Second-Generation Jak2 Inhibitors for Advanced Prostate Cancer: Are We Ready for Clinical Development?
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Clinical Problem
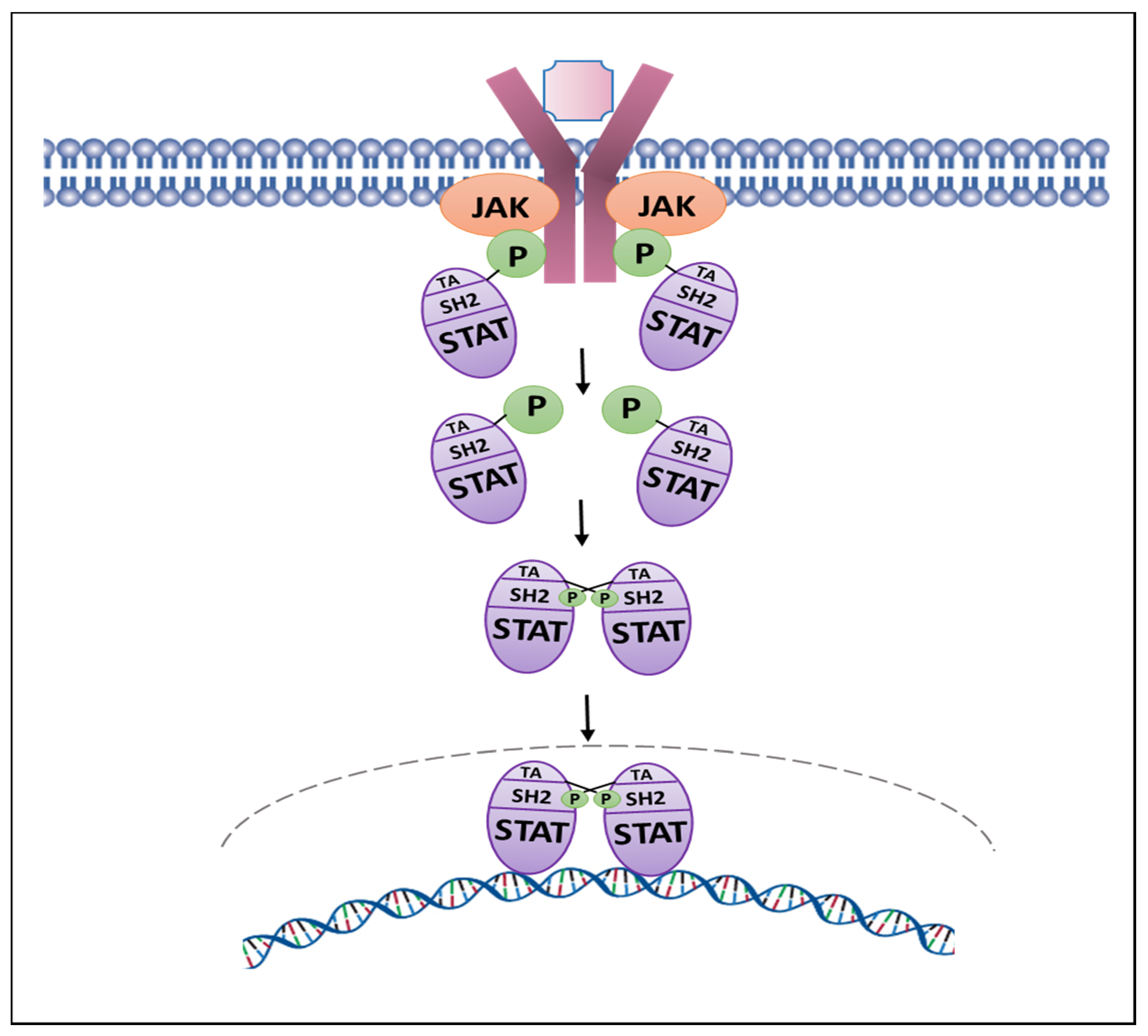
1.2. Jak1/2-Stat Pathway
1.2.1. Jak2-Stat5 Signaling Pathway
1.2.2. Jak1-Stat3 Signaling Pathway
1.2.3. Transcription Factor Stat5 Induction of Prostate Cancer Growth, Metastatic Progression and Anti-Androgen Resistance
1.2.4. Transcription Factor Stat3 and Prostate Cancer Growth
2. Development of Pharmacological Jak2 Inhibitors: Type I and II Inhibitors
The First-Generation Jak1/2 Inhibitor Ruxolitinib (INCB018424/Jakafi; Incyte, Novartis)
3. Next-Generation Type I Jak2-Inhibitors with FDA-Approval or in Clinical Development
3.1. Fedratinib (TG101348/SAR302503/Inrebic; TargeGen, Celgene, Bristol Myers Squibb)
3.2. Pacritinib (SB1518/S-BIO; CTI-BioPharma)
3.3. Baricitinib (LY3009104/INCB028050/Olumiant; Eli Lilly)
3.4. Momelotinib (CYT 387; Gilead)
3.5. Gandotinib (LY2784544; Eli Lilly)
3.6. Peficitinib (ASP015K/Smyraf; Astellas)
3.7. Lestaurtinib (CEP-701; Cephalon, Teva Pharmaceuticals)
3.8. Tofacitinib (CP-690550/Tasocitinib/Xeljanz; Pfizer)
3.9. WP1066/WP 1220 (Moleculin Biotech)
3.10. Atiprimod (SKF 106615; AnorMed Inc.)
3.11. Ilginatib (NS-018; NS Pharma)
3.12. AC430/AC410 (Ambit Biosciences)
3.13. LS104 (AEG 41174; Aegara Bio-Therapeutics)
3.14. Jaktinib (Suzhou Zelgen Biopharmaceuticals)
3.15. AT9283 (Astex Pharmaceuticals)
3.16. Cerdulatinib (PRT062070; Portola Pharmaceuticals)
3.17. Filgotinib (GLPG0634/GS-6034/Jyseleca; Galapagos NV)
3.18. Decernotinib (VX 509; Vertex Pharmaceuticals)
3.19. Erlotinib (Tarceva; Genentech)
3.20. Givinostat (ITF2357; ItalFarmaco)
3.21. Repotrectinib (TPX0005; Turning Point Therapeutics)
3.22. Zotiraciclib (TG02; Adastra Pharmaceuticals)
4. Next-Generation Type I Jak2-Inhibitors Currently in Pre-Clinical Development
4.1. NVP-BSK805 (Novartis)
4.2. CEP-33779 (Cephalon Inc./Teva Pharmaceuticals)
4.3. TG101209 (TargeGen/Sanofi)
4.4. AZ960 (AstraZeneca)
4.5. CHZ868 (Novartis)
4.6. ON044580 (Onconova Therapeutics)
4.7. ZT55 (Molnova)
5. Next-Generation Type I Jak2-Inhibitors with the Clinical Development Terminated
5.1. AZD1480 (AstraZeneca)
5.2. XL019 (Exelixis Inc.)
5.3. BMS-911543 (Bristol-Myers Squibb)
5.4. Tozasertib (MK-0457/VX680; Vertex Pharmaceuticals/Merck)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2019. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html (accessed on 10 April 2021).
- Labrie, F.; Belanger, A.; Simard, J.; Labrie, C.; Dupont, A. Combination therapy for prostate cancer. Endocrine and biologic basis of its choice as new standard first-line therapy. Cancer 1993, 71, 1059–1067. [Google Scholar] [CrossRef]
- Yap, T.A.; Zivi, A.; Omlin, A.; de Bono, J.S. The changing therapeutic landscape of castration-resistant prostate cancer. Nat. Rev. Clin. Oncol. 2011, 8, 597–610. [Google Scholar] [CrossRef]
- Wong, Y.N.; Ferraldeschi, R.; Attard, G.; de Bono, J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat. Rev. Clin. Oncol. 2014, 11, 365–376. [Google Scholar] [CrossRef]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Masiello, D.; Cheng, S.; Bubley, G.J.; Lu, M.L.; Balk, S.P. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J. Biol. Chem. 2002, 277, 26321–26326. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.M.; Pierce, W.; Maher, V.E.; Karuri, S.; Tang, S.H.; Chiu, H.J.; Palmby, T.; Zirkelbach, J.F.; Marathe, D.; Mehrotra, N.; et al. Enzalutamide for treatment of patients with metastatic castration-resistant prostate cancer who have previously received docetaxel: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2013, 19, 6067–6073. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef]
- Ning, Y.M.; Brave, M.; Maher, V.E.; Zhang, L.; Tang, S.; Sridhara, R.; Kim, G.; Ibrahim, A.; Pazdur, R.U.S. Food and Drug Administration Approval Summary: Enzalutamide for the Treatment of Patients With Chemotherapy-Naive Metastatic Castration-Resistant Prostate Cancer. Oncologist 2015, 20, 960–966. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Chong, J.T.; Oh, W.K.; Liaw, B.C. Profile of apalutamide in the treatment of metastatic castration-resistant prostate cancer: Evidence to date. Onco Targets Ther. 2018, 11, 2141–2147. [Google Scholar] [CrossRef]
- Saad, F.; Hotte, S.J. Guidelines for the management of castrate-resistant prostate cancer. Can. Urol. Assoc. J. 2010, 4, 380–384. [Google Scholar] [CrossRef]
- Claessens, F.; Helsen, C.; Prekovic, S.; Van den Broeck, T.; Spans, L.; Van Poppel, H.; Joniau, S. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nat. Rev. Urol. 2014, 11, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Dehm, S.M.; Schmidt, L.J.; Heemers, H.V.; Vessella, R.L.; Tindall, D.J. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008, 68, 5469–5477. [Google Scholar] [CrossRef]
- Arora, V.K.; Schenkein, E.; Murali, R.; Subudhi, S.K.; Wongvipat, J.; Balbas, M.D.; Shah, N.; Cai, L.; Efstathiou, E.; Logothetis, C.; et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013, 155, 1309–1322. [Google Scholar] [CrossRef]
- Joseph, J.D.; Lu, N.; Qian, J.; Sensintaffar, J.; Shao, G.; Brigham, D.; Moon, M.; Maneval, E.C.; Chen, I.; Darimont, B.; et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013, 3, 1020–1029. [Google Scholar] [CrossRef]
- Korpal, M.; Korn, J.M.; Gao, X.; Rakiec, D.P.; Ruddy, D.A.; Doshi, S.; Yuan, J.; Kovats, S.G.; Kim, S.; Cooke, V.G.; et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013, 3, 1030–1043. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; Bianchi-Frias, D.; Dumpit, R.F.; Kaipainen, A.; Corella, A.N.; et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 2017, 32, 474e476–489e476. [Google Scholar] [CrossRef]
- Udhane, V.; Maranto, C.; Hoang, D.T.; Gu, L.; Erickson, A.; Devi, S.; Talati, P.G.; Banerjee, A.; Iczkowski, K.A.; Jacobsohn, K.; et al. Enzalutamide-Induced Feed-Forward Signaling Loop Promotes Therapy-Resistant Prostate Cancer Growth Providing an Exploitable Molecular Target for Jak2 Inhibitors. Mol. Cancer Ther. 2020, 19, 231–246. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen, O.; Hubbard, S.R. Molecular insights into regulation of JAK2 in myeloproliferative neoplasms. Blood 2015, 125, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Rui, H.; Djeu, J.Y.; Evans, G.A.; Kelly, P.A.; Farrar, W.L. Prolactin receptor triggering. Evidence for rapid tyrosine kinase activation. J. Biol. Chem. 1992, 267, 24076–24081. [Google Scholar] [CrossRef]
- Rui, H.; Lebrun, J.J.; Kirken, R.A.; Kelly, P.A.; Farrar, W.L. JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology 1994, 135, 1299–1306. [Google Scholar] [CrossRef]
- Dagvadorj, A.; Collins, S.; Jomain, J.B.; Abdulghani, J.; Karras, J.; Zellweger, T.; Li, H.; Nurmi, M.; Alanen, K.; Mirtti, T.; et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology 2007, 148, 3089–3101. [Google Scholar] [CrossRef]
- Talati, P.G.; Gu, L.; Ellsworth, E.M.; Girondo, M.A.; Trerotola, M.; Hoang, D.T.; Leiby, B.; Dagvadorj, A.; McCue, P.A.; Lallas, C.D.; et al. Jak2-Stat5a/b Signaling Induces Epithelial-to-Mesenchymal Transition and Stem-Like Cell Properties in Prostate Cancer. Am. J. Pathol. 2015, 185, 2505–2522. [Google Scholar] [CrossRef]
- Li, H.; Ahonen, T.J.; Alanen, K.; Xie, J.; LeBaron, M.J.; Pretlow, T.G.; Ealley, E.L.; Zhang, Y.; Nurmi, M.; Singh, B.; et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004, 64, 4774–4782. [Google Scholar] [CrossRef]
- Levy, D.E.; Darnell, J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Quintas-Cardama, A.; Verstovsek, S. Molecular pathways: Jak/STAT pathway: Mutations, inhibitors, and resistance. Clin. Cancer Res. 2013, 19, 1933–1940. [Google Scholar] [CrossRef]
- Ihle, J.N. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 2001, 13, 211–217. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, G.W.; Gouilleux, F.; Groner, B.; Hennighausen, L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl. Acad. Sci. USA 1995, 92, 8831–8835. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, G.W.; Hennighausen, L. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 1996, 10, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Muller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Scheller, J.; Rose-John, S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Investig. 2011, 121, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Pestell, R.G.; Nevalainen, M.T. Prostate Cancer: Signaling Networks, Genetics, and New Treatment Strategies; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar]
