Modern Rotational Radiation Techniques with Volumetric Modulated Arc Therapy or Helical Tomotherapy for Optimal Sparing of the Lung and Heart in Left-Breast Cancer Radiotherapy Plus Regional Nodal Irradiation: A Comparative Dosimetric Analysis
Abstract
:Simple Summary
Abstract
1. Background
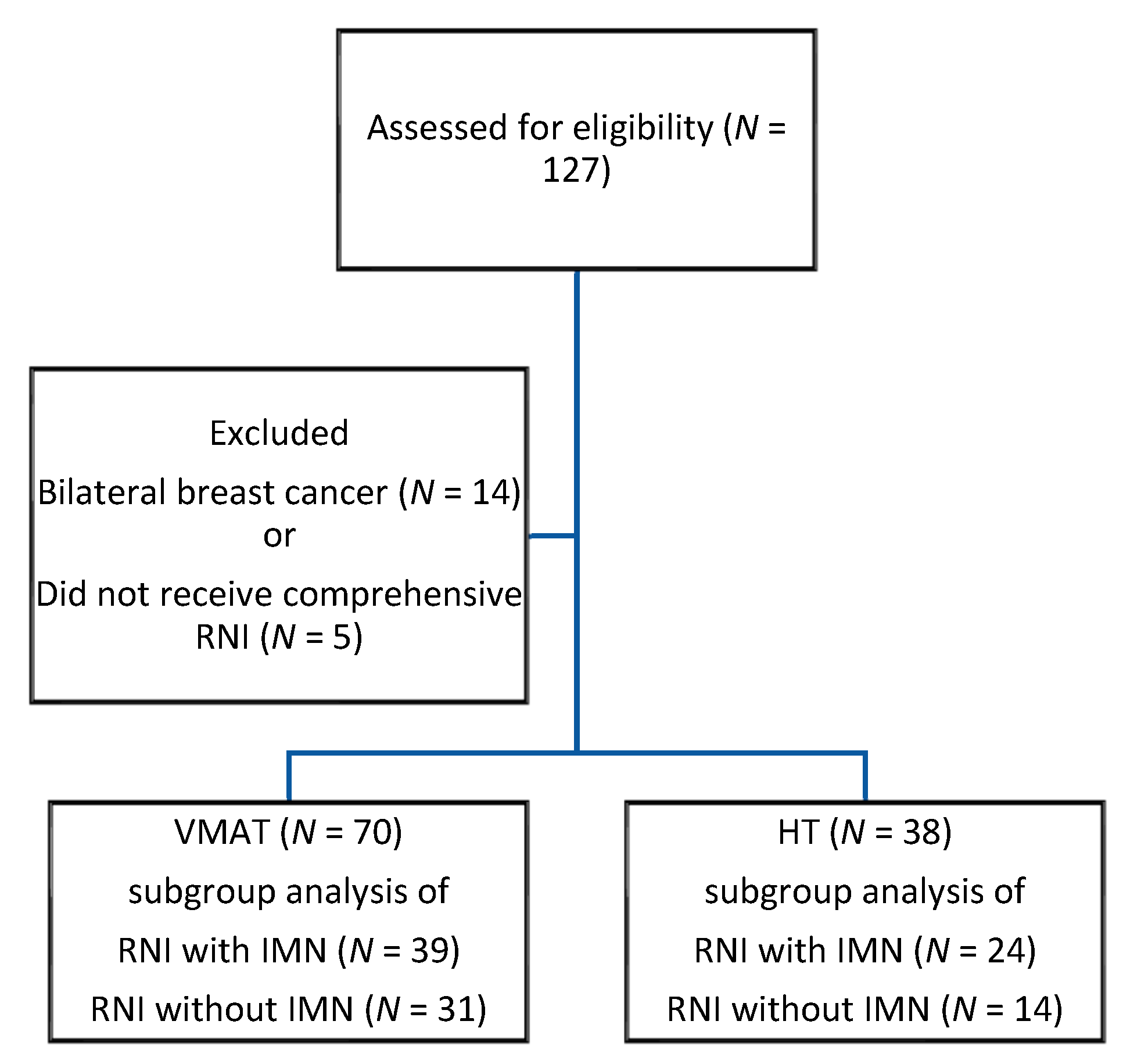
2. Methods
2.1. Patient Population
2.2. RT Treatment Plan
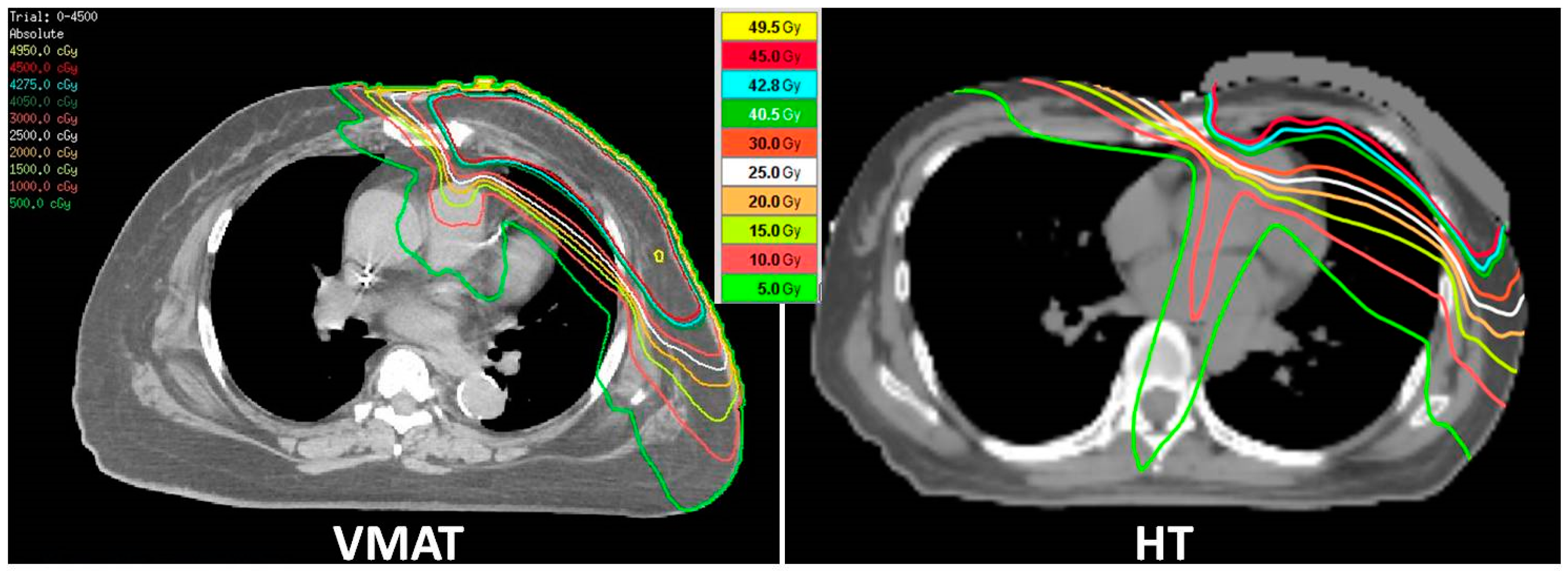
2.3. RT Technique and Dosimetric Evaluation
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Comparisons of Dosimetric Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Tasaka, H.; Yu, K.P.; Murphy, M.L.; Karnofsky, D.A. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 1967, 20, 333–353. [Google Scholar] [CrossRef]
- Cardinale, D. A new frontier: Cardio-oncology. Cardiologia 1996, 41, 887–891. [Google Scholar] [PubMed]
- de Azambuja, E.; Bedard, P.L.; Suter, T.; Piccart-Gebhart, M. Cardiac toxicity with anti-HER-2 therapies-what have we learned so far? Target. Oncol. 2009, 4, 77–88. [Google Scholar] [CrossRef]
- Florido, R.; Smith, K.L.; Cuomo, K.K.; Russell, S.D. Cardiotoxicity from human epidermal growth factor receptor-2 (HER 2) targeted therapies. J. Am. Heart Assoc. 2017, 6, e006915. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Bronnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Poortmans, P.M.; Weltens, C.; Fortpied, C.; Kirkove, C.; Peignaux-Casasnovas, K.; Budach, V.; van der Leij, F.; Vonk, E.; Weidner, N.; Rivera, S.; et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I–III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1602–1610. [Google Scholar] [CrossRef]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R. Estimating the risks of breast cancer radiotherapy: Evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J. Clin. Oncol. 2017, 35, 1641. [Google Scholar] [CrossRef]
- Correa, C.R.; Litt, H.I.; Hwang, W.T.; Ferrari, V.A.; Solin, L.J.; Harris, E.E. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J. Clin. Oncol. 2007, 25, 3031–3037. [Google Scholar] [CrossRef]
- Gutt, R.; Correa, C.R.; Hwang, W.T.; Solin, L.J.; Litt, H.I.; Ferrari, V.A.; Harris, E.E. Cardiac morbidity and mortality after breast conservation treatment in patients with early-stage breast cancer and preexisting cardiac disease. Clin. Breast Cancer 2008, 8, 443–448. [Google Scholar] [CrossRef]
