The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications
Abstract
:Simple Summary
Abstract
1. Introduction
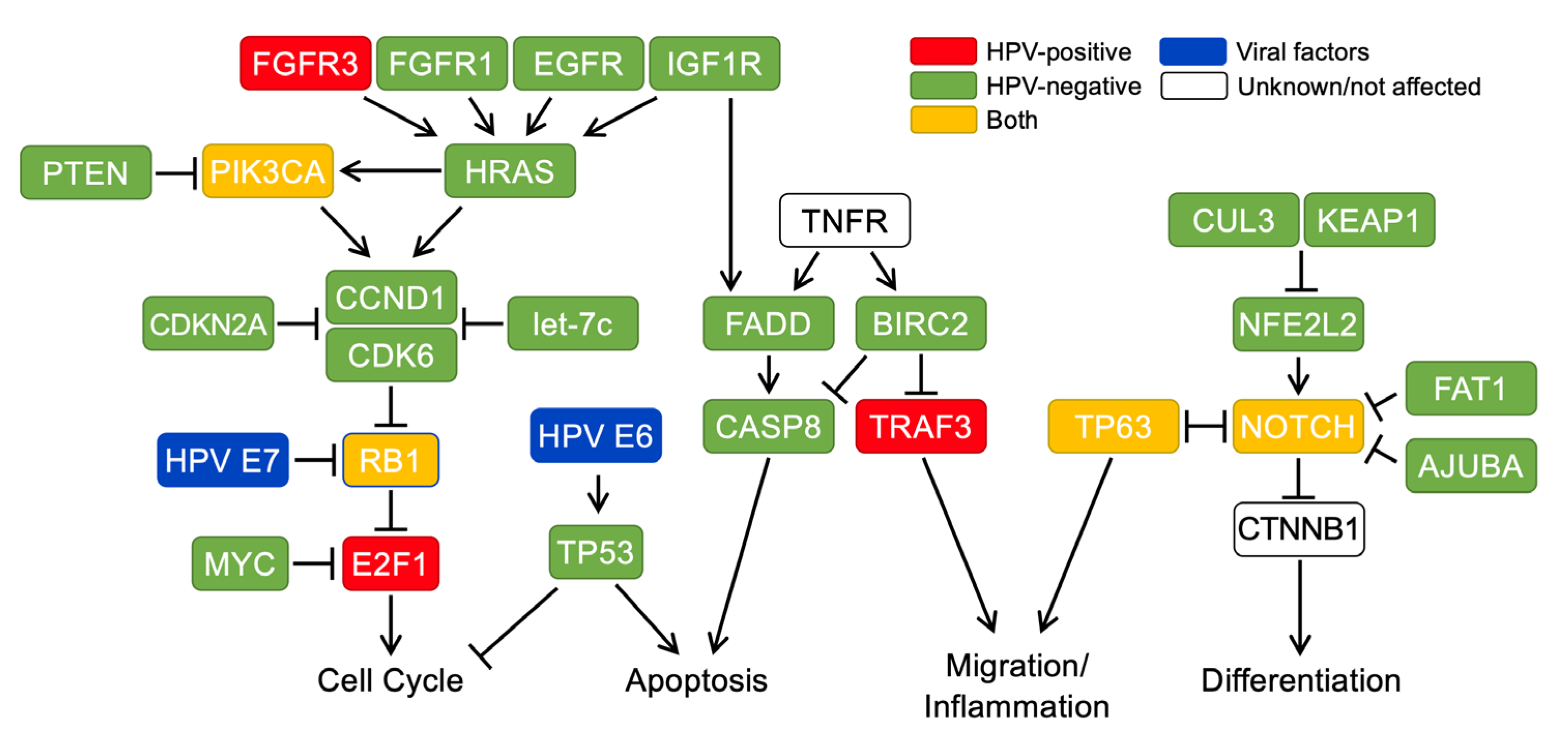
2. Molecular Carcinogenesis Is Distinct between HPV+ and HPV− HNSCCs
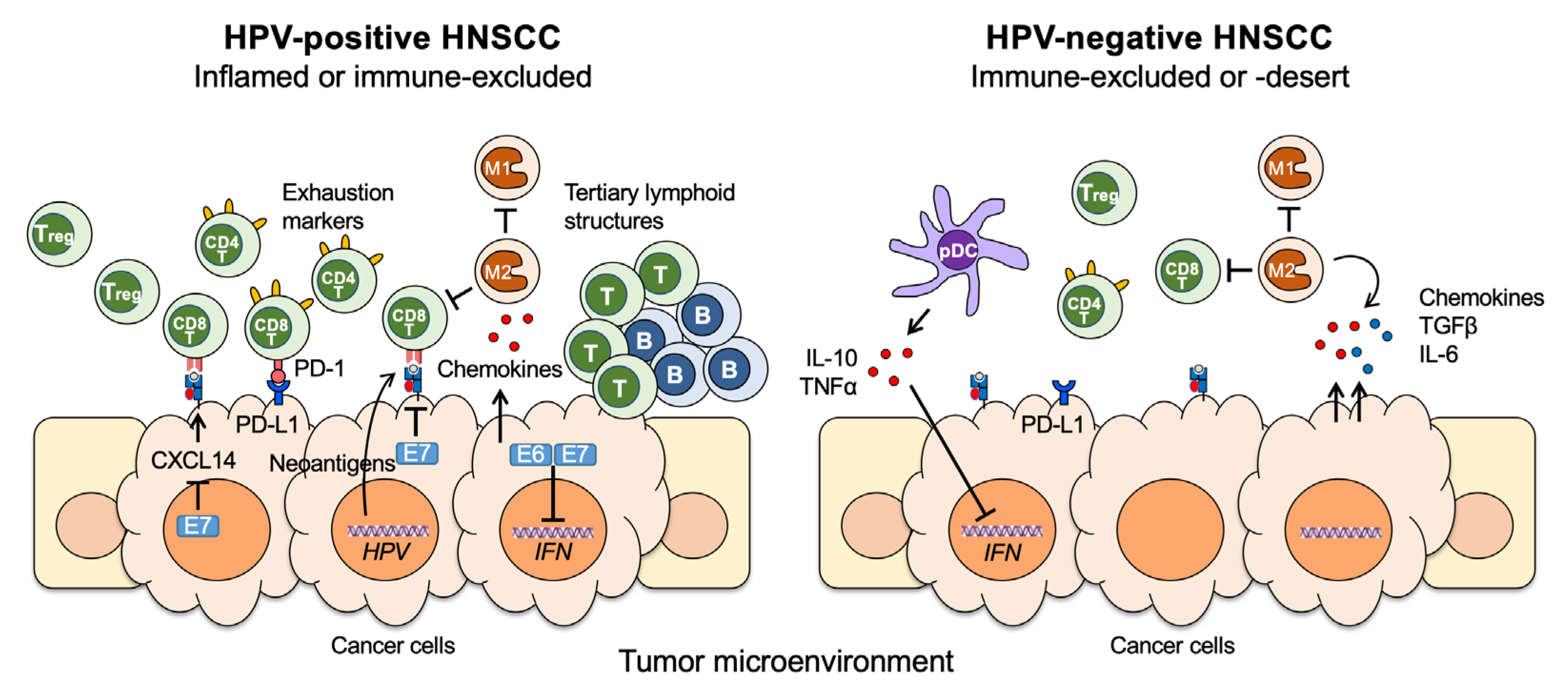
3. Differential Immune Responses between HPV+ and HPV− HNSCCs
4. Differential Contributions of Oral Microbiome to HPV+ and HPV− HNSCCs
5. Race, Sex, and Disparity in HPV+ and HPV− HNSCCs
6. Clinical Management of HNSCC: The Role of HPV
6.1. Diagnosis, Staging, and Treatment
6.2. Surgery
6.3. Multimodality Treatment
6.4. Low and Intermediate-Risk HPV+ HNSCC
6.5. Metastatic Disease: Do HPV+ HNSCC Patients Fare Better?
6.6. Immunotherapy Trials: Impact of HPV Status
7. Current Treatment Approaches for HPV+ and HPV− HNSCC
7.1. De-Intensification or Intensification: Risk Adapted Therapy
7.2. Advances in the Treatment of Recurrent/Metastatic Disease: Implications of HPV Status
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RT | radiation therapy |
| OP | oropharynx |
| RFS | relapse-free survival |
| OS | overall survival |
| PFS | progression-free survival |
| ORR | overall response rate |
| LRF | locoregional failure |
| IC | induction chemotherapy |
| CRT | chemoradiotherapy |
| ISH | in situ hybridization |
| IHC | immunohistochemistry |
| Fx | fractions |
| Gy | Gray |
| BID | twice a day |
| AUC | area under the curve |
| MDADI | MD Anderson Dysphagia Inventory |
| PORT | post-operative radiation therapy |
| POCRT | post-operative chemoradiotherapy |
| IMRT | intensity modulated radiation therapy |
| QoL | quality of life |
| SFX | standard fraction |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, D.; Lee, J.; Koch, W.M.; Kim, M.M.; Trink, B.; Sidransky, D.; Moon, C.S. Habitual risk factors for head and neck cancer. Otolaryngol. Head Neck Surg. 2004, 131, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yao, Y.; Chen, H.; Zhang, S.; Cao, S.M.; Zhang, Z.; Luo, B.; Liu, Z.; Li, Z.; Xiang, T.; et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat. Genet. 2019, 51, 1131–1136. [Google Scholar] [CrossRef]
- Pyeon, D.; Newton, M.A.; Lambert, P.F.; Boon, J.A.D.; Sengupta, S.; Marsit, C.; Woodworth, C.D.; Connor, J.P.; Haugen, T.; Smith, E.M.; et al. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 2007, 67, 4605–4619. [Google Scholar] [CrossRef] [Green Version]
- Gillison, M.L.; Akagi, K.; Xiao, W.; Jiang, B.; Pickard, R.K.L.; Li, J.; Swanson, B.J.; Agrawal, A.D.; Zucker, M.; Stache-Crain, B.; et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. 2019, 29, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Vokes, E.E.; Agrawal, N.; Seiwert, T.Y. HPV-Associated Head and Neck Cancer. J. Natl. Cancer Inst. 2015, 107, djv344. [Google Scholar] [CrossRef] [Green Version]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.-X.; Zhang, J.; Wang, J.; et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011, 333, 1154–1157. [Google Scholar] [CrossRef] [Green Version]
- Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [CrossRef] [Green Version]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Simard, E.P.; Ward, E.M.; Siegel, R.; Jemal, A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 2012, 62, 118–128. [Google Scholar] [CrossRef]
- Ellington, T.D.; Henley, S.J.; Senkomago, V.; O’Neil, M.E.; Wilson, R.J.; Singh, S.; Thomas, C.C.; Wu, M.; Richardson, L.C. Trends in Incidence of Cancers of the Oral Cavity and Pharynx—United States 2007–2016. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 433–438. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Kimple, R.J.; Harari, P.M. The prognostic value of HPV in head and neck cancer patients undergoing postoperative chemoradiotherapy. Ann. Transl. Med. 2015, 3, S14. [Google Scholar] [CrossRef]
- Cillo, A.R.; Kürten, C.H.L.; Tabib, T.; Qi, Z.; Onkar, S.S.; Wang, T.; Liu, A.; Duvvuri, U.; Kim, S.; Soose, R.J.; et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity 2020, 52, 183–199. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; Fernandez, L.; et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef]
- Di Credico, G.; Polesel, J.; Dal Maso, L.; Pauli, F.; Torelli, N.; Luce, D.; Radoï, L.; Matsuo, K.; Serraino, D.; Brennan, P.; et al. Alcohol drinking and head and neck cancer risk: The joint effect of intensity and duration. Br. J. Cancer 2020, 123, 1456–1463. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Denissenko, M.F.; Olivier, M.; Tretyakova, N.; Hecht, S.S.; Hainaut, P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002, 21, 7435–7451. [Google Scholar] [CrossRef] [Green Version]
- Khariwala, S.S.; Ma, B.; Ruszczak, C.; Carmella, S.G.; Lindgren, B.; Hatsukami, D.K.; Hecht, S.S.; Stepanov, I. High Level of Tobacco Carcinogen-Derived DNA Damage in Oral Cells Is an Independent Predictor of Oral/Head and Neck Cancer Risk in Smokers. Cancer Prev. Res. 2017, 10, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Slater, D.N. Dermatological applications of gene silencing RNA technology. Br. J. Derm. 2011, 164, 939. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef]
- Gillison, M.L. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck 2007, 29, 779–792. [Google Scholar] [CrossRef]
- Taioli, E. Gene-environment interaction in tobacco-related cancers. Carcinogenesis 2008, 29, 1467–1474. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Jagirdar, B.R. Synthesis of mesoporous iridium nanosponge: A highly active, thermally stable and efficient olefin hydrogenation catalyst. Dalton. Trans. 2017, 46, 11431–11439. [Google Scholar] [CrossRef]
- Di Credico, G.; Edefonti, V.; Polesel, J.; Pauli, F.; Torelli, N.; Serraino, D.; Negri, E.; Luce, D.; Stucker, I.; Matsuo, K.; et al. Joint effects of intensity and duration of cigarette smoking on the risk of head and neck cancer: A bivariate spline model approach. Oral Oncol. 2019, 94, 47–57. [Google Scholar] [CrossRef]
- Li, Y.C.; Cheng, A.J.; Lee, L.Y.; Huang, Y.C.; Chang, J.T. Multifaceted Mechanisms of Areca Nuts in Oral Carcinogenesis: The Molecular Pathology from Precancerous Condition to Malignant Transformation. J. Cancer 2019, 10, 4054–4062. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Chang, J.T.; Chiu, C.; Lu, Y.C.; Li, Y.L.; Chiang, C.H.; You, G.R.; Lee, L.Y.; Cheng, A.J. Areca nut contributes to oral malignancy through facilitating the conversion of cancer stem cells. Mol. Carcinog. 2016, 55, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, C.; Cigna, D.; Costanzo, G.; Ferraro, M.; Siena, L.; Vitulo, P.; Gjomarkaj, M.; Pace, E. Cigarette smoke alters cell cycle and induces inflammation in lung fibroblasts. Life Sci. 2015, 126, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Koyama, S.; Takamizawa, A.; Masubuchi, T.; Kubo, K.; Robbins, R.A.; Nagai, S.; Izumi, T. Smoke extract stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activities. Am. J. Physiol. 1999, 277, L1149–L1157. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Qin, X.; Yan, M.; Li, R.; Chen, G.; Zhang, J.; Chen, W. Cancer-associated fibroblasts promote cancer cell growth through a miR-7-RASSF2-PAR-4 axis in the tumor microenvironment. Oncotarget 2017, 8, 1290–1303. [Google Scholar] [CrossRef] [Green Version]
