The Dual Role of Innate Lymphoid and Natural Killer Cells in Cancer. from Phenotype to Single-Cell Transcriptomics, Functions and Clinical Uses
Abstract
Simple Summary
Abstract
1. Introduction
2. NK and Helper ILCs in Cancer Control, Escape and Progression
2.1. Physiological Function of NK Cells and Their Receptors
2.2. NK Cell Responses to Cancer
2.3. Classification of Helper ILCs and Their Physiological Roles
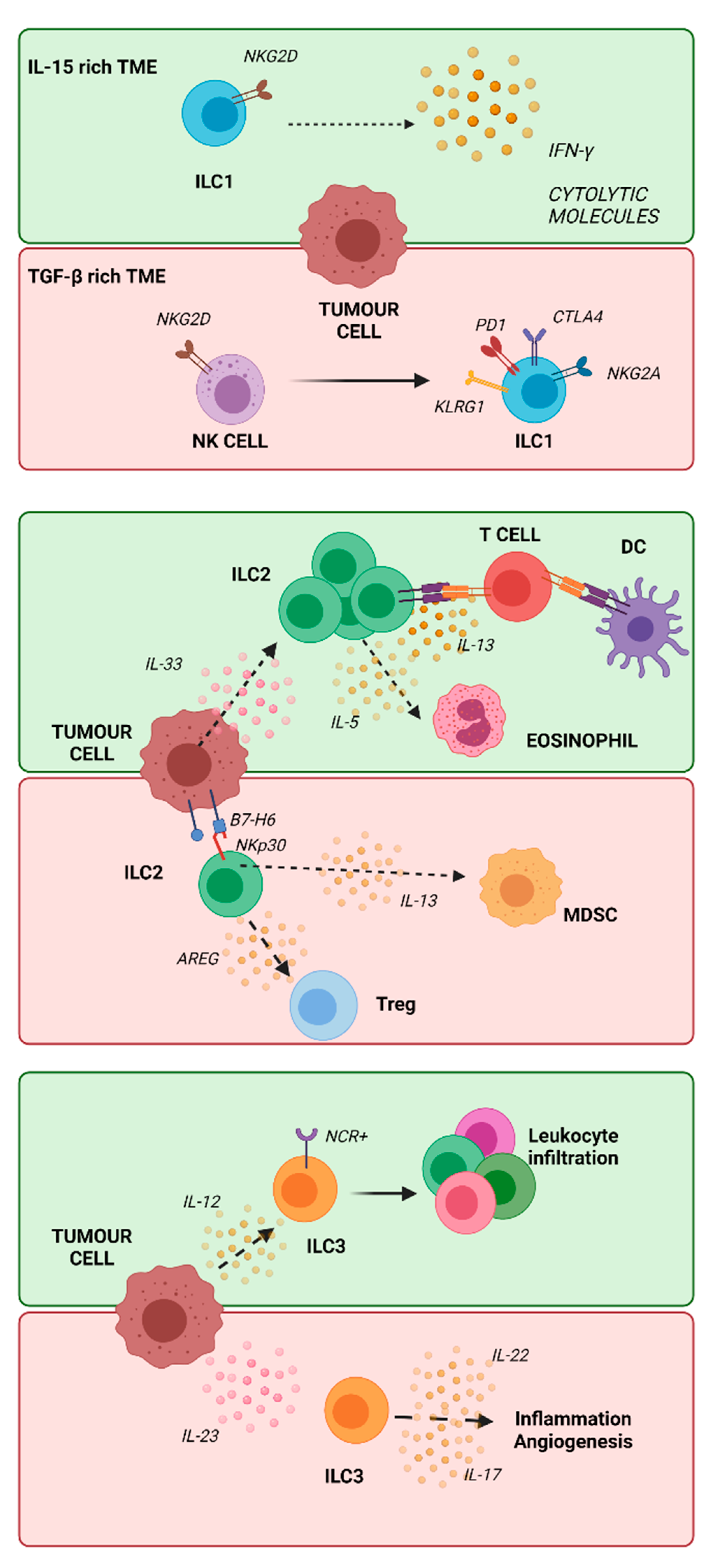
2.4. ILCs in Cancer
2.4.1. ILC1 in Cancer
2.4.2. ILC2 in Cancer
2.4.3. ILC3 in Cancer
3. NK Cells as Cancer Therapeutics
3.1. Clinical-Grade NK Cells Generated from Differentiated or Progenitor Cells
3.2. Autologous vs. Allogeneic Clinical-Grade NK Cells
3.3. Naïve vs. Activated Engineered or Monoclonal-Antibody-Associated NK Cells
4. How Single-Cell RNA Sequencing Studies Have Reshaped the Field and Will Contribute Further
4.1. ScRNA Sequencing State of the Art
4.2. A scRNA-seq Gaze of Helper-like ILCs
4.3. NKs under a Single-Cell RNA Microscope
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vrtílek, M.; Bolnick, D.I. Macroevolutionary foundations of a recently-evolved innate immune defense. Evolution 2021. [Google Scholar] [CrossRef]
- Wendel, P.; Reindl, L.M.; Bexte, T.; Künnemeyer, L.; Särchen, V.; Albinger, N.; Mackensen, A.; Rettinger, E.; Bopp, T.; Ullrich, E. Arming immune cells for battle: A brief journey through the advancements of t and nk cell immunotherapy. Cancers 2021, 13, 1481. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural Innate and Adaptive Immunity to Cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Spits, H.; Di Santo, J.P. The expanding family of innate lymphoid cells: Regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011, 12, 21–27. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Stabile, H.; Fionda, C.; Gismondi, A.; Santoni, A. Role of distinct natural killer cell subsets in anticancer response. Front. Immunol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Cooper, M.A.; Nuovo, G.J.; Cella, M.; Facchetti, F.; Colonna, M.; Caligiuri, M.A. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: A potential new link between adaptive and innate immunity. Blood 2003, 101, 3052–3057. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56BRIGHT SUBSET. Blood 2000, 96, 3146–3151. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Shah, M.H.; Turner, M.J.; VanDeusen, J.B.; Whitman, S.P.; Cooper, M.A.; Suzuki, K.; Wechser, M.; Goodsaid, F.; Caligiuri, M.A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: Implications for the innate immune response. J. Immunol. 1999, 162, 4511–4520. [Google Scholar]
- Cooper, M.A.; Fehniger, T.A.; Ponnappan, A.; Mehta, V.; Wewers, M.D.; Caligiuri, M.A. Interleukin-1β costimulates interferon-γ production by human natural killer cells. Eur. J. Immunol. 2001, 31, 792–801. [Google Scholar] [CrossRef]
- Santoni, A.; Bertaina, A.; Gismondi, A.; Locatelli, F.; Pagliara, D.; Nisti, P.; Stabile, H.; Morrone, S. Multifunctional human CD56low CD16low natural killer cells are the prominent subset in bone marrow of both healthy pediatric donors and leukemic patients. Haematologica 2015, 100, 489–498. [Google Scholar] [CrossRef]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.; Cooley, S.; Verneris, M.; Walcheck, B.; et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013, 121, 3599–3608. [Google Scholar] [CrossRef]
- Grzywacz, B.; Kataria, N.; Verneris, M.R. CD56dimCD16+ NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia 2007, 21, 356–359. [Google Scholar] [CrossRef]
- Srpan, K.; Ambrose, A.; Karampatzakis, A.; Saeed, M.; Cartwright, A.N.R.; Guldevall, K.; De Matos, G.D.S.C.; Önfelt, B.; Davis, D.M. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J. Cell Biol. 2018, 217, 3267–3283. [Google Scholar] [CrossRef]
- Takahashi, E.; Kuranaga, N.; Satoh, K.; Habu, Y.; Shinomiya, N.; Asano, T.; Seki, S.; Hayakawa, M. Induction of CD16+ CD56bright NK cells with antitumour cytotoxicity not only from CD16- CD56bright NK cells but also from CD16-CD56dim NK cells. Scand. J. Immunol. 2007, 65, 126–138. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Yang, B.Y.; Wu, C. You Phenotypically and functionally distinct subsets of natural killer cells in human PBMCs. Cell Biol. Int. 2008, 32, 188–197. [Google Scholar] [CrossRef]
- Thiel, E.; Fischer, L.; Asemissen, A.M.; Scheibenbogen, C.; Uharek, L.; Gentilini, C.; Penack, O. CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia 2005, 19, 835–840. [Google Scholar] [CrossRef]
- Roberto, A.; Di Vito, C.; Zaghi, E.; Mazza, E.M.C.; Capucetti, A.; Calvi, M.; Tentorio, P.; Zanon, V.; Sarina, B.; Mariotti, J.; et al. The early expansion of anergic NKG2Apos/CD56dim /CD16neg natural killer represents a therapeutic target in haploidentical hematopoietic stem cell transplantation. Haematologica 2018, 103, 1390–1402. [Google Scholar] [CrossRef]
- Horowitz, A.; Stegmann, K.A.; Riley, E.M. Activation of natural killer cells during microbial infections. Front. Immunol. 2012, 2, 88. [Google Scholar] [CrossRef]
- Vacca, P.; Montaldo, E.; Croxatto, D.; Moretta, F.; Bertaina, A.; Vitale, C.; Locatelli, F.; Mingari, M.C.; Moretta, L. NK Cells and other innate lymphoid cells in hematopoietic stem cell transplantation. Front. Immunol. 2016, 7, 188. [Google Scholar] [CrossRef]
- Meyer, R.M. Limited-Stage Hodgkin Lymphoma: Clarifying uncertainty. J. Clin. Oncol. 2017, 35, 1760–1763. [Google Scholar] [CrossRef]
- Khalil, M.; Wang, D.; Hashemi, E.; Terhune, S.S.; Malarkannan, S. Implications of a ‘Third Signal’ in NK Cells. Cells 2021, 10, 1955. [Google Scholar] [CrossRef]
- Pallmer, K.; Oxenius, A. Recognition and regulation of T cells by NK cells. Front. Immunol. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Anandappa, A.J.; Wu, C.J.; Ott, P.A. Directing traffic: How to effectively drive t cells into tumors. Cancer Discov. 2020, 10, 185–197. [Google Scholar] [CrossRef]
- Diefenbach, A.; Raulet, D.H. Strategies for target cell recognition by natural killer cells. Immunol. Rev. 2001, 181, 170–184. [Google Scholar] [CrossRef]
- Hammer, Q.; Rückert, T.; Romagnani, C. Natural killer cell specificity for viral infections. Nat. Immunol. 2018, 19, 800–808. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer–natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- Vosshenrich, C.A.J.; Di Santo, J.P. Roles for NK Cells and ILC1 in Inflammation and Infection. Inflamm. Mol. Cell. Mech. Clin. 2017, 315–340. [Google Scholar] [CrossRef]
- Lanier, L.L. NK cell receptors. Annu. Rev. Immunol. 1998, 16, 359–393. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Cantoni, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001, 19, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.-G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2021, 1–12. [Google Scholar] [CrossRef]
- Mariotti, F.R.; Quatrini, L.; Munari, E.; Vacca, P.; Tumino, N.; Pietra, G.; Mingari, M.C.; Moretta, L. Inhibitory checkpoints in human natural killer cells: IUPHAR Review 28. Br. J. Pharmacol. 2020, 177, 2889–2903. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell. Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Kruse, P.H.; Matta, J.; Ugolini, S.; Vivier, E. Natural cytotoxicity receptors and their ligands. Immunol. Cell Biol. 2014, 92, 221–229. [Google Scholar] [CrossRef]
- Zompi, S.; Hamerman, J.A.; Ogasawara, K.; Schweighoffer, E.; Tybulewicz, V.L.J.; Di Santo, J.P.; Lanier, L.L.; Colucci, F. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat. Immunol. 2003, 4, 565–572. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Xin, J.; Wang, J.; Yao, C.; Zhang, Z. Role of NKG2D and its ligands in cancer immunotherapy. Am. J. Cancer Res. 2019, 9, 2064–2078. [Google Scholar]
- Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C.; Grassi, J.; Marcenaro, S.; Reymond, N.; et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 2003, 198, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, A.; Campbell, D.; Hannum, C.; Yssel, H.; Franz-Bacon, K.; McClanahan, T.; Kitamura, T.; Nicholl, J.; Sutherland, G.R.; Lanier, L.L.; et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996, 4, 573–581. [Google Scholar] [CrossRef]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Arooj, S.; Wang, H. NK cell-based immune checkpoint inhibition. Front. Immunol. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Manser, A.R.; Uhrberg, M. Age-related changes in natural killer cell repertoires: Impact on NK cell function and immune surveillance. Cancer Immunol. Immunother. 2016, 65, 417–426. [Google Scholar] [CrossRef]