- Yamasaki, K.; Taga, T.; Hirata, Y.; Yawata, H.; Kawanishi, Y.; Seed, B.; Taniguchi, T.; Hirano, T.; Kishimoto, T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science 1988, 241, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhong, Z.; Darnell, J.E., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 1995, 82, 241–250. [Google Scholar] [CrossRef]
- Ahonen, T.J.; Xie, J.; LeBaron, M.J.; Zhu, J.; Nurmi, M.; Alanen, K.; Rui, H.; Nevalainen, M.T. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J. Biol. Chem. 2003, 278, 27287–27292. [Google Scholar] [CrossRef]
- Dagvadorj, A.; Kirken, R.A.; Leiby, B.; Karras, J.; Nevalainen, M.T. Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin. Cancer Res. 2008, 14, 1317–1324. [Google Scholar] [CrossRef]
- Gu, L.; Dagvadorj, A.; Lutz, J.; Leiby, B.; Bonuccelli, G.; Lisanti, M.P.; Addya, S.; Fortina, P.; Dasgupta, A.; Hyslop, T.; et al. Transcription factor Stat3 stimulates metastatic behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am. J. Pathol. 2010, 176, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Vogiatzi, P.; Puhr, M.; Dagvadorj, A.; Lutz, J.; Ryder, A.; Addya, S.; Fortina, P.; Cooper, C.; Leiby, B.; et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr. Relat. Cancer 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Gu, L.; Vergalli, J.; Mariani, S.A.; De Dominici, M.; Lokareddy, R.K.; Dagvadorj, A.; Purushottamachar, P.; McCue, P.A.; Trabulsi, E.; et al. Structure-Based Screen Identifies a Potent Small Molecule Inhibitor of Stat5a/b with Therapeutic Potential for Prostate Cancer and Chronic Myeloid Leukemia. Mol. Cancer Ther. 2015, 14, 1777–1793. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liao, Z.; Hoang, D.T.; Dagvadorj, A.; Gupta, S.; Blackmon, S.; Ellsworth, E.; Talati, P.; Leiby, B.; Zinda, M.; et al. Pharmacologic inhibition of Jak2-Stat5 signaling By Jak2 inhibitor AZD1480 potently suppresses growth of both primary and castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5658–5674. [Google Scholar] [CrossRef]
- Kazansky, A.V.; Spencer, D.M.; Greenberg, N.M. Activation of signal transducer and activator of transcription 5 is required for progression of autochthonous prostate cancer: Evidence from the transgenic adenocarcinoma of the mouse prostate system. Cancer Res. 2003, 63, 8757–8762. [Google Scholar]
- Hoang, D.T.; Iczkowski, K.A.; Kilari, D.; See, W.; Nevalainen, M.T. Androgen receptor-dependent and-independent mechanisms driving prostate cancer progression: Opportunities for therapeutic targeting from multiple angles. Oncotarget 2017, 8, 3724–3745. [Google Scholar] [CrossRef]
- Thomas, C.; Zoubeidi, A.; Kuruma, H.; Fazli, L.; Lamoureux, F.; Beraldi, E.; Monia, B.P.; MacLeod, A.R.; Thuroff, J.W.; Gleave, M.E. Transcription factor Stat5 knockdown enhances androgen receptor degradation and delays castration-resistant prostate cancer progression in vivo. Mol. Cancer Ther. 2011, 10, 347–359. [Google Scholar] [CrossRef]
- Maranto, C.; Udhane, V.; Jia, J.; Verma, R.; Muller-Newen, G.; LaViolette, P.S.; Pereckas, M.; Sabharwal, L.; Terhune, S.; Pattabiraman, N.; et al. Prospects for Clinical Development of Stat5 Inhibitor IST5-002: High Transcriptomic Specificity in Prostate Cancer and Low Toxicity In Vivo. Cancers 2020, 12, 3412. [Google Scholar] [CrossRef]
- Haddad, B.R.; Gu, L.; Mirtti, T.; Dagvadorj, A.; Vogiatzi, P.; Hoang, D.T.; Bajaj, R.; Leiby, B.; Ellsworth, E.; Blackmon, S.; et al. STAT5A/B gene locus undergoes amplification during human prostate cancer progression. Am. J. Pathol. 2013, 182, 2264–2275. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Glass, A.; Zellweger, T.; Gehan, E.; Bubendorf, L.; Gelmann, E.P.; Nevalainen, M.T. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin. Cancer Res. 2005, 11, 5863–5868. [Google Scholar] [CrossRef] [PubMed]
- Mirtti, T.; Leiby, B.E.; Abdulghani, J.; Aaltonen, E.; Pavela, M.; Mamtani, A.; Alanen, K.; Egevad, L.; Granfors, T.; Josefsson, A.; et al. Nuclear Stat5a/b predicts early recurrence and prostate cancer-specific death in patients treated by radical prostatectomy. Hum. Pathol. 2013, 44, 310–319. [Google Scholar] [CrossRef]
- Haddad, B.R.; Erickson, A.; Udhane, V.; LaViolette, P.S.; Rone, J.D.; Kallajoki, M.A.; See, W.A.; Rannikko, A.; Mirtti, T.; Nevalainen, M.T. Positive STAT5 Protein and Locus Amplification Status Predicts Recurrence after Radical Prostatectomy to Assist Clinical Precision Management of Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Nakonechnaya, A.O.; Jefferson, H.S.; Chen, X.; Shewchuk, B.M. Differential effects of exogenous and autocrine growth hormone on LNCaP prostate cancer cell proliferation and survival. J. Cell Biochem. 2013, 114, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Nakonechnaya, A.O.; Shewchuk, B.M. Growth hormone enhances LNCaP prostate cancer cell motility. Endocr. Res. 2015, 40, 97–105. [Google Scholar] [CrossRef]
- Zhang, Y.; Gc, S.; Patel, S.B.; Liu, Y.; Paterson, A.J.; Kappes, J.C.; Jiang, J.; Frank, S.J. Growth hormone (GH) receptor (GHR)-specific inhibition of GH-Induced signaling by soluble IGF-1 receptor (sol IGF-1R). Mol. Cell Endocrinol. 2019, 492, 110445. [Google Scholar] [CrossRef]
- Goffin, V. Prolactin receptor targeting in breast and prostate cancers: New insights into an old challenge. Pharmacol. Ther. 2017, 179, 111–126. [Google Scholar] [CrossRef]
- Rouet, V.; Bogorad, R.L.; Kayser, C.; Kessal, K.; Genestie, C.; Bardier, A.; Grattan, D.R.; Kelder, B.; Kopchick, J.J.; Kelly, P.A.; et al. Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 15199–15204. [Google Scholar] [CrossRef]
- Sackmann-Sala, L.; Goffin, V. Prolactin-induced prostate tumorigenesis. Adv. Exp. Med. Biol. 2015, 846, 221–242. [Google Scholar] [CrossRef]
- Gan, Y.; Zhang, Y.; Buckels, A.; Paterson, A.J.; Jiang, J.; Clemens, T.L.; Zhang, Z.Y.; Du, K.; Chang, Y.; Frank, S.J. IGF-1R modulation of acute GH-induced STAT5 signaling: Role of protein tyrosine phosphatase activity. Mol. Endocrinol. 2013, 27, 1969–1979. [Google Scholar] [CrossRef][Green Version]
- Jeong, J.Y.; Hoxhaj, G.; Socha, A.L.; Sytkowski, A.J.; Feldman, L. An erythropoietin autocrine/paracrine axis modulates the growth and survival of human prostate cancer cells. Mol. Cancer Res. 2009, 7, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Amorino, G.P.; Deeble, P.D.; Parsons, S.J. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene 2007, 26, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.; Wang, Y.; Rhim, J.S.; Bhattacharya, N.; Loda, M.; Sytkowski, A.J. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate 2006, 66, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, T.J.; Harkonen, P.L.; Rui, H.; Nevalainen, M.T. PRL signal transduction in the epithelial compartment of rat prostate maintained as long-term organ cultures in vitro. Endocrinology 2002, 143, 228–238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahonen, T.J.; Harkonen, P.L.; Laine, J.; Rui, H.; Martikainen, P.M.; Nevalainen, M.T. Prolactin is a survival factor for androgen-deprived rat dorsal and lateral prostate epithelium in organ culture. Endocrinology 1999, 140, 5412–5421. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, M.T.; Valve, E.M.; Ahonen, T.; Yagi, A.; Paranko, J.; Harkonen, P.L. Androgen-dependent expression of prolactin in rat prostate epithelium in vivo and in organ culture. FASEB J. 1997, 11, 1297–1307. [Google Scholar] [CrossRef]
- Nevalainen, M.T.; Valve, E.M.; Ingleton, P.M.; Harkonen, P.L. Expression and hormone regulation of prolactin receptors in rat dorsal and lateral prostate. Endocrinology 1996, 137, 3078–3088. [Google Scholar] [CrossRef]
- Nevalainen, M.T.; Valve, E.M.; Ingleton, P.M.; Nurmi, M.; Martikainen, P.M.; Harkonen, P.L. Prolactin and prolactin receptors are expressed and functioning in human prostate. J. Clin. Investig. 1997, 99, 618–627. [Google Scholar] [CrossRef]
- Nevalainen, M.T.; Valve, E.M.; Makela, S.I.; Blauer, M.; Tuohimaa, P.J.; Harkonen, P.L. Estrogen and prolactin regulation of rat dorsal and lateral prostate in organ culture. Endocrinology 1991, 129, 612–622. [Google Scholar] [CrossRef]
- Mora, L.B.; Buettner, R.; Seigne, J.; Diaz, J.; Ahmad, N.; Garcia, R.; Bowman, T.; Falcone, R.; Fairclough, R.; Cantor, A.; et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: Direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002, 62, 6659–6666. [Google Scholar]
- Campbell, C.L.; Jiang, Z.; Savarese, D.M.; Savarese, T.M. Increased expression of the interleukin-11 receptor and evidence of STAT3 activation in prostate carcinoma. Am. J. Pathol. 2001, 158, 25–32. [Google Scholar] [CrossRef]
- Huang, H.F.; Murphy, T.F.; Shu, P.; Barton, A.B.; Barton, B.E. Stable expression of constitutively-activated STAT3 in benign prostatic epithelial cells changes their phenotype to that resembling malignant cells. Mol. Cancer 2005, 4, 2. [Google Scholar] [CrossRef][Green Version]