- Curry, J.M.; Sprandio, J.; Cognetti, D.; Luginbuhl, A.; Bar-ad, V.; Pribitkin, E.; Tuluc, M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin. Oncol. 2014, 41, 217–234. [Google Scholar] [CrossRef] [Green Version]
- Rademakers, S.E.; Lok, J.; van der Kogel, A.J.; Bussink, J.; Kaanders, J.H. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer 2011, 11, 167. [Google Scholar] [CrossRef] [Green Version]
- Domingo-Vidal, M.; Whitaker-Menezes, D.; Martos-Rus, C.; Tassone, P.; Snyder, C.M.; Tuluc, M.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Cigarette Smoke Induces Metabolic Reprogramming of the Tumor Stroma in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Res. 2019, 17, 1893–1909. [Google Scholar] [CrossRef] [Green Version]
- Stämpfli, M.R.; Anderson, G.P. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat. Rev. Immunol. 2009, 9, 377–384. [Google Scholar] [CrossRef]
- Clement, J.M.; Duan, F.; Srivastava, P.K. Smoking-induced immune deviation contributes to progression of bladder and other cancers. Oncoimmunology 2015, 4, e1019199. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Torres, M.P.; Kaur, S.; Rachagani, S.; Joshi, S.; Johansson, S.L.; Momi, N.; Baine, M.J.; Gilling, C.E.; Smith, L.M.; et al. Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages. Oncogene 2015, 34, 2052–2060. [Google Scholar] [CrossRef] [Green Version]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Wang, F.Q.; Li, H.M.; Yang, J.G.; Ren, J.G.; He, K.F.; Liu, B.; Zhang, W.; Zhao, Y.F. CCL2/EGF positive feedback loop between cancer cells and macrophages promotes cell migration and invasion in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 87037–87051. [Google Scholar] [CrossRef] [Green Version]
- Westrich, J.A.; Warren, C.J.; Pyeon, D. Evasion of Host Immune Defenses by Human Papillomavirus. Virus Res. 2016. [Google Scholar] [CrossRef]
- Momi, N.; Kaur, S.; Rachagani, S.; Ganti, A.K.; Batra, S.K. Smoking and microRNA dysregulation: A cancerous combination. Trends Mol. Med. 2014, 20, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, A.R.; Zheng, H.; Kwok, J.G.; Qu, Y.; Zou, A.E.; Korrapati, A.; Li, P.X.; Califano, J.A.; Hovell, M.F.; Wang-Rodriguez, J.; et al. A comprehensive study of smoking-specific microRNA alterations in head and neck squamous cell carcinoma. Oral Oncol. 2017, 72, 56–64. [Google Scholar] [CrossRef]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef]
- Doukas, S.G.; Vageli, D.P.; Lazopoulos, G.; Spandidos, D.A.; Sasaki, C.T.; Tsatsakis, A. The Effect of NNK, A Tobacco Smoke Carcinogen, on the miRNA and Mismatch DNA Repair Expression Profiles in Lung and Head and Neck Squamous Cancer Cells. Cells 2020, 9, 1031. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.Y.; Hsiao, J.R.; Chou, S.T.; Hsu, Y.M.; Wu, G.H.; Shieh, Y.S.; Shiah, S.G. MiR-944/CISH mediated inflammation via STAT3 is involved in oral cancer malignance by cigarette smoking. Neoplasia 2020, 22, 554–565. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Sun, Q.; Qin, X.; Zhang, Z.; Feng, Y.; Yan, M.; Chen, W. Identification and Confirmation of the miR-30 Family as a Potential Central Player in Tobacco-Related Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 616372. [Google Scholar] [CrossRef]
- Kao, C.J.; Martiniez, A.; Shi, X.B.; Yang, J.; Evans, C.P.; Dobi, A.; deVere White, R.W.; Kung, H.J. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene 2014, 33, 2495–2503. [Google Scholar] [CrossRef] [Green Version]
- Croset, M.; Pantano, F.; Kan, C.W.S.; Bonnelye, E.; Descotes, F.; Alix-Panabières, C.; Lecellier, C.H.; Bachelier, R.; Allioli, N.; Hong, S.S.; et al. miRNA-30 Family Members Inhibit Breast Cancer Invasion, Osteomimicry, and Bone Destruction by Directly Targeting Multiple Bone Metastasis-Associated Genes. Cancer Res. 2018, 78, 5259–5273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, A.D.; Cheng, H.; Martin, S.E.; Si, H.; Ormanoglu, P.; Carlson, S.; Clavijo, P.E.; Yang, X.; Das, R.; Cornelius, S.; et al. Integrated Genomic and Functional microRNA Analysis Identifies miR-30-5p as a Tumor Suppressor and Potential Therapeutic Nanomedicine in Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 2860–2873. [Google Scholar] [CrossRef] [Green Version]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Kalantari, M.; Lee, D.; Calleja-Macias, I.E.; Lambert, P.F.; Bernard, H.U. Effects of cellular differentiation, chromosomal integration and 5-aza-2’-deoxycytidine treatment on human papillomavirus-16 DNA methylation in cultured cell lines. Virology 2008, 374, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Dürst, M.; Kleinheinz, A.; Hotz, M.; Gissmann, L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J. Gen. Virol. 1985, 66, 1515–1522. [Google Scholar] [CrossRef]
- Jeon, S.; Lambert, P.F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: Implications for cervical carcinogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 1654–1658. [Google Scholar] [CrossRef] [Green Version]
- Bernard, B.A.; Bailly, C.; Lenoir, M.C.; Darmon, M.; Thierry, F.; Yaniv, M. The human papillomavirus type 18 (HPV18) E2 gene product is a repressor of the HPV18 regulatory region in human keratinocytes. J. Virol. 1989, 63, 4317–4324. [Google Scholar] [CrossRef] [Green Version]
- Romanczuk, H.; Thierry, F.; Howley, P.M. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J. Virol. 1990, 64, 2849–2859. [Google Scholar] [CrossRef] [Green Version]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef] [Green Version]
