Some evidence has been provided. In particular, microRNA-34 (miR-34a) plays a role as a tumor suppressor, and its overexpression could suppress MMP-1, MMP-9, and tumor cell proliferation, migration, and invasion via acetylation of P53 in CRC [137,138,139]. The upregulation of miR-139 reduces proliferation, migration, and invasion by suppression of the IGF-IR/MEK/ERK signaling and MMP-2 gene in CRC patients [140]. Upregulation of miR-29a increases CRC metastasis via suppression of KLF4 (Kruppel-like factor 4), transcription factor, and upregulation of MMP-2 gene [141]. Also, miR-29b suppresses CRC metastasis, reduces angiogenesis and EMT by targeting the MMP-2 gene [142]. Overexpression of miR-143 can suppress the MMP-7 gene directly and prevent colorectal tumor cell proliferation and invasion [143].

Table 3.
MMPs are Regulated by microRNAs in CRC.
6.4. MMPs Regulation by Long Non-Coding RNAs
Long non-coding RNAs (lncRNAs) can regulate gene expression and have key roles in cell proliferation, migration, invasion, apoptosis, metastasis, and EMT in CRC. In this regard, lncRNA-targeted therapy is today considered a potential promising strategy for CRC treatment [144]. In fact, based on mechanistic studies investigating the complex lncRNA-mediated sponge interactions in CRC, potential therapeutic targets for the treatment of this cancer may be identified. Among the available findings, Tian et al., demonstrated that the suppression of TUG1 by shRNA prevented MMP-14 expression, proliferation, invasion, and EMT in colon cancer [145]. Sun et al., found a significant association between XIST inhibition and suppression of c-Myc, cyclinD1, and MMP-7 expression through inactivation of Wnt/β-catenin signaling pathway [146]. A recent investigation showed a meaningful correlation between the overexpression of LINC00963 and the upregulation of MMP-2 and MMP-9, proliferation, migration, and invasion of CRC cells [147]. Duan et al., revealed that the inhibition of the CCEPR lncRNA reduced the expression levels of MMP-2 and MMP-9, and prevented EMT in CRC cells [148]. Pan et al., realized that the expression level of MMP-2 protein was notably decreased when PCA3 was knocked out. In addition, suppression of PCA3 inhibited colon cancer cell invasion and migration [149].
7. Conclusions
In summary, MMPs genes and proteins, through complex mechanisms involving the induction of many molecular signaling pathways and the EMT process, play a relevant role in the transition from pre-cancerous lesions and polyps to advanced CRC. However, further investigation is needed to understand how MMPs exactly work. This would improve the selectivity of MMPIs that could be exploited in a dual-mode: to treat CRC alone or in combination with targeted agents and/or chemotherapy and to prevent CRC development.
Author Contributions
Conceptualization, investigation, writing—original draft, designed tables and figures Z.P.; editing, validation, and revise, S.N.; writing, design table, and investigation, N.P.; revise, B.S.; investigation, H.N. and H.S.; validation, H.A.-A. and E.M.; supervision, validation, and revise E.N.-M. and M.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Fondo Beneficenza Intesa Sanpaolo S.p.A. (Milan, Italy) and Associazione Giacomo Onlus (Castiglioncello, Italy) to E.M.
Acknowledgments
The authors would like to thank all the staff of the Department of Cancer at the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Nakamura, Y.; White, R.; Smits, A.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kasi, A.; Handa, S.; Bhatti, S.; Umar, S.; Bansal, A.; Sun, W. Molecular Pathogenesis and Classification of Colorectal Carcinoma. Curr. Colorectal Cancer Rep. 2020, 16, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Huck, M.B.; Bohl, J.L. Colonic Polyps: Diagnosis and Surveillance. Clin. Colon Rectal Surg. 2016, 29, 296–305. [Google Scholar] [CrossRef]
- Smit, W.L.; Spaan, C.N.; de Boer, R.J.; Ramesh, P.; Garcia, T.M.; Meijer, B.J.; Vermeulen, J.L.M.; Lezzerini, M.; MacInnes, A.W.; Koster, J.; et al. Driver mutations of the adenoma-carcinoma sequence govern the intestinal epithelial global translational capacity. Proc. Natl. Acad. Sci. USA 2020, 117, 25560. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, J.; Yu, J.; Wu, Y.; Guo, J.; Xu, Z.; Sun, X. Knockdown of MMP--1 inhibits the progression of colorectal cancer by suppressing the PI3K/Akt/c--myc signaling pathway and EMT. Oncol. Rep. 2020, 43, 1103–1112. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, X.; Shi, X.; Wang, H.; Wu, G.; Jiang, C.; Yu, D.; Zhang, W.; Xue, B.; Ding, Y. USP39 promotes colorectal cancer growth and metastasis through the Wnt/β-catenin pathway. Oncol. Rep. 2017, 37, 2398–2404. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Yadav, L.; Puri, N.; Rastogi, V.; Satpute, P.; Ahmad, R.; Kaur, G. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 1085–1091. [Google Scholar] [CrossRef]