- Dhir, R.; Ni, Z.; Lou, W.; DeMiguel, F.; Grandis, J.R.; Gao, A.C. Stat3 activation in prostatic carcinomas. Prostate 2002, 51, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Horinaga, M.; Okita, H.; Nakashima, J.; Kanao, K.; Sakamoto, M.; Murai, M. Clinical and pathologic significance of activation of signal transducer and activator of transcription 3 in prostate cancer. Urology 2005, 66, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, L.H.; Farrar, W.L. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000, 60, 2132–2135. [Google Scholar] [PubMed]
- Matsuda, T.; Junicho, A.; Yamamoto, T.; Kishi, H.; Korkmaz, K.; Saatcioglu, F.; Fuse, H.; Muraguchi, A. Cross-talk between signal transducer and activator of transcription 3 and androgen receptor signaling in prostate carcinoma cells. Biochem. Biophys. Res. Commun. 2001, 283, 179–187. [Google Scholar] [CrossRef]
- Ueda, T.; Bruchovsky, N.; Sadar, M.D. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J. Biol. Chem. 2002, 277, 7076–7085. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Lin, H.K.; Kan, P.Y.; Xie, S.; Tsai, M.Y.; Wang, P.H.; Chen, Y.T.; Chang, C. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem. Biophys. Res. Commun. 2003, 305, 462–469. [Google Scholar] [CrossRef]
- Barton, B.E.; Karras, J.G.; Murphy, T.F.; Barton, A.; Huang, H.F. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: Direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol. Cancer Ther. 2004, 3, 11–20. [Google Scholar]
- DeMiguel, F.; Lee, S.O.; Lou, W.; Xiao, X.; Pflug, B.R.; Nelson, J.B.; Gao, A.C. Stat3 enhances the growth of LNCaP human prostate cancer cells in intact and castrated male nude mice. Prostate 2002, 52, 123–129. [Google Scholar] [CrossRef]
- Lou, W.; Ni, Z.; Dyer, K.; Tweardy, D.J.; Gao, A.C. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate 2000, 42, 239–242. [Google Scholar] [CrossRef]
- Gu, L.; Talati, P.; Vogiatzi, P.; Romero-Weaver, A.L.; Abdulghani, J.; Liao, Z.; Leiby, B.; Hoang, D.T.; Mirtti, T.; Alanen, K.; et al. Pharmacologic suppression of JAK1/2 by JAK1/2 inhibitor AZD1480 potently inhibits IL-6-induced experimental prostate cancer metastases formation. Mol. Cancer Ther. 2014, 13, 1246–1258. [Google Scholar] [CrossRef]
- Abdulghani, J.; Gu, L.; Dagvadorj, A.; Lutz, J.; Leiby, B.; Bonuccelli, G.; Lisanti, M.P.; Zellweger, T.; Alanen, K.; Mirtti, T.; et al. Stat3 promotes metastatic progression of prostate cancer. Am. J. Pathol. 2008, 172, 1717–1728. [Google Scholar] [CrossRef]
- Culig, Z.P.R.; Nevalainen, M.T. Transcription factors Stat5 and Stat3: Survival Factors for Prostate Cancer Cells. In Prostate Cancer: Signaling Networks, Genetics and New Treatment Strategies; Nevalainen, P., Ed.; Springer: Totowa, NJ, USA, 2008; pp. 257–455. [Google Scholar]
- Hobisch, A.; Rogatsch, H.; Hittmair, A.; Fuchs, D.; Bartsch, G., Jr.; Klocker, H.; Bartsch, G.; Culig, Z. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J. Pathol. 2000, 191, 239–244. [Google Scholar] [CrossRef]
- Royuela, M.; Ricote, M.; Parsons, M.S.; Garcia-Tunon, I.; Paniagua, R.; de Miguel, M.P. Immunohistochemical analysis of the IL-6 family of cytokines and their receptors in benign, hyperplastic, and malignant human prostate. J. Pathol. 2004, 202, 41–49. [Google Scholar] [CrossRef]
- Drachenberg, D.E.; Elgamal, A.A.; Rowbotham, R.; Peterson, M.; Murphy, G.P. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate 1999, 41, 127–133. [Google Scholar] [CrossRef]
- George, D.J.; Halabi, S.; Shepard, T.F.; Sanford, B.; Vogelzang, N.J.; Small, E.J.; Kantoff, P.W. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: Results from cancer and leukemia group B 9480. Clin. Cancer Res. 2005, 11, 1815–1820. [Google Scholar] [CrossRef]
- Michalaki, V.; Syrigos, K.; Charles, P.; Waxman, J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br. J. Cancer 2004, 90, 2312–2316. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, J.; Tachibana, M.; Horiguchi, Y.; Oya, M.; Ohigashi, T.; Asakura, H.; Murai, M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin. Cancer Res. 2000, 6, 2702–2706. [Google Scholar] [PubMed]
- Shariat, S.F.; Andrews, B.; Kattan, M.W.; Kim, J.; Wheeler, T.M.; Slawin, K.M. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 2001, 58, 1008–1015. [Google Scholar] [CrossRef]
- Twillie, D.A.; Eisenberger, M.A.; Carducci, M.A.; Hseih, W.S.; Kim, W.Y.; Simons, J.W. Interleukin-6: A candidate mediator of human prostate cancer morbidity. Urology 1995, 45, 542–549. [Google Scholar] [CrossRef]
- Wise, G.J.; Marella, V.K.; Talluri, G.; Shirazian, D. Cytokine variations in patients with hormone treated prostate cancer. J. Urol. 2000, 164, 722–725. [Google Scholar] [CrossRef]
- Hobisch, A.; Ramoner, R.; Fuchs, D.; Godoy-Tundidor, S.; Bartsch, G.; Klocker, H.; Culig, Z. Prostate cancer cells (LNCaP) generated after long-term interleukin-6 treatment express interleukin-6 and acquire an interleukin-6-partially resistant phenotype. Clin. Cancer Res. 2001, 7, 2941–2948. [Google Scholar] [PubMed]
- Levine, R.L.; Loriaux, M.; Huntly, B.J.; Loh, M.L.; Beran, M.; Stoffregen, E.; Berger, R.; Clark, J.J.; Willis, S.G.; Nguyen, K.T.; et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 2005, 106, 3377–3379. [Google Scholar] [CrossRef]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- James, C.; Ugo, V.; Le Couedic, J.P.; Staerk, J.; Delhommeau, F.; Lacout, C.; Garcon, L.; Raslova, H.; Berger, R.; Bennaceur-Griscelli, A.; et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005, 434, 1144–1148. [Google Scholar] [CrossRef]
- Meyer, S.C.; Levine, R.L. Molecular pathways: Molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res. 2014, 20, 2051–2059. [Google Scholar] [CrossRef]
- Meyer, S.C.; Keller, M.D.; Chiu, S.; Koppikar, P.; Guryanova, O.A.; Rapaport, F.; Xu, K.; Manova, K.; Pankov, D.; O’Reilly, R.J.; et al. CHZ868, a Type II JAK2 Inhibitor, Reverses Type I JAK Inhibitor Persistence and Demonstrates Efficacy in Myeloproliferative Neoplasms. Cancer Cell 2015, 28, 15–28. [Google Scholar] [CrossRef]
- Plimack, E.R.; Lorusso, P.M.; McCoon, P.; Tang, W.; Krebs, A.D.; Curt, G.; Eckhardt, S.G. AZD1480: A phase I study of a novel JAK2 inhibitor in solid tumors. Oncologist 2013, 18, 819–820. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Hoffman, R. Ruxolitinib: The first FDA approved therapy for the treatment of myelofibrosis. Clin. Cancer Res. 2012, 18, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, M.; Huber, E.; Kincaid, Z.; Evelyn, C.R.; Biesiada, J.; Rance, M.; Thapa, M.B.; Shah, N.P.; Meller, J.; Zheng, Y.; et al. Targeting substrate-site in Jak2 kinase prevents emergence of genetic resistance. Sci. Rep. 2015, 5, 14538. [Google Scholar] [CrossRef]
- Singer, J.W.; Al-Fayoumi, S.; Ma, H.; Komrokji, R.S.; Mesa, R.; Verstovsek, S. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J. Exp. Pharmacol. 2016, 8, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.A.; Khong, T.; Burns, C.J.; Spencer, A. The novel JAK inhibitor CYT387 suppresses multiple signalling pathways, prevents proliferation and induces apoptosis in phenotypically diverse myeloma cells. Leukemia 2011, 25, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.; Kawabata, T.; Krishnaswami, S.; Clark, J.; Telliez, J.-B.; Dowty, M.; Menon, S.; Lamba, M.; Zwillich, S. The mechanism of action of tofacitinib—An oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 318–328. [Google Scholar]
- Vainchenker, W.; Leroy, E.; Gilles, L.; Marty, C.; Plo, I.; Constantinescu, S.N. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Res 2018, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Cardama, A.; Vaddi, K.; Liu, P.; Manshouri, T.; Li, J.; Scherle, P.A.; Caulder, E.; Wen, X.; Li, Y.; Waeltz, P.; et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: Therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010, 115, 3109–3117. [Google Scholar] [CrossRef]
- Verstovsek, S.; Kantarjian, H.; Mesa, R.A.; Pardanani, A.D.; Cortes-Franco, J.; Thomas, D.A.; Estrov, Z.; Fridman, J.S.; Bradley, E.C.; Erickson-Viitanen, S.; et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010, 363, 1117–1127. [Google Scholar] [CrossRef]
- Moran, N. Incyte comes of age with JAK inhibitor approval. Nat. Biotechnol. 2012, 30, 3–5. [Google Scholar] [CrossRef]
- Deshpande, A.; Reddy, M.M.; Schade, G.O.; Ray, A.; Chowdary, T.K.; Griffin, J.D.; Sattler, M. Kinase domain mutations confer resistance to novel inhibitors targeting JAK2V617F in myeloproliferative neoplasms. Leukemia 2012, 26, 708–715. [Google Scholar] [CrossRef]
