Comparison of Oncologic Outcomes between Carbon Ion Radiotherapy and Stereotactic Body Radiotherapy for Early-Stage Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics of the Entire Cohort
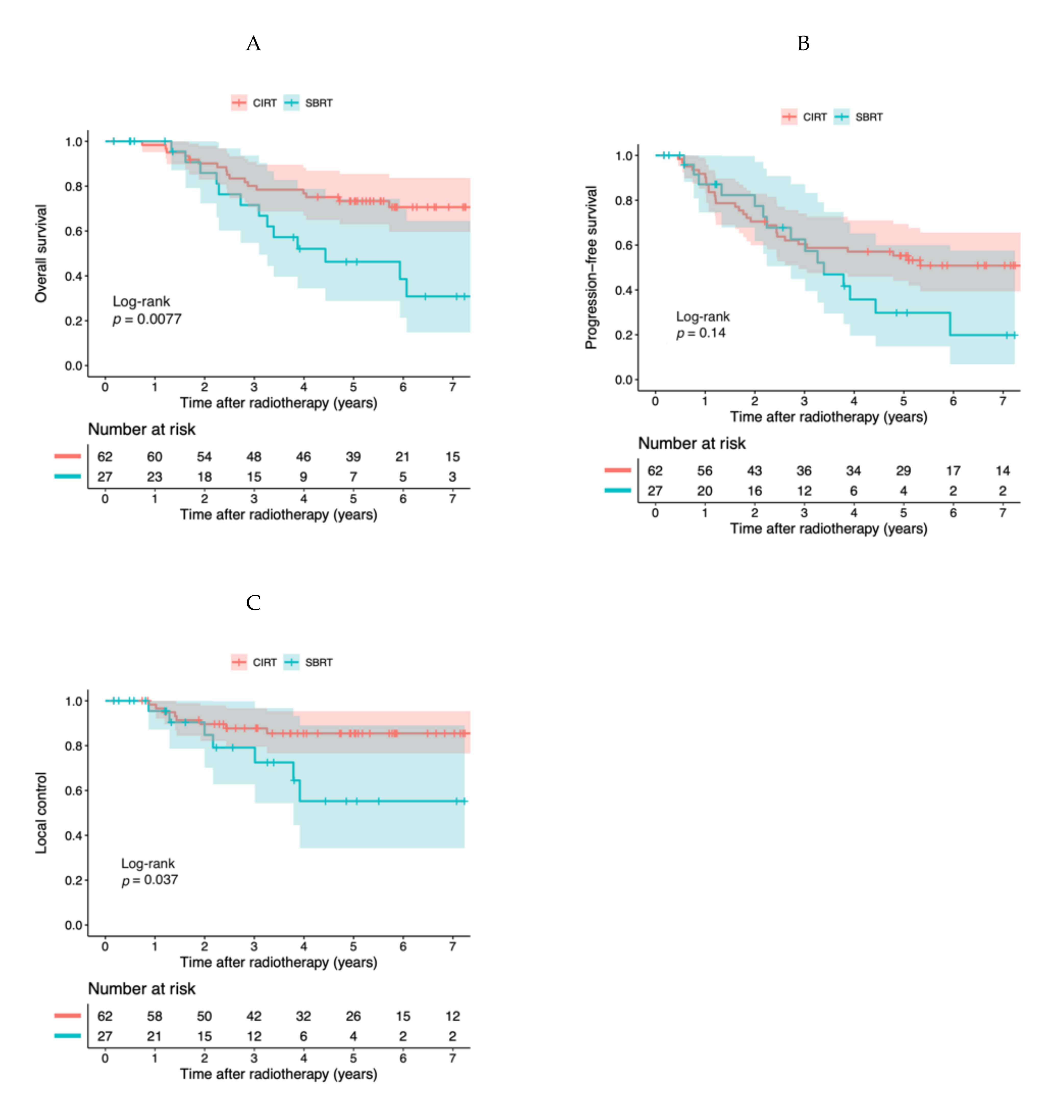
2.2. Clinical Outcomes of the Entire Cohort
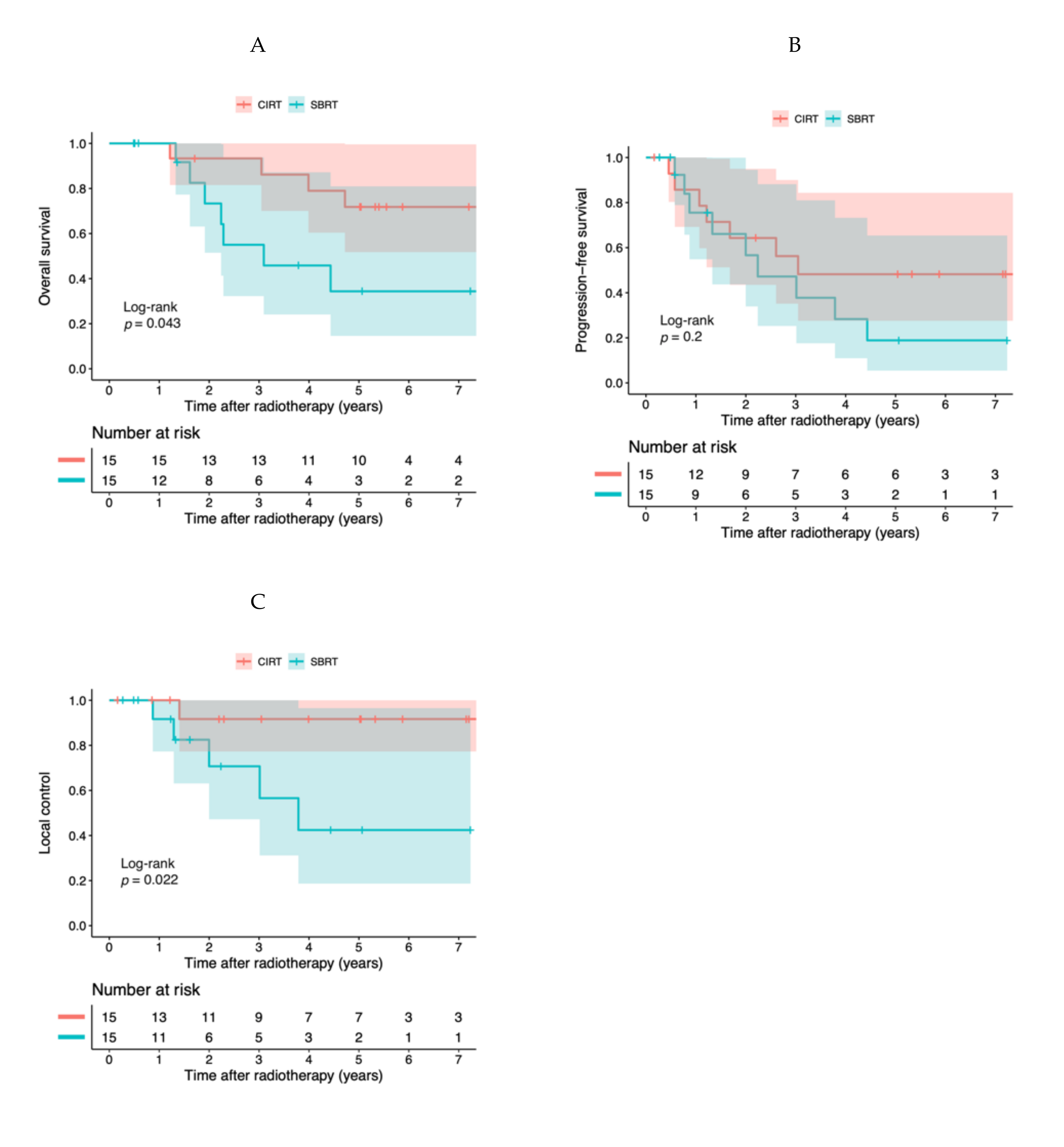
2.3. Propensity Score-Adjusted Analyses
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Patients
4.3. Carbon Ion Radiotherapy
4.4. Stereotactic Body Radiotherapy
4.5. Follow-Up and Evaluation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN) Guidelines; Non-Small Cell Lung Cancer Version 8. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 4 October 2020).