- Parfenov, M.; Pedamallu, C.S.; Gehlenborg, N.; Freeman, S.S.; Danilova, L.; Bristow, C.A.; Lee, S.; Hadjipanayis, A.G.; Ivanova, E.V.; Wilkerson, M.D.; et al. Characterization of HPV and host genome interactions in primary head and neck cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 15544–15549. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Gaykalova, D.A.; Guo, T.; Favorov, A.V.; Fertig, E.J.; Tamayo, P.; Callejas-Valera, J.L.; Allevato, M.; Gilardi, M.; Santos, J.; et al. HPV E2, E4, E5 drive alternative carcinogenic pathways in HPV positive cancers. Oncogene 2020, 39, 6327–6339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, Z.; Myers, J.N. TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response. J. Cell Biochem. 2016, 117, 2682–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeets, S.J.; Braakhuis, B.J.; Abbas, S.; Snijders, P.J.; Ylstra, B.; van de Wiel, M.A.; Meijer, G.A.; Leemans, C.R.; Brakenhoff, R.H. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene 2006, 25, 2558–2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, E.; Meza-Zepeda, L.A.; Kresse, S.H.; Myklebost, O.; Vasstrand, E.N.; Ibrahim, S.O. Chromosomal aberrations in head and neck squamous cell carcinomas in Norwegian and Sudanese populations by array comparative genomic hybridization. Oncol. Rep. 2008, 20, 825–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, D.Z.; Flam, E.L.; Izumchenko, E.; Danilova, L.V.; Wulf, H.A.; Guo, T.; Singman, D.A.; Afsari, B.; Skaist, A.M.; Considine, M.; et al. Integrated Analysis of Whole-Genome ChIP-Seq and RNA-Seq Data of Primary Head and Neck Tumor Samples Associates HPV Integration Sites with Open Chromatin Marks. Cancer Res. 2017, 77, 6538–6550. [Google Scholar] [CrossRef] [Green Version]
- Aldersley, J.; Lorenz, D.R.; Mouw, K.W.; D’Andrea, A.D.; Gabuzda, D. Genomic Landscape of Primary and Recurrent Anal Squamous Cell Carcinomas in Relation to HPV Integration, Copy-Number Variation, and DNA Damage Response Genes. Mol. Cancer Res. 2021, 19, 1308–1321. [Google Scholar] [CrossRef]
- den Boon, J.A.; Pyeon, D.; Wang, S.S.; Horswill, M.; Schiffman, M.; Sherman, M.; Zuna, R.E.; Wang, Z.; Hewitt, S.M.; Pearson, R.; et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc. Natl. Acad. Sci. USA 2015, 112, E3255–E3264. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Faden, D.L.; Ding, F.; Lin, Y.; Zhai, S.; Kuo, F.; Chan, T.A.; Morris, L.G.; Ferris, R.L. APOBEC mutagenesis is tightly linked to the immune landscape and immunotherapy biomarkers in head and neck squamous cell carcinoma. Oral Oncol. 2019, 96, 140–147. [Google Scholar] [CrossRef]
- Warren, C.J.; Xu, T.; Guo, K.; Griffin, L.M.; Westrich, J.a.; Lee, D.; Lambert, P.F.; Santiago, M.L.; Pyeon, D. APOBEC3A Functions as a Restriction Factor of Human Papillomavirus. J. Virol. 2015, 89, 688–702. [Google Scholar] [CrossRef] [Green Version]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef]
- Van Kempen, P.M.; Noorlag, R.; Swartz, J.E.; Bovenschen, N.; Braunius, W.W.; Vermeulen, J.F.; Van Cann, E.M.; Grolman, W.; Willems, S.M. Oropharyngeal squamous cell carcinomas differentially express granzyme inhibitors. Cancer Immunol. Immunother. 2016, 65, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Solomon, B.; Young, R.J.; Bressel, M.; Urban, D.; Hendry, S.; Thai, A.; Angel, C.; Haddad, A.; Kowanetz, M.; Fua, T.; et al. Prognostic Significance of PD-L1(+) and CD8(+) Immune Cells in HPV(+) Oropharyngeal Squamous Cell Carcinoma. Cancer Immunol. Res. 2018, 6, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Welters, M.J.P.; Ma, W.; Santegoets, S.; Goedemans, R.; Ehsan, I.; Jordanova, E.S.; van Ham, V.J.; van Unen, V.; Koning, F.; van Egmond, S.I.; et al. Intratumoral HPV16-Specific T Cells Constitute a Type I-Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin. Cancer Res. 2018, 24, 634–647. [Google Scholar] [CrossRef] [Green Version]
- Nordfors, C.; Grün, N.; Tertipis, N.; Ährlund-Richter, A.; Haeggblom, L.; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Munck-Wikland, E.; et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer. 2013, 49, 2522–2530. [Google Scholar] [CrossRef]
- So, Y.K.; Byeon, S.J.; Ku, B.M.; Ko, Y.H.; Ahn, M.J.; Son, Y.I.; Chung, M.K. An increase of CD8(+) T cell infiltration following recurrence is a good prognosticator in HNSCC. Sci. Rep. 2020, 10, 20059. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013, 73, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8(+) T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef] [Green Version]
- Outh-Gauer, S.; Morini, A.; Tartour, E.; Lépine, C.; Jung, A.C.; Badoual, C. The Microenvironment of Head and Neck Cancers: Papillomavirus Involvement and Potential Impact of Immunomodulatory Treatments. Head Neck Pathol. 2020, 14, 330–340. [Google Scholar] [CrossRef]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, K.H.; Neller, M.A.; Srihari, S.; Crooks, P.; Lekieffre, L.; Aftab, B.T.; Liu, H.; Smith, C.; Kenny, L.; Porceddu, S.; et al. Profiling HPV-16-specific T cell responses reveals broad antigen reactivities in oropharyngeal cancer patients. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Albers, A.; Abe, K.; Hunt, J.; Wang, J.; Lopez-Albaitero, A.; Schaefer, C.; Gooding, W.; Whiteside, T.L.; Ferrone, S.; DeLeo, A.; et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005, 65, 11146–11155. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Canteli, M.; Granda-Díaz, R.; Del Rio-Ibisate, N.; Allonca, E.; López-Alvarez, F.; Agorreta, J.; Garmendia, I.; Montuenga, L.M.; García-Pedrero, J.M.; Rodrigo, J.P. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. 2020, 69, 2089–2100. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Sak, A.; Stuschke, M.; et al. The PD-1/PD-L1 axis and human papilloma virus in patients with head and neck cancer after adjuvant chemoradiotherapy: A multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2017, 141, 594–603. [Google Scholar] [CrossRef]
- Wuerdemann, N.; Gültekin, S.E.; Pütz, K.; Wittekindt, C.; Huebbers, C.U.; Sharma, S.J.; Eckel, H.; Schubotz, A.B.; Gattenlöhner, S.; Büttner, R.; et al. PD-L1 Expression and a High Tumor Infiltrate of CD8+ Lymphocytes Predict Outcome in Patients with Oropharyngeal Squamous Cells Carcinoma. Int. J. Mol. Sci. 2020, 21, 5228. [Google Scholar] [CrossRef]
- Gameiro, S.F.; Ghasemi, F.; Barrett, J.W.; Koropatnick, J.; Nichols, A.C.; Mymryk, J.S.; Maleki Vareki, S. Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology 2018, 7, e1498439. [Google Scholar] [CrossRef] [Green Version]
- Stevanović, S.; Pasetto, A.; Helman, S.R.; Gartner, J.J.; Prickett, T.D.; Howie, B.; Robins, H.S.; Robbins, P.F.; Klebanoff, C.A.; Rosenberg, S.A.; et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 2017, 356, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Cicchini, L.; Blumhagen, R.Z.; Westrich, J.A.; Myers, M.E.; Warren, C.J.; Siska, C.; Raben, D.; Kechris, K.J.; Pyeon, D. High-Risk Human Papillomavirus E7 Alters Host DNA Methylome and Represses HLA-E Expression in Human Keratinocytes. Sci. Rep. 2017, 7, 3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westrich, J.A.; Vermeer, D.W.; Silva, A.; Bonney, S.; Berger, J.N.; Cicchini, L.; Greer, R.O.; Song, J.I.; Raben, D.; Slansky, J.E.; et al. CXCL14 suppresses human papillomavirus-associated head and neck cancer through antigen-specific CD8(+) T-cell responses by upregulating MHC-I expression. Oncogene 2019, 38, 7166–7180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gameiro, S.F.; Zhang, A.; Ghasemi, F.; Barrett, J.W.; Nichols, A.C.; Mymryk, J.S. Analysis of Class I Major Histocompatibility Complex Gene Transcription in Human Tumors Caused by Human Papillomavirus Infection. Viruses 2017, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, B.; Lou, H.; Shen, Z.; Tong, F.; Zhai, A.; Wei, L.; Zhang, F. Immunological network analysis in HPV associated head and neck squamous cancer and implications for disease prognosis. Mol. Immunol. 2018, 96, 28–36. [Google Scholar] [CrossRef]