- Harris, E.E.; Correa, C.; Hwang, W.T.; Liao, J.; Litt, H.I.; Ferrari, V.A.; Solin, L.J. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J. Clin. Oncol. 2006, 24, 4100–4106. [Google Scholar] [CrossRef]
- Hooning, M.J.; Botma, A.; Aleman, B.M.; Baaijens, M.H.; Bartelink, H.; Klijn, J.G.; Taylor, C.W.; van Leeuwen, F.E. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J. Natl. Cancer Inst. 2007, 99, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Jagsi, R.; Griffith, K.A.; Koelling, T.; Roberts, R.; Pierce, L.J. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stage breast cancer. Cancer 2007, 109, 650–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grantzau, T.; Thomsen, M.S.; Væth, M.; Overgaard, J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother. Oncol. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Holmes, T.W.; Afghan, M.K.; Shepard, D.M. Comparison of plan quality provided by intensity-modulated arc therapy and helical tomotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Tyran, M.; Mailleux, H.; Tallet, A.; Fau, P.; Gonzague, L.; Minsat, M.; Moureau-Zabotto, L.; Resbeut, M. Volumetric-modulated arc therapy for left-sided breast cancer and all regional nodes improves target volumes coverage and reduces treatment time and doses to the heart and left coronary artery, compared with a field-in-field technique. J. Radiat. Res. 2015, 56, 927–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, C.C.; Olivotto, I.A.; Beckham, W.A.; Ansbacher, W.; Zavgorodni, S.; Shaffer, R.; Wai, E.S.; Otto, K. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Nobnop, W.; Phakoetsuk, P.; Chitapanarux, I.; Tippanya, D.; Khamchompoo, D. Dosimetric comparison of TomoDirect, helical tomotherapy, and volumetric modulated arc therapy for postmastectomy treatment. J. Appl. Clin. Med. Phys. 2020, 21, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lauche, O.; Kirova, Y.M.; Fenoglietto, P.; Costa, E.; Lemanski, C.; Bourgier, C.; Riou, O.; Tiberi, D.; Campana, F.; Fourquet, A. Helical tomotherapy and volumetric modulated arc therapy: New therapeutic arms in the breast cancer radiotherapy. World J. Radiol. 2016, 8, 735. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.P.; Huang, Y.C.; Wang, L.Y.; Shueng, P.W.; Tien, H.J.; Chang, C.H.; Chou, S.F.; Hsieh, C.H. Helical tomotherapy with a complete-directional-complete block technique effectively reduces cardiac and lung dose for left-sided breast cancer. Br. J. Radiol. 2020, 93, 20190792. [Google Scholar] [CrossRef] [PubMed]