- Ball, D.; Mai, G.T.; Vinod, S.; Babington, S.; Ruben, J.; Kron, T.; Chesson, B.; Herschtal, A.; Vanevski, M.; Rezo, A.; et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019, 20, 494–503. [Google Scholar] [CrossRef]
- Chang, J.; Senan, S.; Paul, A.; Mehran, R.; Louie, A.; Balter, P.; Groen, H.; McRae, S.; Widder, J.; Feng, L.; et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 2017, 16, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-Ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 201–210. [Google Scholar] [CrossRef]
- Koto, M.; Miyamoto, T.; Yamamoto, N.; Nishimura, H.; Yamada, S.; Tsujii, H. Local control and recurrence of stage I non-small cell lung cancer after carbon ion radiotherapy. Radiother. Oncol. 2004, 71, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Baba, M.; Yamamoto, N.; Koto, M.; Sugawara, T.; Yashiro, T.; Kadono, K.; Ezawa, H.; Tsujii, H.; Mizoe, J.E.; et al. Curative treatment of Stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 750–758. [Google Scholar] [CrossRef]
- Miyamoto, T.; Baba, M.; Sugane, T.; Nakajima, M.; Yashiro, T.; Kagei, K.; Hirasawa, N.; Sugawara, T.; Yamamoto, N.; Koto, M.; et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J. Thorac. Oncol. 2007, 2, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, J.I.; Shirai, K.; Mizukami, T.; Abe, T.; Ebara, T.; Ohno, T.; Minato, K.; Saito, R.; Yamada, M.; Nakano, T. Hypofractionated carbon-ion radiotherapy for stage I peripheral nonsmall cell lung cancer (GUNMA0701): Prospective phase II study. Cancer Med. 2019, 8, 6644–6650. [Google Scholar] [CrossRef]
- Sugane, T.; Baba, M.; Imai, R.; Nakajima, M.; Yamamoto, N.; Miyamoto, T.; Ezawa, H.; Yoshikawa, K.; Kandatsu, S.; Kamada, T.; et al. Carbon ion radiotherapy for elderly patients 80 years and older with stage I non-small cell lung cancer. Lung Cancer 2009, 64, 45–50. [Google Scholar] [CrossRef]
- Karube, M.; Yamamoto, N.; Nakajima, M.; Yamashita, H.; Nakagawa, K.; Miyamoto, T.; Tsuji, H.; Fujisawa, T.; Kamada, T. Single-Fraction Carbon-Ion Radiation Therapy for Patients 80 Years of Age and Older with Stage i Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 542–548. [Google Scholar] [CrossRef]
- Nakajima, M.; Yamamoto, N.; Hayashi, K.; Karube, M.; Ebner, D.K.; Takahashi, W.; Anzai, M.; Tsushima, K.; Tada, Y.; Tatsumi, K.; et al. Carbon-ion radiotherapy for non-small cell lung cancer with interstitial lung disease: A retrospective analysis. Radiat. Oncol. 2017, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ebara, T.; Shimada, H.; Kawamura, H.; Shirai, K.; Saito, J.I.; Kawashima, M.; Tashiro, M.; Ohno, T.; Kanai, T.; Nakano, T. Dosimetric analysis between carbon ion radiotherapy and stereotactic body radiotherapy in stage I lung cancer. Anticancer Res. 2014, 34, 5099–5104. [Google Scholar] [PubMed]
