Simple Summary
Molecular characterization of ependymoma has revolutionized its categorization. This new molecular classification has implications particularly in targeted therapeutics. Amongst the ten subgroups of ependymoma currently described, three are found in the spinal compartment, and three in the infratentorial and supratentorial compartments respectively; the subependymoma subgroup is found in all these anatomic compartments. Each subgroup carries unique molecular features that lead to oncogenesis and to disparities in prognosis. Here, the molecular classification, key clinical features, current understanding of tumorigenesis, and potential molecular targets for cranial and spinal ependymoma are discussed.
Abstract
Ependymoma is a biologically diverse tumor wherein molecular classification has superseded traditional histological grading based on its superior ability to characterize behavior, prognosis, and possible targeted therapies. The current, updated molecular classification of ependymoma consists of ten distinct subgroups spread evenly among the spinal, infratentorial, and supratentorial compartments, each with its own distinct clinical and molecular characteristics. In this review, the history, histopathology, standard of care, prognosis, oncogenic drivers, and hypothesized molecular targets for all subgroups of ependymoma are explored. This review emphasizes that despite the varied behavior of the ependymoma subgroups, it remains clear that research must be performed to further elucidate molecular targets for these tumors. Although not all ependymoma subgroups are oncologically aggressive, development of targeted therapies is essential, particularly for cases where surgical resection is not an option without causing significant morbidity. The development of molecular therapies must rely on building upon our current understanding of ependymoma oncogenesis, as well as cultivating transfer of knowledge based on malignancies with similar genomic alterations.
Keywords:
ependymoma; subependymoma; RELA; YAP1; ZFTA; PFA; PFB; MYCN; Group A; Group B; myxopapillary; targeted therapy 1. Introduction
Ependymomas are primary central nervous system (CNS) tumors derived from the ependymal lining of the ventricular system [1,2]. These tumors are found in both the pediatric and adult population and are found throughout the CNS, including the supratentorial (ST) space, the infratentorial (IT) space and the spinal (SP) compartment. They are most commonly found in the ventricular system, but also in the parenchyma.
Based on the Central Brain Tumor Registry of the United States, ependymal tumors represent approximately 1.6% of all primary CNS tumors. Incidence of ependymal tumors ranges from 0.29 to 0.6 per 100,000 person-years and is lowest in the first two decades of life and highest in the 65–74 years old age group [3]. Tumor location is largely dependent on the patient age, with nearly 90% of pediatric ependymal tumors occurring intracranially, and approximately 65% of adult tumors occurring in the spine [4]. Amongst patients with intracranial ependymomas, there is significant morbidity and mortality. In a multicenter study of 282 adult patients, the 5-year overall survival (OS) rate was 62% for ST tumors and 85% for IT tumors [5]. Patients with SP ependymomas fare better with a 5-year OS of 97%.
Management of adult patients with ependymoma involves surgical resection for its cytoreductive effects and, in many cases, to restore normal cerebrospinal fluid (CSF) dynamics [6,7,8]. Postoperative radiotherapy is usually employed, particularly in cases of World Health Organization (WHO) grade II intracranial ependymoma after subtotal resection (STR) and WHO grade III intracranial and spinal anaplastic ependymoma regardless of the extent of resection [5,8,9,10,11]. The use of radiotherapy remains controversial for WHO grade II intracranial ependymoma and spinal ependymoma after gross total resection (GTR) [12]. Chemotherapy is typically reserved for cases of advanced or recurrent ependymoma that cannot be further resected or irradiated [11]. There is very little description of efficacious use of chemotherapies for ependymoma in general. In a study of adult recurrent low-grade and anaplastic SP, ST, and IT ependymoma, a regimen of temozolomide and lapatinib was used with modest results and a median progression-free survival (PFS) of 8 months [13]. Among the small number of studies looking at the use of chemotherapy for ependymoma, there is a lack of any standard regimens with durable results, though use of platinum compounds or temozolomide has been suggested due their favorable safety profiles [11,13,14,15,16,17,18]. There has been limited use of immunotherapy for adult ependymoma, though there are a few documented cases of its use in pediatric recurrent ependymoma [19].
The management of pediatric patients with ependymoma follows a similar paradigm except for several key differences. Pediatric ependymoma management centers around GTR and radiotherapy. Based on National Cancer Institute recommendations, all intracranial pediatric ependymoma cases require surgery and postoperative radiotherapy unless the patient is under 1 year of age [20]. The European Association of Neuro-Oncology (EANO) has similar recommendations except it is recommended that postoperative radiotherapy should be used in children older than 18 months, or in children between 12 and 18 months with significant neurological deficits [11]. Traditionally, chemotherapy regimens were utilized in children younger than 3 years of age with newly diagnosed ependymoma to defer radiation. However, there is mounting evidence that earlier radiation may lead to improved outcomes [21,22,23] In comparing the trials POG-9233 trial and ACNS0121, which had similar protocols except for the use of radiotherapy, there was a 50–60% improvement in survival for patients under 3 years old who were treated with radiation therapy [24,25]. It is recommended that second-look surgery be performed when GTR is achievable, as it leads to better disease control [26]. In STR cases, the benefit of pre-radiation chemotherapy is still being investigated but may be used [27]. Pediatric patients with disease recurrence should undergo resection with postoperative radiotherapy [28]. Chemotherapy has been used in recurrent cases but the response generally lacks durability [29,30]. Chemotherapy regimens should be based on prior exposures, though clinical trials should be explored [11].
The management strategy of adult patients that suffer from relapse is currently evolving as our understanding of the molecular drivers of this disease are being further investigated [31]. Moreover, the most effective management of disseminated versus local recurrence is also not well delineated. Although there is a dearth of randomized clinical data, there is a consensus that recurrent lesions that are non-operable should receive radiotherapy in both the adult and pediatric populations. The utility of chemotherapy and immunotherapy for disease recurrence is also actively being explored. EANO recommends platinum or temozolomide based on their favorable toxicity profiles, though clinical trials should be considered [11,17]. Ongoing research on this topic includes locoregional delivery of chimeric antigen receptor T (CAR T) cells targeting various surface antigens expressed on recurrent ependymomas, which has been shown to be highly efficacious in a mouse model [32]. As our treatment modalities improve for this pathology, and longevity of this patient population subsequentially increases, optimization of treatment regimens for recurrence using a combination of surgery, radiation, and chemo-/immuno-therapies will need to be further investigated.
2. History
Ependymomas have classically been diagnosed based on the WHO histological classification strategy, which was designed to integrate histopathological characteristics to facilitate tumor diagnosis, guide treatment strategy, allow for prognostication, and aid research by allowing for interlaboratory comparisons. In 2016, the WHO classification for CNS tumors incorporated not only light microscopy, immunohistochemical lineage-associated proteins, and ultrastructural characteristics, but also a few molecular markers, namely RELA-fusion ependymoma, to enhance the diagnostic and prognostic utility of this classification strategy [1]. Despite this, controversy in the field remains regarding the use of primarily histology to guide tumor grading. This controversy stems from the notable histopathologic variations seen between and within specimens, the large inter-observer grading discrepancies among neuropathologists, and the lack of consistent prognostication based on WHO grading [33,34,35,36]. There was a clear paucity amongst scientists and clinicians on how to effectively categorize this diverse CNS pathology to better identify, analyze, and prognosticate in order to provide patients with accurate information regarding their diagnosis. Similar to other CNS malignancies, investigators began to focus on molecular signatures as a means to fill this void.
The introduction of molecular markers in the 2016 WHO classification and the expansion in the 2021 WHO classification, was a function of several investigations elucidating the genetic uniqueness within the histologically defined subgroups of ependymomas. These findings began as a series of multi-omics studies that separated genomic groups of ependymomas with clinical and prognostic correlation. Early studies focused on gene expression and copy number profiles, while late studies focused on epigenomics [37,38,39,40,41,42,43,44].
In 2015, a landmark clinical and genomic study by Pajtler et al., utilized DNA methylation profiling of 500 ependymomas to identify nine subgroups [40]. This project solidified and supported previous research emphasizing that despite similar histopathological features among ependymoma tumors, their diverse biological behavior could be better defined and categorized based on genomic markers. The nine subgroups of ependymomas were divided evenly amongst the three major compartments of the CNS. The SP compartment contained the subgroups of SP subependymoma (SP-SE), SP myxopapillary ependymoma (MPE), and SP ependymoma (SPE), all of which have predilection for the adult population. The IT compartment contained the subgroups posterior fossa subependymoma (PF-SE), posterior fossa ependymoma Group A (PFA), and posterior fossa ependymoma Group B (PFB), of which, the PFA alone has a strong preponderance for the pediatric population. Lastly, the ST compartment contained the subgroups of ST subependymoma (ST-SE), ST ependymoma with YAP1 fusion (ST-YAP1), and ST ependymoma with RELA fusion (ST-RELA). All nine subgroups were found to have distinct DNA methylation patterns that correlated with pertinent clinicopathological variables, such as age and sex. The study was also able to demonstrate that risk stratification by molecular subgrouping is superior to the histopathological grading used by the 2007 WHO grading system. However, it is important to note that in this and following studies, many classic histology ependymomas (grade II) were classified into the SE and MPE subgroups [37,40]. Nevertheless, this study served as the foundation for subsequent studies validating and expanding upon these new molecular subgroups of ependymomas.
In 2021, the WHO Classification of Tumors of the CNS will be again updated with its 5th edition attempting to capture all of the recent updates in genomic profiling and to address some of the aforementioned issues. This update introduced the C11orf95/ZFTA-fusion subgroup (same as the former 2016 RELA-fusion subgroup), YAP1-fusion subgroup, PFA subgroup, PFB subgroup, and the SP ependymoma with MYCN amplification subgroup. These were added as unique genomic markers, and transcriptional patterns were identified that could better differentiate ependymomas despite similar histological features [45,46,47,48,49,50]. While this offers additional molecular categories, data is lacking to assign grading based solely on molecular alterations, as is seen in diffuse gliomas [49,50].
The 2021 WHO classification, the Pajtler study, and the subsequent studies that examined these distinct subgroups in the population, their underlying oncogenic drivers, as well as proposed molecular targets, are the subject of this review. Besides, SE and MPE which remain their own histological categories, ependymomas are now primarily defined by anatomic location (SP, PF, ST), histology (grade 2 or 3), and molecular features [49,50]. In this review, we describe the three subgroups of ependymoma found in each of the three anatomic compartments, as well as the SE subgroups found at every location. These 10 subgroups and their characteristics are summarized in Figure 1.
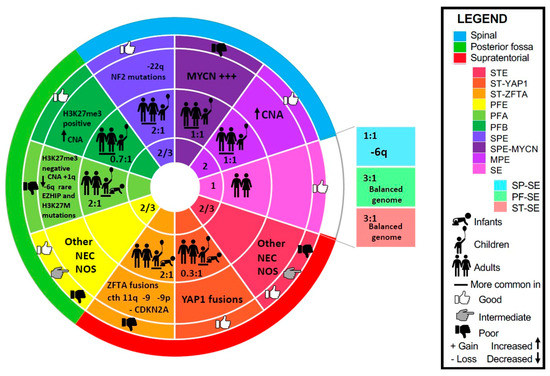
Figure 1.
Ependymoma molecular classification per WHO 2021. Concentric discs represent (inner to outward): WHO grade, age and sex ratio (male to female), characteristic molecular features, outcome, location. Abbreviations: CNA—copy number alterations, cth—chromothripsis, MPE—myxopapillary ependymoma, NEC—not elsewhere classified (other molecular alteration, not described), NOS—not otherwise specified (molecular testing is not available), PFE—posterior fossa ependymoma, PFA—posterior fossa ependymoma Group A, PFB—posterior fossa ependymoma Group B, PF-SE—posterior fossa subependymoma, SE—subependymoma, SP-SE—spinal cord subependymoma, SPE—spinal cord ependymoma, SPE-MYCN—spinal cord ependymoma with MYCN amplification, STE—supratentorial ependymoma, ST-SE—supratentorial subependymoma, ST-YAP1—supratentorial ependymoma with YAP1 fusion, ST-ZFTA—supratentorial ependymoma with ZFTA fusion.
3. Subependymoma (SE)
In the 2021 CNS WHO classification, subependymoma (SE) remains a separate entity (encompassing SP-SE, ST-SE and PF-SE–described below) identified by morphologic criteria (Figure 2A) as there is currently no clinical justification of SE molecular subgroups at each anatomic site [49,50]. Grossly, these tumors are tan-white-gray, nodular, and firm, bulging into the ventricles. The histopathology is defined as clusters of isomorphic nuclei embedded in a dense, fine, glial fibrillary background, mild nuclear pleomorphism, microcystic formations (especially in lateral ventricular tumors), with or without occasional hemorrhage, and/or calcification. SE express glial acidic fibrillary protein (GFAP) and ezrin-radixin-moesin binding protein 50 or Na+/H+ Exchanger Regulatory Factor (NHERF1/EBP50) microlumens, whereas epithelial membrane antigen (EMA) is rarely expressed [1,51,52]. They are considered slow growing WHO grade 1 tumors often presenting with symptoms related to CSF obstruction if intracranially located, given their predilection for the ventricular system. They can present with spinal cord/nerve root compression if located in the spine.
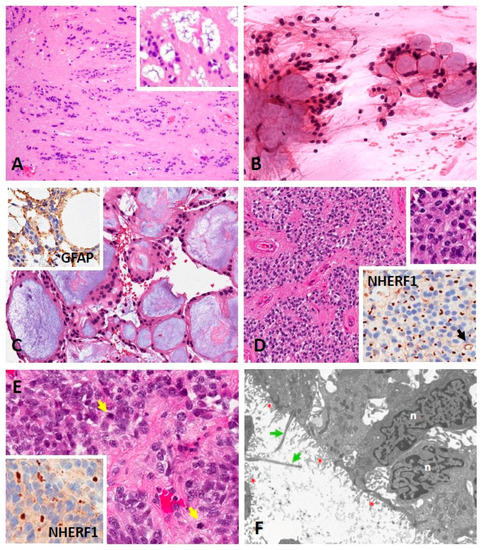
Figure 2.
Morphology of subependymoma and ependymoma. (A) Subependymoma composed of clusters of isomorphic nuclei embedded in a fibrillary matrix of glial cell processes (HE, 100×). Cystic degeneration and nuclear pleomorphism (degeneration-related) can be present in larger tumors (inset) (HE, 200×). (B) Myxopapillary ependymoma showing ependymal cells with bland oval nuclei and fibrillary processes around aggregates of myxoid material (smear, HE, 200×). (C) Tissue section of myxopapillary ependymoma showing bland GFAP positive (inset, GFAP, 200×) ependymal cells around blood vessels with myxoid degeneration (HE, 200×). (D) Ependymoma composed of neoplastic glial cells arranged around blood vessels in pseudorosettes (HE, 100×). The tumor nuclei are round to oval, monomorphic with stippled chromatin (top inset) (HE, 200×). Anti-NHERF1/EBP50 immunohistochemistry showing numerous dot-like microlumens and a rare ring-like structure (arrow) (bottom inset) (200×). (E) Anaplastic ependymoma with enlarged pleomorphic nuclei and mitotic activity (arrows) (HE, 200×). Anti-NHERF1/EBP50 immunohistochemistry showing an area with microlumens (inset) (200×). (F) Electron micrograph showing the lumen of a true rosette lined by ependymal tumor cells with rare cilia (green arrows) and abundant microvilli (red asterisks) (18,000×). Abbreviations: HE—hematoxylin and eosin, n—nucleus Note: Photographs in D-F were kindly provided by Dr. Maria-Magdalena Georgescu, NeuroMarkers PLLC, Houston, TX, USA.
3.1. Spinal Subependymoma (SP-SE)
Spinal subependymomas (SP-SE) have a predilection for the cervical or cervicothoracic junction, and typically present with insidious myelopathy or radicular pain [53]. This subgroup commonly presents in the adult population with a mean age of presentation of 44 years old, and with an even sex distribution, based on the few case series that have been published [54,55].