- Shiau, A.-C.; Hsieh, C.-H.; Tien, H.-J.; Yeh, H.-P.; Lin, C.-T.; Shueng, P.-W.; Wu, L.-J. Left-sided whole breast irradiation with hybrid-IMRT and helical tomotherapy dosimetric comparison. BioMed Res. Int. 2014, 2014, 741326. [Google Scholar] [CrossRef]
- Duane, F.; McGale, P.; Teoh, S.; Mortimer, C.; Broggio, J.; Darby, S.; Dodwell, D.; Lavery, B.; Oliveros, S.; Vallis, K. International variation in criteria for internal mammary chain radiotherapy. Clin. Oncol. 2019, 31, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, T.J.; Olivotto, I.A.; Parulekar, W.R.; Ackerman, I.; Chua, B.H.; Nabid, A.; Vallis, K.A.; White, J.R.; Rousseau, P.; Fortin, A.; et al. Regional nodal irradiation in early-stage breast cancer. N. Engl. J. Med. 2015, 373, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yu, X.L.; Hu, W.G.; Chen, J.Y.; Wang, J.Z.; Ye, J.S.; Guo, X.M. Dosimetric comparison for volumetric modulated arc therapy and intensity-modulated radiotherapy on the left-sided chest wall and internal mammary nodes irradiation in treating post-mastectomy breast cancer. Radiol. Oncol. 2015, 49, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamounas, E.P.; White, J.R.; Bandos, H.; Julian, T.B.; Kahn, A.J.; Shaitelman, S.F.; Torres, M.A.; McCloskey, S.A.; Vicini, F.A.; Ganz, P.A.; et al. NSABP B-51/RTOG 1304: Randomized phase III clinical trial evaluating the role of postmastectomy chest wall and regional nodal XRT (CWRNRT) and post-lumpectomy RNRT in patients (pts) with documented positive axillary (Ax) nodes before neoadjuvant chemotherapy (NC) who convert to pathologically negative Ax nodes after NC. J. Clin. Oncol. 2014, 32, TPS1141. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Jagsi, R.; Griffith, K.A.; Moran, J.M.; Ficaro, E.; Marsh, R.; Dess, R.T.; Chung, E.; Liss, A.L.; Hayman, J.A.; Mayo, C.S.; et al. A randomized comparison of radiation therapy techniques in the management of node-positive breast cancer: Primary outcomes analysis. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1149–1158. [Google Scholar] [CrossRef]
- Yeung, R.; Conroy, L.; Long, K.; Walrath, D.; Li, H.; Smith, W.; Hudson, A.; Phan, T. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiat. Oncol. 2015, 10, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-S.; Chen, C.-H.; Liu, K.-C.; Ho, C.-S.; Chen, M.-F. Selection of patients with left breast cancer for IMRT with deep inspiration breath-hold technique. J. Radiat. Res. 2020, 61, 431–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanguturi, S.K.; Lyatskaya, Y.; Chen, Y.; Catalano, P.J.; Chen, M.H.; Yeo, W.