Improved Prognostic Stratification Using Circulating Tumor Cell Clusters in Patients with Metastatic Castration-Resistant Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Associations between Baseline CTCs and Clinical Outcomes
2.3. Associations between Baseline CTC-Clusters and Clinical Outcomes
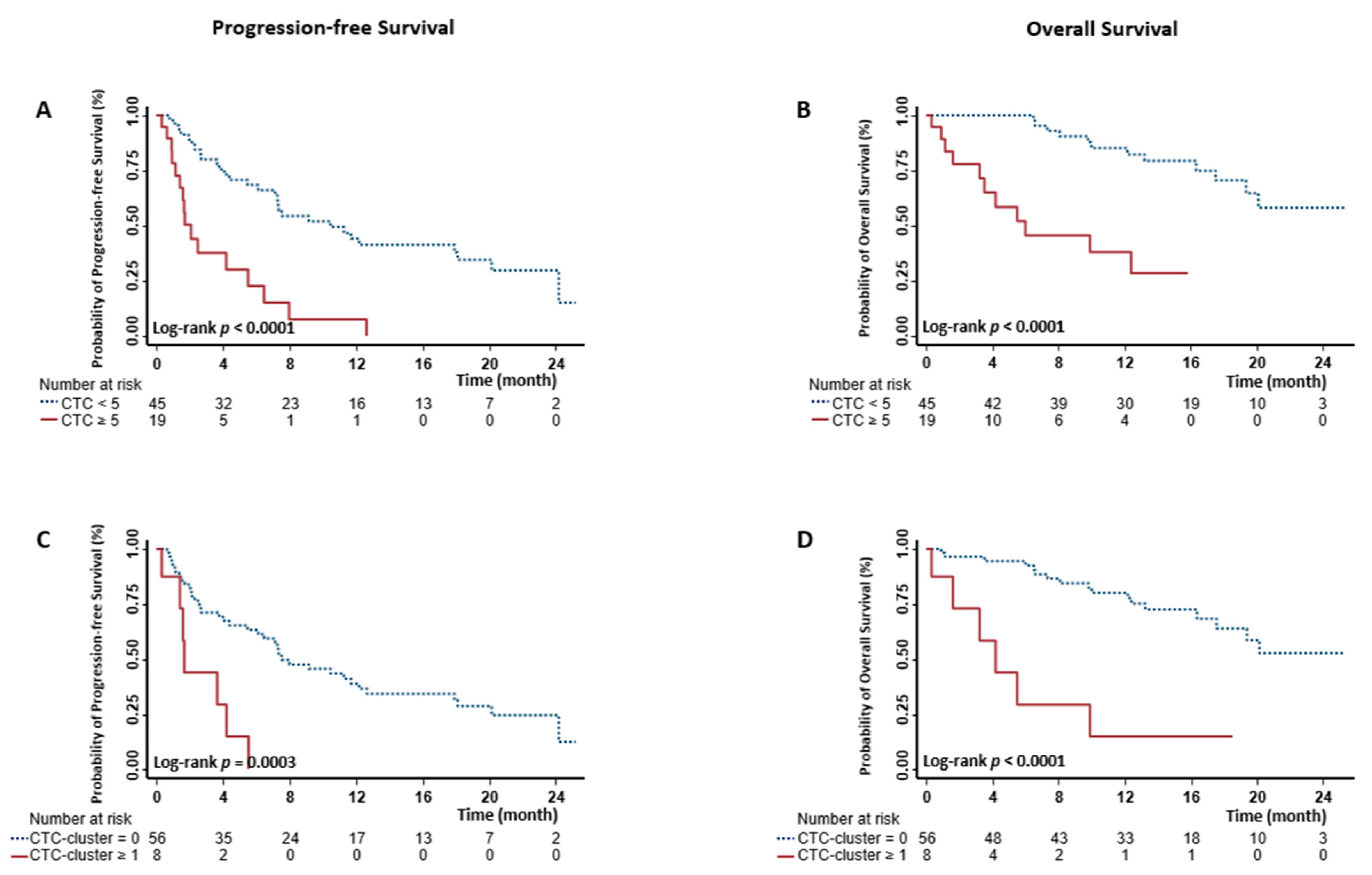
2.4. Prognostic Stratification Using Baseline CTCs and CTC-Clusters
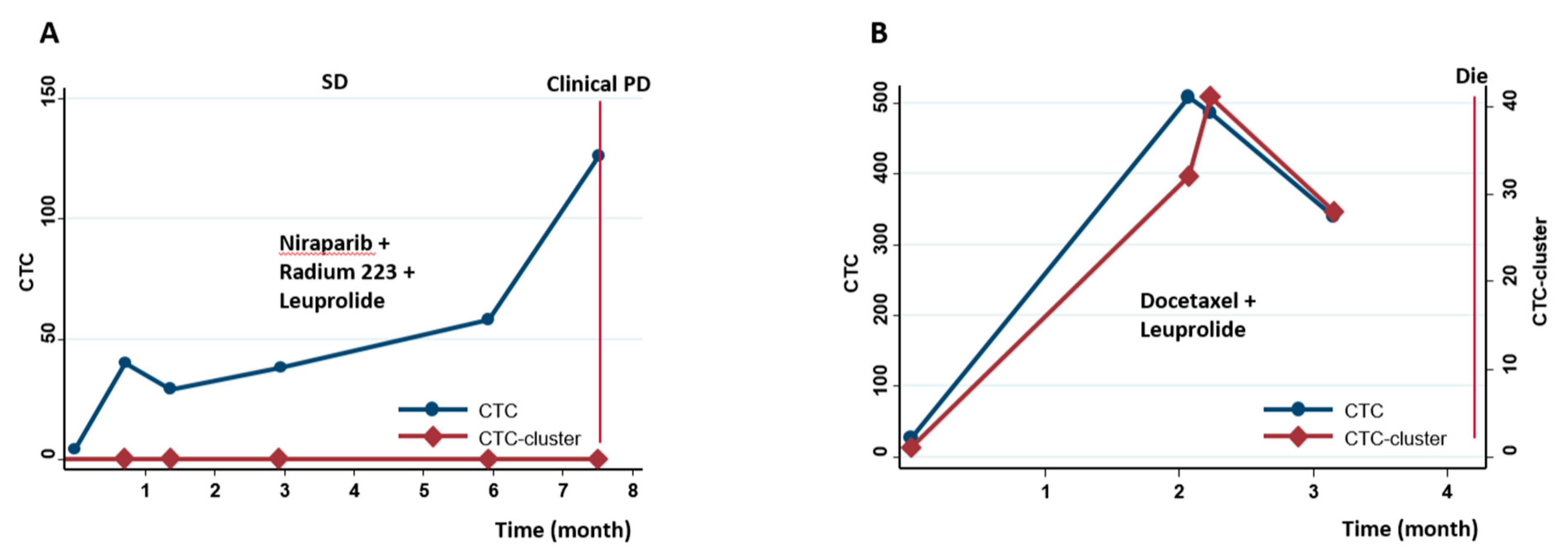
2.5. Prognostic Stratification Using Longitudinal CTCs and CTC-Clusters
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. CTC and CTC-Cluster Enumeration
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Pantel, K.; Hille, C.; Scher, H.I. Circulating Tumor Cells in Prostate Cancer: From Discovery to Clinical Utility. Clin. Chem. 2019, 65, 87–99. [Google Scholar] [CrossRef]
- Wade, C.A.; Kyprianou, N. Profiling Prostate Cancer Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 904. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef]
- Scher, H.I.; Solo, K.; Valant, J.; Todd, M.B.; Mehra, M. Prevalence of Prostate Cancer Clinical States and Mortality in the United States: Estimates Using a Dynamic Progression Model. PLoS ONE 2015, 10, e0139440. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Li, J.; Kemeny, G.; Bitting, R.L.; Beaver, J.; Somarelli, J.A.; Ware, K.E.; Gregory, S.; Armstrong, A.J. Whole Genomic Copy Number Alterations in Circulating Tumor Cells from Men with Abiraterone or Enzalutamide-Resistant Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2017, 23, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Stenehjem, D.; Nussenzveig, R.; Carroll, E.; Bailey, E.; Batten, J.; Maughan, B.L.; Agarwal, N. Evolution of the genomic landscape of circulating tumor DNA (ctDNA) in metastatic prostate cancer over treatment and time. Cancer Treat. Res. Commun. 2019, 19, 100120. [Google Scholar] [CrossRef]
- Boutros, P.C.; Fraser, M.; Harding, N.J.; de Borja, R.; Trudel, D.; Lalonde, E.; Meng, A.; Hennings-Yeomans, P.H.; McPherson, A.; Sabelnykova, V.Y.; et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat. Genet. 2015, 47, 736–745. [Google Scholar] [CrossRef]
- Maheswaran, S.; Haber, D.A. Circulating tumor cells: A window into cancer biology and metastasis. Curr. Opin. Genet. Dev. 2010, 20, 96–99. [Google Scholar] [CrossRef]