- Ligi, D.; Mannello, F. Do matrix metalloproteinases represent reliable circulating biomarkers in colorectal cancer? Br. J. Cancer 2016, 115, 633–634. [Google Scholar] [CrossRef][Green Version]
- Yeh, Y.-C.; Sheu, B.-S. Matrix metalloproteinases and their inhibitors in the gastrointestinal cancers: Current knowledge and clinical potential. Met. Med. 2014, 1, 3–13. [Google Scholar] [CrossRef][Green Version]
- Wu, Z.; Liu, Z.; Ge, W.; Shou, J.; You, L.; Pan, H.; Han, W. Analysis of potential genes and pathways associated with the colorectal normal mucosa-adenoma-carcinoma sequence. Cancer Med. 2018, 7, 2555–2566. [Google Scholar] [CrossRef]
- Bertelson, N.L.; Kalkbrenner, K.A.; Merchea, A.; Dozois, E.J.; Landmann, R.G.; De Petris, G.; Young-Fadok, T.M.; Etzioni, D.A. Colectomy for Endoscopically Unresectable Polyps: How Often Is It Cancer? Dis. Colon Rectum 2012, 55, 1111–1116. [Google Scholar] [CrossRef]
- Shussman, N.; Wexner, S.D. Colorectal polyps and polyposis syndromes. Gastroenterol. Rep. 2014, 2, 1–15. [Google Scholar] [CrossRef]
- Liljegren, A.; Lindblom, A.; Rotstein, S.; Nilsson, B.; Rubio, C.; Jaramillo, E. Prevalence and incidence of hyperplastic polyps and adenomas in familial colorectal cancer: Correlation between the two types of colon polyps. Gut 2003, 52, 1140–1147. [Google Scholar] [CrossRef]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef]
- Kato, S.; Lippman, S.M.; Flaherty, K.T.; Kurzrock, R. The Conundrum of Genetic “Drivers” in Benign Conditions. J. Natl. Cancer Inst. 2016, 108, djw036. [Google Scholar] [CrossRef]
- Mustjoki, S.; Young, N.S. Somatic Mutations in “Benign” Disease. N. Engl. J. Med. 2021, 384, 2039–2052. [Google Scholar] [CrossRef]
- Nojadeh, J.N.; Behrouz Sharif, S.; Sakhinia, E. Microsatellite instability in colorectal cancer. EXCLI J. 2018, 17, 159–168. [Google Scholar] [CrossRef]
- Arabsorkhi, Z.; Sadeghi, H.; Gharib, E.; Rejali, L.; Asadzadeh-Aghdaei, H.; Nazemalhosseini-Mojarad, E. Can hypoxia-inducible factor-1α overexpression discriminate human colorectal cancers with different microsatellite instability? Genes Genet. Syst. 2021, 96, 1–6. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Colussi, D.; Brandi, G.; Bazzoli, F.; Ricciardiello, L. Molecular pathways involved in colorectal cancer: Implications for disease behavior and prevention. Int. J. Mol. Sci. 2013, 14, 16365–16385. [Google Scholar] [CrossRef]
- Grady, W.M.; Markowitz, S.D. The molecular pathogenesis of colorectal cancer and its potential application to colorectal cancer screening. Dig. Dis. Sci. 2015, 60, 762–772. [Google Scholar] [CrossRef]
- Sadanandam, A.; Lyssiotis, C.A.; Homicsko, K.; Collisson, E.A.; Gibb, W.J.; Wullschleger, S.; Ostos, L.C.; Lannon, W.A.; Grotzinger, C.; Del Rio, M.; et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat. Med. 2013, 19, 619–625. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Thanki, K.; Nicholls, M.E.; Gajjar, A.; Senagore, A.J.; Qiu, S.; Szabo, C.; Hellmich, M.R.; Chao, C. Consensus Molecular Subtypes of Colorectal Cancer and their Clinical Implications. Int. Biol. Biomed. J. 2017, 3, 105–111. [Google Scholar]
- McCorry, A.M.; Loughrey, M.B.; Longley, D.B.; Lawler, M.; Dunne, P.D. Epithelial-to-mesenchymal transition signature assessment in colorectal cancer quantifies tumour stromal content rather than true transition. J. Pathol. 2018, 246, 422–426. [Google Scholar] [CrossRef]
- Isella, C.; Brundu, F.; Bellomo, S.E.; Galimi, F.; Zanella, E.; Porporato, R.; Petti, C.; Fiori, A.; Orzan, F.; Senetta, R.; et al. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat. Commun. 2017, 8, 15107. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Morgunova, E.; Tuuttila, A.; Bergmann, U.; Isupov, M.; Lindqvist, Y.; Schneider, G.; Tryggvason, K. Structure of human pro-matrix metalloproteinase-2: Activation mechanism revealed. Science 1999, 284, 1667–1670. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell. Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.H.; Edwards, D.R.; Murphy, G. Metalloproteinase inhibitors: Biological actions and therapeutic opportunities. J. Cell Sci. 2002, 115, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Li, M.; Luo, T.; Yin, Y.; Jiang, Y. Matrix metalloproteinases in tumorigenesis: An evolving paradigm. Cell Mol. Life Sci. 2011, 68, 3853–3868. [Google Scholar] [CrossRef]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019, 56, 100–115. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Herszényi, L.; Hritz, I.; Lakatos, G.; Varga, M.Z.; Tulassay, Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int. J. Mol. Sci. 2012, 13, 13240–13263. [Google Scholar] [CrossRef]
