Simple Summary
The impact of aspirin use after the diagnosis of colorectal cancer is unknown. Among others, PIK3CA mutational status was proposed as a molecular biomarker for the response to adjuvant aspirin therapy. The aim of this study was to retrospectively analyze whether the PIK3CA and KRAS mutational status had an impact on overall survival in patients with colorectal cancer and aspirin use. In a retrospective study, we obtained KRAS and PIK3CA mutational status in a cohort of 153 patients with a first diagnosis of colorectal cancer receiving tumor surgery with curative intent. Clinicopathological data and survival data were assessed using patient records and reporting registers. We observed a significant 10-year overall survival benefit in patients with aspirin use and combined wild-type PIK3CA and mutated-KRAS tumors (HR = 0.38; 95% CI = 0.17–0.87; p = 0.02). Our data indicated a benefit of aspirin usage particularly for patients with combined wild-type PIK3CA and mutated-KRAS tumor characteristics.
Abstract
The impact of aspirin use after the diagnosis of colorectal cancer is unknown. Among others, PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha) mutational status was proposed as a molecular biomarker for the response to adjuvant aspirin therapy. However, prognostic data on aspirin use after a colorectal cancer diagnosis in relation to KRAS mutational status is limited. In a single-center retrospective study, we obtained KRAS and PIK3CA mutational status in a cohort of 153 patients with a first diagnosis of colorectal cancer receiving tumor surgery with curative intent. PIK3CA mutational status was determined by pyrosequencing, and KRAS mutational status was determined by next-generation sequencing. Clinicopathological data and survival data were assessed using patient records and reporting registers. We observed a significant 10-year overall survival benefit in patients with aspirin use and combined wild-type PIK3CA and mutated-KRAS tumors (HR = 0.38; 95% CI = 0.17–0.87; p = 0.02), but not in patients without aspirin use. Our data indicate a benefit of aspirin usage particularly for patients with combined wild-type PIK3CA and mutated-KRAS tumor characteristics.
1. Introduction
With 1.4 million new cases per year, colorectal cancer is one of the most common cancers worldwide. Moreover, colorectal cancer is one of the most common causes of cancer-related death, with almost 700,000 deaths per year [1]. Therefore, it is of interest to understand the molecular mechanisms of cancer development and metastasis to identify new therapeutic approaches.
Retrospective analyses suggest a superior clinical outcome for patients with regular use of aspirin after diagnosis of colorectal cancer [2,3,4,5,6,7]. Aspirin is a commonly prescribed drug in Western societies, and its indication includes prevention and therapy of coronary artery disease and stroke [8,9]. Nevertheless, the long-term use of aspirin may provoke side effects such as intracranial and gastrointestinal bleeding, stomach ulcers, and renal impairment [10]. Therefore, it is highly desirable to identify those colorectal cancer patients with an expectable overweight of benefits [11,12]. Several possible biomarkers have been suggested, including the PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha) mutational status [13,14,15,16,17]. Some studies indicated a favorable survival or longer recurrence-free survival of aspirin users with mutated-PIK3CA tumors [13,14], while others reported increased survival of aspirin users with wild-type PIK3CA tumors [17]. Two other independent studies did not point to survival differences with regard to PIK3CA mutations and aspirin use [15,16]. Prognostic data on aspirin use after diagnosis of colorectal cancer in relation to KRAS mutational status is limited.
The mechanisms by which aspirin could prolong survival in patients with colorectal cancer have not been clarified. The prognosis-improving effect in various solid tumors appears to be mediated on the one hand by inhibition of cyclooxygenase, and on the other hand via cyclooxygenase-independent signaling pathways [18,19]. In animal models, it has been shown that inhibitors of cyclooxygenase hamper both tumor growth and metastasis in colorectal cancers [20,21]. In addition, it has been demonstrated experimentally that inhibition of cyclooxygenase-2, which is overexpressed in a large number of colorectal cancers, leads to reduced production of prostaglandin E2 (PGE2), which favors cell migration, angiogenesis, and reduced apoptosis [22,23,24].