- Ruffin, A.T.; Cillo, A.R.; Tabib, T.; Liu, A.; Onkar, S.; Kunning, S.R.; Lampenfeld, C.; Atiya, H.I.; Abecassis, I.; Kürten, C.H.L.; et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 2021, 12, 3349. [Google Scholar] [CrossRef]
- Hladíková, K.; Koucký, V.; Bouček, J.; Laco, J.; Grega, M.; Hodek, M.; Zábrodský, M.; Vošmik, M.; Rozkošová, K.; Vošmiková, H.; et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8(+) T cells. J. Immunother. Cancer 2019, 7, 261. [Google Scholar] [CrossRef]
- Wood, O.; Woo, J.; Seumois, G.; Savelyeva, N.; McCann, K.J.; Singh, D.; Jones, T.; Peel, L.; Breen, M.S.; Ward, M.; et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 2016, 7, 56781–56797. [Google Scholar] [CrossRef] [Green Version]
- Snietura, M.; Brewczynski, A.; Kopec, A.; Rutkowski, T. Infiltrates of M2-Like Tumour-Associated Macrophages Are Adverse Prognostic Factor in Patients with Human Papillomavirus-Negative but Not in Human Papillomavirus-Positive Oropharyngeal Squamous Cell Carcinoma. Pathobiology 2020, 87, 75–86. [Google Scholar] [CrossRef]
- Santegoets, S.J.; Duurland, C.L.; Jordanova, E.J.; van Ham, V.J.; Ehsan, I.; Loof, N.M.; Narang, V.; Dutertre, C.A.; Ginhoux, F.; van Egmond, S.L.; et al. CD163(+) cytokine-producing cDC2 stimulate intratumoral type 1 T cell responses in HPV16-induced oropharyngeal cancer. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Koucký, V.; Hladíková, K.; Táborská, E.; Bouček, J.; Grega, M.; Špíšek, R.; Fialová, A. The cytokine milieu compromises functional capacity of tumor-infiltrating plasmacytoid dendritic cells in HPV-negative but not in HPV-positive HNSCC. Cancer Immunol. Immunother. 2021, 70, 2545–2557. [Google Scholar] [CrossRef]
- Johnson-Obaseki, S.; Caulley, L.; Corsten, M.; Liu, G.; Dimitroulakos, J.; Goldstein, D.; Irish, J.; Rider, J. C-reactive Protein in HPV-Positive and HPV-Negative Oropharyngeal Cancer. Otolaryngol. Head Neck Surg. 2019, 160, 494–501. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Bauml, J.; Seiwert, T.Y.; Pfister, D.G.; Worden, F.; Liu, S.V.; Gilbert, J.; Saba, N.F.; Weiss, J.; Wirth, L.; Sukari, A.; et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J. Clin. Oncol. 2017, 35, 1542–1549. [Google Scholar] [CrossRef]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, K.; Carey, R.M.; Lin, X.; Seckar, T.D.; Wei, Z.; Chorath, K.; Newman, J.G.; O’Malley, B.W.; Weinstein, G.S.; Feldman, M.D.; et al. The microbiome of HPV-positive tonsil squamous cell carcinoma and neck metastasis. Oral Oncol. 2021, 117, 105305. [Google Scholar] [CrossRef]
- Burr, A.H.P.; Bhattacharjee, A.; Hand, T.W. Nutritional Modulation of the Microbiome and Immune Response. J. Immunol. 2020, 205, 1479–1487. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, D.M.; Hand, T.W.; Han, S.J.; Gerner, M.Y.; Glatman Zaretsky, A.; Byrd, A.L.; Harrison, O.J.; Ortiz, A.M.; Quinones, M.; Trinchieri, G.; et al. Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity. Cell 2015, 163, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell Infect. Microbiol. 2019, 9, 476. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.; Nightingale, K.; Kerr, A.R.; de Lacure, M.D.; Veeramachaneni, R.; et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE 2014, 9, e98741. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef]
- Ganly, I.; Yang, L.; Giese, R.A.; Hao, Y.; Nossa, C.W.; Morris, L.G.T.; Rosenthal, M.; Migliacci, J.; Kelly, D.; Tseng, W.; et al. Periodontal pathogens are a risk factor of oral cavity squamous cell carcinoma, independent of tobacco and alcohol and human papillomavirus. Int. J. Cancer. 2019, 145, 775–784. [Google Scholar] [CrossRef]
- Sharma, A.K.; DeBusk, W.T.; Stepanov, I.; Gomez, A.; Khariwala, S.S. Oral Microbiome Profiling in Smokers with and without Head and Neck Cancer Reveals Variations Between Health and Disease. Cancer Prev. Res. 2020, 13, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Neuzillet, C.; Marchais, M.; Vacher, S.; Hilmi, M.; Schnitzler, A.; Meseure, D.; Leclere, R.; Lecerf, C.; Dubot, C.; Jeannot, E.; et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci. Rep. 2021, 11, 7870. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef] [Green Version]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhao, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Narbad, A.; Chen, W. A mixture of Lactobacillus species isolated from traditional fermented foods promote recovery from antibiotic-induced intestinal disruption in mice. J. Appl. Microbiol. 2018, 124, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Freedman, N.D.; Alekseyenko, A.V.; Wu, J.; Yang, L.; Pei, Z.; et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, E.; Iacovelli, N.A.; Tombolini, V.; Rancati, T.; Polimeni, A.; De Cecco, L.; Valdagni, R.; De Felice, F. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol. 2019, 99, 104453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronzato, J.D.; Bomfim, R.A.; Edwards, D.H.; Crouch, D.; Hector, M.P.; Gomes, B. Detection of Fusobacterium in oral and head and neck cancer samples: A systematic review and meta-analysis. Arch. Oral Biol. 2020, 112, 104669. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.M.; Moran, G.P. The microbiome and oral cancer: More questions than answers. Oral Oncol. 2019, 89, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wong, P.Y.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Wong, S.H.; et al. The Intersection between Oral Microbiota, Host Gene Methylation and Patient Outcomes in Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 3425. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer. 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Chatterjee, S.; Do Kang, S.; Alam, S.; Salzberg, A.C.; Milici, J.; van der Burg, S.H.; Freeman, W.; Meyers, C. Tissue-Specific Gene Expression during Productive Human Papillomavirus 16 Infection of Cervical, Foreskin, and Tonsil Epithelium. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Gazzaz, M.J.; Jeffery, C.; O’Connell, D.; Harris, J.; Seikaly, H.; Biron, V. Association of human papillomavirus related squamous cell carcinomas of the oropharynx and cervix. Papillomavirus Res. 2019, 8, 100188. [Google Scholar] [CrossRef]