- Heitzer, E.; Auer, M.; Ulz, P.; Geigl, J.B.; Speicher, M.R. Circulating tumor cells and DNA as liquid biopsies. Genome Med. 2013, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Danila, D.C.; Fleisher, M.; Scher, H.I. Circulating tumor cells as biomarkers in prostate cancer. Clin. Cancer Res. 2011, 17, 3903–3912. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Lin, C.Y.; Kelly, W.K.; Fizazi, K.S.; Moul, J.W.; Kaplan, E.B.; Morris, M.J.; Small, E.J. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 671–677. [Google Scholar] [CrossRef]
- Goldkorn, A.; Ely, B.; Quinn, D.I.; Tangen, C.M.; Fink, L.M.; Xu, T.; Twardowski, P.; Van Veldhuizen, P.J.; Agarwal, N.; Carducci, M.A.; et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: A phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 1136–1142. [Google Scholar] [CrossRef]
- Heller, G.; Fizazi, K.; McCormack, R.; Molina, A.; MacLean, D.; Webb, I.J.; Saad, F.; de Bono, J.S.; Scher, H.I. The Added Value of Circulating Tumor Cell Enumeration to Standard Markers in Assessing Prognosis in a Metastatic Castration-Resistant Prostate Cancer Population. Clin. Cancer Res. 2017, 23, 1967–1973. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Fizazi, K.; Burke, J.M.; De Wit, R.; Bellmunt, J.; Hutson, T.E.; Crane, E.; Berry, W.R.; Doner, K.; Hainsworth, J.D.; et al. Circulating Tumor Cells in a Phase 3 Study of Docetaxel and Prednisone with or without Lenalidomide in Metastatic Castration-resistant Prostate Cancer. Eur. Urol 2017, 71, 168–171. [Google Scholar] [CrossRef]
- Heller, G.; McCormack, R.; Kheoh, T.; Molina, A.; Smith, M.R.; Dreicer, R.; Saad, F.; de Wit, R.; Aftab, D.T.; Hirmand, M.; et al. Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison With Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials. J. Clin. Oncol. 2018, 36, 572–580. [Google Scholar] [CrossRef]
- Te Boekhorst, V.; Friedl, P. Plasticity of Cancer Cell Invasion-Mechanisms and Implications for Therapy. Adv. Cancer Res. 2016, 132, 209–264. [Google Scholar] [CrossRef]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Wang, C.; Ye, Z.; Austin, L.; Civan, J.; Hyslop, T.; Palazzo, J.P.; Jaslow, R.; Li, B.; Myers, R.E.; et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res. Treat. 2015, 154, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef]