- Grutters, J.P.C.; Kessels, A.G.H.; Pijls-Johannesma, M.; De Ruysscher, D.; Joore, M.A.; Lambin, P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: A meta-analysis. Radiother. Oncol. 2010, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Hiraoka, M.; Shibata, T.; Onishi, H.; Kokubo, M.; Karasawa, K.; Shioyama, Y.; Onimaru, R.; Kozuka, T.; Kunieda, E.; et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 989–996. [Google Scholar] [CrossRef] [PubMed]
- IJsseldijk, M.A.; Shoni, M.; Siegert, C.; Wiering, B.; van Engelenburg, K.C.A.; Lebenthal, A.; ten Broek, R.P.G. Survival After Stereotactic Body Radiation Therapy for Clinically Diagnosed or Biopsy-Proven Early-Stage NSCLC: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2019, 14, 583–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirai, K.; Kawashima, M.; Saitoh, J.I.; Abe, T.; Fukata, K.; Shigeta, Y.; Irie, D.; Shiba, S.; Okano, N.; Ohno, T.; et al. Clinical outcomes using carbon-ion radiotherapy and dose-volume histogram comparison between carbon-ion radiotherapy and photon therapy for T2b-4N0M0 non-small cell lung cancer-A pilot study. PLoS ONE 2017, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Onimaru, R.; Onishi, H.; Ogawa, G.; Hiraoka, M.; Ishikura, S.; Karasawa, K.; Matsuo, Y.; Kokubo, M.; Shioyama, Y.; Matsushita, H.; et al. Final report of survival and late toxicities in the Phase i study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer (JCOG0702). Jpn. J. Clin. Oncol. 2018, 48, 1076–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, Y.; Shibuya, K.; Nagata, Y.; Takayama, K.; Norihisa, Y.; Mizowaki, T.; Narabayashi, M.; Sakanaka, K.; Hiraoka, M. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Wu, A.J.; Adeseye, V.; Din, S.U.; Shaikh, F.; Woo, K.M.; Zhang, Z.; Foster, A.; Rosenzweig, K.E.; Gewanter, R.; et al. Recurrence Patterns and Second Primary Lung Cancers after Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Cancer: Implications for Surveillance This work was presented at the 56th Annual Meeting of the American Society for Radiation Oncology (ASTRO); September 14-17, 2014; San Francisco, CA. Clin. Lung Cancer 2016, 17, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, F.; Li, B.; Li, H.; Liu, J.; Huang, W.; Wang, D.; Yi, Y.; Wang, J. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage i non-small-cell lung cancer? A meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 305–316. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; Van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Instig. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Data File. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 30 May 2020).
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 22 May 2020).