- Huang, X.; Lan, Y.; Li, E.; Li, J.; Deng, Q.; Deng, X. Diagnostic values of MMP-7, MMP-9, MMP-11, TIMP-1, TIMP-2, CEA, and CA19-9 in patients with colorectal cancer. J. Int. Med. Res. 2021, 49, 1–11. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, D.; Ding, X.; Xu, P. Clinical value of microRNA-135a and MMP-13 in colon cancer. Oncol. Lett. 2021, 22, 583. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Waquar, S.; Ain, Q.T.; Rasool, R.; Asif, M.; Anfinan, N.; Haque, A.; Alam, H.; Ahmed, S.; et al. Assessment of clinical variables as predictive markers in the development and progression of colorectal cancer. Bioengineered 2021, 12, 2288–2298. [Google Scholar] [CrossRef]
- Barabás, L.; Hritz, I.; István, G.; Tulassay, Z.; Herszényi, L. The Behavior of MMP-2, MMP-7, MMP-9, and Their Inhibitors TIMP-1 and TIMP-2 in Adenoma-Colorectal Cancer Sequence. Dig. Dis. 2021, 39, 217–224. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Hsieh, S.; Lai, P.Y.; Wang, J.J.; Li, C.C.; Wu, C.C. Carnosine Suppresses Human Colorectal Cell Migration and Intravasation by Regulating EMT and MMP Expression. Am. J. Chin. Med. 2019, 47, 477–494. [Google Scholar] [CrossRef]
- Kıyak, R.; Keles, D.; Bengi, G.; Yalcin, M.; Topalak, Ö.; Oktay, G. The Importance of Fecal and Plasma CEA, COX-2, MMP-7, and TIMP-1 in the Diagnosis of Colorectal Cancer. J. Basic Clin. Health Sci. 2018, 2, 7–14. [Google Scholar] [CrossRef]
- Eiró, N.; Gonzalez, L.; Cid, S.; Andicoechea, A.; Vizoso, F. Matrix metalloproteases expression in different histological types of colorectal polyps. Rev. Esp. Enferm. Dig. 2017, 109, 414–420. [Google Scholar] [CrossRef]
- Pezeshkian, Z.; Forouzesh, F.; Peyravian, N.; Yaghoob-Taleghani, M.; Asadzadeh-Aghdaei, H.; Zali, M.; Nazemalhosseini-Mojarad, E. Clinicopathological correlations of VEGF-A and MMP-7 genes expression in different types of colorectal adenoma polyps. WCRJ 2017, 4, e978. [Google Scholar]
- Wernicke, A.-K.; Churin, Y.; Sheridan, D.; Windhorst, A.; Tschuschner, A.; Gattenlöhner, S.; Roderfeld, M.; Roeb, E. Matrix metalloproteinase-13 refines pathological staging of precancerous colorectal lesions. Oncotarget 2016, 7, 73552–73557. [Google Scholar] [CrossRef][Green Version]
- Gimeno-García, A.Z.; Triñanes, J.; Quintero, E.; Salido, E.; Nicolás-Pérez, D.; Adrián-de-Ganzo, Z.; Alarcón-Fernández, O.; Abrante, B.; Romero, R.; Carrillo, M.; et al. Plasma matrix metalloproteinase 9 as an early surrogate biomarker of advanced colorectal neoplasia. Gastroenterol. Hepatol. 2016, 39, 433–441. [Google Scholar] [CrossRef]
- Annaházi, A.; Ábrahám, S.; Farkas, K.; Rosztóczy, A.; Inczefi, O.; Földesi, I.; Szűcs, M.; Rutka, M.; Theodorou, V.; Eutamene, H.; et al. A pilot study on faecal MMP-9: A new noninvasive diagnostic marker of colorectal cancer. Br. J. Cancer 2016, 114, 787–792. [Google Scholar] [CrossRef]
- Klupp, F.; Neumann, L.; Kahlert, C.; Diers, J.; Halama, N.; Franz, C.; Schmidt, T.; Koch, M.; Weitz, J.; Schneider, M.; et al. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer 2016, 16, 494. [Google Scholar] [CrossRef]
- Otero-Estévez, O.; De Chiara, L.; Rodríguez-Girondo, M.; Rodríguez-Berrocal, F.J.; Cubiella, J.; Castro, I.; Hernández, V.; Martínez-Zorzano, V.S. Serum matrix metalloproteinase-9 in colorectal cancer family-risk population screening. Sci. Rep. 2015, 5, 13030. [Google Scholar] [CrossRef]
- Bengi, G.; Keles, D.; Topalak, Ö.; Yalçin, M.; Kiyak, R.; Oktay, G. Expressions of TIMP-1, COX-2 and MMP-7 in Colon Polyp and Colon Cancer. Euroasian J. Hepatogastroenterol. 2015, 5, 74–79. [Google Scholar] [CrossRef]
- Odabasi, M.; Yesil, A.; Ozkara, S.; Paker, N.; Ozkan, S.; Eris, C.; Yildiz, M.K.; Abuoglu, H.H.; Gunay, E.; Tekeşin, K. Role of human neutrophil gelatinase associated lipocalin (NGAL) and Matrix Metalloproteinase-9 (MMP-9) overexpression in neoplastic colon polyps. Int. J. Clin. Exp. Med. 2014, 7, 2804–2811. [Google Scholar]
- Qasim, B.J.; Ali, H.H.; Hussein, A.G. Immunohistochemical expression of matrix metalloproteinase-7 in human colorectal adenomas using specified automated cellular image analysis system: A clinicopathological study. Saudi J. Gastroenterol. 2013, 19, 23–27. [Google Scholar] [CrossRef]