- Shifrin, N.; Raulet, D.H.; Ardolino, M. NK cell self tolerance, responsiveness and missing self recognition. Semin. Immunol. 2014, 26, 138–144. [Google Scholar] [CrossRef]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011, 89, 216–224. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Tartter, P.I.; Steinberg, B.; Barron, D.M.; Martinelli, G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch. Surg. 1987, 122, 1264–1268. [Google Scholar] [CrossRef]
- Eckl, J.; Buchner, A.; Prinz, P.U.; Riesenberg, R.; Siegert, S.I.; Kammerer, R.; Nelson, P.J.; Noessner, E. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J. Mol. Med. 2012, 90, 55–66. [Google Scholar] [CrossRef]
- Garcia-Iglesias, T.; del Toro-Arreola, A.; Albarran-Somoza, B.; del Toro-Arreola, S.; Sanchez-Hernandez, P.E.; Ramirez-Dueñas, M.; Balderas-Peña, L.M.A.; Bravo-Cuellar, A.; Ortiz-Lazareno, P.C.; Daneri-Navarro, A. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer 2009, 9, 186. [Google Scholar] [CrossRef]
- Cursons, J.; Souza-Fonseca-Guimaraes, F.; Foroutan, M.; Anderson, A.; Hollande, F.; Hediyeh-Zadeh, S.; Behren, A.; Huntington, N.D.; Davis, M.J. A Gene Signature Predicting Natural Killer Cell Infiltration and Improved Survival in Melanoma Patients. Cancer Immunol. Res. 2019, 7, 1162 LP–1174 LP. [Google Scholar] [CrossRef]
- Platonova, S.; Cherfils-Vicini, J.; Damotte, D.; Crozet, L.; Vieillard, V.; Validire, P.; André, P.; Dieu-Nosjean, M.-C.; Alifano, M.; Régnard, J.-F.; et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011, 71, 5412–5422. [Google Scholar] [CrossRef] [PubMed]
- Carrega, P.; Morandi, B.; Costa, R.; Frumento, G.; Forte, G.; Altavilla, G.; Ratto, G.B.; Mingari, M.C.; Moretta, L.; Ferlazzo, G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 2008, 112, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Godet, Y.; Laheurte, C.; Dosset, M.; Galaine, J.; Beziaud, L.; Loyon, R.; Boullerot, L.; Lauret Marie Joseph, E.; Spehner, L.; et al. Circulating NKp46(+) Natural Killer cells have a potential regulatory property and predict distinct survival in Non-Small Cell Lung Cancer. Oncoimmunology 2018, 8, e1527498. [Google Scholar] [CrossRef] [PubMed]
- Messaoudene, M.; Fregni, G.; Enot, D.; Jacquelot, N.; Neves, E.; Germaud, N.; Garchon, H.J.; Boukouaci, W.; Tamouza, R.; Chanal, J.; et al. NKp30 isoforms and NKp46 transcripts in metastatic melanoma patients: Unique NKp30 pattern in rare melanoma patients with favorable evolution. Oncoimmunology 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Rusakiewicz, S.; Perier, A.; Semeraro, M.; Pitt, J.M.; von Strandmann, E.P.; Reiners, K.S.; Aspeslagh, S.; Pipéroglou, C.; Vély, F.; Ivagnes, A.; et al. NKp30 isoforms and NKp30 ligands are predictive biomarkers of response to imatinib mesylate in metastatic GIST patients. Oncoimmunology 2017, 6, e1137418. [Google Scholar] [CrossRef]
- Messaoudene, M.; Frazao, A.; Gavlovsky, P.J.; Toubert, A.; Dulphy, N.; Caignard, A. Patient’s natural killer cells in the era of targeted therapies: Role for tumor killers. Front. Immunol. 2017, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Fend, L.; Rusakiewicz, S.; Adam, J.; Bastien, B.; Caignard, A.; Messaoudene, M.; Iribarren, C.; Cremer, I.; Marabelle, A.; Borg, C.; et al. Prognostic impact of the expression of NCR1 and NCR3 nk cell receptors and PD-L1 on advanced non-small cell lung cancer. Oncoimmunology 2017, 6. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Hu, B.; Wang, P.; Lv, X.; Chen, S.; Shao, Z. Prognostic significance of tumor-infiltrating natural killer cells in solid tumors: A systematic review and meta-analysis. Front. Immunol. 2020, 11, 1242. [Google Scholar] [CrossRef]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vély, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M.; et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 2015, 7, 283ra55. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.C.; Shim, D.H.; Yang, C.M.; Lee, S.-B.; Kim, S.M.; Shin, T.Y.; Kim, K.H.; Yoon, H.G.; Rha, K.H.; Lee, J.M.; et al. Reduction of the CD16(−)CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PLoS ONE 2013, 8, e78049. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Yin, W.-W.; Xia, Y.; Yi, Y.-Y.; He, Q.-F.; Wang, X.; Ren, H.; Zhang, D.-Z. Liver-infiltrating CD11b(−)CD27(−) NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell. Mol. Immunol. 2017, 14, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Galat, V.; Galat4, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Mamessier, E.; Sylvain, A.; Thibult, M.-L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef] [PubMed]
- Gallazzi, M.; Baci, D.; Mortara, L.; Bosi, A.; Buono, G.; Naselli, A.; Guarneri, A.; Dehò, F.; Capogrosso, P.; Albini, A.; et al. Prostate Cancer Peripheral Blood NK Cells Show Enhanced CD9, CD49a, CXCR4, CXCL8, MMP-9 Production and Secrete Monocyte-Recruiting and Polarizing Factors. Front. Immunol. 2020, 11, 3608. [Google Scholar] [CrossRef]
- Carlsten, M.; Baumann, B.C.; Simonsson, M.; Jädersten, M.; Forsblom, A.-M.; Hammarstedt, C.; Bryceson, Y.T.; Ljunggren, H.-G.; Hellström-Lindberg, E.; Malmberg, K.-J. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia 2010, 24, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Epling-Burnette, P.K.; Bai, F.; Painter, J.S.; Rollison, D.E.; Salih, H.R.; Krusch, M.; Zou, J.; Ku, E.; Zhong, B.; Boulware, D.; et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 2007, 109, 4816–4824. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, J.; Grosse-Hovest, L.; Grünebach, F.; Buechele, C.; Nuebling, T.; Raum, T.; Steinle, A.; Salih, H.R. Comprehensive analysis of NKG2D ligand expression and release in leukemia: Implications for NKG2D-mediated NK cell responses. J. Immunol. 2012, 189, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Chretien, A.-S.; Granjeaud, S.; Gondois-Rey, F.; Harbi, S.; Orlanducci, F.; Blaise, D.; Vey, N.; Arnoulet, C.; Fauriat, C.; Olive, D. Increased NK cell maturation in patients with acute myeloid leukemia. Front. Immunol. 2015, 6, 564. [Google Scholar] [CrossRef] [PubMed]
- Fauriat, C.; Just-Landi, S.; Mallet, F.; Arnoulet, C.; Sainty, D.; Olive, D.; Costello, R.T. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCR dull phenotype induction. Blood 2007, 109, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Reiners, K.S.; Topolar, D.; Henke, A.; Simhadri, V.R.; Kessler, J.; Sauer, M.; Bessler, M.; Hansen, H.P.; Tawadros, S.; Herling, M.; et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013, 121, 3658–3665. [Google Scholar] [CrossRef]
- Vari, F.; Arpon, D.; Keane, C.; Hertzberg, M.S.; Talaulikar, D.; Jain, S.; Cui, Q.; Han, E.; Tobin, J.; Bird, R.; et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018, 131, 1809–1819. [Google Scholar] [CrossRef]
- Benson, D.M.J.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef]
- Klanova, M.; Oestergaard, M.Z.; Trněný, M.; Hiddemann, W.; Marcus, R.; Sehn, L.H.; Vitolo, U.; Bazeos, A.; Goede, V.; Zeuner, H.; et al. Prognostic Impact of Natural Killer Cell Count in Follicular Lymphoma and Diffuse Large B-cell Lymphoma Patients Treated with Immunochemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4634–4643. [Google Scholar] [CrossRef]
- Al Sayed, M.F.; Ruckstuhl, C.A.; Hilmenyuk, T.; Claus, C.; Bourquin, J.P.; Bornhauser, B.C.; Radpour, R.; Riether, C.; Ochsenbein, A.F. CD70 reverse signaling enhances NK cell function and immunosurveillance in CD27-expressing B-cell malignancies. Blood 2017, 130, 297–309. [Google Scholar] [CrossRef]
- Warner, K.; Ohashi, P.S. ILC regulation of T cell responses in inflammatory diseases and cancer. Semin. Immunol. 2019, 41, 101284. [Google Scholar] [CrossRef] [PubMed]