| Characteristic | Overall, n = 89 1 | CIRT, n = 62 1 | SBRT, n = 27 1 | p-Value 2 |
|---|---|---|---|---|
| Age | 74 (66,80) | 72 (64,79) | 77 (73,82) | 0.032 |
| Sex | 0.8 | |||
| Male | 63 (71%) | 43 (69%) | 20 (74%) | |
| Female | 26 (29%) | 19 (31%) | 7 (26%) | |
| ECOG PS | >0.9 | |||
| 0 | 42 (47%) | 29 (47%) | 13 (48%) | |
| 1 | 45 (51%) | 31 (50%) | 14 (52%) | |
| 2 | 2 (2.2%) | 2 (3.2%) | 0 (0%) | |
| CCI | 0.12 | |||
| 0 | 36 (40%) | 28 (45%) | 8 (30%) | |
| 1 | 11 (12%) | 8 (13%) | 3 (11%) | |
| 2 | 23 (26%) | 16 (26%) | 7 (26%) | |
| 3 | 10 (11%) | 5 (8.1%) | 5 (19%) | |
| 4 | 6 (6.7%) | 5 (8.1%) | 1 (3.7%) | |
| 5 | 1 (1.1%) | 0 (0%) | 1 (3.7%) | |
| 6 | 2 (2.2%) | 0 (0%) | 2 (7.4%) | |
| Pathological diagnosis | >0.9 | |||
| Adenocarcinoma | 48 (54%) | 33 (53%) | 15 (56%) | |
| Squamous cell carcinoma | 26 (29%) | 19 (31%) | 7 (26%) | |
| Clinical | 15 (17%) | 10 (16%) | 5 (19%) | |
| T stage | 0.009 | |||
| 1a | 41 (46%) | 23 (37%) | 18 (67%) | |
| 1b | 29 (33%) | 21 (34%) | 8 (30%) | |
| 2a | 19 (21%) | 18 (29%) | 1 (3.7%) | |
| Brinkman Index | 572 (0,1,120) | 511 (0,1,085) | 600 (50,1,335) | 0.2 |
| Smoking | 0.3 | |||
| Never | 32 (36%) | 25 (40%) | 7 (26%) | |
| Past/ Current | 57 (64%) | 37 (60%) | 20 (74%) | |
| COPD | 0.7 | |||
| No | 62 (70%) | 42 (68%) | 20 (73%) | |
| Yes | 27 (30%) | 20 (32%) | 7 (26%) | |
| Interstitial pneumonia | 0.2 | |||
| No | 86 (97%) | 61 (98%) | 25 (93%) | |
| Yes | 3 (3.4%) | 1 (1.6%) | 2 (7.4%) | |
| Treatment year | 0.065 | |||
| 2010–2012 | 42 (47%) | 25 (9.7%) | 17 (3.7%) | |
| 2013–2015 | 47 (53%) | 37 (9.7%) | 10 (3.7%) |
| Variables | Subgroup | Overall Survival | Progression-Free Survival | Local Control | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Treatment | SBRT | reference | 0.047 | reference | 0.20 | reference | 0.040 |
| CIRT | 0.41 (0.17–0.99) | 0.61 (0.29–1.3)) | 0.30 (0.097–0.95) | ||||
| T stage | T1a–1b | reference | 0.00095 | reference | 0.0023 | reference | 0.48 |
| T2a | 5.5 (2.0–15) | 3.2 (1.5–6.8) | 1.7 (0.4–6.9) | ||||
| CCI | 0–2 | reference | 0.00015 | reference | 0.019 | ||
| 3–6 | 6.0 (2.4–15.3) | 2.5 (1.2–5.5) | |||||
| Characteristic | Overall, n = 30 1 | CIRT, n = 15 1 | SBRT, n = 15 1 | p-Value 2 |
|---|---|---|---|---|
| Age | 76 (70,82) | 74 (66,82) | 76 (71,79) | >0.9 |
| Sex | >0.9 | |||
| Male | 23 (77%) | 11 (73%) | 12 (80%) | |
| Female | 7 (23%) | 4 (27%) | 3 (20%) | |
| ECOG PS | 0.7 | |||
| 0 | 14 (47%) | 8 (53%) | 6 (40%) | |
| 1 | 16 (53%) | 7 (47%) | 9 (60%) | |
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | |
| CCI | 0.6 | |||
| 0 | 8 (27%) | 4 (27%) | 4 (27%) | |
| 1 | 4 (13%) | 3 (20%) | 1 (6.7%) | |
| 2 | 8 (27%) | 3 (20%) | 5 (33%) | |
| 3 | 7 (23%) | 3 (20%) | 4 (27%) | |
| 4 | 2 (6.7%) | 2 (13%) | 0 (0%) | |