- McDaniel, A.S.; Ferraldeschi, R.; Krupa, R.; Landers, M.; Graf, R.; Louw, J.; Jendrisak, A.; Bales, N.; Marrinucci, D.; Zafeiriou, Z.; et al. Phenotypic diversity of circulating tumour cells in patients with metastatic castration-resistant prostate cancer. BJU Int. 2017, 120, E30–E44. [Google Scholar] [CrossRef]
- Okegawa, T.; Ninomiya, N.; Masuda, K.; Nakamura, Y.; Tambo, M.; Nutahara, K. AR-V7 in circulating tumor cells cluster as a predictive biomarker of abiraterone acetate and enzalutamide treatment in castration-resistant prostate cancer patients. Prostate 2018, 78, 576–582. [Google Scholar] [CrossRef]
- Marshall, J.A.; Scarbro, S.; Shetterly, S.M.; Jones, R.H. Improving power with repeated measures: Diet and serum lipids. Am. J. Clin. Nutr. 1998, 67, 934–939. [Google Scholar] [CrossRef]
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B.; et al. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov. 2019, 9, 96–113. [Google Scholar] [CrossRef]
- Au, S.H.; Storey, B.D.; Moore, J.C.; Tang, Q.; Chen, Y.L.; Javaid, S.; Sarioglu, A.F.; Sullivan, R.; Madden, M.W.; O’Keefe, R.; et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl. Acad. Sci. USA 2016, 113, 4947–4952. [Google Scholar] [CrossRef]
- Molnar, B.; Ladanyi, A.; Tanko, L.; Sreter, L.; Tulassay, Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin. Cancer Res. 2001, 7, 4080–4085. [Google Scholar]
- Fanelli, M.F.; Oliveira, T.B.; Braun, A.C.; Corassa, M.; Abdallah, E.A.; Nicolau, U.R.; da Silva Alves, V.; Garcia, D.; Calsavara, V.F.; Kowalski, L.P.; et al. Evaluation of incidence, significance, and prognostic role of circulating tumor microemboli and transforming growth factor-beta receptor I in head and neck cancer. Head Neck 2017, 39, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Harryman, W.L.; Hinton, J.P.; Rubenstein, C.P.; Singh, P.; Nagle, R.B.; Parker, S.J.; Knudsen, B.S.; Cress, A.E. The Cohesive Metastasis Phenotype in Human Prostate Cancer. Biochim. Biophys. Acta 2016, 1866, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Budna-Tukan, J.; Świerczewska, M.; Mazel, M.; Cieślikowski, W.A.; Ida, A.; Jankowiak, A.; Antczak, A.; Nowicki, M.; Pantel, K.; Azria, D.; et al. Analysis of Circulating Tumor Cells in Patients with Non-Metastatic High-Risk Prostate Cancer before and after Radiotherapy Using Three Different Enumeration Assays. Cancers 2019, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Nagle, R.B.; Cress, A.E. Metastasis Update: Human Prostate Carcinoma Invasion via Tubulogenesis. Prostate Cancer 2011, 2011, 249290. [Google Scholar] [CrossRef] [PubMed]