- Simoni, Y.; Newell, E.W. Dissecting human ILC heterogeneity: More than just three subsets. Immunology 2017, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Simoni, Y.; Fehlings, M.; Kløverpris, H.N.; McGovern, N.; Koo, S.-L.; Loh, C.Y.; Lim, S.; Kurioka, A.; Fergusson, J.R.; Tang, C.-L.; et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 2017, 46, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Hashimoto-Hill, S.; Kim, M. Migration and tissue tropism of innate lymphoid cells. Trends Immunol. 2016, 37, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.I.; Li, Y.; Lopez-Lastra, S.; Stadhouders, R.; Paul, F.; Casrouge, A.; Serafini, N.; Puel, A.; Bustamante, J.; Surace, L.; et al. Systemic Human ILC precursors provide a substrate for tissue ILC Differentiation. Cell 2017, 168, 1086–1100.e10. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J.; et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef]
- Hochdörfer, T.; Winkler, C.; Pardali, K.; Mjösberg, J. Expression of c-Kit discriminates between two functionally distinct subsets of human type 2 innate lymphoid cells. Eur. J. Immunol. 2019, 49, 884–893. [Google Scholar] [CrossRef]
- Luci, C.; Reynders, A.; Ivanov, I.I.; Cognet, C.; Chiche, L.; Chasson, L.; Hardwigsen, J.; Anguiano, E.; Banchereau, J.; Chaussabel, D.; et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Crinier, A.; Vivier, E.; Bléry, M. Helper-like innate lymphoid cells and cancer immunotherapy. Semin. Immunol. 2019, 41, 101274. [Google Scholar] [CrossRef]
- Trabanelli, S.; Curti, A.; Lecciso, M.; Salomé, B.; Riether, C.; Ochsenbein, A.; Romero, P.; Jandus, C. CD127+ innate lymphoid cells are dysregulated in treatment naïve acute myeloid leukemia patients at diagnosis. Haematologica 2015, 100, e257–e260. [Google Scholar] [CrossRef]
- Loyon, R.; Jary, M.; Salomé, B.; Gomez-Cadena, A.; Galaine, J.; Kroemer, M.; Romero, P.; Trabanelli, S.; Adotévi, O.; Borg, C.; et al. Peripheral innate lymphoid cells are increased in first line metastatic colorectal carcinoma patients: A negative correlation with Th1 immune responses. Front. Immunol. 2019, 10, 2121. [Google Scholar] [CrossRef] [PubMed]
- De Weerdt, I.; Van Hoeven, V.; Marius Munneke, J.; Endstra, S.; Hofland, T.; Hazenberg, M.D.; Kater, A.P. Innate lymphoid cells are expanded and functionally altered in chronic lymphocytic leukemia. Haematologica 2016, 101, e461–e464. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Wang, R.; Yao, X.; Li, X.; Wang, X.; Hu, Y.; Chang, X.; Fan, P.; Dong, T.; Ogg, G. Activated innate lymphoid cell populations accumulate in human tumour tissues. BMC Cancer 2018, 18, 341. [Google Scholar] [CrossRef] [PubMed]
- Kini Bailur, J.; Mehta, S.; Zhang, L.; Neparidze, N.; Parker, T.; Bar, N.; Anderson, T.; Xu, M.L.; Dhodapkar, K.M.; Dhodapkar, M.V. Changes in bone marrow innate lymphoid cell subsets in monoclonal gammopathy: Target for IMiD therapy. Blood Adv. 2017, 1, 2343–2347. [Google Scholar] [CrossRef]
- Romano, M.; Sollazzo, D.; Trabanelli, S.; Barone, M.; Polverelli, N.; Perricone, M.; Forte, D.; Luatti, S.; Cavo, M.; Vianelli, N.; et al. Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis. Oncoimmunology 2017, 6, e1345402. [Google Scholar] [CrossRef]
- Dadi, S.; Chhangawala, S.; Whitlock, B.M.; Franklin, R.A.; Luo, C.T.; Oh, S.A.; Toure, A.; Pritykin, Y.; Huse, M.; Leslie, C.S.; et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 2016, 164, 365–377. [Google Scholar] [CrossRef]
- Ercolano, G.; Garcia-Garijo, A.; Salome, B.; Gomez-Cadena, A.; Vanoni, G.; Mastelic-Gavillet, B.; Ianaro, A.; Speiser, D.E.; Romero, P.; Trabanelli, S.; et al. Immunosuppressive mediators impair proinflammatory innate lymphoid cell function in human malignant melanoma. Cancer Immunol. Res. 2020, 8, 556–564. [Google Scholar] [CrossRef]
- Gao, Y.; Souza-Fonseca-Guimaraes, F.; Bald, T.; Ng, S.S.; Young, A.; Ngiow, S.F.; Rautela, J.; Straube, J.; Waddell, N.; Blake, S.J.; et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 2017, 18, 1004–1015. [Google Scholar] [CrossRef]
- Hawke, L.G.; Mitchell, B.Z.; Ormiston, M.L. TGF-β and IL-15 Synergize through MAPK pathways to drive the conversion of human NK cells to an innate lymphoid cell 1-like phenotype. J. Immunol. 2020, 204, 3171–3181. [Google Scholar] [CrossRef]
- Sowell, R.T.; Goldufsky, J.W.; Rogozinska, M.; Quiles, Z.; Cao, Y.; Castillo, E.F.; Finnegan, A.; Marzo, A.L. IL-15 Complexes induce migration of resting memory CD8 T cells into mucosal tissues. J. Immunol. 2017, 199, 2536–2546. [Google Scholar] [CrossRef]
- Bernink, J.H.; Krabbendam, L.; Germar, K.; de Jong, E.; Gronke, K.; Kofoed-Nielsen, M.; Munneke, J.M.; Hazenberg, M.D.; Villaudy, J.; Buskens, C.J.; et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 2015, 43, 146–160. [Google Scholar] [CrossRef]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumor incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef]
- Cella, M.; Gamini, R.; Sécca, C.; Collins, P.L.; Zhao, S.; Peng, V.; Robinette, M.L.; Schettini, J.; Zaitsev, K.; Gordon, W.; et al. Subsets of ILC3-ILC1-like cells generate a diversity spectrum of innate lymphoid cells in human mucosal tissues. Nat. Immunol. 2019, 20, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.-Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumor growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Goc, J.; Lv, M.; Bessman, N.J.; Flamar, A.-L.; Sahota, S.; Suzuki, H.; Teng, F.; Putzel, G.G.; Eberl, G.; Withers, D.R.; et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 2021, 1–16. [Google Scholar] [CrossRef]
- Saranchova, I.; Han, J.; Zaman, R.; Arora, H.; Huang, H.; Fenninger, F.; Choi, K.B.; Munro, L.; Pfeifer, C.G.; Welch, I.; et al. Type 2 innate lymphocytes actuate immunity against tumours and limit cancer metastasis. Sci. Rep. 2018, 8, 2924. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-pereira, S.; Dartiguenave, F.; Fritschi, A.; et al. ILC2-modulated T cell–to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127. [Google Scholar] [CrossRef]
- Ikutani, M.; Yanagibashi, T.; Ogasawara, M.; Tsuneyama, K.; Yamamoto, S.; Hattori, Y.; Kouro, T.; Itakura, A.; Nagai, Y.; Takaki, S.; et al. Identification of innate IL-5–producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 2012, 188, 703–713. [Google Scholar] [CrossRef]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the tumor microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef]
- Wagner, M.; Ealey, K.N.; Tetsu, H.; Kiniwa, T.; Motomura, Y.; Moro, K.; Koyasu, S. Tumor-derived lactic acid contributes to the paucity of intratumoral ILC2s. Cell Rep. 2020, 30, 2743–2757.e5. [Google Scholar] [CrossRef] [PubMed]
- Jacquelot, N.; Seillet, C.; Wang, M.; Pizzolla, A.; Liao, Y.; Hediyeh-zadeh, S.; Grisaru-Tal, S.; Louis, C.; Huang, Q.; Schreuder, J.; et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 2021, 22, 851–864. [Google Scholar] [CrossRef]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D.; et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 2020, 579, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jacquelot, N.; Preaudet, A.; Hediyeh-zadeh, S.; Souza-Fonseca-Guimaraes, F.; McKenzie, A.N.J.; Hansbro, P.M.; Davis, M.J.; Mielke, L.A.; Putoczki, T.L.; et al. Type 2 innate lymphoid cells protect against colorectal cancer progression and predict improved patient survival. Cancers 2021, 13, 559. [Google Scholar] [CrossRef]
- Wan, J.; Wu, Y.; Huang, L.; Tian, Y.; Ji, X.; Abdelaziz, M.H.; Cai, W.; Dineshkumar, K.; Lei, Y.; Yao, S.; et al. ILC2-derived IL-9 inhibits colorectal cancer progression by activating CD8+ T cells. Cancer Lett. 2021, 502, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.W.; van Loosdregt, J.; Gorlani, A.; Bekker, C.P.J.; Gröne, A.; Sibilia, M.; van Bergen en Henegouwen, P.M.P.; Roovers, R.C.; Coffer, P.J.; Sijts, A.J.A.M. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013, 38, 275–284. [Google Scholar] [CrossRef]
- Busser, B.; Sancey, L.; Brambilla, E.; Coll, J.-L.; Hurbin, A. The multiple roles of amphiregulin in human cancer. Biochim. Biophys. Acta 2011, 1816, 119–131. [Google Scholar] [CrossRef]
- Willmarth, N.E.; Ethier, S.P. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J. Biol. Chem. 2006, 281, 37728–37737. [Google Scholar] [CrossRef]
- So, W.K.; Fan, Q.; Lau, M.T.; Qiu, X.; Cheng, J.C.; Leung, P.C.K. Amphiregulin induces human ovarian cancer cell invasion by down-regulating E-cadherin expression. FEBS Lett. 2014, 588, 3998–4007. [Google Scholar] [CrossRef]