Patients with SP-SE have an excellent prognosis when treated with the standard of care treatment strategy of microsurgical resection [55]. GTR with minimal disruption of adjacent vital structures via microsurgery is the gold standard treatment, and is often feasible with the advancement of microsurgical tools and intraoperative neuromonitoring. When GTR is not achievable given proximity to eloquent structures, STR is advised with serial follow-up imaging. A large systematic review assessing outcomes in 105 cases of SP-SE found that 65% of patients underwent GTR and that of these, 57% had worsened function after surgery, while only 41% of the patients that underwent STR or partial resection experienced worsened function [55]. No patients had tumors with malignant transformation; therefore, long-term survival for all patients was expected. This study emphasizes the significant morbidity associated with surgical intervention of this subtype of tumor given the eloquent adjacent structures despite the excellent mortality rates. The authors make the argument for the benefits of cure in this disease. However, when the demarcation margin is not very clear during surgical debulking, the benefits of cure via GTR may not outweigh the risks of causing significant neurological morbidity as disease progression is likely to be very slow. No adjuvant radiotherapy is needed as good clinical outcomes are seen following both gross and STR of SP-SE [56]. Altogether, while SP-SE have an excellent prognosis when treated with microsurgical resection, there is inherent risk taken when surgically removing these tumors from the eloquent spinal cord, which may limit therapeutic reach.
Research into the molecular drivers underlying oncogenesis of SP-SE has been sparse, and likely secondary to its benign clinical course. The two largest genetic analyses of this tumor subgroup both showed loss of 6q [37,40]. In addition, Witt et al. noted an almost 50% copy number reduction in 19p and 19q, while in the seven SP-SE patients in the Pajtler study no chromosome 19 copy number loss was noted. The one, uniformly defining characteristic of this subgroup is deletion of chromosome 6q [40]. Previously, the role of 6q deletion in all subsets of ependymoma has been controversial as studies have reported variable influence on patient prognosis. Specifically, 6q25.3 deletions were found to be markers of good prognosis and survival, as shown by the risk stratification scheme of intracranial ependymomas proposed by Korshunov et al. and confirmed in the pediatric population by Monoranu et al. [57,58]. Conversely, Rajaram et al. found 6q23 deletions to be a marker of disease progression in a mixed population of ependymoma subgroups [59]. While the role of 6q deletion in SE tumorigenesis and behavior requires further research, its involvement and targeting in other malignancies may provide avenues for future treatment strategies. Specifically, the c-myeloblastosis gene (c-MYB), a proto-oncogene encoding a transcription regulator that plays an essential role in regulation of hematopoiesis and located on chromosome 6q23.3, is being explored as a driver in the oncogenesis of this tumor [60]. Additionally, 6q cytogenetic abnormalities are commonly seen in T-cell lymphoblastic leukemia/lymphoma. Research in this field has recently identified EPHA7, a tumor suppressor gene found on 6q that encodes the protein Ephrin type-A receptor 7, a receptor tyrosine kinase that mediates developmental events in the nervous system [61]. EPHA7 may contribute in part to the onset of T-cell lymphomas.
3.2. Posterior Fossa Subependymoma (PF-SE)
The histopathologic origin of SE in the IT compartment remains uncertain. Possible cell origins include ependymal-glial precursor cells, subependymal plate cells, and tanycytes [62,63]. Macroscopically, the tumors are firm, well-delineated, and lobulated masses bulging into the fourth ventricle (IVthV). The most common location for intracranial SE. PF-SE is typically found on the floor or the roof of the IVthV. Approximately 20% of these tumors are calcified. Unlike ST-SE, PF-SE is unlikely to be cystic. Dissimilar to most other ependymoma subgroups, PF-SE occurs in middle-aged to older patients, with the average age around 60 years old [40,62]. PF-SE is more common in the male population with almost a 3:1 ratio [62].
PF-SE carries low pathogenicity and, in fact, is often not discovered until postmortem examination [64,65]. GTR is generally thought to be curative, though recurrences can occur [62,66]. Radiotherapy may be used in cases of STR, but is typically reserved as salvage therapy if progression occurs. Chemotherapy and molecularly targeted therapy have not routinely been employed given the tumor’s slow growth and low risk for recurrence or metastasis. The 5-year PFS and OS are generally high, at 86–90% and 91–100%, respectively [40,62,67].
Genomic analyses of PF-SE have had mixed results regarding copy number variations [37,40]. While the landmark paper by Pajtler et al. did not find any copy number loss or gain, there was over 50% copy number loss in chromosome 19p and 19q in the 24 PF-SE patients studied by Witt et al. Based on examination of the transcription profiles of PF-SE tumors, its tumorigenesis appears to be related to fatty acid metabolism, mast cell and leukocyte processes (KIT), signal transduction pathways (specifically STAT), as well as, chemotaxis [40]. In a study by Kong et al., an SE cell line was created and a tissue microarray analysis was performed demonstrating high tumor expression levels of topoisomerase II-β, HIF-1α, E3 ubiquitin-protein ligase Mdm2, and nucleolin on immunohistochemistry and Western blot analyses [68]. Topoisomerase II-β is encoded by TOP2B and is a key decatenating enzyme [69]. HIF-1α is encoded by HIF1A and functions as a master transcriptional regulator in the response to hypoxia. E3 ubiquitin-protein ligase Mdm2 is encoded by MDM2 and functions to mediate ubiquitination of p53 leading to its degradation. Nucleolin is encoded by NCL and is a major nucleolar protein associated with intranucleolar chromatin and preribosomal particles that induces chromatin decondensation by binding to histone H1. Given the benign course of PF-SE, there are no chemotherapy regimens commonly used, nor are there any current targeted therapies for this disease. At the time of writing, there are no clinical trials for chemotherapies or molecularly targeted therapies for SE. KIT may be a viable target as it is only expressed at high levels in PF-SE tumors but not the other subgroups [40]. Multikinase inhibitors, such as imatinib, have been demonstrated to inhibit the autophosphorylation of c-KIT and could play a role in disease progression [70,71]. Topoisomerase inhibitors and p-STAT3/HIF-1α inhibitors appear to inhibit SE cell line growth suggesting a topoisomerase II inhibitor, such as FDA-approved etoposide and teniposide, could counteract PF-SE disease progression [68].
3.3. Supratentorial Subependymoma (ST-SE)
Supratentorial subependymoma (ST-SE) is often attached to the wall of the ventricular system. While the IVthV is a common intracranial location (40–60% of intracranial cases), SE is most commonly found supratentorially in the lateral ventricles (30–45% of intracranial cases), as subependymal glial cells are thought to play a large role in the origin of this neoplasm [72,73,74]. Similar to its IT counterpart, patients can present with symptoms related to CSF obstruction [67,75]. Presentation is common throughout adulthood but is most frequently seen in the third to sixth decades of life and in males rather than females [40,65,67,76,77].
The current gold standard treatment is maximal resection [67,76]. The use of adjuvant radiotherapy is generally not recommended as a good prognosis with surgery alone is seen if GTR is achieved [40,62,66,67]. Similar to its IT counterpart, there may be a role for radiotherapy if there is recurrence or symptomatic residual disease [67,76]. Unlike PF-SE, GTR may be more easily achievable given ST-SE is not in proximity to the brainstem. OS is excellent, at 96–100% for 1, 5, and 10-year [40,78].
Because of the uncommon nature of this subgroup, as well as the excellent prognosis following GTR, there has been little research regarding molecular targets for ST-SE. Similar to SP-SE and PF-SE, the two largest genomic studies have shown disputing results regarding copy number variations [37,40]. The Pajtler analysis of 21 ST-SE patients demonstrated a balanced genome, while the Witt analysis of 14 ST-SE patients demonstrated a 50% copy number loss in chromosome 19p and 19q. This discrepancy will have to be solved by future studies in all SEs. Regarding target development, the previously mentioned study by Kong et al. sought to identify and prioritize potential therapeutic targets for SE tumors through the use of tissue microarrays, ex vivo analysis, and in vitro cytotoxic assays [68]. This study derived the first-known human SE cell line from a resected SE and has laid the foundation for future research to identify potential molecular targets and develop therapeutic approaches. Of importance, this study demonstrated tumor expression of p53, MDM2, HIF-1α, topoisomerase II-b, p-STAT3 and nucleolin while also showing growth suppression of SE cells ex vivo utilizing a topoisomerase inhibitor (WP744) and the p-STAT3/HIF-1α inhibitors (WP1066 and WP1193). The targets highlighted in this molecular study may be applicable to SP-SE, IT-SE, and ST-SE; however, given the benign course of SE, development of targeted therapy may have a limited role in management in comparison to other ependymoma subgroups that may have malignant transformation. In rare instances of symptomatic residual or inability to resection, particularly in SP-SE cases, there may be a role for targeted therapies, but given the rarity of this, the authors encourage investigation into other ependymoma subgroups.
4. Myxopapillary Ependymoma (MPE)
Myxopapillary ependymoma (MPE) is a tumor originating almost exclusively in the spine (conus medullaris, cauda equina, and filum terminale). MPE was previously classified as WHO grade I in the 2016 WHO CNS classification, but because there is evidence that its outcomes are comparable to those of classic ependymoma, the new 2021 WHO CNS classification recommends assigning grade 2 to MPE [5,49,50]. MPE is defined by slow growth and favorable prognosis and accounts for 27% of all SP ependymoma [79]. Grossly, MPE are often encapsulated, lobulated, tan, and soft. They have a glistening cut surface with or without cyst formation and/or hemorrhage. Histopathologically, MPE is composed of well-differentiated cuboidal to elongated tumor cells radially oriented around hyalinized fibrovascular cores, commonly with degeneration-derived myxoid accumulation (Figure 2B,C) [1,80,81,82]. Patients frequently present with radicular pain and/or back pain [83]. While these tumors are found within all age groups, they are commonly found in the third and fourth decade of life, with a slight male predominance [84].
MPE is retained as a separate entity in WHO 2021 and defined by histological criteria. Methylation analysis by Pajtler et al., separated a MPE methylation subgroup from two SE and six classic ependymoma methylation subgroups [40]; however, that study, and an additional methylation analysis in a different study, grouped many classic histological ependymomas in the MPE and SE methylation subgroups, suggesting that methylation classification might not be reliable [37,40]. Moreover, such classification does not bring additional clinical significance to MPEs [49,50].
Similar to SP-SE, the gold standard treatment modality for MPE is maximal safe resection [85]. A systematic review of 28 articles demonstrated that overall recurrence rate after GTR (16%) was significantly lower than what was demonstrated following STR (33%) in the pooled cohort, with a mean follow-up of 75 months [84]. This review also demonstrated that adjuvant radiotherapy is not necessary as is not associated with a decrease in recurrence. There are reports, however, that radiotherapy may aid in disease control. An MD Anderson Cancer Center study showed that the addition of adjuvant radiotherapy to surgery was associated with significantly higher 10-year PFS rates (75% for surgery and postoperative radiotherapy vs. 37% for surgery alone) and higher 10-year local tumor control rates (86% for surgery and postoperative radiotherapy vs. 46% for surgery alone) [86]. A 10-year OS of 75–100% has been demonstrated with STR followed by radiotherapy [87,88].
The current state of understanding of the oncogenic drivers that lead to MPE is still in its infancy as the excellent prognosis with surgery has not necessitated targeted therapies. While chromosomal instability is considered to be the defining genomic characteristic of this subgroup of ependymoma, as seen by the copy-number variation gains observed by Pajtler et al. in their DNA methylation array of 26 samples of MPE, there are three notable genes that are areas of current research and possible future molecular targets [40]. Overexpression of homeobox B13 (HOXB13), a gene encoding a transcription factor that regulates skin development, has been shown by multiple studies [40,89]. The key involvement of this gene in developmental pathways has led researchers to hypothesize it plays a role in MPE tumorigenesis. Confounding this hypothesis is that the upregulated HOX genes commonly seen in MPE are HOXB13, homeobox A13 (HOXA13), homeobox C10 (HOXC10), and homeobox D10 (HOXD10), all of which are genes overexpressed in the developing lumbar spine [90]. Immunohistochemistry analysis of adult filum terminale did not demonstrate HOXB13 expression, supporting the hypothesis that HOX groups 10–13 are expressed in early development and switched off once segmentation has completed, and its presence in adult MPE represents aberrant expression [89]. Oncogenesis amongst HOX genes is a growing area of research as aberrant HOX expression has been demonstrated in other malignancies including acute myeloid leukemia, breast, cervical, small-cell and non-small cell lung, prostate, skin, and thyroid cancers [89]. HOXB13 protein overexpression must be recognized and further assessed as a potential future therapeutic target [89,91]. Two other genes with overexpression seen in MPE are neurofilament light chain (NEFL), encoding a Class IV intermediate neurofilament expressed in neurons and located on chromosome 8p21.2 in close proximity to transcription factor binding sites of HOX genes, and platelet derived growth factor receptor alpha (PDGFRA), a gene which encodes a tyrosine kinase [89,92]. While the ubiquitous expression of these genes in various processes throughout the CNS has thus far precluded any significant progress in developing targeted therapies, as seen by the lack of clinical trials addressing these overexpressed genes, these are promising targets for future research. Lastly, a recent study assessing DNA methylation and gene expression profiles of pediatric SP ependymomas also identified overexpression of HOXB13, lending further evidence towards the importance of this gene [93]. This study also demonstrated significant overexpression of genes involved in the mitochondrial oxidative phosphorylation respiratory chain, such as cyclooxygenase-2 (COX2), a gene that encodes for cyclooxygenase, which plays a vital role in inflammation and has been shown to be overexpressed in various cancers as it also plays a role in cell proliferation, neovascularization, and tumor metastasis [93,94,95,96]. While this was a small series in the pediatric population, it has provided more insight into the complex molecular amalgam that drives the tumorigenesis of MPE. As surgical resection remains the mainstay treatment of those afflicted by this tumor, further research will attempt to identify key genes that are uniquely expressed that may be future targets.
5. Spinal Ependymoma (SPE)
Spinal ependymoma (SPE) (WHO grade 2 or 3) is a tan, soft, well-circumscribed tumor composed of monomorphic glial cells with round to oval nuclei with speckled chromatin. They form perivascular pseudorosettes and/or true ependymal rosettes. When hypercellularity, cellular atypia, frequent mitoses, abundant endothelial proliferation and/or palisading necrosis are present, the tumor is deemed to be WHO grade 3. Histological patterns (clear cell, papillary, tanycytic) can be seen but do not have clinical significance [1,49,50,97]. Ependymomas express GFAP, S100 protein, EMA, and NHERF1/EBP50. The latter is a protein involved in epithelial morphogenesis and is superior to EMA for diagnosis of complex cases or ambiguous tumors (AO unpublished observations) [51]. Ultrastructurally, ependymomas are characterized by cilia, blepharoblasts, microvilli, and junctional complexes (Figure 2D–F) [1]. SPE is associated with neurofibromatosis type 2 (NF2) mutations or deletions as seen in the molecular study by Pajtler et al., which found 90.5% of samples categorized into the SP ependymoma group demonstrated copy number variations of chromosome 22q where this gene is located [40].
Unlike MPE and SE, which are easily distinguishable entities, the 2016 WHO classification of ependymoma into grades II or III is less consistent [1,98]. Studies have found there to be significant intratumoral heterogeneity and, moreover, significant grade interrater variability among neuropathologists [33,99]. Although there are a number of studies demonstrating prognosis correlating with WHO grade, there is controversy regarding the prognostic utility of WHO grading of ependymoma, as there are also studies demonstrating no significant difference in survival between cases stratified per WHO grade [99,100,101,102,103]. It is for this, and the aforementioned reasons, that the field has moved towards molecular characterization of ependymoma. This tumor can be found anywhere in the spinal column but is often found in the cervical spine, which leads to the common presenting symptoms of radicular pain, myelopathy, and neck pain [97]. Men are found to be more affected than women. This subgroup is frequently diagnosed in patients 30–40 years old [37,40].
The standard treatment modality is microsurgical resection with the goal of GTR with minimal normal tissue disruption; however, the role of adjuvant radiotherapy is a topic of debate. One series of 104 (101 grade II and 3 grade III) SPEs that underwent surgical resection found the median PFS of those that underwent microsurgical resection to be 14.9 years for grade II and 3.7 years for grade III SPEs. Furthermore, they reported no significant change in PFS for patients that underwent adjuvant radiotherapy following STR [104]. Another large case series assessing long-term outcomes of 88 patients with SPE that underwent microsurgical resection, with and without adjuvant radiotherapy, found the surgical extent of resection to be an independent predictor of longer PFS, while postoperative radiotherapy after incomplete resection did not significantly correlate with longer times to recurrence [105]. A more recent study of 69 patients with SPE found for grade II lesions, STR and radiotherapy yielded better outcomes than STR alone, with a 10-year PFS of 77% and 68%, respectively [106]. Altogether, there is moderate evidence that adjuvant radiotherapy should be considered for patients that undergo STR.