-P.; Marques, A.; Truong, L.; Yeh, M.; Orlina, L. Prospective assessment of deep inspiration breath-hold using 3-dimensional surface tracking for irradiation of left-sided breast cancer. Pract. Radiat. Oncol. 2015, 5, 358–365. [Google Scholar] [CrossRef]
- Tanna, N.; McLauchlan, R.; Karis, S.; Welgemoed, C.; Gujral, D.; Cleator, S. Assessment of upfront selection criteria to prioritise patients for breath-hold left-sided breast radiotherapy. Clin. Oncol. 2017, 29, 356–361. [Google Scholar] [CrossRef]
- Gaál, S.; Kahán, Z.; Paczona, V.; Kószó, R.; Drencsényi, R.; Szabó, J.; Rónai, R.; Antal, T.; Deák, B.; Varga, Z. Deep-inspirational breath-hold (DIBH) technique in left-sided breast cancer: Various aspects of clinical utility. Radiat. Oncol. 2021, 16, 89. [Google Scholar] [CrossRef]
- Dell’Oro, M.; Giles, E.; Sharkey, A.; Borg, M.; Connell, C.; Bezak, E. A retrospective dosimetric study of radiotherapy patients with left-sided breast cancer; patient selection criteria for deep inspiration breath hold technique. Cancers 2019, 11, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, L.; Goldsmith, C.; Green, M.M.; Cleator, S.; Price, P.M. An effective deep-inspiration breath-hold radiotherapy technique for left-breast cancer: Impact of post-mastectomy treatment, nodal coverage, and dose schedule on organs at risk. Breast Cancer Targets Ther. 2017, 9, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boice Jr, J.D.; Harvey, E.B.; Blettner, M.; Stovall, M.; Flannery, J.T. Cancer in the contralateral breast after radiotherapy for breast cancer. N. Engl. J. Med. 1992, 326, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Stovall, M.; Smith, S.A.; Langholz, B.M.; Boice, J.D., Jr.; Shore, R.E.; Andersson, M.; Buchholz, T.A.; Capanu, M.; Bernstein, L.; Lynch, C.F. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Alterio, D.; Jereczek-Fossa, B.A.; Franchi, B.; D’Onofrio, A.; Piazzi, V.; Rondi, E.; Ciocca, M.; Gibelli, B.; Grosso, E.; Tradati, N. Thyroid disorders in patients treated with radiotherapy for head-and-neck cancer: A retrospective analysis of seventy-three patients. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Siala, W.; Mnejja, W.; Abid, M.; Ghorbel, A.; Frikha, M.; Daoud, J. Thyroid toxicity after radiotherapy of nasopharyngeal carcinoma. Annal. Endocrinol. 2011, 72, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.A.; Alterio, D.; Jassem, J.; Gibelli, B.; Tradati, N.; Orecchia, R. Radiotherapy-induced thyroid disorders. Cancer Treat. Rev. 2004, 30, 369–384. [Google Scholar] [CrossRef]
