Trends in Phase II Trials for Cancer Therapies
Abstract
Simple Summary
Abstract
1. Introduction
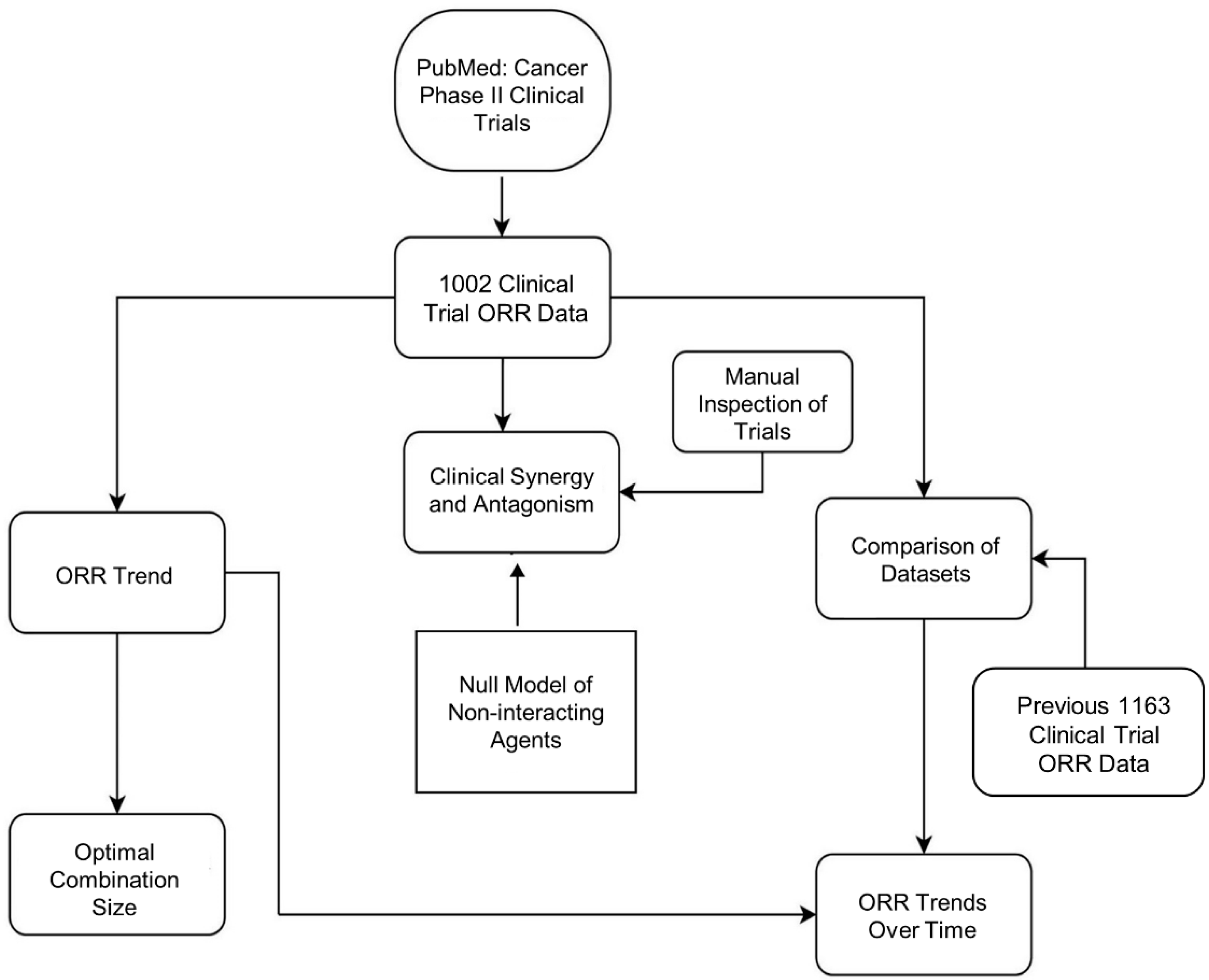
2. Experimental Methods
2.1. Endpoint Clinical Variable
2.2. ORR Data Source and Selection Criteria
2.3. Agent Classification
2.4. Statistical Analysis
2.5. Clinical Synergy and Antagonism
3. Results
3.1. Impact of Combination Size
3.2. One versus Multiple Targeted Agents
3.3. Trends across Time
3.4. Synergistic and Antagonistic Combinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Goubran, H.A.; Kotb, R.R.; Stakiw, J.; Emara, M.E.; Burnouf, T. Regulation of Tumor Growth and Metastasis: The Role of Tumor Microenvironment. Cancer Growth Metastasis 2014, 7, CGM.S11285–18. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Koo, J.S. Role of Tumor-Associated Myeloid Cells in Breast Cancer. Cells 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Shree, T.; Olson, O.C.; Elie, B.T.; Kester, J.C.; Garfall, A.L.; Simpson, K.; Bell-McGuinn, K.M.; Zabor, E.C.; Brogi, E.; Joyce, J.A. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011, 25, 2465–2479. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Q.; Zhou, Y.-B.; Wang, J.; Liu, Q.; Xu, H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 2017, 11, 87. [Google Scholar] [CrossRef]
- Tibau, A.; Molto, C.; Borrell, M.; Del Paggio, J.C.; Barnadas, A.; Booth, C.M.; Amir, E. Magnitude of Clinical Benefit of Cancer Drugs Approved by the US Food and Drug Administration Based on Single-Arm Trials. JAMA Oncol. Am. Med. Assoc. 2018, 4, 1610–1611. [Google Scholar] [CrossRef]
- Wu, M.; Sirota, M.; Butte, A.J.; Chen, B. Characteristics of drug combination therapy in oncology by analyzing clinical trial data on clinicaltrials.gov. Pac. Symp. Biocomput. 2015, 68–79. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Vivot, A.; Jacot, J.; Zeitoun, J.-D.; Ravaud, P.; Créquit, P.; Porcher, R. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000–2015. Ann. Oncol. 2017, 28, 1111–1116. [Google Scholar] [CrossRef]
- Del Paggio, J.C.; Azariah, B.; Sullivan, R.; Hopman, W.M.; James, F.V.; Roshni, S.; Tannock, I.F.; Booth, C.M. Do contemporary randomized controlled trials meet ESMO thresholds for meaningful clinical benefit? Ann. Oncol. 2017, 28, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Tibau, A.; Molto, C.; Ocana, A.; Templeton, A.; Del Carpio, L.P.; Del Paggio, J.C.; Barnadas, A.; Booth, C.M.; Amir, E. Magnitude of Clinical Benefit of Cancer Drugs Approved by the US Food and Drug Administration. J. Natl. Cancer Inst. 2018, 110, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Naci, H.; Gurpinar, E.; Poplavska, E.; Pinto, A.; Aggarwal, A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: Retrospective cohort study of drug approvals 2009–2013. BMJ 2017, 359, j4530. [Google Scholar] [CrossRef] [PubMed]
- Grössmann, N.; Del Paggio, J.; Wolf, S.; Sullivan, R.; Booth, C.; Rosian, K.; Emprechtinger, R.; Wild, C. Five years of EMA-approved systemic cancer therapies for solid tumours—A comparison of two thresholds for meaningful clinical benefit. Eur. J. Cancer 2017, 82, 66–71. [Google Scholar] [CrossRef]