- Sindrewicz, K.; Kędzierska-Kapuza, K.; Jaworowska, E.; Ciechanowski, K. Prevalence of Human Papillomavirus Infection in the Head and Neck Area of Patients After Kidney Transplantation Treated With Immunosuppressive Therapy. Transpl. Proc. 2020, 52, 2388–2393. [Google Scholar] [CrossRef]
- Freedman, R.L.; Sibley, H.; Williams, A.M.; Chang, S.S. Race, not socioeconomic disparities, correlates with survival in human papillomavirus-negative oropharyngeal cancer: A retrospective study. Am. J. Otolaryngol. 2021, 42, 102816. [Google Scholar] [CrossRef]
- Sheth, S.; Farquhar, D.R.; Lenze, N.R.; Mazul, A.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Zevallos, J.P.; Hayes, D.N.; Olshan, F. Decreased overall survival in black patients with HPV-associated oropharyngeal cancer. Am. J. Otolaryngol. 2021, 42, 102780. [Google Scholar] [CrossRef]
- Mazul, A.L.; Naik, A.N.; Zhan, K.Y.; Stepan, K.O.; Old, M.O.; Kang, S.Y.; Nakken, E.R.; Puram, S.V. Gender and race interact to influence survival disparities in head and neck cancer. Oral Oncol. 2021, 112, 105093. [Google Scholar] [CrossRef]
- Stein, E.; Lenze, N.R.; Yarbrough, W.G.; Hayes, D.N.; Mazul, A.; Sheth, S. Systematic review and meta-analysis of racial survival disparities among oropharyngeal cancer cases by HPV status. Head Neck 2020, 42, 2985–3001. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Wang, S.J.; van Zante, A.; Zhang, Y.; Rettig, E.; Yin, L.X.; Ryan, W.R.; Ha, P.K.; Wentz, A.; et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer 2017, 123, 1566–1575. [Google Scholar] [CrossRef] [Green Version]
- Mazul, A.L.; Colditz, G.A.; Zevallos, J.P. Factors associated with HPV testing in oropharyngeal cancer in the National Cancer Data Base from 2013 to 2015. Oral Oncol. 2020, 104, 104609. [Google Scholar] [CrossRef]
- deJong, R.J.B.; Brandwein-Gensler, M.; Brizel, D.M.; Califano, J.A.; Amy, Y.; Chen, M.M.P.H.; Colevas, A.D.; Edge, S.B.; Fury, M.; Ghossein, R.A.; et al. AJCC Cancer Staging System; American College of Surgeons: Chicago, IL, USA, 2017. [Google Scholar]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef]
- Lacas, B.; Carmel, A.; Landais, C.; Wong, S.J.; Licitra, L.; Tobias, J.S.; Burtness, B.; Ghi, M.G.; Cohen, E.E.W.; Grau, C.; et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother. Oncol. 2021, 156, 281–293. [Google Scholar] [CrossRef]
- Heiduschka, G.; Grah, A.; Oberndorfer, F.; Kadletz, L.; Altorjai, G.; Kornek, G.; Wrba, F.; Thurnher, D.; Selzer, E. Improved survival in HPV/p16-positive oropharyngeal cancer patients treated with postoperative radiotherapy. Strahlenther. Onkol. 2015, 191, 209–216. [Google Scholar] [CrossRef]
- Viros Porcuna, D.; Pollan Guisasola, C.; Viña Soria, C.; Cirauqui Cirauqui, B.; Pardo Muñoz, L.; Collurá, F.; Mesia Nin, R. Transoral robotic surgery for squamous cell carcinoma of the oropharynx in a primarily human papillomavirus-negative patient population. Clin. Transl. Oncol. 2020, 22, 1303–1311. [Google Scholar] [CrossRef]
- Sload, R.; Silver, N.; Jawad, B.A.; Gross, N.D. The Role of Transoral Robotic Surgery in the Management of HPV Negative Oropharyngeal Squamous Cell Carcinoma. Curr. Oncol. Rep. 2016, 18, 53. [Google Scholar] [CrossRef]
- Zhan, K.Y.; Puram, S.V.; Li, M.M.; Silverman, D.A.; Agrawal, A.A.; Ozer, E.; Old, M.O.; Carrau, R.L.; Rocco, J.W.; Higgins, K.M.; et al. National treatment trends in human papillomavirus-positive oropharyngeal squamous cell carcinoma. Cancer 2020, 126, 1295–1305. [Google Scholar] [CrossRef]
- Rubek, N.; Channir, H.I.; Charabi, B.W.; Lajer, C.B.; Kiss, K.; Nielsen, H.U.; Bentzen, J.; Friborg, J.; von Buchwald, C. Primary transoral robotic surgery with concurrent neck dissection for early stage oropharyngeal squamous cell carcinoma implemented at a Danish head and neck cancer center: A phase II trial on feasibility and tumour margin status. Eur. Arch. Otorhinolaryngol. 2017, 274, 2229–2237. [Google Scholar] [CrossRef]
- Høxbroe Michaelsen, S.; Grønhøj, C.; Høxbroe Michaelsen, J.; Friborg, J.; von Buchwald, C. Quality of life in survivors of oropharyngeal cancer: A systematic review and meta-analysis of 1366 patients. Eur. J. Cancer 2017, 78, 91–102. [Google Scholar] [CrossRef]
- Nichols, D.S.; Zhao, J.; Boyce, B.J.; Amdur, R.; Mendenhall, W.M.; Danan, D.; Hitchcock, K.; Ning, K.; Keyes, K.; Lee, J.H.; et al. HPV/p16-positive oropharyngeal cancer treated with transoral robotic surgery: The roles of margins, extra-nodal extension and adjuvant treatment. Am. J. Otolaryngol. 2021, 42, 102793. [Google Scholar] [CrossRef]
- Schwartz, S.R.; Yueh, B.; McDougall, J.K.; Daling, J.R.; Schwartz, S.M. Human papillomavirus infection and survival in oral squamous cell cancer: A population-based study. Otolaryngol. Head Neck Surg. 2001, 125, 1–9. [Google Scholar] [CrossRef]
- Licitra, L.; Perrone, F.; Bossi, P.; Suardi, S.; Mariani, L.; Artusi, R.; Oggionni, M.; Rossini, C.; Cantù, G.; Squadrelli, M.; et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2006, 24, 5630–5636. [Google Scholar] [CrossRef] [Green Version]
- Cmelak, A.; Dietrich, M.S.; Li, S.; Ridner, S.; Forastiere, A.; Burtness, B.A.; Cella, D.; Murphy, B.A. ECOG-ACRIN 2399: Analysis of patient related outcomes after Chemoradiation for locally advanced head and neck Cancer. Cancers Head Neck 2020, 5, 12. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Rischin, D.; Young, R.J.; Fisher, R.; Fox, S.B.; Le, Q.-T.; Peters, L.J.; Solomon, B.; Choi, J.; O’Sullivan, B.; Kenny, L.M.; et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J. Clin. Oncol. 2010, 28, 4142–4148. [Google Scholar] [CrossRef] [Green Version]
- Posner, M.R.; Lorch, J.H.; Goloubeva, O.; Tan, M.; Schumaker, L.M.; Sarlis, N.J.; Haddad, R.I.; Cullen, K.J. Survival and human papillomavirus in oropharynx cancer in TAX 324: A subset analysis from an international phase III trial. Ann. Oncol. 2011, 22, 1071–1077. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Huang, S.H.; Su, J.; Garden, A.S.; Sturgis, E.M.; Dahlstrom, K.; Lee, N.; Riaz, N.; Pei, X.; Koyfman, S.A.; et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol. 2016, 17, 440–451. [Google Scholar] [CrossRef]