- Cella, L.; Conson, M.; Caterino, M.; De Rosa, N.; Liuzzi, R.; Picardi, M.; Grimaldi, F.; Solla, R.; Farella, A.; Salvatore, M. Thyroid V30 predicts radiation-induced hypothyroidism in patients treated with sequential chemo-radiotherapy for Hodgkin’s lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1802–1808. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, S.; Refaat, T.; Bacchus, I.D.; Sathiaseelan, V.; Mittal, B.B. Thyroid V50 highly predictive of hypothyroidism in head-and-neck cancer patients treated with intensity-modulated radiotherapy (IMRT). Am. J. Clin. Oncol. 2017, 40, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Chang, J.S.; Byun, H.K.; Son, N.H.; Hong, C.S.; Hong, N.; Park, M.Y.I.; Kim, J.; Kim, J.S.; Kim, Y.B. Risk of hypothyroidism in women after radiation therapy for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 462–472. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | VMAT (n = 70) | HT (n = 38) | p-Value |
|---|---|---|---|
| Lumpectomy: N (%) | 35 (50%) | 23 (60.5%) | 0.30 |
| Mastectomy: N (%) | 35 (50%) | 15 (39.5%) | |
| RT total dose (Gy) (SD) (range) | 56.4 (6.6) (40–74) | 56.5 (5.4) (45–70) | 0.97 |
| Number of fractions (SD) (range) | 28.7 (4.6) (15–37) | 28.8 (4.1) (16–35) | 0.92 |
| RT total dose EQD2 (Gy) | 57.1 | 57.8 | 0.77 |
| Conventional fractionation: N (%) Hypofractionation: N (%) | 63 (90%) 7 (10%) | 34 (89.5%) 4 (10.5%) | 1 |
| CTV (mL) (SD) (range) | 481.7 (263.8) (158.5–1314.4) | 532.3 (383.5) (150.4–1845.6) | 0.42 |
| PTV (mL) (SD) (range) | 974.3 (382.1) (402.2–2137.8) | 1058.8 (544.2) (388.9–2937.1) | 0.35 |
| RT target volume | 0.44 | ||
| Breast_ SCF | 21 (30%) | 10 (26.3%) | |
| Breast_ SCF_IMN | 14 (20%) | 13 (34.2%) | |
| Chest wall CW_ SCF | 10 (14.3%) | 4 (10.5%) | |
| Chest wall CW_ SCF_IMN | 25 (35.7%) | 11 (28.9%) | |
| RNI volume | 0.45 | ||
| IMN uninvolved | 31 (44.3%) | 14 (36.8%) | |
| IMN involved | 39 (55.7%) | 24 (63.2%) |
| Characteristics | Heart | Left Lung | Whole Lung | Other Normal Organs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VMAT (n = 70) | HT (n = 38) | p-Value | VMAT (n = 70) | HT (n = 38) | p-Value | VMAT (n = 70) | HT (n = 38) | p-Value | VMAT (n = 70) | HT (n = 38) | p-Value | ||