- Kim, C.; Prasad, V. Strength of Validation for Surrogate End Points Used in the US Food and Drug Administration’s Approval of Oncology Drugs. Mayo Clin. Proc. 2016, 91, 713–725. [Google Scholar] [CrossRef]
- Kim, C.; Prasad, V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: An analysis of 5 years of us food and drug administration approvals. JAMA Intern. Med. Am. Med. Assoc. 2015, 175, 1992–1994. [Google Scholar] [CrossRef]
- Hotta, K.; Suzuki, E.; Di Maio, M.; Chiodini, P.; Fujiwara, Y.; Takigawa, N.; Ichihara, E.; Reck, M.; Manegold, C.; Pilz, L.; et al. Progression-free survival and overall survival in phase III trials of molecular-targeted agents in advanced non-small-cell lung cancer. Lung Cancer 2013, 79, 20–26. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Chen, E.Y.; Haslam, A.; Prasad, V. FDA Acceptance of Surrogate End Points for Cancer Drug Approval: 1992–2019. JAMA Intern. Med. Am. Med. Assoc. 2020, 180, 912–914. [Google Scholar] [CrossRef]
- Koo, S.-M.; Kim, K.-U.; Kim, Y.-K.; Uh, S.-T. Efficacy of Afatinib, Erlotinib, and Gefitinib on epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC) patients with brain metastasis: A network meta-analysis. Eur. Respir. J. 2018, 52, PA2802. [Google Scholar]
- Camidge, D.R. Targeted therapy vs chemotherapy: Which has had more impact on survival in lung cancer? Does targeted therapy make patients live longer? Hard to prove, but impossible to ignore. Clin. Adv. Hematol. Oncol. 2014, 12, 763–766. [Google Scholar] [PubMed]
- Redana, S.; Donadio, M.; Nolè, F.; Jacomuzzzi, M.E.; Beano, A.; Martinello, R.; Gillio-Tos, A.; Viale, G.; Aglietta, M.; Montemurro, F. Trastuzumab with either docetaxel or vinorelbine as first-line treatment for patients with HER2-positive advanced breast cancer: A retrospective comparison. BMC Cancer 2010, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; Di Gioia, D.; Vehling-Kaiser, U.; Harich, H.-D.; Heinrich, B.; Welt, A.; Ziske, C.; Deutsch, G.; Pihusch, R.; Kölbl, H.; et al. A prospective multicenter phase II study of oral and i.v. vinorelbine plus trastuzumab as first-line therapy in HER2-overexpressing metastatic breast cancer. Ann. Oncol. 2010, 22, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Wilks, S.; Puhalla, S.; O’Shaughnessy, J.; Schwartzberg, L.; Berrak, E.; Song, J.; Cox, D.; Vahdat, L. Phase 2, Multicenter, Single-Arm Study of Eribulin Mesylate With Trastuzumab as First-Line Therapy for Locally Recurrent or Metastatic HER2-Positive Breast Cancer. Clin. Breast Cancer 2014, 14, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, S.; Mantarro, S.; Guarneri, V.; Tagliabue, L.; Pistotti, V.; Moja, L.; D’Amico, R. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst. Rev. 2014, 2014, CD006242. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Pertuzumab: A Review of Its Use for First-Line Combination Treatment of HER2-Positive Metastatic Breast Cancer. Drugs 2013, 73, 1491–1502. [Google Scholar] [CrossRef]