- Hoevenaar-Blom, M.P.; Spijkerman, A.M.; Boshuizen, H.C.; Boer, J.M.; Kromhout, D.; Verschuren, W.M. Effect of using repeated measurements of a Mediterranean style diet on the strength of the association with cardiovascular disease during 12 years: The Doetinchem Cohort Study. Eur. J. Nutr. 2014, 53, 1209–1215. [Google Scholar] [CrossRef]
- Hiew, K.; Hart, C.A.; Ali, A.; Elliott, T.; Ramani, V.; Sangar, V.; Lau, M.; Maddineni, S.; Brown, M.; Clarke, N. Primary Mutational Landscape Linked with Pre-Docetaxel Lactate Dehydrogenase Levels Predicts Docetaxel Response in Metastatic Castrate-Resistant Prostate Cancer. Eur. Urol. Focus 2019, 5, 831–841. [Google Scholar] [CrossRef]
- Ciccarese, C.; Santoni, M.; Brunelli, M.; Buti, S.; Modena, A.; Nabissi, M.; Artibani, W.; Martignoni, G.; Montironi, R.; Tortora, G.; et al. AR-V7 and prostate cancer: The watershed for treatment selection? Cancer Treat. Rev. 2016, 43, 27–35. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. J. Clin. Oncol. 2017, 35, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]

| Variables | n (%) |
|---|---|
| Age (year), median (range) | 71.8 (53.0–93.0) |
| Race | |
| White | 49 (76.6) |
| Black | 12 (18.8) |
| Other | 3 (4.7) |
| ISUP Grade at Diagnosis | |
| 1 | 3 (4.7) |
| 2 | 5 (7.8) |
| 3 | 5 (7.8) |
| 4 | 11 (17.2) |
| 5 | 32 (50.0) |
| Unknown | 8 (12.5) |
| ECOG Performance Status | |
| 0 | 28 (43.8) |
| 1 | 25 (39.1) |
| 2 | 8 (12.5) |
| 3 | 2 (3.1) |
| Unknown | 1 (1.6) |
| Bone Metastasis | |
| No | 4 (6.3) |
| Yes | 60 (93.8) |
| Visceral Metastasis | |
| No | 51 (79.7) |
| Yes | 13 (20.3) |
| Previously Treated by AR Signaling Inhibitors * | |
| No | 29 (45.3) |
| Yes | 35 (54.7) |
| Previous Chemotherapy * | |
| No | 54 (84.4) |
| Yes | 10 (15.6) |
| AR Signaling Inhibitors After Baseline Blood Draw | |
| No | 20 (31.3) |
| Yes | 44 (68.8) |
| Cytotoxic Therapy After Baseline Blood Draw | |
| No | 48 (75.0) |
| Yes | 16 (25.0) |
| Prostate-specific antigen (ng/mL), median (range) | 9.5 (0.1–3256.0) |
| Hemoglobin (g/dL), median (range) | 12.0 (7.4–15.9) |