- Sheth, R.A.; Kunin, A.; Stangenberg, L.; Sinnamon, M.; Hung, K.E.; Kucherlapati, R.; Mahmood, U. In vivo optical molecular imaging of matrix metalloproteinase activity following celecoxib therapy for colorectal cancer. Mol. Imaging 2012, 11, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Murnane, M.J.; Cai, J.; Shuja, S.; McAneny, D.; Klepeis, V.; Willett, J.B. Active MMP-2 effectively identifies the presence of colorectal cancer. Int. J. Cancer 2009, 125, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.; McLean, M.H.; El-Omar, E.M.; Murray, G.I. The matrix metalloproteinase/tissue inhibitor of matrix metalloproteinase profile in colorectal polyp cancers. Histopathology 2009, 54, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A.; Milet, J.; Carayol, J.; Le Corre, D.; Milan, C.; Pariente, A.; Nalet, B.; Lafon, J.; Faivre, J.; Bonithon-Kopp, C.; et al. Genetic polymorphisms of MMP1, MMP3 and MMP7 gene promoter and risk of colorectal adenoma. BMC Cancer 2006, 6, 270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tutton, M.G.; George, M.L.; Eccles, S.A.; Burton, S.; Swift, R.I.; Abulafi, A.M. Use of plasma MMP-2 and MMP-9 levels as a surrogate for tumour expression in colorectal cancer patients. Int. J. Cancer 2003, 107, 541–550. [Google Scholar] [CrossRef]
- Jonsson, A.; Falk, P.; Angenete, E.; Hjalmarsson, C.; Ivarsson, M.-L. Plasma MMP-1 Expression as a Prognostic Factor in Colon Cancer. J. Surg. Res. 2021, 266, 254–260. [Google Scholar] [CrossRef]
- Liang, Y.; Lv, Z.; Huang, G.; Qin, J.; Li, H.; Nong, F.; Wen, B. Prognostic significance of abnormal matrix collagen remodeling in colorectal cancer based on histologic and bioinformatics analysis. Oncol. Rep. 2020, 44, 1671–1685. [Google Scholar] [CrossRef]
- Sunami, E.; Tsuno, N.; Osada, T.; Saito, S.; Kitayama, J.; Tomozawa, S.; Tsuruo, T.; Shibata, Y.; Muto, T.; Nagawa, H. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist 2000, 5, 108–114. [Google Scholar] [CrossRef]
- Yamada, T.; Oshima, T.; Yoshihara, K.; Tamura, S.; Kanazawa, A.; Inagaki, D.; Yamamoto, N.; Sato, T.; Fujii, S.; Numata, K.; et al. Overexpression of MMP-13 gene in colorectal cancer with liver metastasis. Anticancer Res. 2010, 30, 2693–2699. [Google Scholar]
- Leeman, M.F.; McKay, J.A.; Murray, G.I. Matrix metalloproteinase 13 activity is associated with poor prognosis in colorectal cancer. J. Clin. Pathol. 2002, 55, 758–762. [Google Scholar] [CrossRef]
- Merchant, N.; Chalikonda, G.; Nagaraju, G.P. Role of Matrix Metalloproteinases in Colorectal Cancer. In Theranostics Approaches to Gastric and Colon Cancer; Springer: Singapore, 2020; pp. 49–59. [Google Scholar] [CrossRef]
- Yan, Q.; Yuan, Y.; Yankui, L.; Jingjie, F.; Linfang, J.; Yong, P.; Dong, H.; Xiaowei, Q. The Expression and Significance of CXCR5 and MMP-13 in Colorectal Cancer. Cell Biochem. Biophys. 2015, 73, 253–259. [Google Scholar] [CrossRef]
- Korpi, J.T.; Kervinen, V.; Mäklin, H.; Väänänen, A.; Lahtinen, M.; Läärä, E.; Ristimäki, A.; Thomas, G.; Ylipalosaari, M.; Aström, P.; et al. Collagenase-2 (matrix metalloproteinase-8) plays a protective role in tongue cancer. Br. J. Cancer 2008, 98, 766–775. [Google Scholar] [CrossRef]
- Balbín, M.; Fueyo, A.; Tester, A.M.; Pendás, A.M.; Pitiot, A.S.; Astudillo, A.; Overall, C.M.; Shapiro, S.D.; López-Otín, C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 2003, 35, 252–257. [Google Scholar] [CrossRef]
- Sirniö, P.; Tuomisto, A.; Tervahartiala, T.; Sorsa, T.; Klintrup, K.; Karhu, T.; Herzig, K.-H.; Mäkelä, J.; Karttunen, T.J.; Salo, T.; et al. High-serum MMP-8 levels are associated with decreased survival and systemic inflammation in colorectal cancer. Br. J. Cancer 2018, 119, 213–219. [Google Scholar] [CrossRef]
- Beutel, B.; Song, J.; Konken, C.P.; Korpos, E.; Schinor, B.; Gerwien, H.; Vidyadharan, R.; Burmeister, M.; Li, L.; Haufe, G.; et al. New in Vivo Compatible Matrix Metalloproteinase (MMP)-2 and MMP-9 Inhibitors. Bioconjugate Chem. 2018, 29, 3715–3725. [Google Scholar] [CrossRef]
- Murnane, M.J.; Cai, J.; Shuja, S.; McAneny, D.; Willett, J.B. Active matrix metalloproteinase-2 activity discriminates colonic mucosa, adenomas with and without high-grade dysplasia, and cancers. Hum. Pathol. 2011, 42, 688–701. [Google Scholar] [CrossRef]
- Gimeno-García, A.Z.; Santana-Rodríguez, A.; Jiménez, A.; Parra-Blanco, A.; Nicolás-Pérez, D.; Paz-Cabrera, C.; Díaz-González, F.; Medina, C.; Díaz-Flores, L.; Quintero, E. Up-regulation of gelatinases in the colorectal adenoma-carcinoma sequence. Eur. J. Cancer 2006, 42, 3246–3252. [Google Scholar] [CrossRef]