- Swain, S.M.; Kim, S.-B.; Cortés, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Ehrlich, P. Address in Pathology, ON CHEMIOTHERAPY: Delivered before the Seventeenth International Congress of Medicine. BMJ 1913, 2, 353–359. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Drug resistance in leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. In Pharmacology Research and Perspectives; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2015; Volume 3. [Google Scholar]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. In Drug Discovery Today; Elsevier Ltd.: London, UK, 2016; Volume 21, pp. 1189–1195. [Google Scholar]
- Pronzato, P.; Rondini, M. First line chemotherapy of metastatic breast cancer. Ann. Oncol. 2006, 17, v165–v168. [Google Scholar] [CrossRef]
- Xiao, Y.-Y.; Zhan, P.; Yuan, D.-M.; Liu, H.-B.; Lv, T.-F.; Song, Y.; Shi, Y. Chemotherapy plus multitargeted antiangiogenic tyrosine kinase inhibitors or chemotherapy alone in advanced NSCLC: A meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2013, 69, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Rossari, J.R.; Metzger-Filho, O.; Paesmans, M.; Saini, K.S.; Gennari, A.; de Azambuja, E.; Piccart-Gebhart, M. Bevacizumab and breast cancer: A meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J. Oncol. 2012, 2012, 417673. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Han, S.; Duan, C.; Chen, K.; You, Z.; Jia, J.; Lin, S.; Liang, L.; Liu, A.; Long, H.; et al. Role of taxane and anthracycline combination regimens in the management of advanced breast cancer a meta-analysis of randomized trials. Medicine 2015, 94, e803. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; DiPaola, R.S.; Vazquez, A. Inference of synergy/antagonism between anticancer drugs from the pooled analysis of clinical trials. BMC Med. Res. Methodol. 2013, 13, 77. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: The lugano classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised Response Criteria for Malignant Lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Mitchell, E.; Chidiac, T.; Scroggin, C.; Hagenstad, C.; Spigel, D.; Marshall, J.; Cohn, A.; McCollum, D.; Stella, P.; et al. A Randomized Phase IIIB Trial of Chemotherapy, Bevacizumab, and Panitumumab Compared With Chemotherapy and Bevacizumab Alone for Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 27, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.; Koopman, M.; Cats, A.; Rodenburg, C.J.; Creemers, G.J.M.; Schrama, J.G.; Erdkamp, F.L.G.; Vos, A.H.; Van Groeningen, C.J.; Sinnige, H.A.M.; et al. Chemotherapy, Bevacizumab, and Cetuximab in Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Haloupek, N. Precision Medicine Stumbles in Umbrella Trial. Cancer Discov. 2020, 10, 1435. [Google Scholar]
- Yang, J.C.-H.; Wu, Y.-L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Chun-Ming, T.; O’Byrne, K.; Feng, J.; et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Sebastian, M.; Chih-Hsin Yang, J.; Sequist, L.; Schuler, M.; Mok, T.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Geater, S.; Zhou, C.; et al. Analysis of overall survival (OS) in two large open-label phase III studies (LUX-Lung 3 [LL3] and LUX-Lung 6 [LL6]) comparing afatinib with chemotherapy (CT) in patients (pts) with advanced non-small cell lung cancer (NSCLC) harboring common. Eur. Respir. J. 2014, 44, 1929. [Google Scholar]