- Huang, S.H.; Xu, W.; Waldron, J.; Siu, L.; Shen, X.; Tong, L.; Ringash, J.; Bayley, A.; Kim, J.; Hope, A.; et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J. Clin. Oncol. 2015, 33, 836–845. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Huang, S.H.; Siu, L.L.; Waldron, J.; Zhao, H.; Perez-Ordonez, B.; Weinreb, I.; Kim, J.; Ringash, J.; Bayley, A.; et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J. Clin. Oncol. 2013, 31, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Perez-Ordonez, B.; Weinreb, I.; Hope, A.; Massey, C.; Waldron, J.N.; Kim, J.; Bayley, A.J.; Cummings, B.; Cho, B.C.; et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013, 49, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Perez-Ordonez, B.; Liu, F.-F.; Waldron, J.; Ringash, J.; Irish, J.; Cummings, B.; Siu, L.L.; Kim, J.; Weinreb, I.; et al. Atypical Clinical Behavior of p16-Confirmed HPV-Related Oropharyngeal Squamous Cell Carcinoma Treated With Radical Radiotherapy. Int. J. Radiat. Oncol. 2012, 82, 276–283. [Google Scholar] [CrossRef]
- Trosman, S.J.; Koyfman, S.A.; Ward, M.C.; Al-Khudari, S.; Nwizu, T.; Greskovich, J.F.; Lamarre, E.D.; Scharpf, J.; Khan, M.J.; Lorenz, R.R.; et al. Effect of human papillomavirus on patterns of distant metastatic failure in oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Bishop, J.A.; Ogawa, T.; Chang, X.; Illei, P.B.; Gabrielson, E.; Pai, S.I.; Westra, W.H. HPV analysis in distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. Am. J. Surg. Pathol. 2012, 36, 142–148. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Nguyen-Tan, P.F.; Rosenthal, D.; El-Naggar, A.; Garden, A.S.; Soulieres, D.; Trotti, A.; Avizonis, V.; Ridge, J.A.; et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2014, 32, 3365–3373. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Psyrri, A.; Mesía, R.; Peyrade, F.; Beier, F.; de Blas, B.; Celik, I.; Licitra, L. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: Retrospective analysis of the phase III EXTREME trial. Ann. Oncol. 2014, 25, 801–807. [Google Scholar] [CrossRef]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Ferris, R.L.; Spanos, W.C.; Leidner, R.; Gonçalves, A.; Martens, U.M.; Kyi, C.; Sharfman, W.; Chung, C.H.; Devriese, L.A.; Gauthier, H.; et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef] [Green Version]
- Hong, A.M.; Ferguson, P.; Dodds, T.; Jones, D.; Li, M.; Yang, J.; Scolyer, R.A. Significant association of PD-L1 expression with human papillomavirus positivity and its prognostic impact in oropharyngeal cancer. Oral Oncol. 2019, 92, 33–39. [Google Scholar] [CrossRef]
- Hong, A.M.; Vilain, R.E.; Romanes, S.; Yang, J.; Smith, E.; Jones, D.; Scolyer, R.A.; Lee, C.S.; Zhang, M.; Rose, B. PD-L1 expression in tonsillar cancer is associated with human papillomavirus positivity and improved survival: Implications for anti-PD1 clinical trials. Oncotarget 2016, 7, 77010–77020. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Park, T.I.; Ahn, D. Comprehensive Analysis and Clinical Implication of PD-L1 Expression Considering HPV Status in Oropharyngeal Squamous Cell Carcinoma. Anticancer Res. 2020, 40, 4001–4010. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Zeng, Q.; Guo, X.J.; Wang, H.; Liu, H.H.; Dong, Z.Y. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Rep. 2019, 9, 13404. [Google Scholar] [CrossRef] [Green Version]
- Chureemas, T.; Larbcharoensub, N.; Juengsamarn, J.; Layangkool, T.; Jiarpinitnun, C.; Chansriwong, P.; Trachu, N.; Pattaranutaporn, P.; Ngamphaiboon, N. 978P - Prevalence, pattern, and impact of PD-L1 expression and HPV-status in head and neck squamous cell carcinoma. Ann. Oncol. 2016, 27, vi337. [Google Scholar] [CrossRef]
- Patel, J.J.; Levy, D.A.; Nguyen, S.A.; Knochelmann, H.M.; Day, T.A. Impact of PD-L1 expression and human papillomavirus status in anti-PD1/PDL1 immunotherapy for head and neck squamous cell carcinoma-Systematic review and meta-analysis. Head Neck 2020, 42, 774–786. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Katada, C.; Hara, H.; Fujii, H.; Nakajima, T.E.; Ando, T.; Nomura, M.; Kojima, T.; Yamashita, K.; Yokoyama, T.; Sakamoto, Y.; et al. A phase II study of chemoselection with docetaxel, cisplatin, and 5–fluorouracil as a strategy for organ preservation in patients with resectable esophageal cancer (CROC trial). J. Clin. Oncol. 2021, 39, 4027-4027. [Google Scholar] [CrossRef]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): An open-label, phase 2, randomised trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Ringash, J.; Lewin, J.S.; Duvvuri, U.; et al. Updated report of a phase II randomized trial of transoral surgical resection followed by low-dose or standard postoperative therapy in resectable p16+ locally advanced oropharynx cancer: A trial of the ECOG-ACRIN cancer research group (E3311). J. Clin. Oncol. 2021, 39, 6010. [Google Scholar] [CrossRef]
- Yom, S.S.; Torres-Saavedra, P.; Caudell, J.J.; Waldron, J.N.; Gillison, M.L.; Xia, P.; Truong, M.T.; Kong, C.; Jordan, R.; Subramaniam, R.M.; et al. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002). J. Clin. Oncol. 2021, 39, 956–965. [Google Scholar] [CrossRef]
- Nguyen-Tan, P.F.; Zhang, Q.; Ang, K.K.; Weber, R.S.; Rosenthal, D.I.; Soulieres, D.; Kim, H.; Silverman, C.; Raben, A.; Galloway, T.J.; et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long-term report of efficacy and toxicity. J. Clin. Oncol. 2014, 32, 3858–3866. [Google Scholar] [CrossRef]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef]
- Haddad, R.; O’Neill, A.; Rabinowits, G.; Tishler, R.; Khuri, F.; Adkins, D.; Clark, J.; Sarlis, N.; Lorch, J.; Beitler, J.J.; et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 257–264. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.; Lin, J.C.; Razaq, M.A.; et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Powell, S.F.; Gold, K.A.; Gitau, M.M.; Sumey, C.J.; Lohr, M.M.; McGraw, S.C.; Nowak, R.K.; Jensen, A.W.; Blanchard, M.J.; Fischer, C.D.; et al. Safety and Efficacy of Pembrolizumab With Chemoradiotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma: A Phase IB Study. J. Clin. Oncol. 2020, 38, 2427–2437. [Google Scholar] [CrossRef]
- Leeman, J.E.; Li, J.G.; Pei, X.; Venigalla, P.; Zumsteg, Z.S.; Katsoulakis, E.; Lupovitch, E.; McBride, S.M.; Tsai, C.J.; Boyle, J.O.; et al. Patterns of Treatment Failure and Postrecurrence Outcomes Among Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma After Chemoradiotherapy Using Modern Radiation Techniques. JAMA Oncol. 2017, 3, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Wieking, B.G.; Vermeer, D.W.; Spanos, W.C.; Lee, K.M.; Vermeer, P.; Lee, W.T.; Xu, Y.; Gabitzsch, E.S.; Balcaitis, S.; Balint, J.P., Jr.; et al. A non-oncogenic HPV 16 E6/E7 vaccine enhances treatment of HPV expressing tumors. Cancer Gene 2012, 19, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.; Zeng, Q.; Kang, T.H.; Peng, S.; Roosinovich, E.; Pai, S.I.; Hung, C.F. Innovative DNA vaccine for human papillomavirus (HPV)-associated head and neck cancer. Gene 2011, 18, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalley Rumfield, C.; Roller, N.; Pellom, S.T.; Schlom, J.; Jochems, C. Therapeutic Vaccines for HPV-Associated Malignancies. Immunotargets 2020, 9, 167–200. [Google Scholar] [CrossRef]