- Singh, B.; Carpenter, G.; Coffey, R.J. EGF receptor ligands: Recent advances. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.-E.; Van Dyken, S.J.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.C.; Van Dyken, S.J.; von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.-E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Png, S.; Richard, A.C.; Tsyben, A.; Hamm, G.; Stockis, J.; Garcia, C.; Pinaud, S.; Nicholls, A.; Ros, X.R.; et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 2020, 21, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Bie, Q.; Zhang, P.; Su, Z.; Zheng, D.; Ying, X.; Wu, Y.; Yang, H.; Chen, D.; Wang, S.; Xu, H. Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J. Immunol. Res. 2014, 2014, 923135. [Google Scholar] [CrossRef]
- Trabanelli, S.; Chevalier, M.F.; Martinez-Usatorre, A.; Gomez-Cadena, A.; Salomé, B.; Lecciso, M.; Salvestrini, V.; Verdeil, G.; Racle, J.; Papayannidis, C.; et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 2017, 8, 593. [Google Scholar] [CrossRef]
- Lange, K.; Uckert, W.; Blankenstein, T.; Nadrowitz, R.; Bittner, C.; Renauld, J.C.; Van Snick, J.; Feller, A.C.; Merz, H. Overexpression of NPM-ALK induces different types of malignant lymphomas in IL-9 transgenic mice. Oncogene 2003, 22, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Behr, T.; Grünwald, F.; Knapp, W.H.; Trümper, L.; Von Schilling, C. Guideline for radioimmunotherapy of rituximab relapsed or refractory CD20+ follicular B-cell non-Hodgkin’s lymphoma. NuklearMedizin 2004, 43, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lau, G.; Chen, L.; Yuan, Y.F.; Huang, J.; Luk, J.M.; Xie, D.; Guan, X.Y. Interleukin 23 promotes hepatocellular carcinoma metastasis via NF-Kappa B induced matrix metalloproteinase 9 expression. PLoS ONE 2012, 7, e72457. [Google Scholar] [CrossRef]
- Ebbo, M.; Crinier, A.; Vély, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef]
- An, Z.; Flores-Borja, F.; Irshad, S.; Deng, J.; Ng, T. Pleiotropic role and bidirectional immunomodulation of innate lymphoid cells in cancer. Front. Immunol. 2020, 10, 3111. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Lin, D.; Lei, L.; Mei, Y.; Jin, Z.; Gong, H.; Zhu, Y.; Hu, B.; Zhang, Y.; et al. NCR(-) group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development. EBioMedicine 2019, 41, 333–344. [Google Scholar] [CrossRef]
- Punt, S.; Fleuren, G.J.; Kritikou, E.; Lubberts, E.; Trimbos, J.B.; Jordanova, E.S.; Gorter, A. Angels and demons: Th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. Oncoimmunology 2015, 4, e984539. [Google Scholar] [CrossRef]
- Goc, J.; Hepworth, M.R.; Sonnenberg, G.F. Group 3 innate lymphoid cells: Regulating host-commensal bacteria interactions in inflammation and cancer. Int. Immunol. 2016, 28, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bostick, J.W.; Zhou, L. Regulation of innate lymphoid cells by aryl hydrocarbon receptor. Front. Immunol. 2017, 8, 1909. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, H.; Deng, L.; Hou, J.; Shi, R.; Yao, M.; Gao, Y.; Yao, A.; Wang, X.; Yu, L.; et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer 2013, 13, 59. [Google Scholar] [CrossRef]
- Kirchberger, S.; Royston, D.J.; Boulard, O.; Thornton, E.; Franchini, F.; Szabady, R.L.; Harrison, O.; Powrie, F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 2013, 210, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cui, L.; Liang, Z.; Liu, C.; Liu, Y.; Li, J. Elevated serum IL-22 levels correlate with chemoresistant condition of colorectal cancer. Clin. Immunol. 2013, 147, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qu, Y.; Xia, P.; Chen, Y.; Zhu, X.; Zhang, J.; Wang, G.; Tian, Y.; Ying, J.; Fan, Z. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 2020, 30, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xia, P.; Chen, Y.; Qu, Y.; Xiong, Z.; Ye, B.; Du, Y.; Tian, Y.; Yin, Z.; Xu, Z.; et al. Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell 2017, 171, 201–216.e18. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Ogino, T.; Kayama, H.; Okuzaki, D.; Nishimura, J.; Fujino, S.; Miyoshi, N.; Takahashi, H.; Uemura, M.; Matsuda, C.; et al. Human NKp44+ group 3 innate lymphoid cells associate with tumor-associated tertiary lymphoid structures in colorectal cancer. Cancer Immunol. Res. 2020, 8, 724LP–731LP. [Google Scholar] [CrossRef]
- Mortha, A.; Chudnovskiy, A.; Hashimoto, D.; Bogunovic, M.; Spencer, S.P.; Belkaid, Y.; Merad, M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014, 343, 1249288. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Thornton, E.E.; McKenzie, B.; Schaupp, A.L.; Huskens, N.; Griseri, T.; West, N.; Tung, S.; Seddon, B.P.; Uhlig, H.H.; et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife 2016, 5, e10066. [Google Scholar] [CrossRef]
- Bruchard, M.; Ghiringhelli, F. Deciphering the roles of innate lymphoid cells in cancer. Front. Immunol. 2019, 10, 656. [Google Scholar] [CrossRef]
- Irshad, S.; Flores-Borja, F.; Lawler, K.; Monypenny, J.; Evans, R.; Male, V.; Gordon, P.; Cheung, A.; Gazinska, P.; Noor, F.; et al. RORγt+ innate lymphoid cells promote lymph node metastasis of breast cancers. Cancer Res. 2017, 77, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Schleussner, N.; Merkel, O.; Costanza, M.; Liang, H.C.; Hummel, F.; Romagnani, C.; Durek, P.; Anagnostopoulos, I.; Hummel, M.; Jöhrens, K.; et al. The AP-1-BATF and -BATF3 module is essential for growth, survival and TH17/ILC3 skewing of anaplastic large cell lymphoma. Leukemia 2018, 32, 1994–2007. [Google Scholar] [CrossRef]
- Eisenring, M.; Vom Berg, J.; Kristiansen, G.; Saller, E.; Becher, B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat. Immunol. 2010, 11, 1030–1038. [Google Scholar] [CrossRef]
- Carrega, P.; Loiacono, F.; Di Carlo, E.; Scaramuccia, A.; Mora, M.; Conte, R.; Benelli, R.; Spaggiari, G.M.; Cantoni, C.; Campana, S.; et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 2015, 6, 8280. [Google Scholar] [CrossRef]
- Reindl, L.M.; Albinger, N.; Bexte, T.; Müller, S.; Hartmann, J.; Ullrich, E. Immunotherapy with NK cells: Recent developments in gene modification open up new avenues. Oncoimmunology 2020, 9, 1777651. [Google Scholar] [CrossRef]
- Gauthier, M.; Laroye, C.; Bensoussan, D.; Boura, C.; Decot, V. Natural Killer cells and monoclonal antibodies: Two partners for successful antibody dependent cytotoxicity against tumor cells. Crit. Rev. Oncol. Hematol. 2021, 160, 103261. [Google Scholar] [CrossRef]
- Angelo, L.S.; Banerjee, P.P.; Monaco-Shawver, L.; Rosen, J.B.; Makedonas, G.; Forbes, L.R.; Mace, E.M.; Orange, J.S. Practical NK cell phenotyping and variability in healthy adults. Immunol. Res. 2015, 62, 341–356. [Google Scholar] [CrossRef]
- Chabannon, C.; Mfarrej, B.; Guia, S.; Ugolini, S.; Devillier, R.; Blaise, D.; Vivier, E.; Calmels, B. Manufacturing natural killer cells as medicinal products. Front. Immunol. 2016, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Szmania, S.; Lapteva, N.; Garg, T.; Greenway, A.; Lingo, J.; Nair, B.; Stone, K.; Woods, E.; Khan, J.; Stivers, J.; et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J. Immunother. 2015, 38, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Rezvani, K.; Olson, A.; Oran, B.; Hosing, C.; Shah, N.; Parmar, S.; Armitage, S.; Shpall, E.J. Novel techniques for ex vivo expansion of cord blood: Clinical trials. Front. Med. 2015, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Kao, I.T.; Yao, C.L.; Kong, Z.L.; Wu, M.L.; Chuang, T.L.; Hwang, S.M. Generation of natural killer cells from serum-free, expanded human umbilical cord blood CD34+ cells. Stem Cells Dev. 2007, 16, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Spanholtz, J.; Preijers, F.; Tordoir, M.; Trilsbeek, C.; Paardekooper, J.; de Witte, T.; Schaap, N.; Dolstra, H. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS ONE 2011, 6, e20740. [Google Scholar] [CrossRef] [PubMed]
- Woll, P.S.; Martin, C.H.; Miller, J.S.; Kaufman, D.S. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J. Immunol. 2005, 175, 5095–5103. [Google Scholar] [CrossRef]
- Knorr, D.A.; Ni, Z.; Hermanson, D.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.N.; Lee, D.A.; Kaufman, D.S. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl. Med. 2013, 2, 274–283. [Google Scholar] [CrossRef]
- Tabatabaei-Zavareh, N.; Vlasova, A.; Greenwood, C.P.; Takei, F. Characterization of developmental pathway of natural killer cells from embryonic stem cells in vitro. PLoS ONE 2007, 2, e232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grimm, E.A.; Mazumder, A.; Zhang, H.Z.; Rosenberg, S.A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med. 1982, 155, 1823–1841. [Google Scholar] [CrossRef]