- Park, K.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.-H.; Lee, K.H.; Luis, P.-A.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of Computed Tomography and Positron Emission Tomography in Patients With Metastatic Gastrointestinal Stromal Tumor Treated at a Single Institution With Imatinib Mesylate: Proposal of New Computed Tomography Response Criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Benjamin, R.S.; Choi, H.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Charnsangavej, C. We Should Desist Using RECIST, at Least in GIST. J. Clin. Oncol. 2007, 25, 1760–1764. [Google Scholar] [CrossRef]
- Vera, R.; Dorronsoro, M.G.; Lopez-Ben, S.; Viudez, A.; Queralt, B.; Hernandez, I.; Ortiz-Duran, M.R.; Zazpe, C.; Soriano, J.; Amat, I.; et al. Retrospective analysis of pathological response in colorectal cancer liver metastases following treatment with bevacizumab. Clin. Transl. Oncol. 2014, 16, 739–745. [Google Scholar] [CrossRef]
- Aykan, N.F.; Özatlı, T. Objective response rate assessment in oncology: Current situation and future expectations. World J. Clin. Oncol. 2020, 11, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Ribero, D.; Wang, H.; Donadon, M.; Zorzi, D.; Thomas, M.B.; Eng, C.; Chang, D.Z.; Curley, S.A.; Abdalla, E.K.; Ellis, L.M.; et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007, 110, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.; Tamandl, D.; Eipeldauer, S.; Hacker, S.; Herberger, B.; Kaczirek, K.; Dorfmeister, M.; Gruenberger, B.; Gruenberger, B. Bevacizumab Improves Pathological Response of Colorectal Cancer Liver Metastases Treated with XELOX/FOLFOX. Ann. Surg. Oncol. 2010, 17, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Blazer, D.G.; Kishi, Y.; Maru, D.M.; Kopetz, E.S.; Chun, Y.S.; Overman, M.J.; Fogelman, D.R.; Eng, C.; Chang, D.Z.; Wang, H.; et al. Pathologic Response to Preoperative Chemotherapy: A New Outcome End Point After Resection of Hepatic Colorectal Metastases. J. Clin. Oncol. 2008, 26, 5344–5351. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Vauthey, J.N.; Boonsirikamchai, P.; Maru, D.M.; Kopetz, S.; Palavecino, M.; Curley, S.A.; Abdalla, E.K.; Kaur, H.; Charnsangavej, C.; et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA J. Am. Med. Assoc. 2009, 302, 2338–2344. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Giostra, E.; Brezault, C.; Roth, A.; Andres, A.; Audard, V.; Sartoretti, P.; Dousset, B.; Majno, P.; Soubrane, O.; et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann. Oncol. 2006, 18, 299–304. [Google Scholar] [CrossRef]
- Adam, R.; Wicherts, D.A.; De Haas, R.J.; Aloia, T.; Lévi, F.; Paule, B.; Guettier, C.; Kunstlinger, F.; Delvart, V.; Azoulay, D.; et al. Complete Pathologic Response After Preoperative Chemotherapy for Colorectal Liver Metastases: Myth or Reality? J. Clin. Oncol. 2008, 26, 1635–1641. [Google Scholar] [CrossRef]
- Modest, D.P.; Stintzing, S.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar]