- Shi, J.G.; Chen, X.; Lee, F.; Emm, T.; Scherle, P.A.; Lo, Y.; Punwani, N.; Williams, W.V.; Yeleswaram, S. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J. Clin. Pharmacol. 2014, 54, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Waltzman, R.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.; Knoops, L.; et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012, 366, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.N.; Vannucchi, A.M.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Knoops, L.; Cervantes, F.; Jones, M.M.; Sun, K.; McQuitty, M.; et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia 2017, 31, 775. [Google Scholar] [CrossRef]
- Verstovsek, S.; Vannucchi, A.M.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; Kirito, K.; et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016, 101, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.G.; Chen, X.; Emm, T.; Scherle, P.A.; McGee, R.F.; Lo, Y.; Landman, R.R.; McKeever, E.G., Jr.; Punwani, N.G.; Williams, W.V.; et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J. Clin. Pharmacol. 2012, 52, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Shilling, A.D.; Nedza, F.M.; Emm, T.; Diamond, S.; McKeever, E.; Punwani, N.; Williams, W.; Arvanitis, A.; Galya, L.G.; Li, M.; et al. Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans. Drug Metab. Dispos. 2010, 38, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 2015, 372, 1670–1671. [Google Scholar] [CrossRef] [PubMed]
- Vallath, S.; Sage, E.K.; Kolluri, K.K.; Lourenco, S.N.; Teixeira, V.S.; Chimalapati, S.; George, P.J.; Janes, S.M.; Giangreco, A. CADM1 inhibits squamous cell carcinoma progression by reducing STAT3 activity. Sci. Rep. 2016, 6, 24006. [Google Scholar] [CrossRef]
- Yang, P.W.; Huang, P.M.; Yong, L.S.; Chang, Y.H.; Wu, C.W.; Hua, K.T.; Hsieh, M.S.; Lee, J.M. Circulating Interleukin-6 is Associated with Prognosis and Genetic Polymorphisms of MIR608 in Patients with Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2018, 25, 2449–2456. [Google Scholar] [CrossRef]
- Ojha, R.S.S.; Bhattacharyya, S. JAK-mediated autophagy regulates stemness and cell survival in cisplatin resistant bladder cancer cells. Biochim. Biophys. Acta 2016, 1860, 2484–2497. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.L.; Macdonald, A. JAK2 Inhibition Impairs Proliferation and Sensitises Cervical Cancer Cells to Cisplatin-Induced Cell Death. Cancers 2019, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhu, X.H.; Visakorpi, T.; Alanen, K.; Mirtti, T.; Edmonston, T.B.; Nevalainen, M.T. Activating mutation (V617F) in the tyrosine kinase JAK2 is absent in locally-confined or castration-resistant prostate cancer. Anal. Cell Pathol. 2010, 33, 55–59. [Google Scholar] [CrossRef]
- Talpaz, M.; Kiladjian, J.J. Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia 2020, 35, 1–17. [Google Scholar] [CrossRef]
- Wernig, G.; Kharas, M.G.; Okabe, R.; Moore, S.A.; Leeman, D.S.; Cullen, D.E.; Gozo, M.; McDowell, E.P.; Levine, R.L.; Doukas, J.; et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell 2008, 13, 311–320. [Google Scholar] [CrossRef]
- Mullally, A.; Lane, S.W.; Ball, B.; Megerdichian, C.; Okabe, R.; Al-Shahrour, F.; Paktinat, M.; Haydu, J.E.; Housman, E.; Lord, A.M.; et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010, 17, 584–596. [Google Scholar] [CrossRef]
- Geron, I.; Abrahamsson, A.E.; Barroga, C.F.; Kavalerchik, E.; Gotlib, J.; Hood, J.D.; Durocher, J.; Mak, C.C.; Noronha, G.; Soll, R.M.; et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell 2008, 13, 321–330. [Google Scholar] [CrossRef]
- Lasho, T.L.; Tefferi, A.; Hood, J.D.; Verstovsek, S.; Gilliland, D.G.; Pardanani, A. TG101348, a JAK2-selective antagonist, inhibits primary hematopoietic cells derived from myeloproliferative disorder patients with JAK2V617F, MPLW515K or JAK2 exon 12 mutations as well as mutation negative patients. Leukemia 2008, 22, 1790–1792. [Google Scholar] [CrossRef]
- Pardanani, A.; Gotlib, J.R.; Jamieson, C.; Cortes, J.E.; Talpaz, M.; Stone, R.M.; Silverman, M.H.; Gilliland, D.G.; Shorr, J.; Tefferi, A. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J. Clin. Oncol. 2011, 29, 789–796. [Google Scholar] [CrossRef]
- Pardanani, A.; Harrison, C.; Cortes, J.E.; Cervantes, F.; Mesa, R.A.; Milligan, D.; Masszi, T.; Mishchenko, E.; Jourdan, E.; Vannucchi, A.M.; et al. Safety and Efficacy of Fedratinib in Patients With Primary or Secondary Myelofibrosis: A Randomized Clinical Trial. JAMA Oncol. 2015, 1, 643–651. [Google Scholar] [CrossRef]
- Ogasawara, K.; Xu, C.; Kanamaluru, V.; Palmisano, M.; Krishna, G. Effects of repeated oral doses of ketoconazole on a sequential ascending single oral dose of fedratinib in healthy subjects. Cancer Chemother. Pharmacol. 2020, 85, 899–906. [Google Scholar] [CrossRef]
- Harrison, C.N.; Schaap, N.; Vannucchi, A.M.; Kiladjian, J.J.; Tiu, R.V.; Zachee, P.; Jourdan, E.; Winton, E.; Silver, R.T.; Schouten, H.C.; et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): A single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017, 4, e317–e324. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Jaszczur, S.M.; Afifi, S.; Zhao, J.C.; Zeidan, A.M. Beyond Ruxolitinib: Fedratinib and Other Emergent Treatment Options for Myelofibrosis. Cancer Manag. Res. 2019, 11, 10777–10790. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.; Goh, K.C.; Novotny-Diermayr, V.; Hu, C.Y.; Hentze, H.; Tan, Y.C.; Madan, B.; Amalini, C.; Loh, Y.K.; Ong, L.C.; et al. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia 2011, 25, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.; Goh, K.C.; Novotny-Diermayr, V.; Tan, Y.C.; Madan, B.; Amalini, C.; Ong, L.C.; Kheng, B.; Cheong, A.; Zhou, J.; et al. Pacritinib (SB1518), a JAK2/FLT3 inhibitor for the treatment of acute myeloid leukemia. Blood Cancer J. 2011, 1, e44. [Google Scholar] [CrossRef]
- William, A.D.; Lee, A.C.; Blanchard, S.; Poulsen, A.; Teo, E.L.; Nagaraj, H.; Tan, E.; Chen, D.; Williams, M.; Sun, E.T.; et al. Discovery of the macrocycle 11-(2-pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[1 9.3.1.1(2,6). 1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent Janus kinase 2/fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma. J. Med. Chem. 2011, 54, 4638–4658. [Google Scholar] [CrossRef] [PubMed]
- Komrokji, R.S.; Seymour, J.F.; Roberts, A.W.; Wadleigh, M.; To, L.B.; Scherber, R.; Turba, E.; Dorr, A.; Zhu, J.; Wang, L.; et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood 2015, 125, 2649–2655. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Zhao, Q.; Buelow, D.R.; Phelps, M.; Walker, A.R.; Mims, A.S.; Vasu, S.; Behbehani, G.; Blachly, J.; Blum, W.; et al. Preclinical activity and a pilot phase I study of pacritinib, an oral JAK2/FLT3 inhibitor, and chemotherapy in FLT3-ITD-positive AML. Investig. New Drugs 2020, 38, 340–349. [Google Scholar] [CrossRef]
- Verstovsek, S.; Komrokji, R.S. A comprehensive review of pacritinib in myelofibrosis. Future Oncol. 2015, 11, 2819–2830. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Hoffman, R.; Talpaz, M.; Gerds, A.T.; Stein, B.; Gupta, V.; Szoke, A.; Drummond, M.; Pristupa, A.; Granston, T.; et al. Pacritinib vs Best Available Therapy, Including Ruxolitinib, in Patients with Myelofibrosis: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 652–659. [Google Scholar] [CrossRef]
- Jayaraman, R.; Pasha, M.K.; Williams, A.; Goh, K.C.; Ethirajulu, K. Metabolism and Disposition of Pacritinib (SB1518), an Orally Active Janus Kinase 2 Inhibitor in Preclinical Species and Humans. Drug Metab. Lett. 2015, 9, 28–47. [Google Scholar] [CrossRef]
- Jensen, K.V.; Cseh, O.; Aman, A.; Weiss, S.; Luchman, H.A. The JAK2/STAT3 inhibitor pacritinib effectively inhibits patient-derived GBM brain tumor initiating cells in vitro and when used in combination with temozolomide increases survival in an orthotopic xenograft model. PLoS ONE 2017, 12, e0189670. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Scherle, P.A.; Collins, R.; Burn, T.C.; Li, Y.; Li, J.; Covington, M.B.; Thomas, B.; Collier, P.; Favata, M.F.; et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: Preclinical characterization of INCB028050. J. Immunol. 2010, 184, 5298–5307. [Google Scholar] [CrossRef] [PubMed]
- Van Rompaey, L.G.R.; Galien, R.; van der Aar, E.M.; Clement-Lacroix, P.; Nelles, L.; Smets, B.; Lepescheux, L.; Christophe, T.; Conrath, K.; Vandeghinste, N.; et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J. Immunol. 2013, 191, 3568–3577. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Kremer, J.; Zamani, O.; Ludivico, C.; Krogulec, M.; Xie, L.; Beattie, S.D.; Koch, A.E.; Cardillo, T.E.; Rooney, T.P.; et al. Baricitinib in Patients with Refractory Rheumatoid Arthritis. N. Engl. J. Med. 2016, 374, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Harigai, M.; Genovese, M.C.; Lindsey, S.; Takeuchi, T.; Fleischmann, R.; Bradley, J.D.; Byers, N.L.; Hyslop, D.L.; Issa, M.; et al. Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 1290–1297. [Google Scholar] [CrossRef]