- Salem, N.; Kamal, I.; Al-Maghrabi, J.; Abuzenadah, A.; Peer-Zada, A.A.; Qari, Y.; Al-Ahwal, M.; Al-Qahtani, M.; Buhmeida, A. High expression of matrix metalloproteinases: MMP-2 and MMP-9 predicts poor survival outcome in colorectal carcinoma. Future Oncol. 2016, 12, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Langenskiöld, M.; Holmdahl, L.; Falk, P.; Ivarsson, M.-L. Increased plasma MMP-2 protein expression in lymph node-positive patients with colorectal cancer. Int. J. Colorectal Dis. 2005, 20, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, J.; Stasiak, M.; Dziki, L.; Mik, M.; Dziki, A.; Cierniewski, C.S. Matrix metalloproteinase-2 cleavage of the β1 integrin ectodomain facilitates colon cancer cell motility. J. Biol. Chem. 2012, 287, 36556–36566. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Hisanaga, M.; Nagao, M.; Ikeda, N.; Fujii, H.; Koyama, F.; Mukogawa, T.; Matsumoto, H.; Kondo, S.; Takahashi, C.; et al. The membrane-anchored matrix metalloproteinase (MMP) regulator RECK in combination with MMP-9 serves as an informative prognostic indicator for colorectal cancer. Clin. Cancer Res. 2004, 10, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ye, Y.; Yang, Y.; Zhong, M.; Gu, L.; Han, Z.; Qiu, J.; Liu, Z.; Qiu, X.; Zhuang, G. TIPE-mediated up-regulation of MMP-9 promotes colorectal cancer invasion and metastasis through MKK-3/p38/NF-κB pro-oncogenic signaling pathway. Signal Transduct. Target. Ther. 2020, 5, 163. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Jeppsson, S.; Dalmasso, G.; Ghaleb, A.M.; McConnell, B.B.; Yang, V.W.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Notch1 regulates the effects of matrix metalloproteinase-9 on colitis-associated cancer in mice. Gastroenterology 2011, 141, 1381–1392. [Google Scholar] [CrossRef]
- Garg, P.; Sarma, D.; Jeppsson, S.; Patel, N.R.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res. 2010, 70, 792–801. [Google Scholar] [CrossRef]
- Walter, L.; Canup, B.; Pujada, A.; Bui, T.A.; Arbasi, B.; Laroui, H.; Merlin, D.; Garg, P. Matrix metalloproteinase 9 (MMP9) limits reactive oxygen species (ROS) accumulation and DNA damage in colitis-associated cancer. Cell Death Dis. 2020, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cao, S.; Liu, S.; Yao, Z.; Sun, T.; Li, Y.; Li, J.; Zhang, D.; Zhou, Y. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients’ survival? A pilot study on relevant mechanism. Oncotarget 2016, 7, 46158–46172. [Google Scholar] [CrossRef]
- Sipos, F.; Germann, T.M.; Wichmann, B.; Galamb, O.; Spisák, S.; Krenács, T.; Tulassay, Z.; Molnár, B.; Műzes, G. MMP3 and CXCL1 are potent stromal protein markers of dysplasia-carcinoma transition in sporadic colorectal cancer. Eur. J. Cancer Prev. 2014, 23, 336–343. [Google Scholar] [CrossRef]
- Işlekel, H.; Oktay, G.; Terzi, C.; Canda, A.E.; Füzün, M.; Küpelioğlu, A. Matrix metalloproteinase-9,-3 and tissue inhibitor of matrix metalloproteinase-1 in colorectal cancer: Relationship to clinicopathological variables. Cell Biochem. Funct. 2007, 25, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yagi, M.; Akiyama, N.; Hirosaki, T.; Higashi, S.; Lin, C.Y.; Dickson, R.B.; Kitamura, H.; Miyazaki, K. Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci. 2006, 97, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, K.; Ogata, Y.; Nagase, H.; Shirouzu, K. Significance of coexpression of urokinase-type plasminogen activator, and matrix metalloproteinase 3 (stromelysin) and 9 (gelatinase B) in colorectal carcinoma. J. Surg. Res. 2000, 93, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Batra, J.; Robinson, J.; Soares, A.S.; Fields, A.P.; Radisky, D.C.; Radisky, E.S. Matrix metalloproteinase-10 (MMP-10) interaction with tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2: Binding studies and crystal structure. J. Biol. Chem. 2012, 287, 15935–15946. [Google Scholar] [CrossRef]
- Surlin, V.; Ioana, M.; Pleşea, I.E. Genetic patterns of metalloproteinases and their tissular inhibitors—Clinicopathologic and prognostic significance in colorectal cancer. Rom. J. Morphol. Embryol. 2011, 52, 231–236. [Google Scholar]
- Asadzadeh Aghdaei, H.; Pezeshkian, Z.; Abdollahpour-Alitappeh, M.; Nazemalhosseini Mojarad, E.; Zali, M.R. The Role of Angiogenesis in Colorectal Polyps and Cancer, a Review. Med. Lab. J. 2018, 12, 1–6. [Google Scholar] [CrossRef]
- Ii, M.; Yamamoto, H.; Adachi, Y.; Maruyama, Y.; Shinomura, Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp. Biol. Med. 2006, 231, 20–27. [Google Scholar] [CrossRef]