Despite this excellent prognostic profile, the presence of NF2 alterations in other CNS tumors has led to extensive research being performed to better understand the function and oncogenesis that occurs in tumors harboring this genomic variation. NF2 is a tumor suppressor gene, located on chromosome 22q12.2 and codes for the protein Merlin. Deletions or loss-of-function mutations of this gene leads to neurofibromatosis type II, which is inherited in an autosomal dominant pattern [107]. Merlin is a scaffolding protein that links F-actin, transmembrane receptors, and intracellular effectors that modulate receptor mediated signaling pathways controlling cell proliferation and survival. The vast number of signaling pathways that are affected by Merlin emphasize its importance in integrating these pathways to influence cell morphology, motility, proliferation and survival. While Merlin’s role in all of these pathways is still not completely understood, there are three molecular pathways that have been well defined and provide insight into how loss-of-function of Merlin can lead to oncogenesis. First, Merlin serves to inhibit various membrane receptors and the RhoGTPase family signaling cascade. Through binding to the CD44 cell membrane protein, Merlin negatively regulates this protein, which functions to increase cell proliferation. Additionally, through interaction with P21 Activated Kinase (PAK), Merlin inhibits Rac Family Small GTPase 1 (Rac1)/Cell Division Cycle 42 (Cdc42) signaling which leads to downstream inhibition of effectors including Rat sarcoma virus (RAS), Phosphoinositide 3-kinase (PI3K), and Ras-related C3 botulinum toxin (RAC). This ultimately leads to reduction in downstream Rapidly Accelerated Fibrosarcoma (RAF)/Myocyte Enhancer Factor (MEF)/Extracellular Signal-Regulated Kinase (ERK), Mammalian Target of Rapamycin Complex 1 (mTORC1), and Focal Adhesion Kinase (FAK) signaling [108]. Second, through binding PI 3-Kinase Enhance-L (PIKE-L), Merlin regulates and inhibits the PI3K/Protein Kinase B (AKT) pathway, which influences and promotes cellular survival and growth. Lastly, through the mammalian Hippo pathway, Merlin is involved with inhibition of Yes-associated Protein (YAP), a protein responsible for cell proliferation control and which has important regulatory functions in regeneration, organ development and stem cell self-renewal [109]. In this pathway, Merlin functions to promote translocation of Large Tumor Suppressor Kinase 1 and 2 (LATS1/2) to the nucleus while also inhibiting Cullin Ring Ubiquitin Ligase 4 (CRL4), both leading to reduced transcriptional output of YAP and other domain transcription factors [109].
As robust research has allowed for the meticulous understanding of the role of Merlin in oncogenesis, there have been several treatments under investigation targeting these molecular pathways. Small molecular MEK inhibitors are currently under investigation as they seek to target and inhibit the RAF/MEK/ERK pathway for NF2-associated tumors (NCT02639546, NCT03095248 on www.clinicaltrials.gov, (accessed on 9 November 2021)). A phase 2 clinical trial is also looking at NF2 specific molecular targets via a FAK inhibitor on NF2 mutant meningiomas (NCT02523014/A071401). Another NF2 specific molecular pathway target is verteporfin a benzoporphyrin derivative that is currently used as a photosensitizer in macular degeneration, as it has been shown to disrupt YAP oncogenic activity [110,111]. Other research regarding bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, has shown promise for NF2-associated schwannomas, meningiomas, and ependymomas [108,112]. Lastly, further proposed targets include the PD-1/PD-L1 axis, the chemokine receptor C-X-C chemokine receptor type 4 (CXCR4), and Ephrin receptor B2 [61,108,113,114,115]. As the function of Merlin and its interactions with various signaling pathways are better understood, additional molecular targets will be discovered, while clinical trials will continue to move forward to understand the utility of drugs for established targets.
6. Spinal Ependymoma with MYCN Amplification (SPE-MYCN)
Spinal ependymoma with MYCN amplification (SPE-MYCN) has recently been added as a new molecular category of ependymoma based on the updated 2021 CNS WHO classification, which was based upon several studies that have identified the presence of this molecular signature in a subgroup of highly aggressive SPEs. In 2001, Scheil et al. was the first to report on the amplification of Myelocytomatosis-N (MYCN), a gene located on chromosome 2p24.3 encoding a proto-oncogene transcription factor located in the cell nucleus that is critical for normal CNS development. This finding was based on a comparative genomic hybridization study of 26 ependymomas in 22 patients. Two cases of SPE-MYCN were identified with histological characterizations leading to a diagnosis of SP ependymoma WHO Grade III, one of which had relapse and intracranial metastases [116]. Later, Ghasemi et al., using DNA methylation analyses, observed MYCN amplification in a cohort of 13 tumors, of which 10 were WHO Grade III and three were WHO Grade II [117]. This series identified a strikingly worse prognosis than any other SP ependymoma subgroup, with a PFS of 17 months and a median OS of 7.3 years. These tumors were also unique in their location, favoring the cervical and thoracic spine, being predominately intradural and extramedullary. The presence of diffuse leptomeningeal spread, and dissemination was observed in 100% of cases. Further supporting the importance of this molecular marker was the retention of MYCN amplification in all recurrent tumors assessed, as well as the presence of MYCN protein overexpression by immunohistochemistry, suggesting malignant progression being driven by increased MYCN gene expression. Using a similar DNA methylation assay, two other studies recorded similar results, leading to a total of 27 published cases of ependymoma with MYCN amplification, which has led to the categorization of this subset of SPE [118,119]. Altogether, these studies demonstrate a slight predilection for the female sex with a mean age of diagnosis in the third decade of life. While these retrospective studies without prospective confirmation represent only a small number of patients, disallowing any clear incidence or strong epidemiological data to be extrapolated, what has thus been reported has called for this molecular marker to be further studied and used as a prognosticator of poor outcomes. MYCN, a member of the family of MYC oncogenes, plays a large role in neurogenesis and has been implicated in the genesis of various CNS malignancies such as neuroblastoma, pediatric glioblastoma, and medulloblastoma, as well as malignancies outside of the CNS such as leukemia and prostate cancer [120,121,122,123,124]. There are no current clinical trials targeting MYCN; however, there are several reported investigations assessing strategies for MYCN inhibition. Among these promising reports include inhibitors targeting histone deacetylase (HDAC), poly(ADP-ribose) polymerase (PARP), Auro A-kinase (AURKA), and Bromodomain and extraterminal domain (BET) proteins, as well as immunotherapy targeting through DNA vaccination [125].
7. Posterior Fossa Ependymoma (PFE)
Posterior fossa ependymoma (PFE) has been studied and classified by many studies. Initially these studies used gene expression and copy number profiles, and defined two groups of tumors: Groups 1 and 2 by Wani et al., and Groups A and B by Witt et al. and by Hoffman et al. [42,43,44]. The groups identified correlate across studies with Group 1/A associated with younger age and a more aggressive course compared to Group 2/B. With the introduction of DNA methylation profiling technology, additional studies confirmed these findings and made uniform the terminology for posterior fossa ependymoma Group A (PFA) and posterior fossa ependymoma Group B (PFB). These two groups are now part of the WHO 2021 classification [37,38,39,40,49,50,126]. PFE that are not PFA/B can be qualified as “other”, “not otherwise specified (NOS)”, which includes those that are not able to be molecularly analyzed, or “not elsewhere classified (NEC)”, which includes those with other molecular alterations. In an analysis of 35 PFE, 14 (40%) patients had PFA, 17 (48.6%) patients had PFB, and 4 (11.4%) patients were not classified into either, and thus should be presumed to be PFE, NOS/NEC [42]. Many studies characterize PFE based on their unique DNA methylation signatures [40,126,127]. As DNA methylation testing is not widely available, the use of an antibody against the trimethylated histone H3 at lysine 27 (H3K27me3) has been used to differentiate PFA from PFB [127,128,129,130]. There is a reduction or loss of H3K27me3 immunoexpression in PFA and persistent H3K27me3 immunoexpression in PFB [127,128,129,130].
Although the molecular characterization of PFEs has caused significant change in the categorization of these tumors, the DNA methylation results should be interpreted with care and in context with histology. There are many reasons why bioinformatics analyses (of any kind) should be interpreted with care, but this is beyond the scope of this article. Briefly, this starts with the quality of the tissue processed (time stored in formalin vs. fresh), the accuracy of tissue selected (tumor vs something else) and the algorithms used for data normalization. Similarly, the parameters, cut-off scores, and functions used for the analysis proper are of outmost importance. In the case of methylation there is no gold standard as of yet, and although the bioinformatic strategy used in the largest study published so far on brain tumors is now being commercialized, this algorithm was not sufficiently validated. Moreover, although this study included a large number of samples overall, when divided per rare entities the number of samples was small [131]. Therefore, methylation analysis, despite some opinions, is far from being “gold standard” for brain tumor diagnosis. For example, in an adult study, DNA methylation profiling of 38 IT tumors (seven WHO grade I SE, twenty-five WHO grade II ependymoma, and six WHO grade III anaplastic ependymoma) recategorized them as 24 PF-SE subgroup, 1 PFA subgroup, and 13 PFB subgroup; therefore this classification should be questioned as many ependymomas (n = 17 in this study) were classified as SE [37]. Similarly in another study, 11 IT ependymomas were classified by DNA methylation profiling as PF-SE [40].
PFEs are histologically grade 2 or 3, as described above in the SPE section (Figure 2D–F). As the molecular classification of PFE tumors is fairly novel, there are few studies describing specific subgroup location and radiographic characteristics. PFE is characterized as arising from the ependymal lining of the IVthV, often extruding out of the foramina of Luschka and Magendie [132,133]. PFE demonstrates heterogeneous enhancement, and approximately 50% demonstrate calcifications, with early evidence that calcifications primarily occur in PFA. Among patients with PFE, signs and symptoms related to CSF obstruction including headaches, nausea/vomiting, and lethargy are common, given their location in the IVthV [67,77].
8. Posterior Fossa Ependymoma Group A (PFA)
PFA is primarily a pediatric disease, with a median age of 3 years, and age ranging from 6 months to 58 years old across studies [37,40,41,42,127]. Adult cases make up a small percentage of the total cases. In studies with adult and pediatric PFA patients, 1–18.5% of patients are adults while the remainder are pediatric [40,41]. In a study of 134 adult PFE patients, 12% of the cases were H3K27me3-negative and presumed to be PFA [134]. PFA occurs more commonly in males, with a 65% to 35% male to female predominance. Aside from the aforementioned general PFE radiographic and clinical presentation, initial research on PFA demonstrates approximately two-thirds occur laterally and approximately one-third occurs medially [39,43].
In regard to ependymal tumors of the IT region, PFA has the poorest prognosis. Additionally, its prognosis is among the most dismal of all ependymoma subgroups. In comparing patients older than 10 years to those younger, the 5-year PFS and OS was 54% and 71%, which was not significantly different than patients younger than 10 years [39,40]. Although studies of purely adult PFA are uncommon, one such study had similar findings with H3K27me3-negative PFE patients having a 5-year PFS and OS of 44% and 80%, respectively [134]. This study showed that Ki-67 (MIB-1) index <10%, use of first-line radiotherapy, and GTR, were positive prognostic factors.
Overall, PFA tumors appear to have balanced genomes with the most frequent copy number variations being 1q gains (17.3–25%) and 6q losses (6.4–8.6%), both of which are negative prognostic indicators [40,135,136]. It is, rather, epigenetic changes such as the loss of H3K27me3 expression that lead to the PFA tumorigenesis [41,127,128]. There is increasing evidence that pathogenesis may be similar to that of diffuse midline gliomas (DMG) with H3K27M mutations and, in very rare instances (0.6–4.2% of cases), PFAs share this mutation [128,129,130,136,137,138]. In DMG, H3K27M mutations induce derepression of pro-oncogenic transcription factors through global reduction of histone 3 K27 trimethylation, H3K27me3. In addition to sharing this global DNA hypomethylation, PFA tumors have increased H3K27me3 enrichment at select genomic loci similar to that of H3K27M-mutant DMG [128].
An important oncogenic driver in PFA is Cxorf67, or EZH Inhibitory Protein (EZHIP), and its interaction with polycomb repressive complex 2 (PRC2), a histone methyltransferase that primarily methylates H3K27. Both Histone H3K27M and Cxorf67/EZHIP have short sequences that bind and inhibit PRC2 [139]. These sequences bind to the Su(var)3-9/enhancer-of-zeste/trithorax (SET) domain of EZH2, which is part of PRC2 and inhibits its methyltransferase activity, mimicking the function of mutated K27M oncohistones and resulting in loss of methylation at residue K27 of Histone H3 (Figure 3) [136,139,140,141]. PFA ependymomas show increased expression of Cxorf67/EZHIP and absence of H3K27me3 [129,136]. Although rare, Cxorf67/EZHIP missense mutations (<10%) have been reported in PFA [136]. It was shown that these mutations do not alter the protein function [139]. Jain et al. hypothesized that these mutations increase EZHIP expression by altering the cis-acting gene regulatory elements [139]. Importantly, increased Cxorf67/EZHIP expression correlates with loss of H3K27me3 in PFE, and is mutually exclusive with H3K27M mutations [129]. The lack of trimethylation and the decrease in H3K27me3 level cause derepression/upregulation of PRC2 target genes, including genes involved in neurodevelopment, and likely contribute to PFA tumorigenesis [139,140,142]. Rarely, ATRX protein loss by immunohistochemistry (4–25%) has been reported in PFA [129,130]. Alpha-thalassemia, mental retardation, X-linked/death domain–associated protein (ATRX/DAXX) complex is involved in incorporating histone H3.3 at pericentric heterochromatin and telomers. ATRX loss leads to increased DNA damage and genomic instability [143].
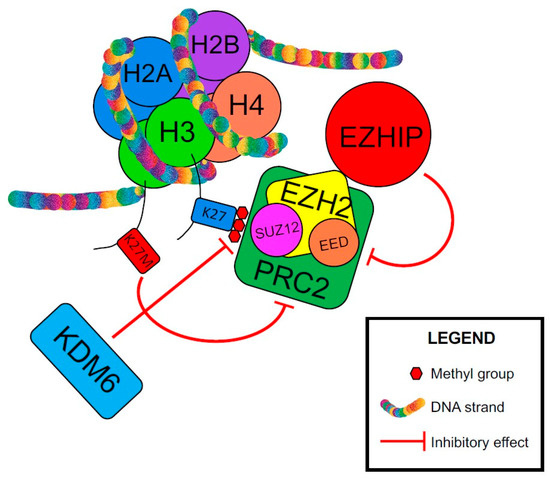
Figure 3.
Mechanism of tumorigenesis in posterior fossa ependymoma Group A (PFA). EED—embryonic ectoderm development, EZH2—enhancer of zeste homolog 2, EZHIP—EZH inhibitory protein or Cxorf67, H2A—histone 2A, H2B—histone 2B, H3—histone 3, H4—histone 4, K27—lysine 27 on histone H3, K27M—methionine substitution for lysine on histone 3, KDM6—lysine-specific demethylase 6, PRC2—polycomb repressive complex 2, SUZ12—suppressor of zeste 12 homolog.
There is evidence that the microenvironment plays a strong role in PFA growth and propagation [144,145,146]. An inflammatory state driven by chronic IL-6 and STAT3 expression differentiates this subtype from PFB [145,146]. The master regulator of IL-6, NF-κB, and its pathway are enriched in PFA tumors. Leucine zipper downregulated in cancer 1 (LDOC1) is a transcriptional repressor of NF-κB and is a key regulator of this pathway. LDOC1 gene expression is decreased in PFA in comparison to other pediatric brain tumors. Moreover, ependymoma cells treated with 5AZA-DC, a DNA methylase transferase inhibitor, upregulate LDOC1 expression and decrease IL-6 secretion. PFA growth is also dependent on hypoxia, and even transient exposure of PFA cells to ambient oxygen causes irreversible cellular death [144]. Hypoxia induces restricted availability of S-adenosyl methionine (SAM), a substrate for methylation including H3K27 methyltransferase EZH2, which leads to the PFA-characteristic globally diminished histone methylation and increased demethylation and acetylation of H3K27. Gene ontology analysis of PFA has demonstrated overexpression of genes associated with wound healing, angiogenesis (VEGF and HIF-1α signaling), and migration and adhesion (integrin signaling and extracellular matrix assembly), a pattern similar to the mesenchymal signature in glioblastoma [43,44]. Telomerase activity has been found to be a significant player in PFA pathogenicity [147]. Epigenetic hypermethylation of hTERT promoter and chromosome 1q gain were both strongly associated with telomerase reactivation in PFA.