- Mogul, A.; Corsi, K.; McAuliffe, L. Baricitinib: The Second FDA-Approved JAK Inhibitor for the Treatment of Rheumatoid Arthritis. Ann. Pharmacother. 2019, 53, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 320. [Google Scholar] [CrossRef]
- Kay, J.; Harigai, M.; Rancourt, J.; Dickson, C.; Melby, T.; Issa, M.; de la Torre, I.; Isaka, Y.; Cardoso, A.; Saifan, C.; et al. Changes in selected haematological parameters associated with JAK1/JAK2 inhibition observed in patients with rheumatoid arthritis treated with baricitinib. RMD Open 2020, 6, e001370. [Google Scholar] [CrossRef]
- Scott, I.C.; Hider, S.L.; Scott, D.L. Thromboembolism with Janus Kinase (JAK) Inhibitors for Rheumatoid Arthritis: How Real is the Risk? Drug Saf. 2018, 41, 645–653. [Google Scholar] [CrossRef]
- Pardanani, A.; Lasho, T.; Smith, G.; Burns, C.J.; Fantino, E.; Tefferi, A. CYT387, a selective JAK1/JAK2 inhibitor: In vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 2009, 23, 1441–1445. [Google Scholar] [CrossRef]
- Pardanani, A.; Laborde, R.R.; Lasho, T.L.; Finke, C.; Begna, K.; Al-Kali, A.; Hogan, W.J.; Litzow, M.R.; Leontovich, A.; Kowalski, M.; et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 2013, 27, 1322–1327. [Google Scholar] [CrossRef]
- Gupta, V.; Mesa, R.A.; Deininger, M.W.; Rivera, C.E.; Sirhan, S.; Brachmann, C.B.; Collins, H.; Kawashima, J.; Xin, Y.; Verstovsek, S. A phase 1/2, open-label study evaluating twice-daily administration of momelotinib in myelofibrosis. Haematologica 2017, 102, 94–102. [Google Scholar] [CrossRef]
- Verstovsek, S.; Courby, S.; Griesshammer, M.; Mesa, R.A.; Brachmann, C.B.; Kawashima, J.; Maltzman, J.D.; Shao, L.; Xin, Y.; Huang, D.; et al. A phase 2 study of momelotinib, a potent JAK1 and JAK2 inhibitor, in patients with polycythemia vera or essential thrombocythemia. Leuk. Res. 2017, 60, 11–17. [Google Scholar] [CrossRef]
- Mesa, R.A.; Kiladjian, J.J.; Catalano, J.V.; Devos, T.; Egyed, M.; Hellmann, A.; McLornan, D.; Shimoda, K.; Winton, E.F.; Deng, W.; et al. SIMPLIFY-1: A Phase III Randomized Trial of Momelotinib Versus Ruxolitinib in Janus Kinase Inhibitor-Naive Patients With Myelofibrosis. J. Clin. Oncol. 2017, 35, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Chen, C.C.; Egyed, M.; Ellis, M.; Fox, L.; Goh, Y.T.; Gupta, V.; Harrison, C.; Kiladjian, J.J.; Lazaroiu, M.C.; et al. MOMENTUM: Momelotinib vs danazol in patients with myelofibrosis previously treated with JAKi who are symptomatic and anemic. Future Oncol. 2021, 17, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.N.; Vannucchi, A.M.; Platzbecker, U.; Cervantes, F.; Gupta, V.; Lavie, D.; Passamonti, F.; Winton, E.F.; Dong, H.; Kawashima, J.; et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): A randomised, open-label, phase 3 trial. Lancet Haematol. 2018, 5, e73–e81. [Google Scholar] [CrossRef]
- Barbie, D.A.; Spira, A.; Kelly, K.; Humeniuk, R.; Kawashima, J.; Kong, S.; Koczywas, M. Phase 1B Study of Momelotinib Combined With Trametinib in Metastatic, Kirsten Rat Sarcoma Viral Oncogene Homolog-Mutated Non-Small-Cell Lung Cancer After Platinum-Based Chemotherapy Treatment Failure. Clin. Lung Cancer 2018, 19, e853–e859. [Google Scholar] [CrossRef]
- Padda, S.K.; Reckamp, K.L. Combination of Immunotherapy and Antiangiogenic Therapy in Cancer-a Rational Approach. J. Thorac. Oncol. 2021, 16, 178–182. [Google Scholar] [CrossRef]
- Ma, L.; Clayton, J.R.; Walgren, R.A.; Zhao, B.; Evans, R.J.; Smith, M.C.; Heinz-Taheny, K.M.; Kreklau, E.L.; Bloem, L.; Pitou, C.; et al. Discovery and characterization of LY2784544, a small-molecule tyrosine kinase inhibitor of JAK2V617F. Blood Cancer J. 2013, 3, e109. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Salama, M.E.; Li, L.; Pitou, C.; Nunes, F.P.; Price, G.L.; Giles, J.L.; D’Souza, D.N.; Walgren, R.A.; et al. A phase 1 study of the Janus kinase 2 (JAK2)(V617F) inhibitor, gandotinib (LY2784544), in patients with primary myelofibrosis, polycythemia vera, and essential thrombocythemia. Leuk. Res. 2017, 61, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.; Palandri, F.; Baer, M.R.; Quick, D.; Kiladjian, J.J.; Martinelli, G.; Verma, A.; Hamid, O.; Walgren, R.; Pitou, C.; et al. Phase 2 study of gandotinib (LY2784544) in patients with myeloproliferative neoplasms. Leuk. Res. 2018, 71, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yamazaki, S.; Yamagami, K.; Kuno, M.; Morita, Y.; Okuma, K.; Nakamura, K.; Chida, N.; Inami, M.; Inoue, T.; et al. A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J. Pharmacol. Sci. 2017, 133, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tanaka, Y.; Tanaka, S.; Kawakami, A.; Song, Y.W.; Chen, Y.H.; Rokuda, M.; Izutsu, H.; Ushijima, S.; Kaneko, Y.; et al. Safety and effectiveness of peficitinib (ASP015K) in patients with rheumatoid arthritis: Interim data (22.7 months mean peficitinib treatment) from a long-term, open-label extension study in Japan, Korea, and Taiwan. Arthritis Res. Ther. 2020, 22, 47. [Google Scholar] [CrossRef]
- Astellas. Oral JAK Inhibitor Smyraf® Tablets Approved in Japan for the Treatment of Rheumatoid Arthritis (Including Prevention of Structural Joint Damage) in Patients Who Have an Inadequate Response to Conventional Therapies. 2019. Available online: https://www.astellas.com/system/files/news/2019-03/190326_eg_pefi_approval_final_0.pdf (accessed on 8 September 2021).
- Levis, M.; Ravandi, F.; Wang, E.S.; Baer, M.R.; Perl, A.; Coutre, S.; Erba, H.; Stuart, R.K.; Baccarani, M.; Cripe, L.D.; et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood 2011, 117, 3294–3301. [Google Scholar] [CrossRef]
- Hexner, E.O.; Mascarenhas, J.; Prchal, J.; Roboz, G.J.; Baer, M.R.; Ritchie, E.K.; Leibowitz, D.; Demakos, E.P.; Miller, C.; Siuty, J.; et al. Phase I dose escalation study of lestaurtinib in patients with myelofibrosis. Leuk. Lymphoma 2015, 56, 2543–2551. [Google Scholar] [CrossRef][Green Version]
- Collins, C.; Carducci, M.A.; Eisenberger, M.A.; Isaacs, J.T.; Partin, A.W.; Pili, R.; Sinibaldi, V.J.; Walczak, J.S.; Denmeade, S.R. Preclinical and clinical studies with the multi-kinase inhibitor CEP-701 as treatment for prostate cancer demonstrate the inadequacy of PSA response as a primary endpoint. Cancer Biol. Ther. 2007, 6, 1360–1367. [Google Scholar] [CrossRef][Green Version]
- Furumoto, Y.; Gadina, M. The arrival of JAK inhibitors: Advancing the treatment of immune and hematologic disorders. BioDrugs 2013, 27, 431–438. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Jesson, M.I.; Li, X.; Lee, J.L.; Ghosh, S.; Alsup, J.W.; Warner, J.D.; Tanaka, M.; Steward-Tharp, S.M.; Gadina, M.; et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J. Immunol. 2011, 186, 4234–4243. [Google Scholar] [CrossRef] [PubMed]
- Seol, M.A.; Kim, J.H.; Oh, K.; Kim, G.; Seo, M.W.; Shin, Y.K.; Sim, J.H.; Shin, H.M.; Seo, B.Y.; Lee, D.S.; et al. Interleukin-7 Contributes to the Invasiveness of Prostate Cancer Cells by Promoting Epithelial-Mesenchymal Transition. Sci. Rep. 2019, 9, 6917. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W.; Study, A.I. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 2012, 367, 616–624. [Google Scholar] [CrossRef]
- Verstovsek, S.; Manshouri, T.; Quintas-Cardama, A.; Harris, D.; Cortes, J.; Giles, F.J.; Kantarjian, H.; Priebe, W.; Estrov, Z. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin. Cancer Res. 2008, 14, 788–796. [Google Scholar] [CrossRef]
- Tsujita, Y.; Horiguchi, A.; Tasaki, S.; Isono, M.; Asano, T.; Ito, K.; Asano, T.; Mayumi, Y.; Kushibiki, T. STAT3 inhibition by WP1066 suppresses the growth and invasiveness of bladder cancer cells. Oncol. Rep. 2017, 38, 2197–2204. [Google Scholar] [CrossRef]
- Horiguchi, A.; Asano, T.; Kuroda, K.; Sato, A.; Asakuma, J.; Ito, K.; Hayakawa, M.; Sumitomo, M.; Asano, T. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br. J. Cancer 2010, 102, 1592–1599. [Google Scholar] [CrossRef]
- Howard, S.; Berdini, V.; Boulstridge, J.A.; Carr, M.G.; Cross, D.M.; Curry, J.; Devine, L.A.; Early, T.R.; Fazal, L.; Gill, A.L.; et al. Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity. J. Med. Chem. 2009, 52, 379–388. [Google Scholar] [CrossRef]
- Qi, W.; Liu, X.; Cooke, L.S.; Persky, D.O.; Miller, T.P.; Squires, M.; Mahadevan, D. AT9283, a novel aurora kinase inhibitor, suppresses tumor growth in aggressive B-cell lymphomas. Int. J. Cancer 2012, 130, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- WP1066. (n.d.). Moleculin Biotech. 2020. Available online: https://www.moleculin.com/technology/wp1066/ (accessed on 23 June 2021).