- Hercend, T.; Farace, F.; Baume, D.; Charpentier, F.; Droz, J.P.; Triebel, F.; Escudier, B. Immunotherapy with lymphokine-activated natural killer cells and recombinant interleukin-2: A feasibility trial in metastatic renal cell carcinoma. J. Biol. Response Mod. 1990, 9, 546–555. [Google Scholar] [PubMed]
- Weisdorf, D.; Miller, J.; Verfaillie, C.; Burns, L.; Wagner, J.; Blazar, B.; Davies, S.; Miller, W.; Hannan, P.; Steinbuch, M.; et al. Cytokine-primed bone marrow stem cells vs. peripheral blood stem cells for autologous transplantation: A randomized comparison of GM-CSF vs. G-CSF. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 1997, 3, 217–223. [Google Scholar]
- Gasteiger, G.; Hemmers, S.; Bos, P.D.; Sun, J.C.; Rudensky, A.Y. IL-2-dependent adaptive control of NK cell homeostasis. J. Exp. Med. 2013, 210, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lim, O.; Kim, T.M.; Ahn, Y.-O.; Choi, H.; Chung, H.; Min, B.; Her, J.H.; Cho, S.Y.; Keam, B.; et al. Phase I study of random healthy donor–Derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol. Res. 2016, 4, 215LP–224LP. [Google Scholar] [CrossRef]
- Williams, B.A.; Law, A.D.; Routy, B.; denHollander, N.; Gupta, V.; Wang, X.H.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef]
- Tonn, T.; Becker, S.; Esser, R.; Schwabe, D.; Seifried, E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J. Hematother. Stem Cell Res. 2001, 10, 535–544. [Google Scholar] [CrossRef]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; DeFor, T.E.; et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef]
- Schmidt-Wolf, I.G.; Negrin, R.S.; Kiem, H.P.; Blume, K.G.; Weissman, I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991, 174, 139–149. [Google Scholar] [CrossRef]
- Introna, M.; Correnti, F. Innovative clinical perspectives for cik cells in cancer patients. Int. J. Mol. Sci. 2018, 19, 358. [Google Scholar] [CrossRef]
- Pievani, A.; Belussi, C.; Klein, C.; Rambaldi, A.; Golay, J.; Introna, M. Enhanced killing of human B-cell lymphoma targets by combined use of cytokine-induced killer cell (CIK) cultures and anti-CD20 antibodies. Blood 2011, 117, 510–518. [Google Scholar] [CrossRef]
- Merker, M.; Salzmann-Manrique, E.; Katzki, V.; Huenecke, S.; Bremm, M.; Bakhtiar, S.; Willasch, A.; Jarisch, A.; Soerensen, J.; Schulz, A.; et al. Clearance of hematologic malignancies by allogeneic cytokine-induced killer cell or donor lymphocyte infusions. Biol. Blood Marrow Transplant. 2019, 25, 1281–1292. [Google Scholar] [CrossRef]
- Zhang, Y.; Schmidt-Wolf, I.G.H. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J. Cell. Physiol. 2020, 235, 9291–9303. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Rajan, A.; Salter, A.I.; Kosasih, P.L.; Wu, Q.; Voutsinas, J.; Jensen, M.C.; Pluckthun, A.; Riddell, S.R. Multispecific targeting with synthetic ankyrin repeat motif chimeric antigen receptors. Clin. Cancer Res. 2019, 25, 7506–7516. [Google Scholar] [CrossRef]
- Lemoine, J.; Ruella, M.; Houot, R. Overcoming intrinsic resistance of cancer cells to CAR T-cell killing. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J. V Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Dosani, T.; Carlsten, M.; Maric, I.; Landgren, O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of myeloma [corrected] and their uses in immunotherapies. Blood Cancer J. 2015, 5, e306. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martinez, D.; Allende-Vega, N.; Orecchioni, S.; Talarico, G.; Cornillon, A.; Vo, D.-N.; Rene, C.; Lu, Z.-Y.; Krzywinska, E.; Anel, A.; et al. Expansion of allogeneic NK cells with efficient antibody-dependent cell cytotoxicity against multiple tumors. Theranostics 2018, 8, 3856–3869. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, Y.; Fang, W.; Lu, C.; Chen, J.; Cui, G.; Diao, H. Cetuximab enhanced the cytotoxic activity of immune cells during treatment of colorectal cancer. Cell. Physiol. Biochem. 2017, 44, 1038–1050. [Google Scholar] [CrossRef]
- Liang, S.; Niu, L.; Xu, K.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Lin, M. Tumor cryoablation in combination with natural killer cells therapy and Herceptin in patients with HER2-overexpressing recurrent breast cancer. Mol. Immunol. 2017, 92, 45–53. [Google Scholar] [CrossRef]
- Method of the Year 2013. Nat. Methods 2013, 11, 1. [CrossRef]
- Saelens, W.; Cannoodt, R.; Todorov, H.; Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 2019, 37, 547–554. [Google Scholar] [CrossRef]
- Lähnemann, D.; Köster, J.; Szczurek, E.; McCarthy, D.J.; Hicks, S.C.; Robinson, M.D.; Vallejos, C.A.; Campbell, K.R.; Beerenwinkel, N.; Mahfouz, A.; et al. Eleven grand challenges in single-cell data science. Genome Biol. 2020, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Mora, F.; Handler, K.; Law, A.M.K.; Salomon, R.; Oakes, S.R.; Ormandy, C.J.; Gallego-Ortega, D. Single-cell transcriptomics in cancer immunobiology: The future of precision oncology. Front. Immunol. 2018, 9, 2582. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Mazutis, L.; Akartuna, I.; Tallapragada, N.; Veres, A.; Li, V.; Peshkin, L.; Weitz, D.A.; Kirschner, M.W. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 2015, 161, 1187–1201. [Google Scholar] [CrossRef]
- Deng, Q.; Ramsköld, D.; Reinius, B.; Sandberg, R. Single-Cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 2014, 343, 193–196. [Google Scholar] [CrossRef]
- Picelli, S.; Faridani, O.R.; Björklund, Å.K.; Winberg, G.; Sagasser, S.; Sandberg, R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014, 9, 171–181. [Google Scholar] [CrossRef]
- Ramsköld, D.; Luo, S.; Wang, Y.-C.; Li, R.; Deng, Q.; Faridani, O.R.; Daniels, G.A.; Khrebtukova, I.; Loring, J.F.; Laurent, L.C.; et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 2012, 30, 777–782. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Li, Z.; Huang, Y.; Correspondence, J.W.; Liu, F.; Chen, Y.; Yao, J.; Wang, J. Comparative analysis of droplet-based ultra-high-throughput single-cell RNA-seq systems molecular cell comparative analysis of droplet-based ultra-high-throughput single-cell RNA-seq systems. Mol. Cell 2019, 73, 130.e5–142.e5. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.A.; Angerer, P.; Theis, F.J. SCANPY: Large-scale single-cell gene expression data analysis. Genome Biol. 2018, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Amezquita, R.A.; Lun, A.T.L.; Becht, E.; Carey, V.J.; Carpp, L.N.; Geistlinger, L.; Marini, F.; Rue-Albrecht, K.; Risso, D.; Soneson, C.; et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 2019, 17, 137–145. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Campbell, K.R.; Lun, A.T.L.; Wills, Q.F. Scater: Pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 2017, 33, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Prete, M.; Huang, N.; van Dongen, S.; Pir, P.; Kiselev, V.Y. Comparison of visualization tools for single-cell RNAseq data. NAR Genom. Bioinform. 2020, 2, lqaa052. [Google Scholar] [CrossRef]
- Hillje, R.; Pelicci, P.G.; Luzi, L. Cerebro: Interactive visualization of scRNA-seq-seq data. Bioinformatics 2020, 36, 2311–2313. [Google Scholar] [CrossRef]
- Harly, C.; Kenney, D.; Ren, G.; Lai, B.; Raabe, T.; Yang, Q.; Cam, M.C.; Xue, H.-H.; Zhao, K.; Bhandoola, A. The transcription factor TCF-1 enforces commitment to the innate lymphoid cell lineage. Nat. Immunol. 2019, 20, 1150–1160. [Google Scholar] [CrossRef]
- Yu, Y.; Tsang, J.C.H.; Wang, C.; Clare, S.; Wang, J.; Chen, X.; Brandt, C.; Kane, L.; Campos, L.S.; Lu, L.; et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature 2016, 539, 102–106. [Google Scholar] [CrossRef]
- Peters, A.L.; Luo, Z.; Li, J.; Mourya, R.; Wang, Y.; Dexheimer, P.; Shivakumar, P.; Aronow, B.; Bezerra, J.A. Single cell RNA sequencing reveals regional heterogeneity of hepatobiliary innate lymphoid cells in a tissue-enriched fashion. PLoS ONE 2019, 14, e0215481. [Google Scholar] [CrossRef]
- Lo, B.C.; Hernaez, D.C.; Scott, R.W.; Hughes, M.R.; Shin, S.B.; Underhill, T.M.; Takei, F.; McNagny, K.M. The transcription factor RORα preserves ILC3 lineage identity and function during chronic intestinal infection. J. Immunol. 2019, 203, 3209–3215. [Google Scholar] [CrossRef]