Currently, there are no established targeted therapies for PFA. As PFA tumorigenesis appears similar to H3K27M-mutant DMG, medications directed at this cancer may also have therapeutic value in PFA [148]. Quantitative high-throughput screening of 2706 approved and investigational drugs, followed by testing on patient-derived xenograft models of H3K27M-mutant DMG, revealed the combination multihistone deacetylase inhibitor panobinostat and the proteasome inhibitor marizomib to have the highest therapeutic value and thus may have applicability in the PFA setting [149]. Given the limited genomic foci of H3K27me3 hypermethylation, DNA methylation inhibitors may be suitable targeted therapies. A study by Michealraj et al. demonstrated this to be the case with inhibition of histone lysine methylation, leading to diminished survival of resected PFA cell lines [144]. In a pilot study, 5-Azacytidine, a DNA methylation inhibitor, was used in pediatric recurrent PFE, and although it was found to be safe, it was not found to limit progression of disease at the study dosage [150]. Lastly, as previously discussed, EZHIP appears to be crucial in the oncogenesis of PFA [136,140]. Interrupting the interaction between EZHIP and EZH2 may block the oncogenic upregulation of PRC2 downstream genes, similar to targeting the residual activity of PRC2 or “detoxification” of Histone H3K27M as attempted for diffuse midline glioma [139]. A recent study showed that tumor cells with increased EZHIP expression suppress DNA repair and respond to PARP inhibitors, especially when associated with radiotherapy [151].
9. Posterior Fossa Ependymoma Group B (PFB)
Posterior fossa ependymoma Group B (PFB) tumors occur in adolescent and adult populations, with a median age of approximately 30 years and an age range of 2 to 65 years across studies [37,40,41,42,127]. These same studies demonstrated a slight predilection of disease towards the female sex. Studies with adult and pediatric PFB patients have demonstrated 75–81% of patients are adult aged, while the remainder are pediatric [40,41]. In the largest study of adult PFE patients, 88% of the cases were H3K27me3-positive and presumably PFB, while the other cases were H3K27me3-negative (PFA) [134]. In addition to the previously described clinical and radiographic findings, over 90% of the PFB subgroup tumors are located medially within the posterior fossa [43]. In comparison to PFA, patients with PFB have a better prognosis [39,40]. These tumors are rarely invasive or metastatic, and are unlikely to recur. Five-year PFS and OS was 83% and 98% in patients older than 10. In a study of adult ependymoma, H3K27me3-positive PFE patients were found to have a 5-year PFS and OS of 87% and 99%, respectively [134]. Similarly to PFA, prognosis of PFB is not affected by age at diagnosis but does benefit from GTR and Ki-67 <10% [38,39,134]. In contrast to PFA, both 5-year PFS and OS in PFB patients do not appear to be affected by first-line radiotherapy.
In comparison to the balanced chromosomal composition of PFA, PFB is characterized by a high degree of chromosomal instability, with many copy number aberrations present [40]; most notably, over 50% copy number losses in 6p and 6q, and over 50% copy number gains in 15q, 18p, and 18q [37,40]. However, thus far, no recurrent mutation has been found in PFB tumors [38,126]. Genomic investigation of PFB has demonstrated this subgroup to be more differentiated with an ependymal-like trajectory [126,152]. It is also characterized by hyperactivity of gene sets involved in sonic hedgehog (Shh) signaling, oxidative metabolism, and ciliogenesis/microtubule assembly (including FOXJ1) [40,43,44,152]. FOXJ1 encodes Forkhead box protein J1, which is an important regulator of motile ciliogenesis, has been associated with Shh signaling, and is highly expressed in ependyma and choroid plexus [153,154]. The expression of FOXJ1 has been associated with several tumors including ependymal and gastric cancers, though this has primarily been decreased expression [154,155]. However, there are several instances of FOXJ1 overexpression in tumors, such as clear cell renal cell carcinoma and colorectal cancer, that merit further investigation [156,157]. In a colorectal cancer study, the overexpression of FOXJ1 significantly promoted nuclear translocation of β-catenin, an important factor in the Wnt-β-catenin pathway and in intestinal tumorigenesis. An adaptor protein NHERF1/EBP50 has been found to suppress the Wnt-β-catenin pathway-driven intestinal neoplasia [158,159]. Interestingly, NHERF1/EBP50 is a strong diagnostic marker for ependymoma in general, is an organizer of polarity structures such as ependymal cilia, and as further discussed in the ST-YAP1 section, has a domain PDZ-2 that binds to both β-catenin and YAP1 [51]. There are several medications that indirectly affect the Wnt-β-catenin pathway, but the development of directed small molecule inhibitors is still in its infancy [160,161,162]. More recently, a first in class NHERF1 PDZ1-domain inhibitor has been developed that addresses the PDZ1-domain role of membrane recruitment/displacement [163]. Although these medications are being developed for other indications, the combination of NHERF1 PDZ1 inhibitors with β-catenin inhibitors may have a role in future care of ependymal tumors.
10. Supratentorial Ependymoma (STE)
The new 2021 WHO Classification of CNS tumors included two main subsets of ependymomas located in the ST space: those that have YAP1-fusions (ST-YAP1) and those that have ZFTA-fusions (ST-ZFTA) [49,50]. The non-YAP1/ZFTA STE are best classified as “other” or “NOS/NEC”, and a histological grade can be offered. Further investigation will lead to other possible molecular subgroups. Additionally, as future studies begin to utilize these subcategories, further prognostic data will likely emerge. Earlier studies assessing prognosis of STE prior to molecular classification likely represent a heterogeneous population which include the now identified molecular subgroups, as well as tumors that would fall into the STE, NOS/NEC subgroup. Therefore, at the moment, no demographic or prognostic data are available for this subgroup, which remains, as of now, a subgroup of exclusion.
11. Supratentorial Ependymoma with YAP1-Fusion (ST-YAP1)
Supratentorial ependymoma with YAP1-fusion (ST-YAP1) is primarily a pediatric subgroup of ependymoma, with the median age of approximately 1 year and an age range from seven months to 51 years across studies [40,164,165]. Its occurrence in the adult population is extremely rare, though documented. Approximately 13–25% of ST-YAP1 cases occur in males [40,165]. Prognosis is significantly better than ST ependymoma with ZFTA-fusion, with most reports of 5-year OS at 100% [40,165]. The genomic origin of this tumor involves the fusion of chromosome 11 YAP1 gene with chromosome X MAMLD1 gene, though fusion with another gene, FAM 118B [40,165]. YAP1 or yes-associated protein 1, the oncoprotein of YAP1 gene, is a downstream effector in the Hippo signaling pathway [69]. YAP interacts with various transcription factors in the nucleus, including TEAD (transcriptional enhancer factor domain) transcription factors, which increases expression of genes involved in cell proliferation [166]. YAP works with another co-activator, TAZ, (also known as protein Tafazzin encoded by gene TAZ) in a similar but non-redundant fashion to complex with TEAD to act on gene targets [167]. Preventing the interaction of YAP with TEAD transcription factors resulted in lack of tumor formation in mice [166]. In another mouse study, ectopic expression of YAP1 led to generation of tumors with molecular and ultrastructural characteristics of human ependymoma [168]. This suggests their interaction is critical in formation of ST-YAP1 tumors, and may be a therapeutic target. YAP1 effects do not lie solely in the Hippo pathway; but have been linked to several pathways including the Wnt-β-catenin pathway and a mechanotransduction pathway [169,170]. YAP also interacts with adaptor molecule NHERF1/EBP50, an in vivo tumor repressor for intestinal adenoma development and an organizer of polarity structures such as ependymal cilia [51,158]. In a primarily adult study by Georgescu et al., NHERF1/EBP50 immunoexpression was shown to be diagnostic in a majority of ependymal tumors but not of other CNS tumors. YAP activity has also been implicated in drug resistance in a number of cancers, including esophageal cancer, oral squamous cell carcinoma, urothelial cell carcinoma, and radiation resistance in glioblastoma and medulloblastoma [171]. YAP has been demonstrated to assist in the immune escape of tumor cells by enhancing programmed death ligand-1 (PD-L1) gene expression, thus attenuating T-cell activation [171,172].
In the setting of breast cancer cell cultures that depend on YAP/TAZ, the use of on-market drugs dasatinib, fluvastatin, and pazopanib inhibit nuclear location of YAP/TAZ [173]. Dasatinib is a tyrosine kinase inhibitor used to treat chronic myeloid leukemia, fluvastatin is a statin class drug that has been used in cell lines with antitumor effects, and pazopanib is a multi-kinase inhibitor of VEFG receptor-1, -2, and -3, PDGFRA, and c-kit used sarcoma and renal cell carcinoma treatment [174,175,176]. Oku et al. demonstrated that all three drugs induce phosphorylation of YAP/TAZ, and pazopanib also induces proteasomal degradation of YAP/TAZ [173]. In addition, a combination of these compounds was shown to reduce cell proliferation in YAP/TAZ-dependent breast cancer cells. As previously discussed in the SPE, Verteporfin has been shown to have anti-oncogenic effects through inhibiting YAP1 activity [110]. In a study using a mouse model with patient gastric cancer xenografts, verteporfin treatment was shown to inhibit cancer growth in vivo [177]. Based on these studies, targeting YAP and/or its co-activators will likely be the mainstay of therapeutic candidates in ST-YAP1 and likely in other cancers as well.
12. Supratentorial Ependymoma with ZFTA-Fusion (ST-ZFTA)
ST-ZFTA is the second main subgroup of ependymoma in the ST compartment and harbors the worst outcome [1]. ST-ZFTA has a predilection for the lateral and third ventricle, with most occurring adjacent to the ventricular system, but it is not uncommon for this subset of ependymoma to be extraventricular and cortically based [178]. While the median age of patients with this tumor subgroup is 8 years, the range is 0 to 69 years, with 23% being adults and a peak incidence in the sixth decade of life for those diagnosed in adulthood [34,40]. There is a sex predilection towards males. Microsurgical resection with radiotherapy is considered the mainstay of treatment [2]. With resection and radiotherapy, this subgroup harbors a dismal prognosis with 10-year PFS of approximately 20% and OS of approximately 50% [40]. The role of chemotherapy for this subgroup is yet to be established; however, the recent introduction of this ependymoma subgroup into the WHO classification of CNS tumors will likely bolster research into the underpinnings of the oncogenesis of this tumor [2].
On immunohistochemistry, L1 Cell Adhesion Molecule (L1CAM)/(CD171) corresponds to the presence of ZFTA fusion but is neither sensitive nor specific, while p65/double immunostaining has a 92% positive predictive value and a 100% negative predictive value [179]. ST-ZFTA was originally classified as ST-RELA based on the presence of the gene REL-associated protein (RELA) fusing with the gene Zinc Finger Translocation Associated (ZFTA), also known as C11orf95, being thought to be the distinguishing molecular characteristic. The reason for this change in nosology is based on several studies showing that both RELA and ZFTA fuse with various genes secondary to chromothripsis. However, ST-ZFTA demonstrates a consistent histomolecular entity following ZFTA fusion with or without RELA, thus underscoring the molecular importance of the presence of ZFTA fusion, as opposed to RELA [50,180,181,182,183,184]. It is reported that about 2/3 of ST ependymomas in children harbor this ZFTA fusion, and Pajtler et al. observed the presence of this genomic fusion in 72% of ST ependymomas [40,181]. While the landscape is vast for the possible fusion partners with ZFTA, the main fusion partners that have been identified and shown to be sufficient for tumorigenesis are Mastermind Like Transcription Coactivator 2 (MAML2), Nuclear Receptor Coactivator 2 (NCOA2), and Nuclear Receptor Coactivator 1 (NCOA1) [182,183,184,185].
The introduction of the ZFTA-fusion subgroup was, in large part, a result of the landmark study by Parker et al. that identified the fusion of RELA with ZFTA in two thirds of ST ependymoma [181]. These tumors were found to involve a chromothripsis event with chromosome 11q13.1, the location of ZFTA. While the fusion of ZFTA has been established as the molecular marker distinguishing this subgroup, the function and role of ZFTA is still not completely understood. However, the introduction of this molecular subgroup has bolstered investigation into the function of ZFTA, and a recent study by Kupp et al. utilized a combination of transcriptomics, chromatin immunoprecipitation sequencing, and proteomics to elucidate the mechanism behind this gene [186]. This work has provided evidence that ZFTA tethers fusion proteins across the genome, modifying chromatin to an active state and enabling its partner transcriptional coactivators to promote uninhibited expression of various genomic targets. The work demonstrating the shuttling and enabling function of ZFTA serves as the foundation for future studies to develop targeted therapies attacking this protein. While the role of ZFTA is only recently being unraveled, RELA, the principal effector of canonical Nuclear factor-κB (NF-κB), has been well studied, and its tumorigenesis mapped out. RELA is normally located in the cytoplasm via nuclear factor of kappa light polypeptide gene enhancer alpha (IkBα)-mediated sequestration; however, upon external pressures, RELA protein is translocated to the nucleus to influence the NF-κB pathway [187,188]. Parker et al. demonstrated in a mouse model that the ZFTA-RELA fusion protein spontaneously translocates to the nucleus of neural stem cells to activate NF-κB target genes, which lead to the transformation of these cells into ependymoma [181]. Therefore, as ZFTA-RELA preferentially localizes to the nucleus, persistent activation of the NF-κB pathway is the proposed mechanism of oncogenesis. The NF-κB transcriptional regulator protein family is closely linked to cellular inflammation, and while constitutive activation of these proteins are often seen in human tumors, the rarity of mutations in members of this pathway has obfuscated research attempts to understand and identify targetable areas [189,190]. Additionally, Arabzade et al. showed through a autochthonous mouse tumor model that in addition to direct activation of canonical NF-κB, that ZFTA-RELA fusion protein binds to thousands of PLAGL (PLAG1 Like Zinc Finger 1)-enriched sites across the genome [191]. Moreover, ZFTA-RELA fusion protein recruits various transcriptional coactivators such as bromodomain-containing protein 4 (Brd4), histone acetyltransferase p300 (Ep300), and CREB-binding protein (Cbp), all of which are possible areas for future pharmacologic inhibition. Another promising target is Fibroblast Growth Factor Receptor 3 (FGFR3). FGFR3, a gene located on chromosome 4 and responsible for bone development, has been shown to be overexpressed in an in-vitro model of ST-ZFTA. This study also demonstrated the efficacy of a broad range of FGFR inhibitors in inducing maturation in their invitro model, a therapeutic concept currently considered of high potential in pediatric cancers [192]. Lastly, immune checkpoint molecules are another possible target currently being investigated. Wang et al. uncovered that expression of PD-L1 independently predicts outcomes in patients with ST, extraventricular ependymomas [193]. T-cell exhaustion induced by overexpression of PD-L1 is a mechanism by which tumors evade immune-mediated clearance [194]. This observed overexpression in high-grade STEs that independently predicts outcomes further emphasizes the importance of this molecular trait and underscores the need for further studies assessing the efficacy of PD-L1 inhibitors in treating ST-ZFTA. While this pathway of oncogenesis is the subject of ongoing research, and has been further elucidated, the driver of chromothripsis causing this fusion is still not understood. It is theorized that in the pediatric setting, the deletion of cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) may play a role, as it influences the TP53 pathway which has been linked to chromothripsis in other tumors [40,195]. Given the established mechanism of oncogenesis, it is vital that inhibitors of RELA pathways, i.e., NF-κB signaling pathways, be further researched as viable therapies [188].
13. Conclusions
The novel description of the molecular origin of ependymoma has led to further understanding of this genetically diverse tumor. When compared to histopathological classification, the molecular categorization of ependymal tumors into 10 distinct subgroups has led to a more refined characterization of clinical course and potential molecular targets.