| Volume (cc) | 536.9 | 505.3 | 0.16 | 952.1 | 933.1 | 0.69 | 2192.0 | 2150.8 | 0.68 | Mean dose (Gy) | |||
| Mean dose (Gy) | 3.82 | 5.13 | <0.001 | 9.99 | 11.16 | 0.054 | 5.57 | 6.32 | 0.006 | Contralateral breast | 2.56 | 3.39 | <0.001 |
| Mean ≤ 4 Gy (%) | 42 (60) | 10 (26.3) | 0.002 | Thyroid | 21.53 | 20.34 | 0.32 | ||||||
| V5 (%) | 13.96 | 28.53 | <0.001 | 40.03 | 52.05 | <0.001 | 20.49 | 27.56 | <0.001 | Trachea | 11.07 | 11.61 | 0.53 |
| V10 (%) | 6.37 | 14.52 | <0.001 | 27.94 | 37.45 | <0.001 | 13.26 | 17.70 | <0.001 | Esophagus | 5.67 | 8.64 | <0.001 |
| V15 (%) | 4.29 | 8.10 | <0.001 | - | - | - | - | - | - | ||||
| V20 (%) | 3.11 | 4.66 | 0.02 | 18.53 | 22.23 | 0.01 | 8.84 | 10.10 | 0.050 | ||||
| V25 (%) | 2.16 | 2.66 | 0.30 | - | - | - | - | - | - | Maximal dose (Gy) | |||
| V30 (%) | 1.46 | 1.49 | 0.92 | - | - | - | - | - | - | Cord | 20.10 | 20.72 | 0.55 |
| Characteristics | Heart | Left Lung | Whole Lung | Other Normal Organs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VMAT (n = 39) | HT (n = 24) | p-Value | VMAT (n = 39) | HT (n = 24) | p-Value | VMAT (n = 39) | HT (n = 24) | p-Value | VMAT (n = 39) | HT (n = 24) | p-Value | ||
| Volume (cc) | 544.5 | 510.8 | 0.30 | 949.2 | 907.1 | 0.73 | 2199.0 | 2089.7 | 0.45 | Mean dose (Gy) | |||
| Mean dose (Gy) | 4.43 | 5.80 | 0.002 | 10.30 | 11.23 | 0.11 | 6.02 | 6.82 | 0.006 | Contralateral breast | 2.92 | 3.71 | <0.001 |
| V5 (%) | 18.31 | 34.44 | <0.001 | 40.80 | 54.56 | <0.001 | 22.31 | 30.48 | <0.001 | Thyroid | 22.08 | 19.58 | 0.02 |
| V10 (%) | 8.18 | 17.11 | <0.001 | 28.80 | 38.93 | <0.001 | 14.36 | 19.14 | <0.001 | Trachea | 10.93 | 10.94 | 0.72 |
| V15 (%) | 5.44 | 9.28 | 0.004 | - | - | - | - | - | - | Esophagus | 5.74 | 8.71 | <0.001 |
| V20 (%) | 3.82 | 5.23 | 0.13 | 19.21 | 22.74 | 0.04 | 9.69 | 10.61 | 0.72 | ||||
| V25 (%) | 2.59 | 2.89 | 0.61 | - | - | - | - | - | - | Maximal dose (Gy) | |||
| V30 (%) | 1.77 | 1.60 | 0.45 | - | - | - | - | - | - | Cord | 20.19 | 20.17 | 0.88 |
| Characteristics | Heart | Left Lung | Whole Lung | Other Normal Organs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VMAT (n = 31) | HT (n = 14) | p-Value | VMAT (n = 31) | HT (n = 14) | p-Value | VMAT (n = 31) | HT (n = 14) | p-Value | VMAT (n = 31) | HT (n = 14) | p-Value | ||
| Volume (cc) | 527.3 | 495.3 | 0.55 | 955.8 | 977.7 | 0.51 | 2183.2 | 2255.6 | 0.62 | Mean dose (Gy) | |||
| Mean dose (Gy) | 3.05 | 3.89 | 0.07 | 9.61 | 11.04 | 0.01 | 5.02 | 5.48 | 0.08 | Contralateral breast | 2.11 | 2.84 | 0.29 |