- Atallah, E.; Verstovsek, S. Prospect of JAK2 inhibitor therapy in myeloproliferative neoplasms. Expert Rev. Anticancer Ther. 2009, 9, 663–670. [Google Scholar] [CrossRef]
- Lakings, D.B. Atiprimod (AnorMED). IDrugs 2000, 3, 329–335. [Google Scholar] [PubMed]
- Quintas-Cardama, A.; Manshouri, T.; Estrov, Z.; Harris, D.; Zhang, Y.; Gaikwad, A.; Kantarjian, H.M.; Verstovsek, S. Preclinical characterization of atiprimod, a novel JAK2 AND JAK3 inhibitor. Investig. New Drugs 2011, 29, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Manshouri, T.; Golemovic, M.; Kantarjian, H.M.M.; Cortes, J.E.; Zhang, Y.; Priebe, W.; Estrov, Z.; Vrstovsek, S. Atiprimod inhibits the JAK-STAT pathway and induces apootosis in human cells carrying the JAK2(V617F) or JAK3 mutation. Blood 2007, 110, 1040a–1041a. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.A.; Han, X.H.; Yang, J.; Qian, J.F.; Hong, S.Y.; Samaniego, F.; Rornaguera, J.; Yi, Q. Atiprimod inhibits the growth of mantle cell lymphoma in vitro and in vivo and induces apoptosis via activating the mitochondrial pathways. Blood 2007, 109, 5455–5462. [Google Scholar] [CrossRef]
- Amit-Vazina, M.; Shishodia, S.; Harris, D.; Van, Q.; Wang, M.; Weber, D.; Alexanian, R.; Talpaz, M.; Aggarwal, B.B.; Estrov, Z. Atiprimod blocks STAT3 phosphorylation and induces apoptosis in multiple myeloma cells. Br. J. Cancer 2005, 93, 70–80. [Google Scholar] [CrossRef]
- Chan, D.; Koren-Michowitz, M. Update on JAK2 Inhibitors in Myeloproliferative Neoplasm. Ther. Adv. Hematol. 2011, 2, 61–71. [Google Scholar] [CrossRef]
- Nakaya, Y.; Naito, H.; Homan, J.; Sugahara, S.; Horio, T.; Niwa, T.; Shide, K.; Shimoda, K. Preferential Inhibition of An Activated Form of Janus Kinase 2 (JAK2) by a Novel JAK2 Inhibitor, NS-018. Blood 2010, 116, 1672. [Google Scholar] [CrossRef]
- Shide, K.; Nakaya, Y.; Kameda, T.; Shimoda, H.; Hidaka, T.; Kubuki, Y.; Katayose, K.; Matsunaga, T.; Homan, J.; Kotera, T.; et al. NS-018, a Potent Novel JAK2 Inhibitor, Effectively Treats Murine MPN Induced by the Janus Kinase 2 (JAK2) V617F Mutant. Blood 2010, 116, 1671–1672. [Google Scholar] [CrossRef]
- Holladay, M.W.; Setti, E. Optically Active Pyrazolylaminoquinazoline, and Pharmaceutical Compositions and Methods of Use Thereof. US patent No. US8703943, 22 April 2014. [Google Scholar]
- Lipka, D.B.; Hoffmann, L.S.; Heidel, F.; Markova, B.; Blum, M.C.; Breitenbuecher, F.; Kasper, S.; Kindler, T.; Levine, R.L.; Huber, C.; et al. LS104, a non-ATP-competitive small-molecule inhibitor of JAK2, is potently inducing apoptosis in JAK2V617F-positive cells. Mol. Cancer Ther. 2008, 7, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lv, B.; Yin, H.; Zhu, X.; Wei, H.; Ding, Y. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Ascending Dose and Food Effect Study to Evaluate the Tolerance, Pharmacokinetics of Jaktinib, a New Selective Janus Kinase Inhibitor in Healthy Chinese Volunteers, CLINICAL TRIAL. Front. Pharmacol. 2020, 11, 604314. [Google Scholar] [CrossRef]
- Santo, L.; Hideshima, T.; Cirstea, D.; Bandi, M.; Nelson, E.A.; Gorgun, G.; Rodig, S.; Vallet, S.; Pozzi, S.; Patel, K.; et al. Antimyeloma activity of a multitargeted kinase inhibitor, AT9283, via potent Aurora kinase and STAT3 inhibition either alone or in combination with lenalidomide. Clin. Cancer Res. 2011, 17, 3259–3271. [Google Scholar] [CrossRef] [PubMed]
- Foran, J.; Ravandi, F.; Wierda, W.; Garcia-Manero, G.; Verstovsek, S.; Kadia, T.; Burger, J.; Yule, M.; Langford, G.; Lyons, J.; et al. A phase I and pharmacodynamic study of AT9283, a small-molecule inhibitor of aurora kinases in patients with relapsed/refractory leukemia or myelofibrosis. Clin. Lymphoma Myeloma Leuk. 2014, 14, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Lu, P.; Coffey, G.; Conley, P.; Pandey, A.; Wang, L.Y. Dual SYK/JAK inhibition overcomes ibrutinib resistance in chronic lymphocytic leukemia: Cerdulatinib, but not ibrutinib, induces apoptosis of tumor cells protected by the microenvironment. Oncotarget 2017, 8, 12953–12967. [Google Scholar] [CrossRef] [PubMed]
- Traves, P.G.; Murray, B.; Campigotto, F.; Galien, R.; Meng, A.; Di Paolo, J.A. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann. Rheum. Dis. 2021, 80, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Shien, K.; Papadimitrakopoulou, V.A.; Ruder, D.; Behrens, C.; Shen, L.; Kalhor, N.; Song, J.; Lee, J.J.; Wang, J.; Tang, X.; et al. JAK1/STAT3 Activation through a Proinflammatory Cytokine Pathway Leads to Resistance to Molecularly Targeted Therapy in Non–Small Cell Lung Cancer. Mol. Cancer Ther. 2017, 16, 2234–2245. [Google Scholar] [CrossRef]
- Dhillon, S.; Keam, S.J. Filgotinib: First Approval. Drugs 2020, 80, 1987–1997. [Google Scholar] [CrossRef]
- European Medicine Agency. Filgotinib (Jyseleca®): Summary of product characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jyseleca (accessed on 30 September 2020).
- Eisai. Jyseleca® (Filgotinib) Approved in Japan for Rheumatoid Arthritis [Press Release]. Available online: https://www.eisai.com/news/2020/news202075.html (accessed on 25 September 2020).
- Gilead Sciences Inc. Gilead Receives Complete Response Letter for Filgotinib for the Treatment of Moderately to Severely Active Rheumatoid Arthritis [Press Release]. Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2020/8/gilead-receives-complete-response-letter-for-filgotinib-for-the-treatment-of-moderately-to-severely-active-rheumatoid-arthritis (accessed on 18 August 2020).
- Genovese, M.C.; Kalunian, K.; Gottenberg, J.E.; Mozaffarian, N.; Bartok, B.; Matzkies, F.; Gao, J.; Guo, Y.; Tasset, C.; Sundy, J.S.; et al. Effect of Filgotinib vs Placebo on Clinical Response in Patients With Moderate to Severe Rheumatoid Arthritis Refractory to Disease-Modifying Antirheumatic Drug Therapy: The FINCH 2 Randomized Clinical Trial. JAMA 2019, 322, 315–325. [Google Scholar] [CrossRef]
- Mahajan, S.; Hogan, J.K.; Shlyakhter, D.; Oh, L.; Salituro, F.G.; Farmer, L.; Hoock, T.C. VX-509 (decernotinib) is a potent and selective janus kinase 3 inhibitor that attenuates inflammation in animal models of autoimmune disease. J. Pharmacol. Exp. Ther. 2015, 353, 405–414. [Google Scholar] [CrossRef]
- Genovese, M.C.; van Vollenhoven, R.F.; Pacheco-Tena, C.; Zhang, Y.; Kinnman, N. VX-509 (Decernotinib), an Oral Selective JAK-3 Inhibitor, in Combination With Methotrexate in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 46–55. [Google Scholar] [CrossRef]
- Li, Z.; Xu, M.; Xing, S.; Ho, W.T.; Ishii, T.; Li, Q.; Fu, X.; Zhao, Z.J. Erlotinib effectively inhibits JAK2V617F activity and polycythemia vera cell growth. J. Biol. Chem. 2007, 282, 3428–3432. [Google Scholar] [CrossRef]
- Nabhan, C.; Lestingi, M.T.; Galvez, A.; Tolzien, K.; Kelby, K.S.; Tsarwhas, D.; Newman, S.; Bitran, D.J. Erlotinib Has Moderate Single-agent Activity in Chemotherapy-naïve Castration-resistant Prostate Cancer: Final Results of a Phase II Trial. Urology 2009, 74, 665–671. [Google Scholar] [CrossRef]
- Chmielinska, J.J.; Kramer, J.H.; Mak, I.T.; Spurney, C.F.; Weglicki, W.B. Substance P receptor blocker, aprepitant, inhibited cutaneous and other neurogenic inflammation side effects of the EGFR1-TKI, erlotinib. Mol. Cell Biochem. 2020, 465, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, A.; Dellacasa, C.M.; Finazzi, G.; Carobbio, A.; Ferrari, M.L.; Guglielmelli, P.; Gattoni, E.; Salmoiraghi, S.; Finazzi, M.C.; Di Tollo, S.; et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br. J. Haematol. 2010, 150, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Savino, A.M.; Sarno, J.; Trentin, L.; Vieri, M.; Fazio, G.; Bardini, M.; Bugarin, C.; Fossati, G.; Davis, K.L.; Gaipa, G.; et al. The histone deacetylase inhibitor givinostat (ITF2357) exhibits potent anti-tumor activity against CRLF2-rearranged BCP-ALL. Leukemia 2017, 31, 2365–2375. [Google Scholar] [CrossRef]
- Zhai, D.; Deng, W.; Huang, Z.; Rogers, E.; Cui, J.J. Abstract 2132: The novel, rationally-designed, ALK/SRC inhibitor TPX-0005 overcomes multiple acquired resistance mechanisms to current ALK inhibitors. Cancer Res. 2016, 76, 2016–2132. [Google Scholar] [CrossRef]
- Karachaliou, N.; Chaib, I.; Cardona, A.F.; Berenguer, J.; Bracht, J.W.P.; Yang, J.; Cai, X.; Wang, Z.; Hu, C.; Drozdowskyj, A.; et al. Common Co-activation of AXL and CDCP1 in EGFR-mutation-positive Non-smallcell Lung Cancer Associated With Poor Prognosis. EBioMedicine 2018, 29, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Ou, S.H.I.; Cho, B.C.; Kim, D.W.; Lees, J.; Lin, J.J.; Zhu, V.W.; Ahns, M.J.; Camidge, D.R.; Nguyen, J.; et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibit ROS1/TRK/ALK Solvent-Front Mutations. Cancer Discov. 2018, 8, 1227–1236. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, Y.; Long Priel, D.A.; Fink, D.; Peer, C.J.; Sissung, T.M.; Su, Y.T.; Pang, Y.; Yu, G.; Butler, M.K.; et al. Phase I Study of Zotiraciclib in Combination with Temozolomide for Patients with Recurrent High-grade Astrocytomas. Clin. Cancer Res. 2021, 27, 3298–3306. [Google Scholar] [CrossRef] [PubMed]