The aim of this work was to examine whether PIK3CA mutational status and KRAS mutational status could indeed augment the effect of aspirin on the survival of patients with colorectal cancer.
2. Materials and Methods
2.1. Patient Cohort
We performed a retrospective single-center analysis at the university clinic in Marburg, Germany. Therefore, we used a database consisting of 336 patients with histologically proven diagnoses of sporadic colorectal cancer that underwent curative surgical resection at the university clinic of Marburg in 2003/2004. For 266 of those 336 patients, tissue for DNA analysis was available; 113 of these patients had to be excluded because they had been diagnosed with stage IV (UICC classification) with no resectable metastases, had a secondary tumor, or did not have sufficient clinical information (information on aspirin intake/survival data). Finally, the study cohort consisted of 153 patients (Figure 1).
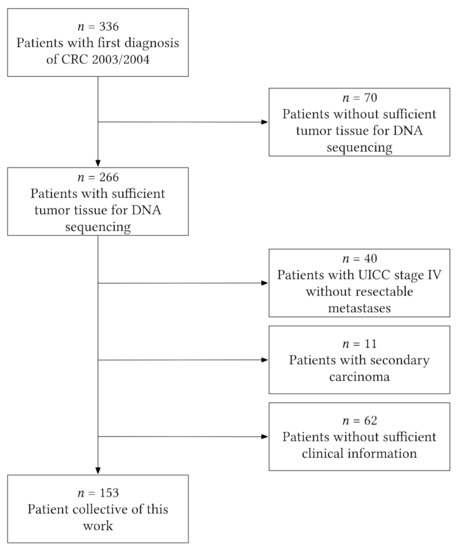
Figure 1.
Flowchart illustrating patient-exclusion criteria leading to the final study cohort of 153 patients. CRC = colorectal cancer.
Information on aspirin use was obtained from the case files or the primary care physician; and information on survival data from the case file, primary care physician, or register of residence. Aspirin use was defined as regular use of low-dose aspirin (100–300 mg) during most weeks after a diagnosis of colorectal cancer, whereas nonuse of aspirin was defined as no regular use of aspirin during most weeks. The indications for aspirin use included primary or secondary prevention of cardiovascular disease.
Paraffin-embedded tissue blocks were provided by the pathology archive of the Institute of Pathology of the university clinic of Marburg. The study was performed according to the guidelines of the local ethics committee (no. 98/20).
2.2. Detection of PIK3CA Mutations
Genomic DNA was isolated from paraffin-embedded tumor tissue samples (QIAamp DNA Mini Kit, Germantown, MD, USA), and DNA amplification was performed by polymerase chain reaction (PCR) with primers from Nosho et al. [25]. Point mutations in exon 9 and 20 of PIK3CA were analyzed by pyrosequencing. The PCR was performed in a 50 µL volume containing 4 µL of template DNA (50 ng/µL), 1×PCR-Buffer, MgCl2, 10 mM (each 2.5 mM) deoxynucleoside triphosphate (dNTP), 10 µM forward-biotin primer, 10 µM reverse primer, and 1.25 U Platinum Taq DNA polymerase (Life Technologies, Carlsbad, CA, USA). PCR conditions were as follows: 95 °C, 11 min; 45 cycles of 95 °C, 30 s; 54 °C, 30 s; 72 °C, 30 s; then 72 °C, 5 min. PCR products were sequenced by pyrosequencing (PyroMark® Q24, Qiagen, Germantown, MD, USA) using the Therascreen® kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions with primers from Nosho et al. [25]. The different pyrosequencing primers for PIK3CA exon 9 were 9-RS1, 5′-ccatagaaaatctttctcct-3′ (mutations: c.1634A>G, c.1636C>A); 9-RS2, 5′-tagaaaatctttctcctgct-3′ (mutations: c.1633G>A, c.1624G>A)′; 9-RS3, 5′-ttctccttgcttcagtgattt-3′ (mutation c.1624G>A); and for PIK3CA exon 20: 20-RS, 5′-gttgtccagccacca-3′ (mutations:c.3140A>G, c.3140A>T, c.3129G>T, c.3139C>T).