| Alkaline phosphatase (IU/L), median (range) | 88.5 (31.0–1709.0) |
| Albumin (g/dL), median (range) | 4.1 (2.6–4.7) |
| Lactate dehydrogenase (IU/L), median (range) # | 216 (149–560) |
| Variables | Event/Total | MST (mo) | Univariate Analyses | Multivariate Analyses * | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |||
| Association With PFS | ||||||
| CTC | ||||||
| <5 | 29/45 | 10.5 | 1.00 | 1.00 | ||
| ≥5 | 16/19 | 2.1 | 4.03 (2.09–7.77) | <0.0001 | 3.01 (1.30–6.95) | 0.0101 |
| CTC-Cluster | ||||||
| 0 | 38/56 | 7.5 | 1.00 | 1.00 | ||
| ≥1 | 7/8 | 1.7 | 4.33 (1.81–10.37) | 0.0010 | 2.36 (0.70–7.95) | 0.1666 |
| Risk Group | ||||||
| CTC < 5 without CTC-cluster | 28/44 | 11.3 | 1.00 | 1.00 | ||
| CTC ≥ 5 without CTC-cluster | 10/12 | 2.1 | 3.53 (1.66–7.51) | 0.0011 | 3.51 (1.38–8.89) | 0.0082 |
| CTC ≥ 5 with CTC-cluster | 6/7 | 1.7 | 6.30 (2.40–16.53) | 0.0002 | 2.57 (0.62–10.59) | 0.1909 |
| Association With OS | ||||||
| CTC | ||||||
| <5 | 12/45 | NR | 1.00 | 1.00 | ||
| ≥5 | 11/19 | 6.0 | 6.90 (2.72–17.47) | <0.0001 | 4.66 (1.38–15.74) | 0.0134 |
| CTC-Cluster | ||||||
| 0 | 17/56 | NR | 1.00 | 1.00 | ||
| ≥1 | 6/8 | 4.2 | 6.58 (2.50–17.33) | 0.0001 | 5.52 (1.18–25.79) | 0.0299 |
| Risk Group | ||||||
| CTC < 5 without CTC-cluster | 12/44 | NR | 1.00 | 1.00 | ||
| CTC ≥ 5 without CTC-cluster | 5/12 | 12.4 | 3.96 (1.29–12.21) | 0.0165 | 1.42 (0.32–6.33) | 0.6497 |
| CTC ≥ 5 with CTC-cluster | 6/7 | 4.2 | 21.53 (6.64–69.85) | <0.0001 | 19.84 (2.24–175.32) | 0.0072 |
| Variables | Univariate Analyses | Multivariate Analyses * | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Association with PFS | ||||
| CTC | ||||
| 0 | 1.00 | 1.00 | ||
| 1–4 | 4.25 (1.98–9.13) | 0.0002 | 4.98 (2.11–11.74) | 0.0002 |
| ≥5 | 9.72 (4.38–21.58) | <0.0001 | 8.07 (2.91–22.41) | <0.0001 |
| CTC-Cluster | ||||
| 0 | 1.00 | 1.00 | ||
| ≥1 | 4.97 (2.15–11.50) | 0.0002 | 2.59 (0.85–7.88) | 0.0947 |
| Risk Group | ||||
| 0 CTC | 1.00 | 1.00 | ||
| 1–4 CTCs without CTC-cluster | 4.09 (1.86–8.97) | 0.0004 | 4.55 (1.85–11.18) | 0.0010 |
| ≥5 CTCs without CTC-cluster | 8.47 (3.41–21.04) | <0.0001 | 8.83 (2.79–27.92) | 0.0002 |
| ≥5 CTCs with CTC-cluster | 12.11 (4.47–32.80) | <0.0001 | 7.59 (1.82–31.73) | 0.0055 |
| 1–4 CTCs without CTC-cluster | 1.00 | 1.00 | ||