- Cheng, K.; Xie, G.; Raufman, J.P. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem. Pharmacol. 2007, 73, 1001–1012. [Google Scholar] [CrossRef]
- Xie, G.; Cheng, K.; Shant, J.; Raufman, J.P. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G755–G763. [Google Scholar] [CrossRef]
- Decock, J.; Thirkettle, S.; Wagstaff, L.; Edwards, D.R. Matrix metalloproteinases: Protective roles in cancer. J. Cell Mol. Med. 2011, 15, 1254–1265. [Google Scholar] [CrossRef]
- Asano, T.; Tada, M.; Cheng, S.; Takemoto, N.; Kuramae, T.; Abe, M.; Takahashi, O.; Miyamoto, M.; Hamada, J.; Moriuchi, T.; et al. Prognostic values of matrix metalloproteinase family expression in human colorectal carcinoma. J. Surg. Res. 2008, 146, 32–42. [Google Scholar] [CrossRef]
- Yang, W.; Arii, S.; Gorrin-Rivas, M.J.; Mori, A.; Onodera, H.; Imamura, M. Human macrophage metalloelastase gene expression in colorectal carcinoma and its clinicopathologic significance. Cancer 2001, 91, 1277–1283. [Google Scholar] [CrossRef]
- Shi, H.; Xu, J.M.; Hu, N.Z.; Wang, X.L.; Mei, Q.; Song, Y.L. Transfection of mouse macrophage metalloelastase gene into murine CT-26 colon cancer cells suppresses orthotopic tumor growth, angiogenesis and vascular endothelial growth factor expression. Cancer Lett. 2006, 233, 139–150. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, H.; Li, Q.; Mei, Q.; Bao, J.; Shen, Y.; Xu, J. Mouse macrophage metalloelastase generates angiostatin from plasminogen and suppresses tumor angiogenesis in murine colon cancer. Oncol. Rep. 2008, 20, 81–88. [Google Scholar] [CrossRef]
- Beurden, P.; Von den Hoff, J. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. BioTechniques 2005, 38, 73–83. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Liu, X.; Lu, J.; He, X.; Wang, Q.; Li, J.; Du, X. Identification of high-risk stage II and stage III colorectal cancer by analysis of MMP-21 expression. J. Surg. Oncol. 2011, 104, 787–791. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Chu, D.; Zheng, J.; Ji, G.; Li, M.; Zhang, H.; Wang, W.; Du, J.; Li, J. Overexpression of matrix metalloproteinase-21 is associated with poor overall survival of patients with colorectal cancer. J. Gastrointest. Surg. 2011, 15, 1188–1194. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Q.; Yan, W.; Wang, Y.; He, X.; Zhao, Z. Overexpression of MMP21 and MMP28 is associated with gastric cancer progression and poor prognosis. Oncol. Lett. 2018, 15, 7776–7782. [Google Scholar] [CrossRef]
- Pahwa, S.; Stawikowski, M.J.; Fields, G.B. Monitoring and Inhibiting MT1-MMP during Cancer Initiation and Progression. Cancers 2014, 6, 416–435. [Google Scholar] [CrossRef]
- Devy, L.; Huang, L.; Naa, L.; Yanamandra, N.; Pieters, H.; Frans, N.; Chang, E.; Tao, Q.; Vanhove, M.; Lejeune, A.; et al. Selective Inhibition of Matrix Metalloproteinase-14 Blocks Tumor Growth, Invasion, and Angiogenesis. Cancer Res. 2009, 69, 1517–1526. [Google Scholar] [CrossRef]
- Duan, F.; Peng, Z.; Yin, J.; Yang, Z.; Shang, J. Expression of MMP-14 and prognosis in digestive system carcinoma: A meta-analysis and databases validation. J. Cancer 2020, 11, 1141–1150. [Google Scholar] [CrossRef]
- Yang, B.; Gao, J.; Rao, Z.; Shen, Q. Clinicopathological and prognostic significance of α5β1-integrin and MMP-14 expressions in colorectal cancer. Neoplasma 2013, 60, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Cai, F.; Ding, Z.; Gao, L. MMP14 predicts a poor prognosis in patients with colorectal cancer. Hum. Pathol. 2019, 83, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L. How the matrix metalloproteinase MMP14 contributes to the progression of colorectal cancer. J. Clin. Investig. 2020, 130, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Decock, J.; Paridaens, R.; Ye, S. Genetic polymorphisms of matrix metalloproteinases in lung, breast and colorectal cancer. Clin. Genet. 2008, 73, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Langers, A.M.; Verspaget, H.W.; Hommes, D.W.; Sier, C.F. Single-nucleotide polymorphisms of matrix metalloproteinases and their inhibitors in gastrointestinal cancer. World J. Gastrointest. Oncol. 2011, 3, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Hinoda, Y.; Okayama, N.; Takano, N.; Fujimura, K.; Suehiro, Y.; Hamanaka, Y.; Hazama, S.; Kitamura, Y.; Kamatani, N.; Oka, M. Association of functional polymorphisms of matrix metalloproteinase (MMP)-1 and MMP-3 genes with colorectal cancer. Int. J. Cancer 2002, 102, 526–529. [Google Scholar] [CrossRef]