| V5 (%) | 8.48 | 17.63 | 0.007 | 39.07 | 47.76 | 0.001 | 18.19 | 22.54 | 0.003 | Thyroid | 20.80 | 21.73 | 0.61 |
| V10 (%) | 4.10 | 9.73 | 0.004 | 26.87 | 34.91 | 0.001 | 11.87 | 15.24 | 0.001 | Trachea | 11.25 | 12.76 | 0.22 |
| V15 (%) | 2.84 | 5.89 | 0.03 | - | - | - | - | - | - | Esophagus | 5.58 | 8.53 | 0.002 |
| V20 (%) | 2.23 | 3.62 | 0.12 | 17.68 | 21.36 | 0.02 | 7.77 | 9.24 | 0.02 | ||||
| V25 (%) | 1.61 | 2.24 | 0.14 | - | - | - | - | - | - | Maximal dose (Gy) | |||
| V30 (%) | 1.06 | 1.29 | 0.16 | - | - | - | - | - | - | Cord | 19.97 | 21.65 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, P.-Y.; Hsieh, C.-H.; Wu, L.-J.; Hsu, C.-X.; Kuo, D.-Y.; Lu, Y.-F.; Tien, H.-J.; Hsiao, H.-W.; Shueng, P.-W.; Hsu, S.-M. Modern Rotational Radiation Techniques with Volumetric Modulated Arc Therapy or Helical Tomotherapy for Optimal Sparing of the Lung and Heart in Left-Breast Cancer Radiotherapy Plus Regional Nodal Irradiation: A Comparative Dosimetric Analysis. Cancers 2021, 13, 5043. https://doi.org/10.3390/cancers13205043
Hou P-Y, Hsieh C-H, Wu L-J, Hsu C-X, Kuo D-Y, Lu Y-F, Tien H-J, Hsiao H-W, Shueng P-W, Hsu S-M. Modern Rotational Radiation Techniques with Volumetric Modulated Arc Therapy or Helical Tomotherapy for Optimal Sparing of the Lung and Heart in Left-Breast Cancer Radiotherapy Plus Regional Nodal Irradiation: A Comparative Dosimetric Analysis. Cancers. 2021; 13(20):5043. https://doi.org/10.3390/cancers13205043
Chicago/Turabian StyleHou, Pei-Yu, Chen-Hsi Hsieh, Le-Jung Wu, Chen-Xiong Hsu, Deng-Yu Kuo, Yueh-Feng Lu, Hui-Ju Tien, Hsiu-Wen Hsiao, Pei-Wei Shueng, and Shih-Ming Hsu. 2021. "Modern Rotational Radiation Techniques with Volumetric Modulated Arc Therapy or Helical Tomotherapy for Optimal Sparing of the Lung and Heart in Left-Breast Cancer Radiotherapy Plus Regional Nodal Irradiation: A Comparative Dosimetric Analysis" Cancers 13, no. 20: 5043. https://doi.org/10.3390/cancers13205043
APA StyleHou, P.-Y., Hsieh, C.-H., Wu, L.-J., Hsu, C.-X., Kuo, D.-Y., Lu, Y.-F., Tien, H.-J., Hsiao, H.-W., Shueng, P.-W., & Hsu, S.-M. (2021). Modern Rotational Radiation Techniques with Volumetric Modulated Arc Therapy or Helical Tomotherapy for Optimal Sparing of the Lung and Heart in Left-Breast Cancer Radiotherapy Plus Regional Nodal Irradiation: A Comparative Dosimetric Analysis. Cancers, 13(20), 5043. https://doi.org/10.3390/cancers13205043