| ≥5 CTCs without CTC-cluster | 2.07 (0.77–5.54) | 0.1471 | 1.94 (0.56–6.72) | 0.2959 |
| ≥5 CTCs with CTC-cluster | 2.96 (1.02–8.57) | 0.0451 | 1.67 (0.42–6.56) | 0.4638 |
| Association with OS | ||||
| CTC | ||||
| 0 | 1.00 | 1.00 | ||
| 1–4 | 3.60 (0.72–17.90) | 0.1180 | 2.51 (0.38–16.43) | 0.3378 |
| ≥5 | 32.84 (8.42–128.20) | <0.0001 | 28.47 (3.85–210.36) | 0.0010 |
| CTC-Cluster | ||||
| 0 | 1.00 | 1.00 | ||
| ≥1 | 9.16 (3.64–23.04) | <0.0001 | 3.90 (1.07–14.20) | 0.0387 |
| Risk Group | ||||
| 0 CTC | 1.00 | 1.00 | ||
| 1–4 CTCs without CTC-cluster | 3.66 (0.73–18.20) | 0.1130 | 2.87 (0.42–19.77) | 0.2847 |
| ≥5 CTCs without CTC-cluster | 26.56 (6.35–111.00) | <0.0001 | 28.15 (3.62–218.61) | 0.0014 |
| ≥5 CTCs with CTC-cluster | 46.35 (10.65–201.80) | <0.0001 | 48.17 (4.10–566.62) | 0.0021 |
| 1–4 CTCs without CTC-cluster | 1.00 | 1.00 | ||
| ≥5 CTCs without CTC-cluster | 7.26 (1.70–31.01) | 0.0074 | 9.81 (1.70–56.62) | 0.0107 |
| ≥5 CTCs with CTC-cluster | 12.67 (2.83–56.84) | 0.0009 | 16.79 (2.30–122.73) | 0.0054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, Z.; Chong, W.; Luo, R.; Myers, R.E.; Gu, J.; Lin, J.; Wei, Q.; Li, B.; Rebbeck, T.R.; et al. Improved Prognostic Stratification Using Circulating Tumor Cell Clusters in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 268. https://doi.org/10.3390/cancers13020268
Wang C, Zhang Z, Chong W, Luo R, Myers RE, Gu J, Lin J, Wei Q, Li B, Rebbeck TR, et al. Improved Prognostic Stratification Using Circulating Tumor Cell Clusters in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers. 2021; 13(2):268. https://doi.org/10.3390/cancers13020268
Chicago/Turabian StyleWang, Chun, Zhenchao Zhang, Weelic Chong, Rui Luo, Ronald E. Myers, Jian Gu, Jianqing Lin, Qiang Wei, Bingshan Li, Timothy R. Rebbeck, and et al. 2021. "Improved Prognostic Stratification Using Circulating Tumor Cell Clusters in Patients with Metastatic Castration-Resistant Prostate Cancer" Cancers 13, no. 2: 268. https://doi.org/10.3390/cancers13020268
APA StyleWang, C., Zhang, Z., Chong, W., Luo, R., Myers, R. E., Gu, J., Lin, J., Wei, Q., Li, B., Rebbeck, T. R., Lu-Yao, G., Kelly, W. K., & Yang, H. (2021). Improved Prognostic Stratification Using Circulating Tumor Cell Clusters in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers, 13(2), 268. https://doi.org/10.3390/cancers13020268