- Kouhkan, F.; Motovali-Bashi, M.; Hojati, Z. The influence of interstitial collagenas-1 genotype polymorphism on colorectal cancer risk in Iranian population. Cancer Investig. 2008, 26, 836–842. [Google Scholar] [CrossRef]
- Langers, A.M.J.; Sier, C.F.M.; Hawinkels, L.J.A.C.; Kubben, F.J.G.M.; van Duijn, W.; van der Reijden, J.J.; Lamers, C.B.H.W.; Hommes, D.W.; Verspaget, H.W. MMP-2 geno-phenotype is prognostic for colorectal cancer survival, whereas MMP-9 is not. Br. J. Cancer 2008, 98, 1820–1823. [Google Scholar] [CrossRef]
- Ting, W.-C.; Chen, L.-M.; Pao, J.-B.; Yang, Y.-P.; You, B.-J.; Chang, T.-Y.; Lan, Y.-H.; Lee, H.-Z.; Bao, B.-Y. Genetic Polymorphisms of Matrix Metalloproteinases and Clinical Outcomes in Colorectal Cancer Patients. Int. J. Med. Sci. 2013, 10, 1022–1027. [Google Scholar] [CrossRef]
- Dziki, L.; Przybyłowska, K.; Majsterek, I.; Trzciński, R.; Mik, M.; Sygut, A. A/G Polymorphism of the MMP-7 Gene Promoter Region in Colorectal Cancer. Pol. Przegl. Chir. 2011, 83, 622–626. [Google Scholar] [CrossRef]
- Fang, W.-L.; Liang, W.; He, H.; Zhu, Y.; Li, S.-L.; Gao, L.-B.; Zhang, L. Association of Matrix Metalloproteinases 1, 7, and 9 Gene Polymorphisms with Genetic Susceptibility to Colorectal Carcinoma in a Han Chinese Population. DNA Cell Biol. 2010, 29, 657–661. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, S.J.; Kim, K.H.; Kim, J.C. Clinical characteristics of TIMP2, MMP2, and MMP9 gene polymorphisms in colorectal cancer. J. Gastroenterol. Hepatol. 2011, 26, 391–397. [Google Scholar] [CrossRef]
- Van Nguyen, S.; Skarstedt, M.; LÖFgren, S.; Zar, N.; Andersson, R.E.; Lindh, M.; Matussek, A.; Dimberg, J.A.N. Gene Polymorphism of Matrix Metalloproteinase-12 and -13 and Association with Colorectal Cancer in Swedish Patients. Anticancer Res. 2013, 33, 3247–3250. [Google Scholar]
- Tai, J.; Sun, D.; Wang, X.; Kang, Z. Matrix metalloproteinase-8 rs11225395 polymorphism correlates with colorectal cancer risk and survival in a Chinese Han population: A case-control study. Aging 2020, 12, 19618–19627. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Caton, J.G. Evaluation of Periostat for patient management. Compend. Contin. Educ. Dent. 1999, 20, 451, 458–460. [Google Scholar]
- Bissett, D.; O’Byrne, K.J.; von Pawel, J.; Gatzemeier, U.; Price, A.; Nicolson, M.; Mercier, R.; Mazabel, E.; Penning, C.; Zhang, M.H.; et al. Phase III study of matrix metalloproteinase inhibitor prinomastat in non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 842–849. [Google Scholar] [CrossRef]
- Scatena, R. Prinomastat, a hydroxamate-based matrix metalloproteinase inhibitor. A novel pharmacological approach for tissue remodelling-related diseases. Expert Opin. Investig. Drugs 2000, 9, 2159–2165. [Google Scholar] [CrossRef]
- Hande, K.R.; Collier, M.; Paradiso, L.; Stuart-Smith, J.; Dixon, M.; Clendeninn, N.; Yeun, G.; Alberti, D.; Binger, K.; Wilding, G. Phase I and pharmacokinetic study of prinomastat, a matrix metalloprotease inhibitor. Clin. Cancer Res. 2004, 10, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Lin, C.-W.; Su, S.-C.; Yang, S.-F. Pharmacodynamic considerations in the use of matrix metalloproteinase inhibitors in cancer treatment. Expert Opin. Drug Metab. Toxicol. 2015, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Starodub, A.; Sharma, S.; Berlin, J.; Patel, M.; Wainberg, Z.A.; Chaves, J.; Gordon, M.; Windsor, K.; Brachmann, C.B.; et al. Andecaliximab/GS-5745 Alone and Combined with mFOLFOX6 in Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: Results from a Phase I Study. Clin. Cancer Res. 2018, 24, 3829–3837. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Bhandari, B.R.; Randall, C.; Younes, Z.H.; Romanczyk, T.; Xin, Y.; Wendt, E.; Chai, H.; McKevitt, M.; Zhao, S.; et al. Andecaliximab [Anti-matrix Metalloproteinase-9] Induction Therapy for Ulcerative Colitis: A Randomised, Double-Blind, Placebo-Controlled, Phase 2/3 Study in Patients With Moderate to Severe Disease. J. Crohn’s Colitis 2018, 12, 1021–1029. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef]
- Li, K.; Tay, F.R.; Yiu, C.K.Y. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol. Ther. 2020, 207, 107465. [Google Scholar] [CrossRef]
- Melendez-Zajgla, J.; Del Pozo, L.; Ceballos, G.; Maldonado, V. Tissue Inhibitor of Metalloproteinases-4. The road less traveled. Mol. Cancer 2008, 7, 85. [Google Scholar] [CrossRef]
- Hayden, D.M.; Forsyth, C.; Keshavarzian, A. The role of matrix metalloproteinases in intestinal epithelial wound healing during normal and inflammatory states. J. Surg. Res. 2011, 168, 315–324. [Google Scholar] [CrossRef]