Given the unique profile of each ependymoma subgroup, future research must rely on molecular characterization rather than classical histopathological categorization alone. Genomic profiling of ependymal tumors is essential in the further elucidation of molecular targets. While many of ependymoma subgroups are aggressive, even the benign subgroups urgently need targeted therapies, as many tumors are not amenable to surgical resection without causing substantial disability. Research must continue to build on studies that have elucidated ependymoma oncogenesis, as well as investigate novel targets based on malignancies with similar gene ontology. An up-to-date clinical trial list regarding targeted treatments for ependymoma is available on “clinicaltrials.gov” (accessed on 9 November 2021). As the molecular underpinnings of ependymomas are further unraveled through the groundbreaking work of investigators in the field, it is expected that this list will continue to grow.
Author Contributions
Concept and design A.O.; Supervision S.R.L., A.O.; literature review, data acquisition and interpretation, manuscript writing T.L., B.F.S.; critical review of manuscript T.L., B.F.S., S.R.L., A.O.; figure acquisition and design A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from NOMIX Laboratories LLC, Denver, CO, USA.
Acknowledgments
This work is dedicated to all ependymoma patients out there. The authors kindly thank Maria-Magdalena Georgescu, NeuroMarkers PLLC, Houston, TX, USA for providing photographs for Figure 2D–F.
Conflicts of Interest
Authors declare no conflict of interest.
Abbreviations
Note of Nomenclature: As the new WHO classification of tumors of the CNS is currently being published and could be released at any moment, it is noteworthy to mention that in this paper we keep WHO 2016 grade terminology (aka roman numerals) when we refer (or cite) to previously published studies that used 2016 classification criteria.
| IVthV | Fourth ventricle |
| 5AZA-DC | DNA methylase transferase inhibitor 5-aza-2′-deoxycytidine |
| AKT | Protein kinase B |
| ATRX/DAXX | Alpha-thalassemia, mental retardation, X-linked/death domain–associated protein complex |
| AURKA | Auro A-kinase |
| BET | Bromodomain and extraterminal domain |
| Brd4 | Bromodomain-containing protein 4 |
| c-KIT | Proto-oncogene encoding tyrosine-protein kinase KIT, CD117, or stem cell growth factor receptor |
| c-MYB | c-myeloblastosis |
| CAR T | Chimeric antigen receptor T cell |
| Cbp | CREB-binding protein |
| Cdc42 | Cell division cycle 42 |
| COX2 | Cyclooxygenase-2 |
| CNS | Central nervous system |
| CRL4 | Cullin Ring Ubiquitin Ligase 4 |
| CSF | Cerebrospinal fluid |
| CXCR4 | C-X-C chemokine receptor type 4 |
| EANO | The European Association of Neuro-Oncology |
| EED | Embryonic ectoderm development |
| EMA | Epithelial membrane antigen |
| Ep300 | Histone acetyltransferase p300 |
| EPHA7 | Ephrin type-A receptor 7 |
| ERK | Extracellular Signal-Regulated Kinase |
| ERM | Ezrin, radixin, moesin binding protein |
| EZHIP | EZH Inhibitory Protein or CXorf67 |
| EZH2 | Enhancer of zeste homolog 2 |
| FAK | Focal Adhesion Kinase |
| FAM 118B | Family with sequence similarity 118 member B |
| FGFR3 | Fibroblast growth factor receptor 3 |
| FOXJ1 | Forkhead box protein J1 |
| GFAP | Glial fibrillary acidic protein |
| GTR | Gross total resection |
| H3K27M | Histone H3 lysine 27 to methionine mutation |
| H3K27me3 | Trimethylated histone H3 at lysine 27 |
| HDAC | Histone deacetylase |
| HE | Hematoxylin and eosin |
| HIF-1a | Hypoxia inducible factor 1 alpha |
| HOXA13 | Homeobox A13 |
| HOXB13 | Homeobox B13 |
| HOXC10 | Homeobox C10 |
| HOXD10 | Homeobox D10 |
| hTERT | Human telomerase reverse transcriptase |
| IkBα | Nuclear factor of kappa light polypeptide gene enhancer in B cells alpha |
| IL-6 | Interleukin-6 |
| IT | Infratentorial |
| KDM6 | Lysine-specific demethylase 6 |
| KIT | Tyrosine protein kinase or stem cell growth factor receptor or CD117 |
| L1CAM | L1 Cell Adhesion Molecule |
| LATS1/2 | Large Tumor Suppressor Kinase 1 and 2 |
| LDOC | Leucine zipper downregulated in cancer 1 |
| MAML2 | Mastermind like transcription coactivator 2 |
| MAMLD1 | Mastermind like domain containing 1 |
| MDM2 | Mouse double minute 2 homolog or E3 ubiquitin-protein ligase Mdm2 |
| MEF | Myocyte enhancer factor |
| MPE | Spinal myxopapillary ependymoma |
| mTORC1 | Mammalian Target of Rapamycin Complex 1 |
| MYCN | Myelocytomatosis-N |
| NCOA1 | Nuclear receptor coactivator 1 |
| NCOA2 | Nuclear receptor coactivator 2 |
| NEFL | Neurofilament light chain |
| NEC | Not elsewhere classified |
| NF-κB | Nuclear factor-κB |
| NF2 | Neurofibromatosis type 2 |
| NHERF1/EBP50 | Na+/H+ exchanger regulatory factor/ezrin-radixin-moesin binding protein 50 |
| NOS | Not otherwise specified |
| OS | Overall survival |
| PAK | P21 activated kinase |
| PARP | Poly (ADP-ribose) polymerase |
| PDGFRA | Platelet derived growth factor alpha |
| PFA | Posterior fossa ependymoma Group A |
| PFB | Posterior fossa ependymoma Group B |
| PFE | Posterior fossa ependymoma |
| PF-SE | Posterior fossa subependymoma |
| PFS | Progression-free survival |
| PI3K | Phosphoinositide 3-kinase |
| PIKE-L | Phosphoinositide 3-Kinase Enhancer-brain specific isoform |
| PLAGL | PLAG1 like zinc finger 1 |
| PRC2 | Polycomb repressive complex 2 |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| RAC | Ras-related C3 botulinum toxin |
| RAF | Rapidly accelerated fibrosarcoma |
| Ras | Rat sarcoma virus |
| RELA | REL-associated protein |
| SAM | S-adenosyl methionine |
| SE | Subependymoma |
| SET | Su(var)3-9/enhancer-of-zeste/trithorax |
| Shh | Sonic hedgehog |
| SP | Spinal |
| SPE | Spinal ependymoma |
| SPE-MYCN | Spinal ependymoma with MYCN amplification |
| SP-SE | Spinal subependymoma |
| ST | Supratentorial |
| ST-RELA | Supratentorial ependymoma with RELA fusion |
| ST-SE | Supratentorial subependymoma |
| ST-YAP1 | Supratentorial ependymoma with YAP1 fusion |
| STAT | Signal transducer and activator of transcription |
| STE | Supratentorial ependymoma |
| STR | Subtotal resection |
| SUZ12 | Suppressor of zeste 12 homolog |
| TEAD | Transcriptional enhancer factor domain |
| TAZ | Gene encoding protein Tafazzin |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
| WP744 | 4′-O-benzylated doxorubicin analog |
| WP1066 | JAK2/STAT3 inhibitor |
| WP1193 | JAK2/STAT3 inhibitor |
| YAP1 | Yes1 associated transcriptional regulator or YAP or YAP65 |
| ZFTA | Zinc finger translocation-associated |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Wu, J.; Armstrong, T.S.; Gilbert, M.R. Biology and management of ependymomas. Neuro-Oncology 2016, 18, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, IV1–IV96. [Google Scholar] [CrossRef] [PubMed]
- McGuire, C.S.; Sainani, K.L.; Fisher, P.G. Incidence patterns for ependymoma: A Surveillance, Epidemiology, and End Results study—Clinical article. J. Neurosurg. 2009, 110, 725–729. [Google Scholar] [CrossRef]
- Vera-Bolanos, E.; Aldape, K.; Yuan, Y.; Wu, J.; Wani, K.; Necesito-Reyes, M.J.; Colman, H.; Dhall, G.; Lieberman, F.S.; Metellus, P.; et al. Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro-Oncology 2015, 17, 440–447. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Mack, S.C.; Ramaswamy, V.; Smith, C.A.; Witt, H.; Smith, A.; Hansford, J.R.; von Hoff, K.; Wright, K.D.; Hwang, E.; et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017, 133, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Reni, M.; Gatta, G.; Mazza, E.; Vecht, C. Ependymoma. Crit. Rev. Oncol. Hematol. 2007, 63, 81–89. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Central Nervous System Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed on 9 September 2021).
- Metellus, P.; Guyotat, J.; Chinot, O.; Durand, A.; Barrie, M.; Giorgi, R.; Jouvet, A.; Figarella-Branger, D. Adult intracranial WHO grade II ependymomas: Long-term outcome and prognostic factor analysis in a series of 114 patients. Neuro-Oncology 2010, 12, 976–984. [Google Scholar] [CrossRef]
- Rogers, L.; Pueschel, J.; Spetzler, R.; Shapiro, W.; Coons, S.; Thomas, T.; Speiser, B. Is gross-total resection sufficient treatment for posterior fossa ependymomas? J. Neurosurg. 2005, 102, 629–636. [Google Scholar] [CrossRef]
- Rudà, R.; Reifenberger, G.; Frappaz, D.; Pfister, S.M.; Laprie, A.; Santarius, T.; Roth, P.; Tonn, J.C.; Soffietti, R.; Weller, M.; et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro-Oncology 2018, 20, 445–456. [Google Scholar] [CrossRef]
- Aizer, A.A.; Ancukiewicz, M.; Nguyen, P.L.; MacDonald, S.M.; Yock, T.I.; Tarbell, N.J.; Shih, H.A.; Loeffler, J.S.; Oh, K.S. Natural history and role of radiation in patients with supratentorial and infratentorial WHO grade II ependymomas: Results from a population-based study. J. Neurooncol. 2013, 115, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Yuan, Y.; Wu, J.; Mendoza, T.; Vera, E.; Omuro, A.; Lieberman, F.; Robins, H.I.; Gerstner, E.R.; Wu, J.; et al. A phase II study of dose-dense temozolomide and lapatinib for recurrent low-grade and anaplastic supratentorial, infratentorial, and spinal cord ependymoma. Neuro-Oncology 2021, 23, 468–477. [Google Scholar] [CrossRef]
- Brandes, A.A.; Cavallo, G.; Reni, M.; Tosoni, A.; Nicolardi, L.; Scopece, L.; Franceschi, E.; Sotti, G.; Talacchi, A.; Turazzi, S.; et al. A multicenter retrospective study of chemotherapy for recurrent intracranial ependymal tumors in adults by the Gruppo Italiano Cooperative di Neuro-Oncologia. Cancer 2005, 104, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Johnston, S.K. Temozolomide for recurrent intracranial supratentorial platinum-refractory ependymoma. Cancer 2009, 115, 4775–4782. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Cloughesy, T.F.; Stupp, R.; Deangelis, L.M.; Woyshner, E.A.; Ney, D.E.; Lassman, A.B. Bevacizumab for recurrent ependymoma. Neurology 2009, 73, 1677–1680. [Google Scholar] [CrossRef]
- Rudà, R.; Bosa, C.; Magistrello, M.; Franchino, F.; Pellerino, A.; Fiano, V.; Trevisan, M.; Cassoni, P.; Soffietti, R. Temozolomide as salvage treatment for recurrent intracranial ependymomas of the adult: A retrospective study. Neuro-Oncology 2016, 18, 261–268. [Google Scholar] [CrossRef]
- Gramatzki, D.; Roth, P.; Felsberg, J.; Hofer, S.; Rushing, E.J.; Hentschel, B.; Westphal, M.; Krex, D.; Simon, M.; Schnell, O.; et al. Chemotherapy for intracranial ependymoma in adults. BMC Cancer 2016, 16, 287. [Google Scholar] [CrossRef]
- Kieran, M.W.; Goumnerova, L.; Manley, P.; Chi, S.N.; Marcus, K.J.; Manzanera, A.G.; Polanco, M.L.S.; Guzik, B.W.; Aguilar-Cordova, E.; Diaz-Montero, C.M.; et al. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro-Oncology 2019, 21, 537–546. [Google Scholar] [CrossRef]
- National Cancer Institute. Childhood Ependymoma Treatment (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/brain/hp/child-ependymoma-treatment-pdq (accessed on 30 November 2021).