- William, A.D.; Lee, A.C.; Goh, K.C.; Blanchard, S.; Poulsen, A.; Teo, E.L.; Nagaraj, H.; Lee, C.P.; Wang, H.; Williams, M.; et al. Discovery of kinase spectrum selective macrocycle (16E)-14-methyl-20-oxa-5,7,14,26-tetraazatetracyclo[19.3.1.1(2,6).1(8,12)]heptaco sa-1(25),2(26),3,5,8(27),9,11,16,21,23-decaene (SB1317/TG02), a potent inhibitor of cyclin dependent kinases (CDKs), Janus kinase 2 (JAK2), and fms-like tyrosine kinase-3 (FLT3) for the treatment of cancer. J. Med. Chem. 2012, 55, 169–196. [Google Scholar] [CrossRef]
- Baffert, F.; Regnier, C.H.; De Pover, A.; Pissot-Soldermann, C.; Tavares, G.A.; Blasco, F.; Brueggen, J.; Chene, P.; Drueckes, P.; Erdmann, D.; et al. Potent and selective inhibition of polycythemia by the quinoxaline JAK2 inhibitor NVP-BSK805. Mol. Cancer Ther. 2010, 9, 1945–1955. [Google Scholar] [CrossRef]
- Stump, K.L.; Lu, L.D.; Dobrzanski, P.; Serdikoff, C.; Gingrich, D.E.; Dugan, B.J.; Angeles, T.S.; Albom, M.S.; Ator, M.A.; Dorsey, B.D.; et al. A highly selective, orally active inhibitor of Janus kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R68. [Google Scholar] [CrossRef] [PubMed]
- Seavey, M.M.; Lu, L.D.; Stump, K.L.; Wallace, N.H.; Hockeimer, W.; O’Kane, T.M.; Ruggeri, B.A.; Dobrzanski, P. Therapeutic efficacy of CEP-33779, a novel selective JAK2 inhibitor, in a mouse model of colitis-induced colorectal cancer. Mol. Cancer Ther. 2012, 11, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Hood, J.; Lasho, T.; Levine, R.L.; Martin, M.B.; Noronha, G.; Finke, C.; Mak, C.C.; Mesa, R.; Zhu, H.; et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia 2007, 21, 1658–1668. [Google Scholar] [CrossRef]
- Pardanani, A.; Hood, J.; Lasho, T.; Noronha, G.; Finke, C.; Mak, C.C.; Mesa, R.; Zhu, H.; Soll, R.; Tefferi, A. TG101209, a selective JAK2 kinase inhibitor, suppresses endogenous and cytokine-supported colony formation from hematopoietic progenitors carrying JAK2V617F or MPLW515K/L mutations. Blood 2006, 108, 758a. [Google Scholar] [CrossRef]
- Sun, Y.; Moretti, L.; Giacalone, N.J.; Schleicher, S.; Speirs, C.K.; Carbone, D.P.; Lu, B. Inhibition of JAK2 signaling by TG101209 enhances radiotherapy in lung cancer models. J. Thorac. Oncol. 2011, 6, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yi, Y.; Xie, S.; Yu, H.; Peng, H.; Zhang, G. The effect of the JAK2 inhibitor TG101209 against T cell acute lymphoblastic leukemia (T-ALL) is mediated by inhibition of JAK-STAT signaling and activation of the crosstalk between apoptosis and autophagy signaling. Oncotarget 2017, 8, 106753–106763. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T.; Kojima, S.; Furihata, M.; Yang, J.; Nishioka, C.; Takeuchi, A.; Isaka, M.; Koeffler, H.P.; Yokoyama, A. Expression of p-JAK2 predicts clinical outcome and is a potential molecular target of acute myelogenous leukemia. Int. J. Cancer 2011, 129, 2512–2521. [Google Scholar] [CrossRef]
- Gozgit, J.M.; Bebernitz, G.; Patil, P.; Ye, M.; Parmentier, J.; Wu, J.; Su, N.; Wang, T.; Ioannidis, S.; Davies, A.; et al. Effects of the JAK2 inhibitor, AZ960, on Pim/BAD/BCL-xL survival signaling in the human JAK2 V617F cell line SET-2. J. Biol. Chem. 2008, 283, 32334–32343. [Google Scholar] [CrossRef]
- Florian, R.; Greten, M.K. Peering into the aftermath: JAKi rips STAT3 in cancer. Nat. Med. 2010, 16, 1085–1087. [Google Scholar]
- Shiels, M.S.; Pfeiffer, R.M.; Gail, M.H.; Hall, H.I.; Li, J.; Chaturvedi, A.K.; Bhatia, K.; Uldrick, T.S.; Yarchoan, R.; Goedert, J.J.; et al. Cancer burden in the HIV-infected population in the United States. J. Natl. Cancer Inst. 2011, 103, 753–762. [Google Scholar] [CrossRef]
- Tvorogov, D.; Thomas, D.; Liau, N.P.D.; Dottore, M.; Barry, E.F.; Lathi, M.; Kan, W.L.; Hercus, T.R.; Stomski, F.; Hughes, T.P.; et al. Accumulation of JAK activation loop phosphorylation is linked to type I JAK inhibitor withdrawal syndrome in myelofibrosis. Sci. Adv. 2018, 4, eaat3834. [Google Scholar] [CrossRef]
- Jatiani, S.S.; Cosenza, S.C.; Reddy, M.V.; Ha, J.H.; Baker, S.J.; Samanta, A.K.; Olnes, M.J.; Pfannes, L.; Sloand, E.M.; Arlinghaus, R.B.; et al. A Non-ATP-Competitive Dual Inhibitor of JAK2 and BCR-ABL Kinases: Elucidation of a Novel Therapeutic Spectrum Based on Substrate Competitive Inhibition. Genes Cancer 2010, 1, 331–345. [Google Scholar] [CrossRef]
- Samanta, A.K.; Chakraborty, S.N.; Wang, Y.; Schlette, E.; Reddy, E.P.; Arlinghaus, R.B. Destabilization of Bcr-Abl/Jak2 Network by a Jak2/Abl Kinase Inhibitor ON044580 Overcomes Drug Resistance in Blast Crisis Chronic Myelogenous Leukemia (CML). Genes Cancer 2010, 1, 346–359. [Google Scholar] [CrossRef]
- Hu, M.; Xu, C.; Yang, C.; Zuo, H.; Chen, C.; Zhang, D.; Shi, G.; Wang, W.; Shi, J.; Zhang, T. Discovery and evaluation of ZT55, a novel highly-selective tyrosine kinase inhibitor of JAK2V617F against myeloproliferative neoplasms. J. Exp. Clin. Cancer Res. 2019, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hedvat, M.; Huszar, D.; Herrmann, A.; Gozgit, J.M.; Schroeder, A.; Sheehy, A.; Buettner, R.; Proia, D.; Kowolik, C.M.; Xin, H.; et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 2009, 16, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Scuto, A.; Krejci, P.; Popplewell, L.; Wu, J.; Wang, Y.; Kujawski, M.; Kowolik, C.; Xin, H.; Chen, L.; Wang, Y.; et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia 2011, 25, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Derenzini, E.; Lemoine, M.; Buglio, D.; Katayama, H.; Ji, Y.; Davis, R.E.; Sen, S.; Younes, A. The JAK inhibitor AZD1480 regulates proliferation and immunity in Hodgkin lymphoma. Blood Cancer J. 2011, 1, e46. [Google Scholar] [CrossRef]
- Forsyth, T.; Kearney, P.C.; Kim, B.G.; Johnson, H.W.; Aay, N.; Arcalas, A.; Brown, D.S.; Chan, V.; Chen, J.; Du, H.; et al. SAR and in vivo evaluation of 4-aryl-2-aminoalkylpyrimidines as potent and selective Janus kinase 2 (JAK2) inhibitors. Bioorg Med. Chem. Lett. 2012, 22, 7653–7658. [Google Scholar] [CrossRef]
- Verstovsek, S.; Tam, C.S.; Wadleigh, M.; Sokol, L.; Smith, C.C.; Bui, L.A.; Song, C.; Clary, D.O.; Olszynski, P.; Cortes, J.; et al. Phase I evaluation of XL019, an oral, potent, and selective JAK2 inhibitor. Leuk. Res. 2014, 38, 316–322. [Google Scholar] [CrossRef][Green Version]
- Pardanani, A.; Roberts, A.W.; Seymour, J.F.; Burbury, K.; Verstovsek, S.; Kantarjian, H.M.; Begna, K.; Yoshitsugu, H.; Gestone, T.A.; Phillips, P.; et al. BMS-911543, A Selective JAK2 Inhibitor: A Multicenter Phase 1/2a Study In Myelofibrosis. Blood 2013, 122, 664. [Google Scholar] [CrossRef]
- Wan, H.; Schroeder, G.M.; Hart, A.C.; Inghrim, J.; Grebinski, J.; Tokarski, J.S.; Lorenzi, M.V.; You, D.; McDevitt, T.; Penhallow, B.; et al. Discovery of a Highly Selective JAK2 Inhibitor, BMS-911543, for the Treatment of Myeloproliferative Neoplasms. ACS Med. Chem. Lett. 2015, 6, 850–855. [Google Scholar] [CrossRef]
- Purandare, A.V.; McDevitt, T.M.; Wan, H.; You, D.; Penhallow, B.; Han, X.; Vuppugalla, R.; Zhang, Y.; Ruepp, S.U.; Trainor, G.L.; et al. Characterization of BMS-911543, a functionally selective small-molecule inhibitor of JAK2. Leukemia 2012, 26, 280–288. [Google Scholar] [CrossRef]
- Martens, S.; Goossens, V.; Devisscher, L.; Hofmans, S.; Claeys, P.; Vuylsteke, M.; Takahashi, N.; Augustyns, K.; Vandenabeele, P. RIPK1-dependent cell death: A novel target of the Aurora kinase inhibitor Tozasertib (VX-680). Cell Death Dis. 2018, 9, 211. [Google Scholar] [CrossRef]
- Harrington, E.A.; Bebbington, D.; Moore, J.; Rasmussen, R.K.; Ajose-Adeogun, A.O.; Nakayama, T.; Graham, J.A.; Demur, C.; Hercend, T.; Diu-Hercend, A.; et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat. Med. 2004, 10, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Arlot-Bonnemains, Y.; Baldini, E.; Martin, B.; Delcros, J.G.; Toller, M.; Curcio, F.; Ambesi-Impiombato, F.S.; D’Armiento, M.; Ulisse, S. Effects of the Aurora kinase inhibitor VX-680 on anaplastic thyroid cancer-derived cell lines. Endocr. Relat. Cancer 2008, 15, 559–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salah, E.; Ugochukwu, E.; Barr, A.J.; von Delft, F.; Knapp, S.; Elkins, J.M. Crystal structures of ABL-related gene (ABL2) in complex with imatinib, tozasertib (VX-680), and a type I inhibitor of the triazole carbothioamide class. J. Med. Chem. 2011, 54, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
| Drug Name | Mechanism | Indication | NCT # | Trial Phase | Outcome |
|---|---|---|---|---|---|
| Ruxolitinib * (FDA approved) | Jak1 > Jak2 > Tyk2 > Jak3 inhibitor (Type 1) [105] | Prostate cancer | NCT 03274778 | N/A | Withdrawn: Recruitment difficulty |
| Refractory malignant solid neoplasm | NCT 03878524 | 1 | Recruiting | ||
| Androgen-independent prostate cancer | NCT 00638378 | 2 | Terminated: Low efficacy | ||
| Prostate cancer | NCT 03274778 | N/A | Withdrawn: Recruitment difficulty | ||
| Solid tumors (including prostate) | NCT 02711137 | 1/2 | Terminated | ||
| Fedratinib * (FDA approved) | Jak2 inhibitor (Type 1) [106] | Solid tumors | NCT 01836705 | 1 | Completed |
| Solid tumors | NCT 01585623 | 1 | Completed | ||
| Pacritinib * | Jak2 (Type 1) [107] | MF, PCV, post-essential thrombocythemia MF | NCT 02055781 | 3 | Terminated (FDA concerns) |