- Song, G.; Xu, S.; Zhang, H.; Wang, Y.; Xiao, C.; Jiang, T.; Wu, L.; Zhang, T.; Sun, X.; Zhong, L.; et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J. Exp. Clin. Cancer Res. 2016, 35, 148. [Google Scholar] [CrossRef]
- Noël, A.; Jost, M.; Maquoi, E. Matrix metalloproteinases at cancer tumor-host interface. Semin. Cell Dev. Biol. 2008, 19, 52–60. [Google Scholar] [CrossRef]
- Lu, X.; Duan, L.; Xie, H.; Lu, X.; Lu, D.; Lu, D.; Jiang, N.; Chen, Y. Evaluation of MMP-9 and MMP-2 and their suppressor TIMP-1 and TIMP-2 in adenocarcinoma of esophagogastric junction. OncoTargets Ther. 2016, 9, 4343–4349. [Google Scholar] [CrossRef][Green Version]
- Groblewska, M.; Mroczko, B.; Gryko, M.; Pryczynicz, A.; Guzińska-Ustymowicz, K.; Kędra, B.; Kemona, A.; Szmitkowski, M. Serum levels and tissue expression of matrix metalloproteinase 2 (MMP-2) and tissue inhibitor of metalloproteinases 2 (TIMP-2) in colorectal cancer patients. Tumor Biol. 2014, 35, 3793–3802. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Xiang, L.; Lv, M.; Tao, L.; Ni, T.; Deng, J.; Gu, X.; Masatara, S.; Liu, Y.; et al. TIMP-2 inhibits metastasis and predicts prognosis of colorectal cancer via regulating MMP-9. Cell Adhes. Migr. 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Huang, H.-L.; Liu, Y.-M.; Sung, T.-Y.; Huang, T.-C.; Cheng, Y.-W.; Liou, J.-P.; Pan, S.-L. TIMP3 expression associates with prognosis in colorectal cancer and its novel arylsulfonamide inducer, MPT0B390, inhibits tumor growth, metastasis and angiogenesis. Theranostics 2019, 9, 6676–6689. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Y.; Wang, H.; Xu, D.; Meng, X.; Shao, Y.; Lin, C.; Ye, Y.; Qian, H.; Wang, S. Tissue inhibitor of metalloproteinases-3 transfer suppresses malignant behaviors of colorectal cancer cells. Cancer Gene Ther. 2012, 19, 845–851. [Google Scholar] [CrossRef]
- Soheilifar, M.H.; Grusch, M.; Keshmiri Neghab, H.; Amini, R.; Maadi, H.; Saidijam, M.; Wang, Z. Angioregulatory microRNAs in Colorectal Cancer. Cancers 2019, 12, 71. [Google Scholar] [CrossRef]
- Wu, J.; Wu, G.; Lv, L.; Ren, Y.; Zhang, X.; Xue, Y.; Li, G.; Lu, X.; Sun, Z.; Tang, K. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1. Carcinogenesis 2012, 33, 519–528. [Google Scholar] [CrossRef]
- Abba, M.; Patil, N.; Allgayer, H. MicroRNAs in the Regulation of MMPs and Metastasis. Cancers 2014, 6, 625–645. [Google Scholar] [CrossRef]
- Shen, K.; Liang, Q.; Xu, K.; Cui, D.; Jiang, L.; Yin, P.; Lu, Y.; Li, Q.; Liu, J. MiR-139 inhibits invasion and metastasis of colorectal cancer by targeting the type I insulin-like growth factor receptor. Biochem. Pharmacol. 2012, 84, 320–330. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, Y.; Gao, J.; Fu, J.; Liu, C.; Liu, Y.; Song, C.; Zhu, S.; Leng, Y.; Wang, G.; et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br. J. Cancer 2014, 110, 450–458. [Google Scholar] [CrossRef]
- Leng, Y.; Chen, Z.; Ding, H.; Zhao, X.; Qin, L.; Pan, Y. Overexpression of microRNA-29b inhibits epithelial-mesenchymal transition and angiogenesis of colorectal cancer through the ETV4/ERK/EGFR axis. Cancer Cell Int. 2021, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, X.; Chang, H. MicroRNA-143 inhibits colorectal cancer cell proliferation by targeting MMP7. Minerva Med. 2017, 108, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Schwarzmueller, L.; Bril, O.; Vermeulen, L.; Léveillé, N. Emerging Role and Therapeutic Potential of lncRNAs in Colorectal Cancer. Cancers 2020, 12, 3843. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhao, Z.F.; Xie, L.; Zhu, J.P. Taurine up-regulated 1 accelerates tumorigenesis of colon cancer by regulating miR-26a-5p/MMP14/p38 MAPK/Hsp27 axis in vitro and in vivo. Life Sci. 2019, 239, 117035. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, G.; Liu, Y. Long non-coding RNA XIST sponges miR-34a to promotes colon cancer progression via Wnt/β-catenin signaling pathway. Gene 2018, 665, 141–148. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, D.; Liu, G. LncRNA LINC00963 promotes colorectal cancer cell proliferation and metastasis by regulating miR--1281 and TRIM65. Mol. Med. Rep. 2021, 24, 781. [Google Scholar] [CrossRef]
- Duan, Y.; Fang, Z.; Shi, Z.; Zhang, L. Knockdown of lncRNA CCEPR suppresses colorectal cancer progression. Exp. Ther. Med. 2019, 18, 3534–3542. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, L.; Pu, J.; Wang, W.; Qian, W. lncRNA PCA3 plays a key role in colon cancer occurrence and development. Arch. Med. Sci. 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).