- Björklund, A.K.; Forkel, M.; Picelli, S.; Konya, V.; Theorell, J.; Friberg, D.; Sandberg, R.; Mjösberg, J. The heterogeneity of human CD127+ innate lymphoid cells revealed by single-cell RNA sequencing. Nat. Immunol. 2016, 17, 451–460. [Google Scholar] [CrossRef]
- Qi, J.; Crinier, A.; Escalière, B.; Ye, Y.; Wang, Z.; Zhang, T.; Batista, L.; Liu, H.; Hong, L.; Wu, N.; et al. Single-cell transcriptomic landscape reveals tumor specific innate lymphoid cells associated with colorectal cancer progression. Cell Rep. Med. 2021, 100353. [Google Scholar] [CrossRef]
- Mazzurana, L.; Czarnewski, P.; Jonsson, V.; Wigge, L.; Ringnér, M.; Williams, T.C.; Ravindran, A.; Björklund, Å.K.; Säfholm, J.; Nilsson, G.; et al. Tissue-specific transcriptional imprinting and heterogeneity in human innate lymphoid cells revealed by full-length single-cell RNA-sequencing. Cell Res. 2021, 31, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Ohne, Y.; Teunissen, M.B.M.; Wang, J.; Wu, J.; Krabbendam, L.; Guntermann, C.; Volckmann, R.; Koster, J.; van Tol, S.; et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat. Immunol. 2019, 20, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Shen, Z.Y.; Orangi, M.; Martinez-Gonzalez, I.; Wei, L.; Lu, X.; Das, A.; Heravi-Moussavi, A.; Marra, M.A.; Bhandoola, A.; et al. Single-cell analysis of RORα tracer mouse lung reveals ILC progenitors and effector ILC2 subsets. J. Exp. Med. 2020, 217, e20182293. [Google Scholar] [CrossRef] [PubMed]
- Zeis, P.; Lian, M.; Fan, X.; Herman, J.S.; Hernandez, D.C.; Gentek, R.; Elias, S.; Symowski, C.; Knöpper, K.; Peltokangas, N.; et al. In situ maturation and tissue adaptation of type 2 innate lymphoid cell progenitors. Immunity 2020, 53, 775.e9–792.e9. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Mahlakõ, T.; Shih, H.-Y.; Kanno, Y.; Artis, D.; O’, J.J.; Correspondence, S.; Gov, J.O. Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity 2019, 51, 682–695. [Google Scholar] [CrossRef]
- Crinier, A.; Milpied, P.; Escalière, B.; Piperoglou, C.; Galluso, J.; Balsamo, A.; Spinelli, L.; Cervera-Marzal, I.; Ebbo, M.; Girard-Madoux, M.; et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK cell subsets in humans and mice. Immunity 2018, 49, 971.e5–986.e5. [Google Scholar] [CrossRef]
- McFarland, A.P.; Yalin, A.; Wang, S.-Y.; Cortez, V.S.; Landsberger, T.; Sudan, R.; Peng, V.; Miller, H.L.; Ricci, B.; David, E.; et al. Multi-tissue single-cell analysis deconstructs the complex programs of mouse natural killer and type 1 innate lymphoid cells in tissues and circulation. Immunity 2021, 54, 1320–1337.e4. [Google Scholar] [CrossRef] [PubMed]
- Sciumè, G.; Mikami, Y.; Jankovic, D.; Nagashima, H.; Villarino, A.V.; Morrison, T.; Yao, C.; Signorella, S.; Sun, H.W.; Brooks, S.R.; et al. Rapid enhancer remodeling and transcription factor repurposing enable high magnitude gene induction upon acute activation of NK cells. Immunity 2020, 53, 745–758.e4. [Google Scholar] [CrossRef]
- Park, E.; Patel, S.; Wang, Q.; Andhey, P.; Zaitsev, K.; Porter, S.; Hershey, M.; Bern, M.; Plougastel-Douglas, B.; Collins, P.; et al. Toxoplasma gondii infection drives conversion of NK cells into ILC1-like cells. eLife 2019, 8, e47605. [Google Scholar] [CrossRef]
- Ni, J.; Wang, X.; Stojanovic, A.; Zhang, Q.; Wincher, M.; Bühler, L.; Arnold, A.; Correia, M.P.; Winkler, M.; Koch, P.S.; et al. Single-Cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1α unleashes NK cell activity. Immunity 2020, 52, 1075.e8–1087.e8. [Google Scholar] [CrossRef]
- de Andrade, L.F.; Lu, Y.; Luoma, A.; Ito, Y.; Pan, D.; Pyrdol, J.W.; Yoon, C.H.; Yuan, G.-C.; Wucherpfennig, K.W. Discovery of specialized NK cell populations infiltrating human melanoma metastases. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Crinier, A.; Dumas, P.-Y.; Escalière, B.; Piperoglou, C.; Gil, L.; Villacreces, A.; Vély, F.; Ivanovic, Z.; Milpied, P.; Narni-Mancinelli, É.; et al. Single-cell profiling reveals the trajectories of natural killer cell differentiation in bone marrow and a stress signature induced by acute myeloid leukemia. Cell. Mol. Immunol. 2020, 18, 1290–1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mo, S.; Li, X.; He, Y.; Yang, J. Single-cell RNA-seq reveals the immune escape and drug resistance mechanisms of mantle cell lymphoma. Cancer Biol. Med. 2020, 17, 726–739. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019, 575, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Aizarani, N.; Saviano, A.; Sagar; Mailly, L.; Durand, S.; Herman, J.S.; Pessaux, P.; Baumert, T.F.; Grün, D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 2019, 572, 199–204. [Google Scholar] [CrossRef] [PubMed]
- MacParland, S.A.; Liu, J.C.; Ma, X.-Z.; Innes, B.T.; Bartczak, A.M.; Gage, B.K.; Manuel, J.; Khuu, N.; Echeverri, J.; Linares, I.; et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 2018, 9, 4383. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Liu, Y.; He, X.; Qu, M.; Xu, G.; Wang, H.; Huang, M.; Pan, J.; Liu, Z.; et al. Single-cell RNA sequencing reveals the heterogeneity of liver-resident immune cells in human. Cell Discov. 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Wan, L.; Wang, Z.; Wang, H.; Ge, C.; Liu, Y.; Hao, Y.; Zhang, D.; Shi, G.; et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Han, Y.; Kim, S.I.; Lee, J.; Jo, H.; Wang, W.; Cho, U.; Park, W.-Y.; Rando, T.A.; Dhanasekaran, D.N.; et al. Computational modeling of malignant ascites reveals CCL5–SDC4 interaction in the immune microenvironment of ovarian cancer. Mol. Carcinog. 2021, 60, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Schelker, M.; Feau, S.; Du, J.; Ranu, N.; Klipp, E.; MacBeath, G.; Schoeberl, B.; Raue, A. Estimation of immune cell content in tumor tissue using single-cell RNA-seq data. Nat. Commun. 2017, 8, 2032. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fan, J.; Huang, J.; Guo, E.; Fu, Y.; Liu, S.; Xiao, R.; Liu, C.; Lu, F.; Qin, T.; et al. Clinical and molecular characteristics of COVID-19 patients with persistent SARS-CoV-2 infection. Nat. Commun. 2021, 12, 3501. [Google Scholar] [CrossRef]
- Wilk, A.J.; Rustagi, A.; Zhao, N.Q.; Roque, J.; Martínez-Colón, G.J.; McKechnie, J.L.; Ivison, G.T.; Ranganath, T.; Vergara, R.; Hollis, T.; et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020, 26, 1070–1076. [Google Scholar] [CrossRef]
- Huang, L.; Shi, Y.; Gong, B.; Jiang, L.; Zhang, Z.; Liu, X.; Yang, J.; He, Y.; Jiang, Z.; Zhong, L.; et al. Dynamic blood single-cell immune responses in patients with COVID-19. Signal Transduct. Target. Ther. 2021, 6, 110. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, X.; Zheng, L.; Zhang, Y.; Li, Y.; Fang, Q.; Gao, R.; Kang, B.; Zhang, Q.; Huang, J.Y.; et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 2018, 564, 268–272. [Google Scholar] [CrossRef]
- Moreno-Nieves, U.Y.; Tay, J.K.; Saumyaa, S.; Horowitz, N.B.; Shin, J.H.; Mohammad, I.A.; Luca, B.; Mundy, D.C.; Gulati, G.S.; Bedi, N.; et al. Landscape of innate lymphoid cells in human head and neck cancer reveals divergent NK cell states in the tumor microenvironment. Proc. Nat. Acad. Sci. USA 2021, 118, e2101169118. [Google Scholar] [CrossRef]
- Michieletto, M.F.; Henao-Mejia, J. Ontogeny and heterogeneity of innate lymphoid cells and the noncoding genome. Immunol. Rev. 2021, 300, 152–166. [Google Scholar] [CrossRef]
- Villanova, F.; Flutter, B.; Tosi, I.; Grys, K.; Sreeneebus, H.; Perera, G.K.; Chapman, A.; Smith, C.H.; Meglio, P. Di; Nestle, F.O. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in Psoriasis. J. Investig. Dermatol. 2014, 134, 984–991. [Google Scholar] [CrossRef]
- Bielecki, P.; Riesenfeld, S.J.; Hütter, J.-C.; Torlai Triglia, E.; Kowalczyk, M.S.; Ricardo-Gonzalez, R.R.; Lian, M.; Amezcua Vesely, M.C.; Kroehling, L.; Xu, H.; et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 2021, 592, 128–132. [Google Scholar] [CrossRef]