| MF, PCV, post-essential thrombocythemia MF | NCT 03165734 | 3 | Recruiting | ||
| COVID | NCT 04404361 | 3 | Recruiting | ||
| MF, PCV, post-essential thrombocythemia MF | NCT 02055781 | 3 | Terminated (FDA concerns) | ||
| MF, PCV, post-essential thrombocythemia MF | NCT 01773187 | 3 | Terminated (FDA concerns) | ||
| NSCLC | NCT 02342353 | 1 | Terminated (drug shortage) | ||
| Refractory colorectal cancer | NCT 02277093 | 2 | Terminated (increased side effects) | ||
| Baricitinib (FDA approved) | Jak1, Jak2 inhibitor (Type 1) | RA | NCT 01711359 | 3 | Completed |
| RA | NCT 01721057 | 3 | Completed | ||
| COVID-19 | NCT 04358614 | 2/3 | Completed | ||
| RA | NCT 01710358 | 3 | Completed | ||
| Momelotinib * | Jak1, Jak2 inhibitor (Type 1) [108] | Symptomatic anemic MF | NCT 04173494 | 3 | Recruiting |
| Thrombocytopenia and MF | NCT 02101268 | 3 | Completed | ||
| MF | NCT 01969838 | 3 | Completed | ||
| Untreated metastatic pancreatic ductal adenocarcinoma | NCT 02101021 | 3 | Terminated (sponsor withdrew) | ||
| Adjuvant capecitabine and oxaliplatin in pancreatic ductal adenocarcinoma | NCT 02244489 | 1 | Terminated | ||
| NSCLC | NCT 02206763 | 1 | Terminated | ||
| Safety/Efficacy in PCV | NCT 01998828 | 2 | Terminated | ||
| NSCLC | NCT 02258607 | 1 | Terminated | ||
| Gandotinib | Jak2 inhibitor (Type 1) | MF, PCV, ET | NCT 01594723 | 2 | Active |
| MF, PCV, ET | NCT 01520220 | 1 | Completed | ||
| Healthy males | NCT 01577355 | 1 | Completed | ||
| MF, PCV, ET | NCT 01134120 | 1 | Completed | ||
| Peficitinib (FDA approved) | Pan-Jak inhibitor (Type 1) | RA | NCT 01638013 | 3 | Completed |
| RA | NCT 02308163 | 3 | Completed | ||
| RA | NCT 02305849 | 3 | Completed | ||
| Lestaurtinib * | FTL3 inhibitor (Jak2 off target inhibitor) | Acute lymphoblastic leukemia | NCT 00557193 | 3 | Active, not yet recruiting |
| Asymptomatic hormone-refractory prostate cancer | NCT 00081601 | 2 | Completed | ||
| Acute lymphoblastic leukemia | NCT 00557193 | 3 | Active, not yet recruiting | ||
| High risk neuroblastoma | NCT 00084422 | 1 | Completed | ||
| Tofacitinib * (FDA approved) | Jak3 > Jak2 > Jak1 inhibitor (Type 1) [109] | Previously treated pancreatic adenocarcinoma, cholangiocarcinoma and other mesothelin expressing solid tumors | NCT 04034238 | 1 | Recruiting |
| Jak3 > Jak2 > Jak1 inhibitor (Type 1) [109] | Relapsed and refractory extranodal NK/T-cell lymphoma | NCT 03598959 | 2 | Not yet recruiting | |
| WP 1066 * | Jak2 inhibitor | Recurrent/progressive pediatric brain tumor | NCT 04334863 | 1 | Recruiting |
| Recurrent malignant glioma or Progressive metastatic brain melanoma (18+) | NCT 01904123 | 1 | Recruiting | ||
| Atiprimod * | Jak2, Jak3 inhibitor | Neuroendocrine carcinoma | NCT 00388063 | 2 | Completed |
| Neuroendocrine carcinoma | NCT 00663429 | 2 | Completed | ||
| Multiple myeloma | NCT 00086216 | 1/2 | Completed | ||
| Advanced cancer | NCT 00430014 | 1 | Terminated: Sponsor withdrew | ||
| Advanced cancer | NCT 00214838 | 1/2 | Unknown, not recruiting. | ||
| Ilginatib (NS-018) | Jak2 inhibitor (Type 1) | MF, PCV and post-ET MF | NCT 01423851 | 1/2 | Active |
| AC430 | Jak2 inhibitor | Safety in healthy subjects | NCT 01287858 | 1 | Completed |
| LS104 | Jak2 inhibitor (allosteric) | Hematological malignancies | Unavailable | 1 | Unknown |
| Hematological malignancies | Unavailable | 1 | Unknown | ||
| Jaktinib | Jak2 inhibitor | Safety trial in healthy volunteers | NCT 03314402 | 1 | Completed |
| MF post Ruxolitinib Intolerance | NCT 04217993 | 2 | Recruiting | ||
| Intermediate and high-risk MF | NCT 03886415 | 2 | Recruiting | ||
| AT9283 * | Aurora kinase inhibitor (Jak2 off target) | Non-Hodgkin’s lymphoma and solid tumors | NCT 00443976 | 1 | Completed |
| Relapsed or refractory multiple myeloma | NCT 01145989 | 2 | Completed | ||
| Relapsed or refractory acute leukemia | NCT 01431664 | 1 | Completed | ||
| Relapsed or refractory solid tumors in pediatric patients | NCT 00985868 | 1 | Completed | ||
| Leukemia dose escalation | NCT 00522990 | 1/2 | Terminated (phase II dose determined) | ||
| Cerdulatinib * | SYK and JAK inhibitor | Vitiligo | NCT 04103060 | 2 | Recruiting |
| Chronic lymphocytic leukemia, Non-Hodgkin lymphoma | NCT 01994382 | 1/2 | Recruiting | ||
| Peripheral T-cell lymphoma | NCT 04021082 | 2/3 | Withdrawn by sponsor, not initiated | ||
| Filgotinib (FDA approved) | Jak1 inhibitor (Type 1) | Ulcerative colitis | NCT 02914522 | 3 | Completed |
| RA | NCT 02873936 | 3 | Completed | ||
| RA | NCT 02886728 | 3 | Completed | ||
| RA | NCT 02889796 | 3 | Completed | ||
| Testicular safety | NCT 03201445 | 2 | Recruiting | ||
| Decernotinib | Jak3 inhibitor (Type 1) | RA | NCT 01830985 | 2/3 | Completed |
| Jak3 inhibitor (Type 1) | Healthy subjects | NCT 01886209 | 1 | Completed | |
| RA | NCT 01886209 | 2 | Completed | ||
| RA | NCT 01590459 | 2 | Completed | ||
| Healthy subjects | NCT 00789126 | 1 | Completed | ||
| RA | NCT 01052194 | 2 | Completed | ||
| Erlotinib * | EGFR inhibitor (Jak2 off target inhibitor) | Chemo-naive, androgen independent prostate cancer | NCT 00272038 | 2 | Completed |
| Adjuvant bevacizumab in prostate cancer | NCT 00203424 | 2 | Completed | ||
| Non-metastatic prostate cancer with rising PSA | NCT 00148772 | 2 | Completed | ||
| Adjuvant docetaxel in older patients with prostate cancer | NCT 00087035 | 2 | Completed | ||
| Solid tumors and liver/kidney dysfunction | NCT 00030498 | 1 | Completed | ||
| Dose escalation study | NCT 00739453 | 1b | Completed | ||
| Drug combination study in various cancers | NCT 03878524 | 1 | |||
| Adjuvant bevacizumab in hormone refractory prostate cancer | NCT00996502 | 1/2 | Terminated | ||
| Givinostat * | HDAC inhibitor | R/R Hodgkin’s lymphoma | NCT 00792467 | 1/2 | Completed |
| Jak2 V617F positive chronic myeloproliferative diseases | NCT 00606307 | 2 | Completed | ||
| Chronic myeloproliferative neoplasms | NCT 01761968 | 2 | Active | ||
| R/R Hodgkin’s lymphoma | NCT 00496431 | 1/2 | Terminated: Well-tolerated with low efficacy | ||
| Repotrectinib * | ROS1 inhibitor with Jak2 (off target) | Solid tumors | NCT 03093116 | 1/2 | Recruiting |
| Zotiraciclib * | CDK and Jak1,2 inhibitor | Adults with recurrent anaplastic astrocytoma and glioblastoma | NCT 02942264 | 1/2 | Recruiting |
| Drug Name | Mechanism | Indication | NCT # | Trial Phase | Outcome |
|---|---|---|---|---|---|
| NVP-BSK805 | Jak2 inhibitor (Type 1) | 0 | |||
| CEP-33779 | Jak2 > Jak3 inhibitor (Type 1) | 0 | |||
| TG101209 | Jak2 inhibitor (type I) | 0 | |||
| AZ960 | Jak2 inhibitor (Type 1) | 0 | |||
| CHZ868 | Jak2 inhibitor (Type 2) | 0 | |||
| ON044580 | Jak2/BCR-ABL dual inhibitor (allosteric) | 0 | |||
| ZT55 | Jak2 inhibitor | 0 |
| Drug Name | Mechanism | Indication | NCT # | Trial Phase | Outcome |
|---|---|---|---|---|---|
| AZD1480 | Jak1/2 inhibitor (Type I) [110] | Primary myelofibrosis and post-PCV/ET-MF | NCT 00910728 | 1 | Terminated (toxicity) |
| Solid tumors, gastric cancer, HCC, NSCLC | NCT 01219543 | 1 | Terminated (toxicity) | ||
| Solid tumors | NCT 01112397 | 1 | Terminated (toxicity) | ||
| XL019 | Jak2 inhibitor (Type 1) | PCV | NCT 00595829 | 1 | Terminated (toxicity) |
| MF | NCT 00522574 | 1 | Terminated (toxicity) | ||
| BMS-911543 | Jak2 inhibitor | MF | NCT 01236352 | 1/2 | Terminated (business decision) |
| MK-0457/VX680 | Aurora kinase inhibitor | NCT 00111683 | 1 | Terminated (toxicity) | |
| NCT 02532868 | 1 | Terminated (toxicity) | |||
| NCT 00290550 | 2a | Terminated (toxicity) | |||
| NCT 00405054 | 2 | Terminated (toxicity) | |||
| NCT 00099346 | 1 | Terminated (toxicity) | |||
| NCT 00500006 | 1 | Terminated (toxicity) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beinhoff, P.; Sabharwal, L.; Udhane, V.; Maranto, C.; LaViolette, P.S.; Jacobsohn, K.M.; Tsai, S.; Iczkowski, K.A.; Wang, L.; Hall, W.A.; et al. Second-Generation Jak2 Inhibitors for Advanced Prostate Cancer: Are We Ready for Clinical Development? Cancers 2021, 13, 5204. https://doi.org/10.3390/cancers13205204
Beinhoff P, Sabharwal L, Udhane V, Maranto C, LaViolette PS, Jacobsohn KM, Tsai S, Iczkowski KA, Wang L, Hall WA, et al. Second-Generation Jak2 Inhibitors for Advanced Prostate Cancer: Are We Ready for Clinical Development? Cancers. 2021; 13(20):5204. https://doi.org/10.3390/cancers13205204
Chicago/Turabian StyleBeinhoff, Paul, Lavannya Sabharwal, Vindhya Udhane, Cristina Maranto, Peter S. LaViolette, Kenneth M. Jacobsohn, Susan Tsai, Kenneth A. Iczkowski, Liang Wang, William A. Hall, and et al. 2021. "Second-Generation Jak2 Inhibitors for Advanced Prostate Cancer: Are We Ready for Clinical Development?" Cancers 13, no. 20: 5204. https://doi.org/10.3390/cancers13205204
APA StyleBeinhoff, P., Sabharwal, L., Udhane, V., Maranto, C., LaViolette, P. S., Jacobsohn, K. M., Tsai, S., Iczkowski, K. A., Wang, L., Hall, W. A., Dehm, S. M., Kilari, D., & Nevalainen, M. T. (2021). Second-Generation Jak2 Inhibitors for Advanced Prostate Cancer: Are We Ready for Clinical Development? Cancers, 13(20), 5204. https://doi.org/10.3390/cancers13205204