- Duffner, P.K.; Horowitz, M.E.; Krischer, J.P.; Friedman, H.S.; Burger, P.C.; Cohen, M.E.; Sanford, R.A.; Mulhern, R.K.; James, H.E.; Freeman, C.R.; et al. Postoperative Chemotherapy and Delayed Radiation in Children Less Than Three Years of Age with Malignant Brain Tumors. N. Engl. J. Med. 1993, 328, 1725–1731. [Google Scholar] [CrossRef]
- Snider, C.A.; Yang, K.; Mack, S.C.; Suh, J.H.; Chao, S.T.; Merchant, T.E.; Murphy, E.S. Impact of radiation therapy and extent of resection for ependymoma in young children: A population-based study. Pediatr. Blood Cancer 2018, 65, e26880. [Google Scholar] [CrossRef]
- Grundy, R.G.; Wilne, S.A.; Weston, C.L.; Robinson, K.; Lashford, L.S.; Ironside, J.; Cox, T.; Chong, W.K.; Campbell, R.H.; Bailey, C.C.; et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: The UKCCSG/SIOP prospective study. Lancet Oncol. 2007, 8, 696–705. [Google Scholar] [CrossRef]
- Strother, D.R.; Lafay-Cousin, L.; Boyett, J.M.; Burger, P.; Aronin, P.; Constine, L.; Duffner, P.; Kocak, M.; Kun, L.E.; Horowitz, M.E.; et al. Benefit from prolonged dose-intensive chemotherapy for infants with malignant brain tumors is restricted to patients with ependymoma: A report of the pediatric oncology group randomized controlled trial 9233/34. Neuro-Oncology 2014, 16, 457–465. [Google Scholar] [CrossRef]
- Merchant, T.E.; Bendel, A.E.; Sabin, N.D.; Burger, P.C.; Shaw, D.W.; Chang, E.; Wu, S.; Zhou, T.; Eisenstat, D.D.; Foreman, N.K.; et al. Conformal radiation therapy for pediatric ependymoma, chemotherapy for incompletely resected ependymoma, and observation for completely resected, supratentorial ependymoma. J. Clin. Oncol. 2019, 37, 974–983. [Google Scholar] [CrossRef]
- Massimino, M.; Solero, C.L.; Garrè, M.L.; Biassoni, V.; Cama, A.; Genitori, L.; Di Rocco, C.; Sardi, I.; Viscardi, E.; Modena, P.; et al. Second-look surgery for ependymoma: The Italian experience—Clinical article. J. Neurosurg. Pediatr. 2011, 8, 246–250. [Google Scholar] [CrossRef]
- Garvin, J.H.; Selch, M.T.; Holmes, E.; Berger, M.S.; Finlay, J.L.; Flannery, A.; Goldwein, J.W.; Packer, R.J.; Rorke-Adams, L.B.; Shiminski-Maher, T.; et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma. Children’s Cancer Group protocol 9942: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2012, 59, 1183–1189. [Google Scholar] [CrossRef]
- Zacharoulis, S.; Ashley, S.; Moreno, L.; Gentet, J.C.; Massimino, M.; Frappaz, D. Treatment and outcome of children with relapsed ependymoma: A multi-institutional retrospective analysis. Childs Nerv. Syst. 2010, 26, 905–911. [Google Scholar] [CrossRef]
- Jakacki, R.I.; Foley, M.A.; Horan, J.; Wang, J.; Kieran, M.W.; Bowers, D.C.; Bouffet, E.; Zacharoulis, S.; Gill, S.C. Single-agent erlotinib versus oral etoposide in patients with recurrent or refractory pediatric ependymoma: A randomized open-label study. J. Neurooncol. 2016, 129, 131–138. [Google Scholar] [CrossRef]
- Bouffet, E.; Capra, M.; Bartels, U. Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv. Syst. 2009, 25, 1293–1301. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Lewis, J. An overview of the management of adult ependymomas with emphasis on relapsed disease. Clin. Oncol. 2013, 25, 726–733. [Google Scholar] [CrossRef]
- Donovan, L.K.; Delaidelli, A.; Joseph, S.K.; Bielamowicz, K.; Fousek, K.; Holgado, B.L.; Manno, A.; Srikanthan, D.; Gad, A.Z.; Van Ommeren, R.; et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020, 26, 720–731. [Google Scholar] [CrossRef]
- Ellison, D.W.; Kocak, M.; Figarella-Branger, D.; Felice, G.; Catherine, G.; Pietsch, T.; Frappaz, D.; Massimino, M.; Grill, J.; Boyett, J.M.; et al. Histopathological grading of pediatric ependymoma: Reproducibility and clinical relevance in European trial cohorts. J. Negat. Results Biomed. 2011, 10, 7. [Google Scholar] [CrossRef]
- Hübner, J.-M.; Kool, M.; Pfister, S.M.; Pajtler, K.W. Epidemiology, molecular classification and WHO grading of ependymoma. J. Neurosurg. Sci. 2018, 62, 46–50. [Google Scholar] [CrossRef]
- Tihan, T.; Zhou, T.; Holmes, E.; Burger, P.C.; Ozuysal, S.; Rushing, E.J. The prognostic value of histological grading of posterior fossa ependymomas in children: A Children’s Oncology Group study and a review of prognostic factors. Mod. Pathol. 2008, 21, 165–177. [Google Scholar] [CrossRef]
- Xi, S.; Sai, K.; Hu, W.; Wang, F.; Chen, Y.; Wang, J.; Zeng, J.; Chen, Z. Clinical significance of the histological and molecular characteristics of ependymal tumors: A single institution case series from China. BMC Cancer 2019, 19, 717. [Google Scholar] [CrossRef]
- Witt, H.; Gramatzki, D.; Hentschel, B.; Pajtler, K.W.; Felsberg, J.; Schackert, G.; Löffler, M.; Capper, D.; Sahm, F.; Sill, M.; et al. DNA methylation-based classification of ependymomas in adulthood: Implications for diagnosis and treatment. Neuro-Oncology 2018, 20, 1616–1624. [Google Scholar] [CrossRef]
- Cavalli, F.M.G.; Hübner, J.M.; Sharma, T.; Luu, B.; Sill, M.; Zapotocky, M.; Mack, S.C.; Witt, H.; Lin, T.; Shih, D.J.H.; et al. Heterogeneity within the PF-EPN-B ependymoma subgroup. Acta Neuropathol. 2018, 136, 227–237. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Hielscher, T.; Mack, S.C.; Lassaletta, A.; Lin, T.; Pajtler, K.W.; Jones, D.T.W.; Luu, B.; Cavalli, F.M.G.; Aldape, K.; et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: A retrospective multicohort analysis. J. Clin. Oncol. 2016, 34, 2468–2477. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.W.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef]
- Mack, S.C.; Witt, H.; Piro, R.M.; Gu, L.; Zuyderduyn, S.; Stütz, A.M.; Wang, X.; Gallo, M.; Garzia, L.; Zayne, K.; et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 2014, 506, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Donson, A.M.; Nakachi, I.; Griesinger, A.M.; Birks, D.K.; Amani, V.; Hemenway, M.S.; Liu, A.K.; Wang, M.; Hankinson, T.C.; et al. Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol. 2014, 127, 731–745. [Google Scholar] [CrossRef]
- Witt, H.; Mack, S.C.; Ryzhova, M.; Bender, S.; Sill, M.; Isserlin, R.; Benner, A.; Hielscher, T.; Milde, T.; Remke, M.; et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 2011, 20, 143–157. [Google Scholar] [CrossRef]
- Wani, K.; Armstrong, T.S.; Vera-Bolanos, E.; Raghunathan, A.; Ellison, D.; Gilbertson, R.; Vaillant, B.; Goldman, S.; Packer, R.J.; Fouladi, M.; et al. A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol. 2012, 123, 727–738. [Google Scholar] [CrossRef]
- Carter, M.; Nicholson, J.; Ross, F.; Crolla, J.; Allibone, R.; Balaji, V.; Perry, R.; Walker, D.; Gilbertson, R.; Ellison, D.W. Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br. J. Cancer 2002, 86, 929–939. [Google Scholar] [CrossRef]
- Puget, S.; Grill, J.; Valent, A.; Bieche, I.; Dantas-Barbosa, C.; Kauffmann, A.; Dessen, P.; Lacroix, L.; Geoerger, B.; Job, B.; et al. Candidate genes on chromosome 9q33-34 involved in the progression of childhood ependymomas. J. Clin. Oncol. 2009, 27, 1884–1892. [Google Scholar] [CrossRef]
- Korshunov, A.; Hielscher, T.; Ryzhova, M. Molecular Staging of Intracranial Ependymoma in Children and Adults Structure and Mechanism of Key Nonsense-Mediated mRNA Decay Factor Complexes View project EURAT: Ethical and legal aspects of genome sequencing View project. J. Clin. Oncol. 2010, 28, 3182–3190. [Google Scholar] [CrossRef]
- Modena, P.; Lualdi, E.; Facchinetti, F.; Veltman, J.; Reid, J.F.; Minardi, S.; Janssen, I.; Giangaspero, F.; Forni, M.; Finocchiaro, G.; et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J. Clin. Oncol. 2006, 24, 5223–5233. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ellison, D.W.; Aldape, K.D.; Capper, D.; Fouladi, M.; Gilbert, M.R.; Gilbertson, R.J.; Hawkins, C.; Merchant, T.E.; Pajtler, K.; Venneti, S.; et al. cIMPACT-NOW update 7: Advancing the molecular classification of ependymal tumors. Brain Pathol. 2020, 30, 863–866. [Google Scholar] [CrossRef]
- Georgescu, M.M.; Yell, P.; Mobley, B.C.; Shang, P.; Georgescu, T.; Wang, S.H.J.; Canoll, P.; Hatanpaa, K.J.; White, C.L.; Raisanen, J.M. NHERF1/EBP50 is an organizer of polarity structures and a diagnostic marker in ependymoma. Acta Neuropathol. Commun. 2015, 3, 11. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Praver, M.; Zanazzi, G.J.; Englander, Z.K.; Sims, J.S.; Samanamud, J.L.; Ogden, A.T.; McCormick, P.C.; Feldstein, N.A.; McKhann, G.M.; et al. Subependymomas Are Low-Grade Heterogeneous Glial Neoplasms Defined by Subventricular Zone Lineage Markers. World Neurosurg. 2017, 107, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Ishizawa, K.; Horiguchi, H.; Shimada, T.; Hirose, T. Subependymoma of the spinal cord and review of the literature. Pathol. Int. 2003, 53, 169–173. [Google Scholar] [CrossRef]
- Krishnan, S.S.; Panigrahi, M.; Pendyala, S.; Rao, S.I.; Varma, D.R. Cervical Subependymoma: A rare case report with possible histogenesis. J. Neurosci. Rural Pract. 2012, 3, 366–369. [Google Scholar] [CrossRef]
- Soleiman, H.A.; Ironside, J.; Kealey, S.; Demetriades, A.K. Spinal subependymoma surgery: Do no harm. Little may be more! Neurosurg. Rev. 2020, 43, 1047–1053. [Google Scholar] [CrossRef]
- Wu, L.; Yang, T.; Deng, X.; Yang, C.; Zhao, L.; Fang, J.; Wang, G.; Yang, J.; Xu, Y. Surgical outcomes in spinal cord subependymomas: An institutional experience. J. Neurooncol. 2014, 116, 99–106. [Google Scholar] [CrossRef]
- Korshunov, A.; Neben, K.; Wrobel, G.; Tews, B.; Benner, A.; Hahn, M.; Golanov, A.; Lichter, P. Gene Expression Patterns in Ependymomas Correlate with Tumor Location, Grade, and Patient Age. Am. J. Pathol. 2003, 163, 1721–1727. [Google Scholar] [CrossRef]
- Monoranu, C.M.; Huang, B.; Zangen, I.L.; Rutkowski, S.; Vince, G.H.; Gerber, N.U.; Puppe, B.; Roggendorf, W. Correlation between 6q25.3 deletion status and survival in pediatric intracranial ependymomas. Cancer Genet. Cytogenet. 2008, 182, 18–26. [Google Scholar] [CrossRef]
- Rajaram, V.; Gutmann, D.H.; Prasad, S.K.; Mansur, D.B.; Perry, A. Alterations of protein 4.1 family members in ependymomas: A study of 84 cases. Mod. Pathol. 2005, 18, 991–997. [Google Scholar] [CrossRef]
- George, O.L.; Ness, S.A. Situational awareness: Regulation of the myb transcription factor in differentiation, the cell cycle and oncogenesis. Cancers 2014, 6, 2049–2071. [Google Scholar] [CrossRef]
- López-Nieva, P.; Vaquero, C.; Fernández-Navarro, P.; González-Sánchez, L.; Villa-Morales, M.; Santos, J.; Esteller, M.; Fernández-Piqueras, J. EPHA7, a new target gene for 6q deletion in T-cell lymphoblastic lymphomas. Carcinogenesis 2012, 33, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.S.; Doan, N.; Gelsomino, M.; Shabani, S. Intracranial Subependymoma: A SEER Analysis 2004–2013. World Neurosurg. 2017, 101, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Friede, R.L.; Pollak, A. The cytogenetic basis for classifying ependymomas. J. Neuropathol. Exp. Neurol. 1978, 37, 103–118. [Google Scholar] [CrossRef]
- Ragel, B.T.; Osborn, A.G.; Whang, K.; Townsend, J.J.; Jensen, R.L.; Couldwell, W.T. Subependymomas: An analysis of clinical and imaging features. Neurosurgery 2006, 58, 881–889. [Google Scholar] [CrossRef]
- Rushing, E.J.; Cooper, P.B.; Quezado, M.; Begnami, M.; Crespo, A.; Smirniotopoulos, J.G.; Ecklund, J.; Olsen, C.; Santi, M. Subependymoma revisited: Clinicopathological evaluation of 83 cases. J. Neuro-Oncol. 2007, 85, 297–305. [Google Scholar] [CrossRef]
- Leeper, H.; Felicella, M.M.; Walbert, T. Recent Advances in the Classification and Treatment of Ependymomas. Curr. Treat. Options Oncol. 2017, 18, 55. [Google Scholar] [CrossRef]
- Bi, Z.; Ren, X.; Zhang, J.; Jia, W. Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J. Neurosurg. 2015, 122, 49–60. [Google Scholar] [CrossRef]
- Kong, L.Y.; Wei, J.; Haider, A.S.; Liebelt, B.D.; Ling, X.; Conrad, C.A.; Fuller, G.N.; Levine, N.B.; Priebe, W.; Sawaya, R.; et al. Therapeutic targets in subependymoma. J. Neuroimmunol. 2014, 277, 168–175. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bursteinas, B.; et al. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Glivec (ST1571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 2002, 1, 493–502. [Google Scholar] [CrossRef]
- Donson, A.; Werner, E.; Amani, V.; Griesinger, A.; Witt, D.; Nellan, A.; Vibhakar, R.; Hankinson, T.; Handler, M.; Dorris, K.; et al. Tyrosine kinase inhibitors axitinib, imatinib and pazopanib are selectively potent in ependymoma. Neuro-Oncology 2017, 19, iv17. [Google Scholar] [CrossRef]
- Newton, H.B.; Ray-Chaudhury, A. Overview of Brain Tumor Epidemiology and Histopathology. Handb. Brain Tumor Chemother. 2006, 3–20. [Google Scholar] [CrossRef]
- Kleihues, P.; Burger, P.C.; Scheithauer, B.W. Histological Typing of Tumours of the Central Nervous System; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar] [CrossRef]
- Kweh, B.T.S.; Rosenfeld, J.V.; Hunn, M.; Tee, J.W. Tumor characteristics and surgical outcomes of intracranial subependymomas: A systematic review and meta-analysis. J. Neurosurg. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Jooma, R.; Torrens, M.J.; Bradshaw, J.; Brownell, B. Subependymomas of the fourth ventricle. Surgical treatment in 12 cases. J. Neurosurg. 1985, 62, 508–512. [Google Scholar] [CrossRef]
- Varma, A.; Giraldi, D.; Mills, S.; Brodbelt, A.R.; Jenkinson, M.D. Surgical management and long-term outcome of intracranial subependymoma. Acta Neurochir. 2018, 160, 1793–1799. [Google Scholar] [CrossRef]
- Scheithauer, B.W. Symptomatic subependymoma. Report of 21 cases with review of the literature. J. Neurosurg. 1978, 49, 689–696. [Google Scholar] [CrossRef]
- Hou, Z.; Wu, Z.; Zhang, J.; Zhang, L.; Tian, R.; Liu, B.; Wang, Z. Lateral ventricular subependymomas: An analysis of the clinical features of 27 adult cases at a single institute. Neurol. India 2012, 60, 379. [Google Scholar] [CrossRef]
- Limaiem, F.; Das, J.M. Myxopapillary Ependymoma. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32644598 (accessed on 7 July 2021).