- Seillet, C.; Mielke, L.A.; Amann-Zalcenstein, D.B.; Su, S.; Gao, J.; Almeida, F.F.; Shi, W.; Ritchie, M.E.; Naik, S.H.; Huntington, N.D.; et al. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 2016, 17, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Gonzalez, R.R.; Van Dyken, S.J.; Schneider, C.; Lee, J.; Nussbaum, J.C.; Liang, H.-E.; Vaka, D.; Eckalbar, W.L.; Molofsky, A.B.; Erle, D.J.; et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 2018, 19, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Freud, A.G.; Caligiuri, M.A. Location and cellular stages of natural killer cell development. Trends Immunol. 2013, 34, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Hilton, H.G.; Rubinstein, N.D.; Janki, P.; Ireland, A.T.; Bernstein, N.; Fong, N.L.; Wright, K.M.; Smith, M.; Finkle, D.; Martin-McNulty, B.; et al. Single-cell transcriptomics of the naked mole-rat reveals unexpected features of mammalian immunity. PLoS Biol. 2019, 17, e3000528. [Google Scholar] [CrossRef] [PubMed]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. NK cell metabolism and tumor microenvironment. Front. Immunol. 2019, 10, 2278. [Google Scholar] [CrossRef]
- Baltimore, D. Discovering NF-κB. Cold Spring Harb. Perspect. Biol. 2009, 1, a000026. [Google Scholar] [CrossRef]
- Phadke, M.S.; Chen, Z.; Li, J.; Mohamed, E.; Davies, M.A.; Smalley, I.; Duckett, D.R.; Palve, V.; Czerniecki, B.J.; Forsyth, P.A.; et al. Targeted therapy given after anti–PD-1 leads to prolonged responses in mouse melanoma models through sustained antitumor immunity. Cancer Immunol. Res. 2021, 9, 554–567. [Google Scholar] [CrossRef]
- Costello, R.T.; Sivori, S.; Marcenaro, E.; Lafage-Pochitaloff, M.; Mozziconacci, M.-J.; Reviron, D.; Gastaut, J.-A.; Pende, D.; Olive, D.; Moretta, A. Defective expression and function of natural killer cell–triggering receptors in patients with acute myeloid leukemia. Blood 2002, 99, 3661–3667. [Google Scholar] [CrossRef]
- Le Bouteiller, P.; Tabiasco, J.; Polgar, B.; Kozma, N.; Giustiniani, J.; Siewiera, J.; Berrebi, A.; Aguerre-Girr, M.; Bensussan, A.; Jabrane-Ferrat, N. CD160: A unique activating NK cell receptor. Immunol. Lett. 2011, 138, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Rocca, Y.S.; Roberti, M.P.; Arriaga, J.M.; Amat, M.; Bruno, L.; Pampena, M.B.; Huertas, E.; Loria, F.S.; Pairola, A.; Bianchini, M.; et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 2013, 19, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125. [Google Scholar] [CrossRef]
- Sun, G.; Li, Z.; Rong, D.; Zhang, H.; Shi, X.; Yang, W.; Zheng, W.; Sun, G.; Wu, F.; Cao, H.; et al. Single-cell RNA sequencing in cancer: Applications, advances, and emerging challenges. Mol. Ther. Oncolytics 2021, 21, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, T.; Jin, Y.; Huang, B.; Zhang, Y. Progress and clinical application of single-cell transcriptional sequencing technology in cancer research. Front. Oncol. 2021, 10, 3367. [Google Scholar] [CrossRef]
| Type of Cancer | NK Cell Role or Phenotype | Impact on Tumor | References |
|---|---|---|---|
| NSCLC | Low cytotoxicity of CD16+ and CD56+ NKs NKp46 expression | Not known Immune-suppressive environment | [57] [59] |
| BC | NK infiltrate with high expression of NKG2A, low expression of Nkp30, Nkp46 and NKG2D | Impaired cytotoxicity | [68,69] |
| Gastric cancer | NK with lower cytotoxicity | Low TNF-α and IFN-γ | [66] |
| CRC | Low NK number | Association with cancer recurrence after resection | [53] |
| Renal cancer | Tumor-infiltrating NK | Better prognosis | [54] |
| Cervical cancer | NK cells with low activating receptor expression | Contributes to cancer progression | [55] |
| Metastatic cutaneous melanoma | High infiltration of NK cells | Better prognosis and survival | [56] |
| Hepatocellular carcinoma | High expression NKG2A, low expression of Nkp30, NKG2D andNkp46 | Impairment of cell cytotoxicity | [67] |
| Myelodysplastic syndrome | Low expression of DNAM-1 | Lower blast killing, higher blast infiltration; high-risk disease | [71] |
| AML | Low NKG2D High NKG2A | Impairment of cell cytotoxicity, low IFN-γ production | [73] |
| CML | Downregulated Nkp30 and Nkp46 | Impaired survival | [75] |
| HL | PD-1 expression | Immune evasion | [77] |
| Multiple myeloma | PD-1 expression | Not known | [78] |
| Cell Populations | Specific Cluster Name 1 | Organism | Tissue | Cell Source | Gene Marker 2 | Reference |
|---|---|---|---|---|---|---|
| ILCP | ALP | Mouse | BM of Tcf7EGFP mice | Lin−cKit+2B4+α4β7−Flt3hiIL-7Rα+ | Tcf7 Flt3 H2dma Sp1 Mef2c | [198] |
| sEILP | BM of Tcf7EGFP mice | Lin−cKit+2B4+α4β7+Tcf7-GFP+ | Tcf7 Irf8 Runx3 | [198] | ||
| cEILP | BM of Tcf7EGFPmice | Lin−cKit+2B4+α4β7+Tcf7-GFP+ | Tcf7 Runx3 Tox | [198] | ||
| General | BM of Tcf7EGFP mice | Lin−cKit+2B4+α4β7+Tcf7-GFP+ | Zbtb16 Lmo4 Rora | [198] | ||
| General | BM | Lin−Flt3lo/−IL−7Rαlo/+α4β7+ | Zbtb16 Il7r Kit Itga4 Itgb7 but low Myc | [199] | ||
| General | BM | Lin−Flt3lo/−IL−7Rαlo/+α4β7+ | Tcf7 Il18r1 | [199] | ||
| ILC1 | General | Mouse | Colon-tumor-infiltrating ILCs | CD45+Lin−CD127+ | Xcl1 Tbx21 Klrb1c Ncr1 Ifng Klrd Klrk1 Klrc1 Ctsw | [138] |
| ILC1s and/or cNKs | BM | Lin−Flt3lo/−IL−7Rαlo/+α4β7+ | Cish | [199] | ||
| Bile duct-ILC1 | Liver or EHDB after Il-33 | CD45+Lin− | Cd28 Il12rb2 Thy1 Cd93 Tbx21 Ifngr1 Il2 Il10 | [200] | ||
| General | Salmonella-infected ceca of WT or Rorasg/sg BM-transplanted (BMT) chimeric mice | CD45.2+Lin−CD90+ | Xcl1 | [201] | ||
| General | Human | Tonsil | Lin−CD127+NKG2A−CD16−CD117−CRTH2− | CXCR3 IFNG | [202] | |
| General | CRC tissue | Lin−CD45+CD127+ | CD3D CD3G CCL4 IFNG IKZF3 PRDM1 | [203] | ||
| General | Lung, blood, colon and tonsil | Lin−CD45+CD127+CD117−CRTH2− | IL7R | [204] | ||
| General | PB | Lin−CD45+CD127+CD117−CRTH2− | ETS1 TBX21 EOMES IFNG BCL11BTCF7 | [205] | ||
| ILC2 | General | Mouse | BM | Lin−Flt3lo/−IL-7Rαlo/+α4β7+ | Pdcd1 Bcl11b Icos Rora Gata3 | [199] |
| General (from clusters c8 to c10) | BM | Lin−Flt3lo/−IL-7Rαlo/+α4β7+ | Il2ra Il1rl1 Bmp7 Pparg | [199] | ||
| General (cluster c8) | BM | Lin−Flt3lo/−IL-7Rαlo/+α4β7+ | Itgb3 Pbxip1 1700113H08Rik | [199] | ||
| General (cluster c9) | BM | Lin−Flt3lo/−IL-7Rαlo/+α4β7+ | Ccr8 Gclc | [199] | ||
| General | Colon tumor infiltrating ILCs | Lin−CD45+CD127+ | Gata3 Il4 Il5 Klrg1 Il1rl1 Il13 Il17rb Fosb Hes1 Itga4 | [138] | ||
| Liver-specific ILC2 | Liver or EHDB after Il-33 | CD45+Lin− | Ccr4 Tnfsf9 Il12rb1 IL10 NFil3 Idi1 IL9r | [200] | ||
| Canonical ILC2 | Liver or EHDB after Il-33 | CD45+Lin− | Rora Gata3 Arg1 Ccr8 Klrg1 Icos Il17Rb Ccr2 Il5 Il13 | [200] | ||
| General | Adult and neonatal lung | CD45lo/+Linlo RORγt−YFP−YFP+ | Areg Il7r Rora and Gata3 and the lack of expression of stromal NK/ILC1 B and T cell genes | [206] | ||
| Conventional ILC2 | Adult and neonatal lung | CD45lo/+Linlo RORγt−YFP−YFP+ | Il1rl1 Tnfrsf18 Areg and Arg1 | [206] | ||
| General | Adult and neonatal lung | CD45lo/+LinloRORγt−YFP−and YFP+ | Cd7 Runx3 Cd2 Tcf7 Il18r1 | [206] | ||
| ILC2 mature | Infected lung | (Lin−) CD45+Il7ra+Thy1+/lowNK1.1− | Gata3 Il1rl1 Klrg1 Bcl11b | [207] | ||
| ILC2 effector | Infected lung | (Lin−) CD45+Il7ra+Thy1+/lowNK1.1− | Il5 Il13 Il17a | [207] | ||
| Natural ILC2 | Lung | Lin−CD3−TCRβ−Thy1+ | Cd69 Nfkbid Fos Il13 Il1r2 Cxcl3 Calca Cxcl2 Il5 Csf2 Ccl1 | [208] | ||
| Inflammatory ILC2 | Lung | Lin−CD3−TCRβ−Thy1+ | Gzma Lgals1 Stmn1 Ccr9 Lgals3 Klrg1 | [208] | ||
| General | Salmonella-infected ceca of WT or Rorasg/sg BM-transplanted (BMT) chimeric mice | CD45.2+Lin2CD90+ | Gata3 Il4 Il17rb Il1rl1 Rora Il7r | [201] | ||
| General | Human | Lung, blood, colon and tonsil | CD3−CD4−Lin−CD45+CD127+CD117+/−CRTH2+ | IL7R GATA3 MAF PTGRD2 HPGDS | [204] | |
| c-kit- ILC2 | Skin | CD45+Lin−CD127+CRTH2+CD117−/+ | GATA3 CRLF2 IL17RB CCR6 RORA BCL11B | [205] | ||
| c-kit+ ILC2 | Skin | CD45+Lin−CD127+CRTH2+CD117−/+ | CRLF2 IL17RB CCR6 RORA BCL11B ZBTB16 RORC | [205] | ||
| General | Tonsil | Lin−CD127+NKG2A−CD16−CRTH2+ | GATA3 IL1RL1 IL17RB PTGDR2 | [202] | ||
| ILC3 | General | Mouse | BM | Lin−Flt3lo/−IL-7Rαlo/+α4β7+ | Tbx21 Il2rb Ncr1 Cxcr3 Ctsw | [199] |
| General | Colon-tumor-infiltrating ILCs | Lin−CD45+CD127+ | Rorc Il22 Ncr1 Sepp1 Ccr7 Fcer1g Cd74 | [138] | ||
| ILCreg | Colon-tumor-infiltrating ILCs | Lin−CD45+CD127+ | Id3-Il10-Ctla4-Klf2-Tnfrsf18-Tnfrs8-Tnfrs9 | [138] | ||
| General | Salmonella-infected ceca of WT or Rorasg/sg BM-transplanted (BMT) chimeric mice | CD45.2+Lin2CD90+ | Rora Il7r Thy1 Gzmb Xcl1 Ncr1 Ifng Cxcr6 Cd4 | [201] | ||
| General | Human | Lung, blood, colon and tonsil | CD45+Lin−CD127+CD117+CRTH2− | KIT IL1R1 IL23R RORC | [204] | |
| General | Tonsil | expression of NKp44 CD62L HLA-DR | IL1R1 IL23R RORC AHR NCR2 | [202] | ||