- Kirtane, K.; Massarelli, E.; Hanna, G.J.; Klebanoff, C.A.; Blumenschein, G.; Bishop, M.R.; Lee, S.; Niu, J.; Adhikary, S.; Astrow, S.H.; et al. KITE-439: A phase I study of HPV16 E7 T cell receptor-engineered T cells in patients with relapsed/refractory HPV16-positive cancers. J. Clin. Oncol. 2020, 38, TPS3149-TPS3149. [Google Scholar] [CrossRef]
- Guo, Y.; Ahn, M.J.; Chan, A.; Wang, C.H.; Kang, J.H.; Kim, S.B.; Bello, M.; Arora, R.S.; Zhang, Q.; He, X.; et al. Afatinib versus methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): An open-label, randomised phase III trial. Ann. Oncol. 2019, 30, 1831–1839. [Google Scholar] [CrossRef] [Green Version]
- Gougis, P.; Moreau Bachelard, C.; Kamal, M.; Gan, H.K.; Borcoman, E.; Torossian, N.; Bièche, I.; Le Tourneau, C. Clinical Development of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. JNCI Cancer Spectr. 2019, 3, pkz055. [Google Scholar] [CrossRef]
- Nichols, A.C.; Palma, D.A.; Chow, W.; Tan, S.; Rajakumar, C.; Rizzo, G.; Fung, K.; Kwan, K.; Wehrli, B.; Winquist, E.; et al. High frequency of activating PIK3CA mutations in human papillomavirus-positive oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Dunn, L.A.; Riaz, N.; Fury, M.G.; McBride, S.M.; Michel, L.; Lee, N.Y.; Sherman, E.J.; Baxi, S.S.; Haque, S.S.; Katabi, N.; et al. A Phase 1b Study of Cetuximab and BYL719 (Alpelisib) Concurrent with Intensity Modulated Radiation Therapy in Stage III-IVB Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 564–570. [Google Scholar] [CrossRef]
- Gilardi, M.; Wang, Z.; Proietto, M.; Chillà, A.; Calleja-Valera, J.L.; Goto, Y.; Vanoni, M.; Janes, M.R.; Mikulski, Z.; Gualberto, A.; et al. Tipifarnib as a Precision Therapy for HRAS-Mutant Head and Neck Squamous Cell Carcinomas. Mol. Cancer 2020, 19, 1784–1796. [Google Scholar] [CrossRef]
- Adkins, D.; Ley, J.; Neupane, P.; Worden, F.; Sacco, A.G.; Palka, K.; Grilley-Olson, J.E.; Maggiore, R.; Salama, N.N.; Trinkaus, K.; et al. Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: A multicentre, multigroup, phase 2 trial. Lancet Oncol. 2019, 20, 1295–1305. [Google Scholar] [CrossRef]
- Moutafi, M.; Economopoulou, P.; Rimm, D.; Psyrri, A. PARP inhibitors in head and neck cancer: Molecular mechanisms, preclinical and clinical data. Oral Oncol. 2021, 117, 105292. [Google Scholar] [CrossRef]
- Gold, K.A.; Sacco, A.; Bykowski, J.; Daniels, G.; Pittman, E.; Messer, K.; Chen, R.; Cohen, E. Abstract CT134: A phase I study of avelumab, palbociclib, and cetuximab (APC) in recurrent or metastatic head and neck squamous cell carcinoma (RM HNSCC). Cancer Res. 2021, 81, CT134. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Wei, J.; Meng, L.; Zhang, Q.; Qu, C.; Xin, Y.; Jiang, X. Molecular mechanisms underlying increased radiosensitivity in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int. J. Biol. Sci. 2020, 16, 1035–1043. [Google Scholar] [CrossRef]
- Li, H.; Torabi, S.J.; Yarbrough, W.G.; Mehra, S.; Osborn, H.A.; Judson, B. Association of Human Papillomavirus Status at Head and Neck Carcinoma Subsites With Overall Survival. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Mahal, B.A.; Catalano, P.J.; Haddad, R.I.; Hanna, G.J.; Kass, J.I.; Schoenfeld, J.D.; Tishler, R.B.; Margalit, D.N. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1660–1667. [Google Scholar] [CrossRef] [Green Version]
- Nauta, I.H.; Heideman, D.A.M.; Brink, A.; van der Steen, B.; Bloemena, E.; Koljenović, S.; Baatenburg de Jong, R.J.; Leemans, C.R.; Brakenhoff, R.H. The unveiled reality of human papillomavirus as risk factor for oral cavity squamous cell carcinoma. Int. J. Cancer 2021, 149, 420–430. [Google Scholar] [CrossRef]
- Stephen, J.K.; Divine, G.; Chen, K.M.; Chitale, D.; Havard, S.; Worsham, M.J. Significance of p16 in Site-specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer Clin. Oncol. 2013, 2, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Vitzthum, L.K.; Mell, L.K. The role of p16 as a biomarker in nonoropharyngeal head and neck cancer. Oncotarget 2018, 9, 33247–33248. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Windon, M.J.; Waterboer, T.; Hillel, A.T.; Chien, W.; Best, S.; Stewart, C.; Akst, L.; Troy, T.; Bender, N.; Miles, B.; et al. Sex differences in HPV immunity among adults without cancer. Hum. Vaccin. Immunother. 2019, 15, 1935–1941. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Dotto, G.P. Sex Hormones and Anticancer Immunity. Clin. Cancer Res. 2019, 25, 4603–4610. [Google Scholar] [CrossRef] [Green Version]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef]
| Type | Samples | Population | Major Findings |
|---|---|---|---|
| Longitudinal Cohort Study | 59 (from 42 patients) | 17 HNSCC (7 HPV+ OPSCC, 4 HPV− OPSCC, 6 HPV− OSCC), 25 control | Shift in bacterial abundance in HPV+ OPSCC following treatment; microbial diversity may be used as a diagnostic for HNSCC [111] |
| 100 (from 50 patients) | Tumor and non-tumor sites from OSCC patients | Increased richness and diversity in OSCC tumor sites; higher prevalence of Prevotellaceae, Fusobacteriaceae, Flavobacteriaceae, Lachnospiraceae, Peptostreptococcaceae, and Campylobacteraceae in OSCC [110] | |
| 83 | Tumor and anatomically matched normal tissue from oral cancer and pre-cancer | Reduction of Firmicutes and Actinobacteria in cancer [112] | |
| Prospective study | 383 | 129 HNSCC and 254 matched controls | Corynebacterium and Kingella are associated with a lower risk of HNSCC [113] |
| 38 | 18 oral cavity squamous cell cancer (OCSCC), 8 pre-malignant lesions, 12 control | Fusobacterium, Prevotella, Alloprevotella-enriched in OCSCC [114] | |
| 51 | 27 smokers with and 24 without HNSCC | Higher relative abundance of bacteria involved in xenobiotic and amine degradation in HNSCC [115] | |
| Retrospective analysis | 151 | Oral squamous cell carcinoma (OSCC) patients | F. nucleatum-associated OSCC is associated with favorable prognosis [116] |
| 325 | Esophageal Cancer (300 SCC, 12 adenocarcinomas, 13 others) | F. nucleatum is a potential biomarker for esophageal cancer is associated with poor prognosis [117] |
| Source | Parent Study | Years | Treatment | Design | Number of Patients in Analysis | Disease Sites | HPV Assessment | % HPV+/HPV− | Outcome (HPV+ vs. HPV−) |
|---|---|---|---|---|---|---|---|---|---|