- Sonneland, P.R.L.; Scheithauer, B.W.; Onofrio, B.M. Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer 1985, 56, 883–893. [Google Scholar] [CrossRef]
- Mørk, S.J.; Løken, A.C. Ependymoma. A follow-up study of 101 cases. Cancer 1977, 40, 907–915. [Google Scholar] [CrossRef]
- Kraetzig, T.; McLaughlin, L.; Bilsky, M.H.; Laufer, I. Metastases of spinal myxopapillary ependymoma: Unique characteristics and clinical management. J. Neurosurg. Spine 2018, 28, 201–208. [Google Scholar] [CrossRef]
- Bagley, C.A.; Wilson, S.; Kothbauer, K.F.; Bookland, M.J.; Epstein, F.; Jallo, G.I. Long term outcomes following surgical resection of myxopapillary ependymomas. Neurosurg. Rev. 2009, 32, 321–334. [Google Scholar] [CrossRef]
- Feldman, W.B.; Clark, A.J.; Safaee, M.; Ames, C.P.; Parsa, A.T. Tumor control after surgery for spinal myxopapillary ependymomas: Distinct outcomes in adults versus children. J. Neurosurg. Spine 2013, 19, 471–476. [Google Scholar] [CrossRef]
- Liu, T.; Yang, C.; Deng, X.; Li, A.; Xin, Y.; Yang, J.; Xu, Y. Clinical characteristics and surgical outcomes of spinal myxopapillary ependymomas. Neurosurg. Rev. 2020, 43, 1351–1356. [Google Scholar] [CrossRef]
- Akyurek, S.; Chang, E.L.; Yu, T.K.; Little, D.; Allen, P.K.; McCutcheon, I.; Mahajan, A.; Maor, M.H.; Woo, S.Y. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J. Neurooncol. 2006, 80, 177–183. [Google Scholar] [CrossRef]
- Waldron, J.N.; Laperriere, N.J.; Jaakkimainen, L.; Simpson, W.J.; Payne, D.; Milosevic, M.; Wong, C.S. Spinal cord ependymomas: A retrospective analysis of 59 cases. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 223–229. [Google Scholar] [CrossRef]
- Whitaker, S.J.; Bessell, E.M.; Ashley, S.E.; Bloom, H.J.G.; Bell, B.A.; Brada, M. Postoperative radiotherapy in the management of spinal cord ependymoma. J. Neurosurg. 1991, 74, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.N.; Donson, A.M.; Kleinschmidt-Demasters, B.K.; Birks, D.K.; Handler, M.H.; Foreman, N.K. Unique molecular characteristics of pediatric myxopapillary ependymoma. Brain Pathol. 2010, 20, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Wellik, D.M. Hox patterning of the vertebrate axial skeleton. Dev. Dyn. 2007, 236, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, R.A.; Pandha, H.S.; Simpson, G.R.; Pettengell, R.; Poterlowicz, K.; Thompson, A.; Harrington, K.; El-Tanani, M.; Morgan, R. Inhibition of HOX/PBX dimer formation leads to necroptosis in acute myeloid leukemia cells. Oncotarget 2017, 8, 89566–89579. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Hamada, J.I.; Murakawa, K.; Takada, M.; Tada, M.; Nogami, I.; Hayashi, N.; Nakamori, S.; Monden, M.; Miyamoto, M.; et al. Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Exp. Cell Res. 2004, 293, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.; Chapman, R.; Storer, L.C.; Luo, L.; Heath, P.R.; Resar, L.; Cohen, K.J.; Grundy, R.G.; Lourdusamy, A. Integrative molecular characterization of pediatric spinal ependymoma: The UK Children’s Cancer and Leukaemia Group study. Neuro-Oncol. Adv. 2021, 3, vdab043. [Google Scholar] [CrossRef]
- Toomey, D.P.; Murphy, J.F.; Conlon, K.C. COX-2, VEGF and tumour angiogenesis. Surgeon 2009, 7, 174–180. [Google Scholar] [CrossRef]
- Schonthal, A.H. Exploiting Cyclooxygenase-(in)Dependent Properties of COX-2 Inhibitors for Malignant Glioma Therapy. Anticancer Agents Med. Chem. 2012, 10, 450–461. [Google Scholar] [CrossRef]
- Axelsson, H.; Lönnroth, C.; Wang, W.; Svanberg, E.; Lundholm, K. Cyclooxygenase inhibition in early onset of tumor growth and related angiogenesis evaluated in EP1 and EP3 knockout tumor-bearing mice. Angiogenesis 2006, 8, 339–348. [Google Scholar] [CrossRef]
- Celano, E.; Salehani, A.; Malcolm, J.G.; Reinertsen, E.; Hadjipanayis, C.G. Spinal cord ependymoma: A review of the literature and case series of ten patients. J. Neurooncol. 2016, 128, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Salunke, P. Understanding Ependymoma Oncogenesis: An Update on Recent Molecular Advances and Current Perspectives. Mol. Neurobiol. 2017, 54, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Guyotat, J.; Metellus, P.; Giorgi, R.; Barrie, M.; Jouvet, A.; Fevre-Montange, M.; Chinot, O.; Durand, A.; Figarella-Branger, D. Infratentorial ependymomas: Prognostic factors and outcome analysis in a multi-center retrospective series of 106 adult patients. Acta Neurochir. 2009, 151, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, P.C.; Pollack, I.F.; Martínez, A.J.; Lo, K.H.; Janosky, J.; Albright, A.L. Intracranial ependymomas of childhood lack of correlation of histopathology and clinical outcome. Pathol. Res. Pract. 1996, 192, 515–522. [Google Scholar] [CrossRef]
- Schiffer, D.; Chiò, A.; Giordana, M.T.; Migheli, A.; Palma, L.; Pollo, B.; Soffietti, R.; Tribolo, A. Histologic prognostic factors in ependymoma. Childs Nerv. Syst. 1991, 7, 177–182. [Google Scholar] [CrossRef]
- Korshunov, A.; Golanov, A.; Sycheva, R.; Timirgaz, V. The Histologic Grade Is a Main Prognostic Factor for Patients with Intracranial Ependymomas Treated in the Microneurosurgical Era: An Analysis of 258 Patients. Cancer 2004, 100, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rivera, V.; Dono, A.; Abdelkhaleq, R.; Sheth, S.A.; Chen, P.R.; Chandra, A.; Ballester, L.Y.; Esquenazi, Y. Treatment trends and overall survival in patients with grade II/III ependymoma: The role of tumor grade and location. Clin. Neurol. Neurosurg. 2020, 199, 106282. [Google Scholar] [CrossRef]
- Tarapore, P.E.; Modera, P.; Naujokas, A.; Oh, M.C.; Amin, B.; Tihan, T.; Parsa, A.T.; Ames, C.P.; Chou, D.; Mummaneni, P.V.; et al. Pathology of spinal ependymomas: An institutional experience over 25 years in 134 patients. Neurosurgery 2013, 73, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chung, C.K.; Kim, C.H.; Yoon, S.H.; Hyun, S.J.; Kim, K.J.; Kim, E.S.; Eoh, W.; Kim, H.J. Long-term outcomes of surgical resection with or without adjuvant radiation therapy for treatment of spinal ependymoma: A retrospective multicenter study by the Korea Spinal Oncology Research Group. Neuro-Oncology 2013, 15, 921–929. [Google Scholar] [CrossRef]
- Savoor, R.; Sita, T.L.; Dahdaleh, N.S.; Helenowski, I.; Kalapurakal, J.A.; Marymont, M.H.; Lukas, R.; Kruser, T.J.; Smith, Z.A.; Koski, T.; et al. Long-term outcomes of spinal ependymomas: An institutional experience of more than 60 cases. J. Neuro-Oncol. 2020, 151, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, A.M.; Fernández-Valle, C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016, 35, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Coy, S.; Rashid, R.; Stemmer-Rachamimov, A.; Santagata, S. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2020, 139, 643–665. [Google Scholar] [CrossRef] [PubMed]
- Abylkassov, R.; Xie, Y. Role of yes-associated protein in cancer: An update (Review). Oncol. Lett. 2016, 12, 2277–2282. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Gan, H.K.; Blagden, S.P.; Plummer, R.; Arkenau, H.T.; Ranson, M.; Evans, T.R.J.; Zalcman, G.; Bahleda, R.; Hollebecque, A.; et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann. Oncol. 2016, 27, 2268–2274. [Google Scholar] [CrossRef]
- Sato, T.; Sekido, Y. NF2/merlin inactivation and potential therapeutic targets in mesothelioma. Int. J. Mol. Sci. 2018, 19, 988. [Google Scholar] [CrossRef]
- Chen, P.; Rossi, N.; Priddy, S.; Pierson, C.R.; Studebaker, A.W.; Johnson, R.A. EphB2 activation is required for ependymoma development as well as inhibits differentiation and promotes proliferation of the transformed cell. Sci. Rep. 2015, 5, 9248. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 724–742. [Google Scholar]
- Pavon, L.F.; Sibov, T.T.; de Toledo, S.R.C.; de Oliveira, D.M.; Cabral, F.R.; de Souza, J.G.; Boufleur, P.; Marti, L.C.; Malheiros, J.M.; da Cruz, E.F.; et al. Establishment of primary cell culture and an intracranial xenograft model of pediatric ependymoma: A prospect for therapy development and understanding of tumor biology. Oncotarget 2018, 9, 21731. [Google Scholar] [CrossRef][Green Version]
- Scheil, S.; Brüderlein, S.; Eicker, M.; Herms, J.; Herold-Mende, C.; Steiner, H.H.; Barth, T.F.E.; Möller, P. Low frequency of chromosomal imbalances in anaplastic ependymomas as detected by comparative genomic hybridization. Brain Pathol. 2001, 11, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, D.R.; Sill, M.; Okonechnikov, K.; Korshunov, A.; Yip, S.; Schutz, P.W.; Scheie, D.; Kruse, A.; Harter, P.N.; Kastelan, M.; et al. MYCN amplification drives an aggressive form of spinal ependymoma. Acta Neuropathol. 2019, 138, 1075–1089. [Google Scholar] [CrossRef]
- Raffeld, M.; Abdullaev, Z.; Pack, S.D.; Xi, L.; Nagaraj, S.; Briceno, N.; Vera, E.; Pittaluga, S.; Lopes Abath Neto, O.; Quezado, M.; et al. High level MYCN amplification and distinct methylation signature define an aggressive subtype of spinal cord ependymoma. Acta Neuropathol. Commun. 2020, 8, 101. [Google Scholar] [CrossRef]
- Swanson, A.A.; Raghunathan, A.; Jenkins, R.B.; Messing-Jünger, M.; Pietsch, T.; Clarke, M.J.; Kaufmann, T.J.; Giannini, C. Spinal cord ependymomas with MYCN amplification show aggressive clinical behavior. J. Neuropathol. Exp. Neurol. 2019, 78, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, A.; Vendemini, F.; Urbini, M.; Melchionda, F.; Masetti, R.; Franzoni, M.; Libri, V.; Serravalle, S.; Togni, M.; Paone, G.; et al. MYCN is a novel oncogenic target in pediatric T-cell Acute Lymphoblastic Leukemia. Oncotarget 2014, 5, 120–130. [Google Scholar] [CrossRef]
- Barone, G.; Anderson, J.; Pearson, A.D.J.; Petrie, K.; Chesler, L. New strategies in neuroblastoma: Therapeutic targeting of MYCN and ALK. Clin. Cancer Res. 2013, 19, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Schrimpf, D.; Ryzhova, M.; Sturm, D.; Chavez, L.; Hovestadt, V.; Sharma, T.; Habel, A.; Burford, A.; Jones, C.; et al. H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 2017, 134, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Phillips, J.W.; Smith, B.A.; Park, J.W.; Stoyanova, T.; McCaffrey, E.F.; Baertsch, R.; Sokolov, A.; Meyerowitz, J.G.; Mathis, C.; et al. N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells. Cancer Cell 2016, 29, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pérez, M.V.; Henley, A.B.; Arsenian-Henriksson, M. The MYCN protein in health and disease. Genes 2017, 8, 113. [Google Scholar] [CrossRef]
- Stermann, A.; Huebener, N.; Seidel, D.; Fest, S.; Eschenburg, G.; Stauder, M.; Schramm, A.; Eggert, A.; Lode, H.N. Targeting of MYCN by means of DNA vaccination is effective against neuroblastoma in mice. Cancer Immunol. Immunother. 2015, 64, 1215–1227. [Google Scholar] [CrossRef]
- Mack, S.C.; Pajtler, K.W.; Chavez, L.; Okonechnikov, K.; Bertrand, K.C.; Wang, X.X.; Erkek, S.; Federation, A.; Song, A.; Lee, C.; et al. Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature 2018, 553, 101–105. [Google Scholar] [CrossRef]
- Panwalkar, P.; Clark, J.; Ramaswamy, V.; Hawes, D.; Yang, F.; Dunham, C.; Yip, S.; Hukin, J.; Sun, Y.; Schipper, M.J.; et al. Immunohistochemical analysis of H3K27me3 demonstrates global reduction in group-A childhood posterior fossa ependymoma and is a powerful predictor of outcome. Acta Neuropathol. 2017, 134, 705–714. [Google Scholar] [CrossRef]
- Bayliss, J.; Mukherjee, P.; Lu, C.; Jain, S.U.; Chung, C.; Martinez, D.; Sabari, B.; Margol, A.S.; Panwalkar, P.; Parolia, A.; et al. Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci. Transl. Med. 2016, 8, 366ra161. [Google Scholar] [CrossRef]
- Nambirajan, A.; Sharma, A.; Rajeshwari, M.; Boorgula, M.T.; Doddamani, R.; Garg, A.; Suri, V.; Sarkar, C.; Sharma, M.C. EZH2 inhibitory protein (EZHIP/Cxorf67) expression correlates strongly with H3K27me3 loss in posterior fossa ependymomas and is mutually exclusive with H3K27M mutations. Brain Tumor Pathol. 2021, 38, 30–40. [Google Scholar] [CrossRef]
- Tanrıkulu, B.; Danyeli, A.E.; Özek, M.M. Is H3K27me3 status really a strong prognostic indicator for pediatric posterior fossa ependymomas? A single surgeon, single center experience. Childs Nerv. Syst. 2020, 36, 941–949. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Yuh, E.L.; Barkovich, A.J.; Gupta, N. Imaging of ependymomas: MRI and CT. Childs Nerv. Syst. 2009, 25, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, U.; Karlowee, V.; Amatya, V.J.; Takayasu, T.; Takano, M.; Takeshima, Y.; Sugiyama, K.; Kurisu, K.; Yamasaki, F. Radiology Profile as a Potential Instrument to Differentiate Between Posterior Fossa Ependymoma (PF-EPN) Group A and B. World Neurosurg. 2020, 140, e320–e327. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, T.; Wang, L.M.; Zhang, J.; Zhang, H.; Li, S.W.; Zhang, S.; Li, P.; Wang, B.; Luo, L.; et al. Survival and Prognostic Factors of Adult Intracranial Ependymoma: A Single-institutional Analysis of 236 Patients. Am. J. Surg. Pathol. 2021, 45, 979–987. [Google Scholar] [CrossRef]
- Baroni, L.V.; Sundaresan, L.; Heled, A.; Coltin, H.; Pajtler, K.W.; Lin, T.; Merchant, T.E.; McLendon, R.; Faria, C.; Buntine, M.; et al. Ultra high-risk PFA ependymoma is characterized by loss of chromosome 6q. Neuro-Oncology 2021, 23, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Pajtler, K.W.; Wen, J.; Sill, M.; Lin, T.; Orisme, W.; Tang, B.; Hübner, J.M.; Ramaswamy, V.; Jia, S.; Dalton, J.D.; et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol. 2018, 136, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Ryall, S.; Guzman, M.; Elbabaa, S.K.; Luu, B.; Mack, S.C.; Zapotocky, M.; Taylor, M.D.; Hawkins, C.; Ramaswamy, V. H3 K27M mutations are extremely rare in posterior fossa group A ependymoma. Childs Nerv. Syst. 2017, 33, 1047–1051. [Google Scholar] [CrossRef]
- De Sousa, G.R.; Lira, R.C.P.; de Almeida Magalhães, T.; da Silva, K.R.; Nagano, L.F.P.; Saggioro, F.P.; Baroni, M.; Marie, S.K.N.; Oba-Shinjo, S.M.; Brandelise, S.; et al. A coordinated approach for the assessment of molecular subgroups in pediatric ependymomas using low-cost methods. J. Mol. Med. 2021, 99, 1101–1113. [Google Scholar] [CrossRef]
- Jain, S.U.; Do, T.J.; Lund, P.J.; Rashoff, A.Q.; Diehl, K.L.; Cieslik, M.; Bajic, A.; Juretic, N.; Deshmukh, S.; Venneti, S.; et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat. Commun. 2019, 10, 2146. [Google Scholar] [CrossRef] [PubMed]