| NK | mNK | Mouse | Blood | CD3−CD19−CD45.2NK1.1+NKp46+ | Hbb-bs Smad7 Tgfb1 Qrfp Ifngr1 | [209] |
| mNK_Bl1 | Blood | CD3−CD19−CD45.2NK1.1+NKp46+ | Gzmb Klrg1 Ly6c2 Cma1 Klra9 Ncr1 Emp3 Fgl2 | [209] | ||
| mNK_Bl2 | Blood | CD3−CD19−CD45.2NK1.1+NKp46+ | Ctla2a Emb Ccr2 Socs3 Xlc1 Cd27 Cd7 Ltb | [209] | ||
| General | Blood | CD45+Lin−NK1.1+NKp46+ | Ly6c2 Klrg1 Klra4 | [210] | ||
| NK (non-tissue- specific) | Blood, spleen, inguinal lymph node, liver, VAT, small intestine IEL/LPL, salivary gland and uterus | CD45+Lin−NK1.1+NKp46+ | Itgam S1pr5 Cma1 Zeb2 | [210] | ||
| General | Inguinal lymph node | CD45+Lin−NK1.1+NKp46+ | Il7r Ly6c2 Klra8 Klra4 Il18r1 | [210] | ||
| General | Liver | CD45+Lin−NK1.1+NKp46+ | Itga1 | [210] | ||
| NK (tissue-specific) | Liver, VAT, salivary gland, uterus, small intestine IEL/LPL and tumor | CD45+Lin−NK1.1+NKp46+ | Kit Itga1 Cd160 Asb2 S100a4m Fgl2 Gzmb Cd7m Ctla2 CCr2 Itgb1 Capg Tnfrsf9 Anxa2 Ldha Irf8 | [210] | ||
| NK (early Pec) | Peritoneal exudates | CD3ε−NKp46+CD49b+ | Furin Ctla2a, Ifitm1 Ifng | [211] | ||
| NK (late Pec) | Peritoneal exudates | CD3ε−NKp46+CD49b+ | Spp1 Gzmb Lag3 Ly6a | [211] | ||
| General | Salivary gland and uterus | CD45+Lin−NK1.1+NKp46+ | Klra4 Klra8 Itga1 Il7r | [210] | ||
| General | Salmonella-infected ceca of WT or Rorasg/sg BM-transplanted (BMT) chimeric mice | CD45.2+Lin2CD90+ | Ncr1 Sell Eomes Gzma | [201] | ||
| General | Small-intestine IEL/LPL | CD45+Lin−NK1.1+NKp46+ | Kit Itga1 Il7r | [210] | ||
| mNK | Spleen | CD3−CD19−CD45.2NK1.1+NKp46+ | Jun Ccl3 Fos Ccl4 Fosb Klf2 | [209] | ||
| mNK_Sp1 | Spleen | CD3−CD19−CD45.2NK1.1+NKp46+ | Lgals1 Cma1 Gzmb Fgl2 Klra9 Lys6c2 Irf8 Klrg1 | [209] | ||
| mNK_Sp2 | Spleen | CD3−CD19−CD45.2NK1.1+NKp46+ | Ctla2a Ltb Emb Cd28 Xcl1 Cd7 Spry2 Fosb Cd27 Cebpb | [209] | ||
| mNK_Sp3 | Spleen | CD3−CD19−CD45.2NK1.1+NKp46+ | Nfkbia Nr4a1 Pim1 Prr7 Ccl4 | [209] | ||
| General | Spleen | CD45+Lin−NK1.1+NKp46+ | Ly6c2 Klrg1 Klra4 Klra8 Il18r1 | [210] | ||
| General | Spleen | CD3−CD19−NK1.1+NKp46+Eomes-GFP+CD49a− | Eomes Itgam Zeb2 (no expression of Cd27 Itga1) | [212] | ||
| NK (early Spl) | Spleen | CD3ε−NKp46+CD49b+ | Xcl1 Ncr1 Jun Fos | [211] | ||
| NK (resting Spl) | Spleen | CD3ε−NKp46+CD49b+ | Cma1 Klf2 Zeb2 Itga4 Klrc2 | [211] | ||
| NK (proliferating Pec/Spl) | Spleen/peritoneal exudates | CD3ε−NKp46+CD49b+ | Mki67 Top2a Stmn1 | [211] | ||
| Cd11bˡᵒʷCd27ʰᶦᵍʰ | TME | CD3ε−NK1.1+ | Kit Pdcd1 Tigit Ctla4 | [213] | ||
| Cd11bʰᶦᵍʰCd27ˡᵒʷ | TME | CD3ε−NK1.1+ | Klrg1 Ncr1 Klrb1c Itga2 Klra3 Klra9 Prf1 Gzma Gzmb | [213] | ||
| NK(Hif1a−/−) | TME | CD3ε−NK1.1+ | Ifng Cd69 Ccl4 Ccl3 Nr4a1 | [213] | ||
| General | VAT | CD45+Lin−NK1.1+NKp46+ | Itga1 Il7r Ly6c2 | [210] | ||
| hNK | Human | Blood | CD3−CD14−CD19−CD45+CD56low/+ | PTMA S100A6 TGFB1 GNLY LDHA SCL75A | [209] | |
| hNK_Bl1 | Blood | anti-CD3, -CD14, -CD19, -CD45, -CD56 | FGFBP2 GZMB GZMA SPON2 CST7 FGCR3A GTF3C1 | [209] | ||
| hNK_Bl2 | Blood | anti-CD3, -CD14, -CD19, -CD45, -CD56 | GZMK CD44 CXCR3 SCML1 NCF1 XCL1 SCML1 ZFP36L2 | [209] | ||
| General | Blood | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | GZMK JUNB LTB LNFS | [214] | ||
| bNK0 | Blood | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | FCGR3A FCGR3B PRF1 GZMB | [214] | ||
| bNK1 | Blood | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | GZMK SELL | [214] | ||
| bNK2 | Blood | CD45+CD56+CD3ε−CD4−CD8a−CD14–CD15−CD163− | SELL IL7R XCL1/2 | [214] | ||
| bNK3 | Blood | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | PCNA MKI67 | [214] | ||
| h_NK_Bm1 | BM | CD3−CD14−CD19−CD45+CD56low/+ | FGFBP2 GZMB GZMH PRF1 FCGR3A | [215] | ||
| h_NK_Bm2 | BM | CD3−CD14−CD19−CD45+CD56low/+ | CCL3 CCL4 XCL1 XCL2 GZMK CCL3L1 AREG CD160 CD69 | [215] | ||
| h_NK_Bm3 | BM | CD3−CD14−CD19−CD45+CD56low/+ | GZMK XCL1 XCL2 AREG LTB SELL CD44 | [215] | ||
| h_NK_Bm4 | BM | CD3−CD14−CD19−CD45+CD56low/+ | CCL5 GZMM GZMH ZEB2 | [215] | ||
| General | BM | Not provided | GZMH GZMK GNLY | [216] | ||
| General | Bronchoalveolar lavage fluid | Not provided | TYROBP KLRD1 NKG7 FCER1G GNLY PRF1 GZMB KLRF1 XCL1/2 CCL3/4 | [217] | ||
| General | Liver/cirrhotic liver | CD45+ | KLRF1 CCL3 XCL1 IL2RB XCL2 CD160 KDLR1 CLEBPD CLIC3 LAT2 CCL3L3 | [218] | ||
| cNK | Liver/cirrhotic liver | CD45+ | KLRF1 GNLY PRF1 FGFBP3 SPON2 | [218] | ||
| General | Liver | Viable cells | NKG7 CCL4 CCL5 KLRD1 KLRK1 GZMA GZMB GZMH | [219] | ||
| General | Liver | Viable cells | CD7 KLRB1 NKG7 | [220] | ||
| trNK | Liver | CD45+ | KLRB1 TRDC CXCR6 EOMES GZMK KLRF1 | [221] | ||
| cNK | Liver | CD45+ | TBX21 CX3CR1 GZMB TRDC FGCR3A KLRF1 | [221] | ||
| NK-GZMH | Intrahepatic cholangiocarcinoma | Viable cells | KLRF1 KLRB1 IL7R LTB CCR6 NCR3 | [222] | ||
| NK-GZMK | Intrahepatic cholangiocarcinoma | Viable cells | KLRF1 TYROBP FCER1G GZMK CD160 | [222] | ||
| General | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15CD163− | FGFBP2 FCGR3A PRF1 S1PR5 GZMB | [214] | ||
| tNK0 | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | AREG SELL XCL1/2 FOS | [214] | ||
| tNK1 | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | CCL3/4L2/5 CD160 TIGIT | [214] | ||
| tNK2 | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | FGFBP2 FGCR3A PRF1 GZMB | [214] | ||
| tNK3 | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | SELL SPTSSB KIR2DL4 | [214] | ||
| tNK4 | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD16− | LST1 LTB | [214] | ||
| tNK5 | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | CCL5 HLA-DRBA GZMH | [214] | ||
| tNK6 | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | PCNA MKI67 | [214] | ||
| tNK7 | Melanoma metastasis | CD45+CD56+CD3ε−CD4−CD8a−CD14−CD15−CD163− | ISG15 IFI6 | [214] | ||
| General | Ovarian cancer ascites | CD45+ | KLRB1 KLRF1 | [223] | ||
| General | Ovarian cancer ascites | CD45+ | FCGR3A FCGR3B NCAM1 KLRB1 KLRB1 KLRC1 KLRD1 KLRF1 KLRK1 | [224] | ||
| General | PBMC | Not provided | KLRF1 FCGR3A | [225] | ||
| General | PBMC | Not provided | FCGR3A NCAM1 GZMB | [226] | ||
| General | PBMC | Not provided | KLRF1 | [227] | ||
| hNK | Spleen | CD3−CD14−CD19−CD45+CD56low/+ | KLF6 NFKBIA TSC22D3 ADGRE5 NCL1 CD69 ANXA1 | [209] | ||
| hNK_Sp1 | Spleen | CD3−CD14−CD19−CD45+CD56low/+ | FGFBP2 GZMB CST7 FGCR3A MYOM2 GNLY S100A4 CYBA PRF1 | [209] | ||
| hNK_Sp2 | Spleen | CD3−CD14−CD19−CD45+CD56low/+ | GZMK XCL1 COTL1 CD160 TOX2 RGS1 LIF KLRB1 KLRB1 BCL2A1 SPRY2 | [209] | ||
| hNK_Sp3 | Spleen | CD3−CD14−CD19−CD45+CD56low/+ | IL7R DUSP4 CD52 GRP183 SELL CD44 CAPG LTB YBX3 | [209] | ||
| hNK_Sp4 | Spleen | CD3−CD14−CD19−CD45+CD56low/+ | TTC30B TXNIP ARRDC3 SDF2 INPP5F | [209] | ||
| General | Tonsil | CD45+Lin−CD127−CD56+NKG2A+ | KLRC1 GNLY GZMA EOMES | [202] | ||
| General | TME/PBMC | CD3CD4CD8CD25 | CD160 XCL1 XCL2 MYADAM CAPG RORA NR4A1/2/3 KLRC1/2/3 IKZF2 EDNTPD1 CD69 ITGAE | [228] | ||
| NK-1 | Tumor/blood | CD3CD19CD20− | IFIT1/3 IFI6 CD8a | [229] | ||
| CD14−CD34−CD68−CD56+/−CD127+/− | ||||||
| NK-2 | Tumor/blood | CD3−CD19−CD20−CD14−CD34−CD68−CD56+/−CD127+/− | NR4A2 REL CXCR4 | [229] | ||
| NK-ILC1 | Tumor/blood | CD3−CD19−CD20−CD14−CD34−CD68−CD56+/−CD127+/− | CD83 IL7R SELL | [229] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roma, S.; Carpen, L.; Raveane, A.; Bertolini, F. The Dual Role of Innate Lymphoid and Natural Killer Cells in Cancer. from Phenotype to Single-Cell Transcriptomics, Functions and Clinical Uses. Cancers 2021, 13, 5042. https://doi.org/10.3390/cancers13205042
Roma S, Carpen L, Raveane A, Bertolini F. The Dual Role of Innate Lymphoid and Natural Killer Cells in Cancer. from Phenotype to Single-Cell Transcriptomics, Functions and Clinical Uses. Cancers. 2021; 13(20):5042. https://doi.org/10.3390/cancers13205042
Chicago/Turabian StyleRoma, Stefania, Laura Carpen, Alessandro Raveane, and Francesco Bertolini. 2021. "The Dual Role of Innate Lymphoid and Natural Killer Cells in Cancer. from Phenotype to Single-Cell Transcriptomics, Functions and Clinical Uses" Cancers 13, no. 20: 5042. https://doi.org/10.3390/cancers13205042
APA StyleRoma, S., Carpen, L., Raveane, A., & Bertolini, F. (2021). The Dual Role of Innate Lymphoid and Natural Killer Cells in Cancer. from Phenotype to Single-Cell Transcriptomics, Functions and Clinical Uses. Cancers, 13(20), 5042. https://doi.org/10.3390/cancers13205042