| 5 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 6 | 1 (3.3%) | 0 (0%) | 1 (6.7%) | |
| Pathological diagnosis | >0.9 | |||
| Adenocarcinoma | 15 (50%) | 8 (53%) | 7 (47%) | |
| Squamous cell carcinoma | 11 (37%) | 5 (33%) | 6 (40%) | |
| Clinical | 4 (13%) | 2 (13%) | 2 (13%) | |
| T stage | >0.9 | |||
| 1a | 15 (50%) | 7 (47%) | 8 (53%) | |
| 1b | 13 (43%) | 7 (47%) | 6 (40%) | |
| 2a | 2 (6.7%) | 1 (6.7%) | 1 (6.7%) | |
| Brinkman Index | 778 (0, 1,100) | 760 (33, 950) | 900 (0, 1,415) | 0.6 |
| Smoking | >0.9 | |||
| Never | 9 (30%) | 4 (27%) | 5 (33%) | |
| Past/Current | 21 (70%) | 11 (73%) | 10 (67%) | |
| COPD | >0.9 | |||
| No | 22 (73%) | 11 (73%) | 11 (73%) | |
| Yes | 8 (27%) | 4 (27%) | 4 (27%) | |
| Interstitial pneumonia | >0.9 | |||
| No | 30 (100%) | 15 (100%) | 15 (100%) | |
| Yes | 0 (0%) | 0 (0%) | 0 (0%) | |
| Treatment year | 0.26 | |||
| 2010–2012 | 12 (40%) | 4 (33%) | 8 (67%) | |
| 2013–2015 | 18 (60%) | 11 (61%) | 7 (39%) |
| Variables | Subgroup | Overall Survival | Progression-Free Survival | Local Control | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Treatment | SBRT | reference | 0.018 | reference | 0.022 | reference | 0.0051 |
| CIRT | 0.34 (0.14–0.83) | 0.41 (0.19–0.88) | 0.19 (0.059–0.61) | ||||
| Propensity scores | 1.4 (0.31–6.4) | 0.66 | 3.3 (0.86–13) | 0.082 | 8.0 (0.79–82) | 0.78 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyasaka, Y.; Komatsu, S.; Abe, T.; Kubo, N.; Okano, N.; Shibuya, K.; Shirai, K.; Kawamura, H.; Saitoh, J.-i.; Ebara, T.; et al. Comparison of Oncologic Outcomes between Carbon Ion Radiotherapy and Stereotactic Body Radiotherapy for Early-Stage Non-Small Cell Lung Cancer. Cancers 2021, 13, 176. https://doi.org/10.3390/cancers13020176
Miyasaka Y, Komatsu S, Abe T, Kubo N, Okano N, Shibuya K, Shirai K, Kawamura H, Saitoh J-i, Ebara T, et al. Comparison of Oncologic Outcomes between Carbon Ion Radiotherapy and Stereotactic Body Radiotherapy for Early-Stage Non-Small Cell Lung Cancer. Cancers. 2021; 13(2):176. https://doi.org/10.3390/cancers13020176
Chicago/Turabian StyleMiyasaka, Yuhei, Shuichiro Komatsu, Takanori Abe, Nobuteru Kubo, Naoko Okano, Kei Shibuya, Katsuyuki Shirai, Hidemasa Kawamura, Jun-ichi Saitoh, Takeshi Ebara, and et al. 2021. "Comparison of Oncologic Outcomes between Carbon Ion Radiotherapy and Stereotactic Body Radiotherapy for Early-Stage Non-Small Cell Lung Cancer" Cancers 13, no. 2: 176. https://doi.org/10.3390/cancers13020176
APA StyleMiyasaka, Y., Komatsu, S., Abe, T., Kubo, N., Okano, N., Shibuya, K., Shirai, K., Kawamura, H., Saitoh, J.-i., Ebara, T., & Ohno, T. (2021). Comparison of Oncologic Outcomes between Carbon Ion Radiotherapy and Stereotactic Body Radiotherapy for Early-Stage Non-Small Cell Lung Cancer. Cancers, 13(2), 176. https://doi.org/10.3390/cancers13020176