- Hübner, J.M.; Müller, T.; Papageorgiou, D.N.; Mauermann, M.; Krijgsveld, J.; Russell, R.B.; Ellison, D.W.; Pfister, S.M.; Pajtler, K.W.; Kool, M. EZHIP/CXorf67 mimics K27M mutated oncohistones and functions as an intrinsic inhibitor of PRC2 function in aggressive posterior fossa ependymoma. Neuro-Oncology 2019, 21, 878–889. [Google Scholar] [CrossRef]
- Krug, B.; Harutyunyan, A.S.; Deshmukh, S.; Jabado, N. Polycomb repressive complex 2 in the driver’s seat of childhood and young adult brain tumours. Trends Cell Biol. 2021, 31, 814–828. [Google Scholar] [CrossRef]
- Jain, S.U.; Rashoff, A.Q.; Krabbenhoft, S.D.; Hoelper, D.; Do, T.J.; Gibson, T.J.; Lundgren, S.M.; Bondra, E.R.; Deshmukh, S.; Harutyunyan, A.S.; et al. H3 K27M and EZHIP Impede H3K27-Methylation Spreading by Inhibiting Allosterically Stimulated PRC2. Mol. Cell 2020, 80, 726–735. [Google Scholar] [CrossRef]
- Dyer, M.A.; Qadeer, Z.A.; Valle-Garcia, D.; Bernstein, E. ATRX and DAXX: Mechanisms and mutations. Cold Spring Harb. Perspect. Med. 2017, 7, a026567. [Google Scholar] [CrossRef]
- Michealraj, K.A.; Kumar, S.A.; Kim, L.J.Y.; Cavalli, F.M.G.; Przelicki, D.; Wojcik, J.B.; Delaidelli, A.; Bajic, A.; Saulnier, O.; MacLeod, G.; et al. Metabolic Regulation of the Epigenome Drives Lethal Infantile Ependymoma. Cell 2020, 181, 1329–1345. [Google Scholar] [CrossRef]
- Griesinger, A.M.; Witt, D.A.; Grob, S.T.; Georgio Westover, S.R.; Donson, A.M.; Sanford, B.; Mulcahy Levy, J.M.; Wong, R.; Moreira, D.C.; Desisto, J.A.; et al. NF-κB upregulation through epigenetic silencing of LDOC1 drives tumor biology and specific immunophenotype in Group A ependymoma. Neuro-Oncology 2017, 19, 1350–1360. [Google Scholar] [CrossRef]
- Griesinger, A.M.; Josephson, R.J.; Donson, A.M.; Levy, J.M.M.; Amani, V.; Birks, D.K.; Hoffman, L.M.; Furtek, S.L.; Reigan, P.; Handler, M.H.; et al. Interleukin-6/STAT3 pathway signaling drives an inflammatory phenotype in group a ependymoma. Cancer Immunol. Res. 2015, 3, 1165–1174. [Google Scholar] [CrossRef]
- Gojo, J.; Lötsch, D.; Spiegl-Kreinecker, S.; Pajtler, K.W.; Neumayer, K.; Korbel, P.; Araki, A.; Brandstetter, A.; Mohr, T.; Hovestadt, V.; et al. Telomerase activation in posterior fossa group A ependymomas is associated with dismal prognosis and chromosome 1q gain. Neuro-Oncology 2017, 19, 1183–1194. [Google Scholar] [CrossRef]
- Meel, M.H.; Kaspers, G.J.L.; Hulleman, E. Preclinical therapeutic targets in diffuse midline glioma. Drug Resist. Updates 2019, 44, 15–25. [Google Scholar] [CrossRef]
- Lin, G.L.; Wilson, K.M.; Ceribelli, M.; Stanton, B.Z.; Woo, P.J.; Kreimer, S.; Qin, E.Y.; Zhang, X.; Lennon, J.; Nagaraja, S.; et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Sandberg, D.I.; Yu, B.; Patel, R.; Hagan, J.; Miesner, E.; Sabin, J.; Smith, S.; Fletcher, S.; Shah, M.N.; Sirianni, R.W.; et al. Infusion of 5-Azacytidine (5-AZA) into the fourth ventricle or resection cavity in children with recurrent posterior Fossa Ependymoma: A pilot clinical trial. J. Neurooncol. 2019, 141, 449–457. [Google Scholar] [CrossRef]
- Han, J.; Yu, M.; Bai, Y.; Yu, J.; Jin, F.; Li, C.; Zeng, R.; Peng, J.; Li, A.; Song, X.; et al. Elevated CXorf67 Expression in PFA Ependymomas Suppresses DNA Repair and Sensitizes to PARP Inhibitors. Cancer Cell 2020, 38, 844–856. [Google Scholar] [CrossRef]
- Gojo, J.; Englinger, B.; Jiang, L.; Hübner, J.M.; Shaw, M.L.; Hack, O.A.; Madlener, S.; Kirchhofer, D.; Liu, I.; Pyrdol, J.; et al. Single-Cell RNA-Seq Reveals Cellular Hierarchies and Impaired Developmental Trajectories in Pediatric Ependymoma. Cancer Cell 2020, 38, 44–59. [Google Scholar] [CrossRef]
- Cruz, C.; Ribes, V.; Kutejova, E.; Cayuso, J.; Lawson, V.; Norris, D.; Stevens, J.; Davey, M.; Blight, K.; Bangs, F.; et al. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to sonic hedgehog signalling. Development 2010, 137, 4271–4282. [Google Scholar] [CrossRef]
- Abedalthagafi, M.S.; Wu, M.P.; Merrill, P.H.; Du, Z.; Woo, T.; Sheu, S.H.; Hurwitz, S.; Ligon, K.L.; Santagata, S. Decreased FOXJ1 expression and its ciliogenesis programme in aggressive ependymoma and choroid plexus tumours. J. Pathol. 2016, 238, 584–597. [Google Scholar] [CrossRef]
- Wang, J.; Cai, X.; Xia, L.; Zhou, J.; Xin, J.; Liu, M.; Shang, X.; Liu, J.; Li, X.; Chen, Z.; et al. Decreased Expression of FOXJ1 is a Potential Prognostic Predictor for Progression and Poor Survival of Gastric Cancer. Ann. Surg. Oncol. 2015, 22, 685–692. [Google Scholar] [CrossRef]
- Zhu, P.; Piao, Y.; Dong, X.; Jin, Z. Forkhead box J1 expression is upregulated and correlated with prognosis in patients with clear cell renal cell carcinoma. Oncol. Lett. 2015, 10, 1487–1494. [Google Scholar] [CrossRef][Green Version]
- Liu, K.; Fan, J.; Wu, J. Forkhead box protein J1 (FOXJ1) is overexpressed in colorectal cancer and promotes nuclear translocation of β-catenin in SW620 cells. Med. Sci. Monit. 2017, 23, 856–866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Georgescu, M.M.; Gagea, M.; Cote, G. NHERF1/EBP50 Suppresses Wnt-β-Catenin Pathway–Driven Intestinal Neoplasia. Neoplasia 2016, 18, 512–523. [Google Scholar] [CrossRef]
- Kreimann, E.L.; Morales, F.C.; De Orbeta-Cruz, J.; Takahashi, Y.; Adams, H.; Liu, T.J.; McCrea, P.D.; Georgescu, M.M. Cortical stabilization of β-catenin contributes to NHERF1/EBP50 tumor suppressor function. Oncogene 2007, 26, 5290–5299. [Google Scholar] [CrossRef] [PubMed]
- Dihlmann, S.; Von Knebel Doeberitz, M. Wnt/β-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int. J. Cancer 2005, 113, 515–524. [Google Scholar] [CrossRef]
- Jang, G.B.; Hong, I.S.; Kim, R.J.; Lee, S.Y.; Park, S.J.; Lee, E.S.; Park, J.H.; Yun, C.H.; Chung, J.U.; Lee, K.J.; et al. Wnt/β-catenin small-molecule inhibitor CWP232228 preferentially inhibits the growth of breast cancer stem-like cells. Cancer Res. 2015, 75, 1691–1702. [Google Scholar] [CrossRef]
- Pak, S.; Park, S.; Kim, Y.; Park, J.H.; Park, C.H.; Lee, K.J.; Kim, C.S.; Ahn, H. The small molecule WNT/β-catenin inhibitor CWP232291 blocks the growth of castration-resistant prostate cancer by activating the endoplasmic reticulum stress pathway. J. Exp. Clin. Cancer Res. 2019, 38, 342. [Google Scholar] [CrossRef]
- Coluccia, A.; La Regina, G.; Naccarato, V.; Nalli, M.; Orlando, V.; Biagioni, S.; De Angelis, M.L.; Baiocchi, M.; Gautier, C.; Gianni, S.; et al. Drug Design and Synthesis of First in Class PDZ1 Targeting NHERF1 Inhibitors as Anticancer Agents. ACS Med. Chem. Lett. 2019, 10, 499–503. [Google Scholar] [CrossRef]
- Lester, A.; McDonald, K.L. Intracranial ependymomas: Molecular insights and translation to treatment. Brain Pathol. 2020, 30, 3–12. [Google Scholar] [CrossRef]
- Andreiuolo, F.; Varlet, P.; Tauziède-Espariat, A.; Jünger, S.T.; Dörner, E.; Dreschmann, V.; Kuchelmeister, K.; Waha, A.; Haberler, C.; Slavc, I.; et al. Childhood supratentorial ependymomas with YAP1-MAMLD1 fusion: An entity with characteristic clinical, radiological, cytogenetic and histopathological features. Brain Pathol. 2019, 29, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Pajtler, K.W.; Wei, Y.; Okonechnikov, K.; Silva, P.B.G.; Vouri, M.; Zhang, L.; Brabetz, S.; Sieber, L.; Gulley, M.; Mauermann, M.; et al. YAP1 subgroup supratentorial ependymoma requires TEAD and nuclear factor I-mediated transcriptional programmes for tumorigenesis. Nat. Commun. 2019, 10, 3914. [Google Scholar] [CrossRef] [PubMed]
- Kristal Kaan, H.Y.; Chan, S.W.; Tan, S.K.J.; Guo, F.; Lim, C.J.; Hong, W.; Song, H. Crystal structure of TAZ-TEAD complex reveals a distinct interaction mode from that of YAP-TEAD complex. Sci. Rep. 2017, 7, 2035. [Google Scholar] [CrossRef]
- Eder, N.; Roncaroli, F.; Dolmart, M.C.; Horswell, S.; Andreiuolo, F.; Flynn, H.R.; Lopes, A.T.; Claxton, S.; Kilday, J.P.; Collinson, L.; et al. YAP1/TAZ drives ependymoma-like tumour formation in mice. Nat. Commun. 2020, 11, 2380. [Google Scholar] [CrossRef]
- Rosenbluh, J.; Nijhawan, D.; Cox, A.G.; Li, X.; Neal, J.T.; Schafer, E.J.; Zack, T.I.; Wang, X.; Tsherniak, A.; Schinzel, A.C.; et al. β-catenin driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 2012, 151. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Szulzewsky, F.; Holland, E.C.; Vasioukhin, V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev. Biol. 2021, 475, 205–221. [Google Scholar] [CrossRef]
- Pan, Z.; Tian, Y.; Cao, C.; Niu, G. The emerging role of YAP/TAZ in tumor immunity. Mol. Cancer Res. 2019, 17, 1777–1786. [Google Scholar] [CrossRef]
- Oku, Y.; Nishiya, N.; Shito, T.; Yamamoto, R.; Yamamoto, Y.; Oyama, C.; Uehara, Y. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS Open Bio 2015, 5, 542–549. [Google Scholar] [CrossRef]
- Lindauer, M.; Hochhaus, A. Dasatinib. Recent Results Cancer Res. 2018, 212, 29–68. [Google Scholar]
- Miyamoto, S.; Kakutani, S.; Sato, Y.; Hanashi, A.; Kinoshita, Y.; Ishikawa, A. Drug review: Pazopanib. Jpn. J. Clin. Oncol. 2018, 48, 503–513. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakazato, Y.; Morito, N.; Sagi, M.; Fujita, T.; Anzai, N.; Chida, M. Fluvastatin is effective against thymic carcinoma. Life Sci. 2020, 240, 117110. [Google Scholar] [CrossRef]
- Giraud, J.; Molina-Castro, S.; Seeneevassen, L.; Sifré, E.; Izotte, J.; Tiffon, C.; Staedel, C.; Boeuf, H.; Fernandez, S.; Barthelemy, P.; et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int. J. Cancer 2020, 146, 2255–2267. [Google Scholar] [CrossRef]
- Sallam, Y.T.; Zhang, Q.; Pandey, S.K. Cortically based cystic supratentorial RELA fusion-positive ependymoma: A case report with unusual presentation and appearance and review of literature. Radiol. Case Rep. 2020, 15, 2495–2499. [Google Scholar] [CrossRef]
- Gessi, M.; Giagnacovo, M.; Modena, P.; Elefante, G.; Gianno, F.; Buttarelli, F.R.; Arcella, A.; Donofrio, V.; Diomedi Camassei, F.; Nozza, P.; et al. Role of immunohistochemistry in the identification of supratentorial C11ORF95-RELA fused ependymoma in routine neuropathology. Am. J. Surg. Pathol. 2019, 43, 56–63. [Google Scholar] [CrossRef]
- Tauziède-Espariat, A.; Siegfried, A.; Nicaise, Y.; Kergrohen, T.; Sievers, P.; Vasiljevic, A.; Roux, A.; Dezamis, E.; Benevello, C.; Machet, M.C.; et al. Supratentorial non-RELA, ZFTA-fused ependymomas: A comprehensive phenotype genotype correlation highlighting the number of zinc fingers in ZFTA-NCOA1/2 fusions. Acta Neuropathol. Commun. 2021, 9, 135. [Google Scholar] [CrossRef]
- Parker, M.; Mohankumar, K.M.; Punchihewa, C.; Weinlich, R.; Dalton, J.D.; Li, Y.; Lee, R.; Tatevossian, R.G.; Phoenix, T.N.; Thiruvenkatam, R.; et al. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 2014, 506, 451–455. [Google Scholar] [CrossRef]
- Zschernack, V.; Jünger, S.T.; Mynarek, M.; Rutkowski, S.; Garre, M.L.; Ebinger, M.; Neu, M.; Faber, J.; Erdlenbruch, B.; Claviez, A.; et al. Supratentorial ependymoma in childhood: More than just RELA or YAP. Acta Neuropathol. 2021, 141, 455–466. [Google Scholar] [CrossRef]
- Tomomasa, R.; Arai, Y.; Kawabata-Iwakawa, R.; Fukuoka, K.; Nakano, Y.; Hama, N.; Nakata, S.; Suzuki, N.; Ishi, Y.; Tanaka, S.; et al. Ependymoma-like tumor with mesenchymal differentiation harboring C11orf95-NCOA1/2 or -RELA fusion: A hitherto unclassified tumor related to ependymoma. Brain Pathol. 2021, 31, e12943. [Google Scholar] [CrossRef]
- Tamai, S.; Nakano, Y.; Kinoshita, M.; Sabit, H.; Nobusawa, S.; Arai, Y.; Hama, N.; Totoki, Y.; Shibata, T.; Ichimura, K.; et al. Ependymoma with C11orf95-MAML2 fusion: Presenting with granular cell and ganglion cell features. Brain Tumor Pathol. 2021, 38, 64–70. [Google Scholar] [CrossRef]
- Zheng, T.; Ghasemi, D.R.; Okonechnikov, K.; Korshunov, A.; Sill, M.; Maass, K.K.; Benites Goncalves da Silva, P.; Ryzhova, M.; Gojo, J.; Stichel, D.; et al. Cross-Species Genomics Reveals Oncogenic Dependencies in ZFTA/C11orf95 Fusion–Positive Supratentorial Ependymomas. Cancer Discov. 2021, 11, 2230–2247. [Google Scholar] [CrossRef]
- Kupp, R.; Ruff, L.; Terranova, S.; Nathan, E.; Ballereau, S.; Stark, R.; Sekhar Reddy Chilamakuri, C.; Hoffmann, N.; Wickham-Rahrmann, K.; Widdess, M.; et al. ZFTA Translocations Constitute Ependymoma Chromatin Remodeling and Transcription Factors. Cancer Discov. 2021, 11, 2216–2229. [Google Scholar] [CrossRef]
- Ozawa, T.; Arora, S.; Szulzewsky, F.; Juric-Sekhar, G.; Miyajima, Y.; Bolouri, H.; Yasui, Y.; Barber, J.; Kupp, R.; Dalton, J.; et al. A De Novo Mouse Model of C11orf95-RELA Fusion-Driven Ependymoma Identifies Driver Functions in Addition to NF-κB. Cell Rep. 2018, 23, 3787–3797. [Google Scholar] [CrossRef]
- Ozawa, T.; Kaneko, S.; Szulzewsky, F.; Qiao, Z.; Takadera, M.; Narita, Y.; Kondo, T.; Holland, E.C.; Hamamoto, R.; Ichimura, K. C11orf95-RELA fusion drives aberrant gene expression through the unique epigenetic regulation for ependymoma formation. Acta Neuropathol. Commun. 2021, 9, 36. [Google Scholar] [CrossRef]
- Didonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. The diverse and complex roles of NF-κB subunits in cancer. Nat. Rev. Cancer 2012, 12, 121–132. [Google Scholar] [CrossRef]
- Arabzade, A.; Zhao, Y.; Varadharajan, S.; Chen, H.-C.; Jessa, S.; Rivas, B.; Stuckert, A.J.; Solis, M.; Kardian, A.; Tlais, D.; et al. ZFTA–RELA Dictates Oncogenic Transcriptional Programs to Drive Aggressive Supratentorial Ependymoma. Cancer Discov. 2021, 11, 2200–2215. [Google Scholar] [CrossRef]
- Lötsch, D.; Kirchhofer, D.; Englinger, B.; Jiang, L.; Okonechnikov, K.; Senfter, D.; Laemmerer, A.; Gabler, L.; Pirker, C.; Donson, A.M.; et al. Targeting fibroblast growth factor receptors to combat aggressive ependymoma. Acta Neuropathol. 2021, 142, 339–360. [Google Scholar] [CrossRef]
- Wang, L.; Han, S.; Yan, C.; Yang, Y.; Li, Z.; Yang, Z. The role of clinical factors and immunocheckpoint molecules in the prognosis of patients with supratentorial extraventricular ependymoma: A single-center retrospective study. J. Cancer Res. Clin. Oncol. 2021, 147, 1259–1270. [Google Scholar] [CrossRef]
- Pauken, K.E.; Wherry, E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef]
- Rausch, T.; Jones, D.T.W.; Zapatka, M.; Stütz, A.M.; Zichner, T.; Weischenfeldt, J.; Jäger, N.; Remke, M.; Shih, D.; Northcott, P.A.; et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell 2012, 148, 59–71. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).