- Saltz, L.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.-S.; Rivera, F.; et al. Bevacizumab in Combination With Oxaliplatin-Based Chemotherapy As First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Grothey, A.; Hedrick, E.E.; Mass, R.D.; Sarkar, S.; Suzuki, S.; Ramanathan, R.K.; Hurwitz, H.I.; Goldberg, R.M.; Sargent, D.J. Response-Independent Survival Benefit in Metastatic Colorectal Cancer: A Comparative Analysis of N9741 and AVF2107. J. Clin. Oncol. 2008, 26, 183–189. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]




| Drug Class | Targeted Agents | Non-Targeted Agents |
|---|---|---|
| Cytotoxic drugs | Doxorubicin, Cisplatin, Nab-Paclitaxel | |
| Synthetic hormonal agents | Abiraterone, Fulvestrant, Anastrozole | |
| Monoclonal antibodies | Bevacizumab, Trastuzumab, Rituximab | |
| Tyrosine kinase inhibitors | Sunitinib, Ibrutinib, Erdafitinib | |
| Proteasome inhibitors | Bortezomib, Carfilzomib, Ixazomib | |
| Modern immunotherapies | Pembrolizumab, Nivolumab, CAR-T cell | |
| Other | Interleukin-2, Everolimus, Temsirolimus | Pomalidomide, Lenalidomide |
| Synergistic Combinations | ||||||
|---|---|---|---|---|---|---|
| Agent 1 | Agent 2 | Expected ORRE (%) | Observed ORRO (%) | psynergy | Cancer Subtype | Null Model |
| Doxorubicin | Carboplatin | 27 | 58 | 9.33 × 10−3 | Ovarian cancer | Kang et al. [38] |
| Carboplatin | Nab-Paclitaxel | 28 | 59 | 4.87 × 10−3 | Lung (NSCLC *), Oropharyngeal, Breast cancer (TNBC **) | |
| Rituximab | Ibrutinib | 86 | 94 | 1.71 × 10−3 | Chronic lymphocytic leukaemia | |
| S-1 | Nab-Paclitaxel | 31 | 58 | 2.59 × 10−2 | Gastric, Pancreatic cancer | |
| Antagonistic Combinations | ||||||
|---|---|---|---|---|---|---|
| Agent 1/Combination 1 | Agent 2 | Expected ORRE (%) | Observed ORRO (%) | pantagonism | Cancer Subtype | Null Model |
| Afatinib | Bevacizumab | 35 | 18 | 1.88 × 10−2 | Lung cancer (NSCLC *, EGFR Mutant) | Kang et al. [38] |
| Carboplatin | Gemcitabine | 88 | 43 | 5.33 × 10−3 | Ovarian, Breast (TNBC **), Lung cancer (Squamous NSCLC *) | |
| Ibrutinib | Durvalumab | 86 | 26 | 1.30 × 10−3 | Non-Hodgkin lymphoma | |
| Erlotinib | Bevacizumab | 36 | 10 | 1.67 × 10−4 | Hepatocellular carcinoma | |
| Erlotinib | Gemcitabine | 89 | 13 | 4.96 × 10−3 | Metastatic pancreatic cancer | |
| Nab-Paclitaxel | Gemcitabine | 88 | 33 | 8.87 × 10−6 | Pancreatic, Breast, Bile duct cancer | |
| Gemcitabine | Paclitaxel | 89 | 39 | 3.87 × 10−2 | Metastatic breast cancer | |
| Trastuzumab | Neratinib | 54 | 27 | 3.27 × 10−2 | Breast cancer (HER2+) *** | |
| Irinotecan | Cetuximab | 45 | 28 | 1.94 × 10−2 | Metastatic colorectal cancer (KRASwt, BRAFwt) **** | |
| FOLFOXIRI # | Cetuximab | 56 | 34 | 4.00 × 10−3 | Metastatic colorectal cancer | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, F.; Vazquez, A. Trends in Phase II Trials for Cancer Therapies. Cancers 2021, 13, 178. https://doi.org/10.3390/cancers13020178
Azam F, Vazquez A. Trends in Phase II Trials for Cancer Therapies. Cancers. 2021; 13(2):178. https://doi.org/10.3390/cancers13020178
Chicago/Turabian StyleAzam, Faruque, and Alexei Vazquez. 2021. "Trends in Phase II Trials for Cancer Therapies" Cancers 13, no. 2: 178. https://doi.org/10.3390/cancers13020178
APA StyleAzam, F., & Vazquez, A. (2021). Trends in Phase II Trials for Cancer Therapies. Cancers, 13(2), 178. https://doi.org/10.3390/cancers13020178