| Licitra, et al., 2006 [146] | NA | 1990–1999 | Surgery followed by RT | Single-arm, retrospective | 90 | OP | HPV 16/18 DNA PCR | 19/81 | 5-year OS (79% vs. 46%), 5-year incidence tumor relapse (21% vs. 53%), 5-year incidence second primary (0 vs. 12%) |
| Fakhry, et al., 2008 [148] | E2399 | 2001–2004 | IC followed by CRT | Single-arm Phase II | 96 | OP, Larynx | HPV types 16, 33, 35 DNA ISH | 39.6/60.4 | ORR to IC (82% vs. 55%, p = 0.01) and CRT (84% vs. 57% p = 0.007, 2-year OS (95% vs 62%, p = 0.005), |
| Ang, et al., 2010 [14] | RTOG 0129 | 2002–2005 | CRT (accelerated fx vs. standard RT) | Randomized phase III | 323 | OP | HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 DNA ISH | 63.8/36.2 | 3-year OS (82.4%, vs. 57.1%, p < 0.001), 3-year PFS (73.7% vs. 43.4%, p < 0.001) |
| Rischin, et al., 2010 [149] | TROG 02.02 | 2002–2005 | CRT | Randomized phase III | 182 | OP | P16 IHC | 57.3/42.7 | 2-year OS (91% vs. 74%, p = 0.004), 2-year FFS (87% vs. 72%, p = 0.003) |
| Posner, et al., 2011 [150] | TAX 324 | 1999–2003 | IC followed by CRT | Randomized Phase III | 111 | OP | HPV E6/E7 PCR | 50/50 | * OS (79% vs. 31%, p < 0.0001, PFS (73% vs. 29%, p < 0.0001), LRF (13% vs. 42%, p = 0.0006) |
| Trial | Design (No. of Patients) | Patient Population | De-Escalation Strategy and Regimens (* De-Escalation Arm) | Primary Outcome Measure | Status | Summary of Findings (if Completed) |
|---|---|---|---|---|---|---|
| RTOG 1016 [170] | Randomized, noninferiority phase 3 (n = 987) | AJCC 7th ed. T1-T2, N2-3 or T3-T4, N0-N3; any smoking history | Chemotherapy
| OS | Completed | Cetuximab was not shown to be non-inferior to cisplatin |
| De-ESCALaTe [171] | Randomized phase 3 (n = 334) | AJCC 7th ed. T3-T4, N0 or T1-T4, N1-N3; <10 PYH | Chemotherapy
| OS and late toxicity | Completed | Mean number of severe events and all grade toxicity the same in both groups |
| TROG 12.01 [172] | Randomized phase 3 (n = 189) | AJCC 7th ed. Stage III (excluding T1-2N1) or stage IVA-B (excluding T4 and/or N3 and/or N2b-c); ≤10 PYH of smoking | Chemotherapy
| Difference in AUC of MDADI from baseline to 13 weeks post-therapy | Completed | No difference in AUC of MDADI between groups; Worse 3-year FFS in cetuximab (80% [95% CI: 70–87%]) vs. cisplatin (93% [95% CI: 86–97%]) |
| ORATOR [173] | Randomized Phase II (n = 68) | AJCC 7th ed. T1-T2, N0-2 (≤4 cm); any smoking history | Surgery
| 1-year swallowing QoL by MDADI | Completed | 1-year MDADI score higher in RT group |
| ECOG 3311 [174] | Randomized phase II (n = 519 overall, 209 Arms B and C) | AJCC 7th ed. stage III-IVA resected, intermediate pathologic risk (close margins [<3 mm], 2–4+ nodes or 1 node >3 cm and ≤6 cm, ENE ≤ 1 mm, or PNI/LVI); any smoking history | PORT
| 2-year PFS | Completed | 2-year PFS similar in Arm B 95.0% (90% CI = 91.4%, 98.6%) to ARM C 95.9% (90% CI = 92.6%, 99.3%) |
| NRG-HN002 [175] | randomized, phase II trial (n = 306) | AJCC 7th ed. T1-T2 N1-N2b M0, or T3 N0-N2b M0; ≤10 PYH of smoking | No chemotherapy
| 2-year PFS and 1-year swallowing QoL by MDADI | Completed | Similar 2-year PFS (88% RT vs. 91% CRT), but RT alone did not meet pre-specified 2-year PFS goal |
| PATHOS [NCT02215265] | Randomized phase II/II (n = up to 1100) | AJCC 7th ed. T1-T3, N0-N2b 8th edition stage T1-T3, N0-N1; Any smoking history (except current smokers with N2b disease) | PORT
| 1-year MDADI and Overall survival | Ongoing | NA |
| NRG-HN005 [NCT03952585] | Randomized phase II/III (n = up to 711) | AJCC 8th ed. 8th T1-2N1M0 or T3N0-N1M0; ≤ 10 PYH of smoking | Radiation and chemotherapy
| Phase II, PFSPhase III, PFSand QoL by the MDADI global score | Ongoing | NA |
| DART-HPV [NCT02908477] | Randomized phase III (n = 227) | TORS resected primary disease with either AJCC 8th ed. T3/4 or N2b disease, and/or ENE, LVI, PNI | POCRT
| Adverse event rate (late grade 3–5 toxicities) | Ongoing | NA |
| Trial | Design (No. of Patients) | Patient Population | Intensification Strategy and Regimens (* Intensification Arm) | Primary Outcome Measure | Status | Summary of Findings (if Completed) |
|---|---|---|---|---|---|---|
| RTOG 0129 [176] | Randomized Phase III (n = 721) | AJCC 6th ed. Stage III-IVB OC, OP, HP, Larynx; any HPV risk group | Accelerated fraction (AFX) RT
| OS | Completed | No difference in OS (HR, 0.96; 95% CI, 0.79 to 1.18; p = 0.37). |
| RTOG 0522 [177] | Randomized Phase III (n = 891) | AJCC 6th ed. stage III-IVB OC, OP, HP, Larynx; any HPV risk group | Combination chemotherapy
| PFS | Completed | No difference in PFS, OS, LRF. Higher acute toxicities with the addition of cetuximab |
| PARADIGM [178] | Randomized Phase III (n = 145) | AJCC 6th ed. Stage IVA-IVB (T3/T4 or N2/N3, but not T1/N2) OC, OP, Larynx; any HPV risk group | Induction chemotherapy
| OS | Completed | No difference in OS (HR, 1.09; 95% CI 0.59–2.03). poor accrual (145 of 300 planned) |
| JAVELIN-Head and Neck 100 [179] | Randomized Phase III (n = 697) | AJCC 7th ed. HPV- Stage III, IVA, IVb disease; non-OP HPV+Stage III, IVA, IVB disease; HPV+ OP T4 or N2c or N3 disease | Combination PD-1 blockade + CRT
| PFS | Completed | Median PFS was not reached in either group, however stratified hazard ratio (1.21 [95% CI 0.93–1.57]) favored the placebo group (one-sided p = 0.92) |
| KEYNOTE-412 [NCT03040999] | Randomized Phase III (n = 780 planned) | AJCC 7th ed OP HPV+ (any T4 or N3), OP HPV− (any T3-T4 or N2a-N3), or larynx/HP/OC (any T3-T4 or N2a-N3) | Combination PD-1 blockade + CRT
| Event Free Survival (EFS) | Ongoing | NA |
| KEYNOTE-689 [NCT03765918] | Randomized Phase III (n = 704 planned) | AJCC 8th ed. resectable, stage III/IVA HPV− or T4N0-2 HPV+ | Neoadjuvant PD-1 blockade
| Major Pathological Response (mPR) and EFS | Ongoing | NA |
| RTOG 1216 [NCT01810913] | Randomized phase II/III (n = 480 planned) | Resected AJCC 7th ed Stage III-IVB HPV+ or HPV− disease with high-risk features (ENE or positive margins) | Adjuvant POCRT + PD-L1 blockade
| Phase II-DFSPhase III-OS | Ongoing | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, S.F.; Vu, L.; Spanos, W.C.; Pyeon, D. The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers 2021, 13, 5206. https://doi.org/10.3390/cancers13205206
Powell SF, Vu L, Spanos WC, Pyeon D. The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers. 2021; 13(20):5206. https://doi.org/10.3390/cancers13205206
Chicago/Turabian StylePowell, Steven F., Lexi Vu, William C. Spanos, and Dohun Pyeon. 2021. "The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications" Cancers 13, no. 20: 5206. https://doi.org/10.3390/cancers13205206
APA StylePowell, S. F., Vu, L., Spanos, W. C., & Pyeon, D. (2021). The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers, 13(20), 5206. https://doi.org/10.3390/cancers13205206