2.3. Detection of KRAS Mutations
The human KRAS locus corresponding to codons 4 to 16 was amplified from genomic DNA isolated from tumor microdissections. Each sample was tagged with a unique 8-nucleotide barcode combination using 12 different forward (5′-AAT[barcode] TTATAAGGCCTGCTGAAAATGACTGAA-3′) and eight different reverse (5′-AAT[barcode]TGAATTAGCTGTATCGTCAAGGCACT-3′) primers per 96-well plate. PCR products from a single 96-well plate were pooled and purified, and an indexed sequencing library was prepared using the IonXpress Plus Fragment Library Prep Kit in combination with the IonXpress Barcode Adapters 1–16 Kit (Life Technologies, Carlsbad, CA, USA). The quality of sequencing libraries was verified on a Bioanalyzer DNA High Sensitivity chip (Agilent, Santa Clara, CA, USA); subsequently, libraries were pooled and quantified by qPCR. Sequencing was carried out on the IonTorrent PGM (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s recommendations. After demultiplexing and removal of the tag sequences using Cutadapt v1.7 (TU Dortmund, Dortmund, DE, Germany), reads were aligned to Ensembl v70 (European Bioinformatics Institute, Hinxton, UK) using Bowtie 2 (version 2.0.0-beta7, JHU, Baltimore, MD, USA). Variants in the KRAS coding region were obtained using VarScan 2 (version 2.3.5, WUSTL, St. Louis, MO, USA), using a minimum variant frequency of 2.5% and minimum read coverage of 2000.
2.4. Statistical Analysis
Statistical analysis was performed using Statistical Package for Social Sciences software (SPSS® v23 (IBM, Armonk, NY, USA)) and Prism Software version 6.0 (GraphPad Software, San Diego, CA, USA). Age (years), sex, tumor location, histological grade, T-size, lymph nodes, metastasis, UICC stage, PIK3CA status, KRAS status, survival at 5 years, survival at 10 years, survival time (days), and presence of two mutations were checked for significant differences between ASS users and ASS nonusers by use of a Mann–Whitney test. Fisher’s exact test and a logrank (Mantel-Cox) test were used to test for differences in overall survival; p < 0.05 was considered statistically significant.
3. Results
3.1. Patients
The study group consisted of 153 patients with histologically proven diagnosis of colorectal cancer. The follow-up of the study group was until death or up to 120 months. Table 1 summarizes the baseline characteristics of the patients with colorectal cancer according to aspirin use or nonuse after diagnosis, and the presence or absence of tumor PIK3CA mutation.

Table 1.
Baseline characteristics of patients with colorectal cancer, according to PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha) mutational status and regular use or nonuse of aspirin after diagnosis.
Mutations in the PIK3CA gene were found in 15.7% of all patients. Of all mutations in the PIK3CA gene, there were 6 mutations in exon 20 and 18 mutations in exon 9. There were no double mutations (exon 9 and exon 20). The most common mutation was the c.1633G>A mutation in exon 9, followed by the c.1624G>A mutation in exon 9 and the c.3140A>G mutation in exon 20. Mutations in the KRAS gene were found in 56.9% of all patients. The most common mutation was the glycine/aspartate substitution in codon 13 (p.G13D, 31%), followed by the glycine/aspartate substitution in codon 12 (p.G12D, 26%). Aspirin was taken by 34% of the patients in daily dosages of 100–300 mg. The 10-year overall survival of the entire study cohort was 43.8%.
Tumor mutations in the KRAS gene were equally distributed between patients with wild-type PIK3CA tumors and patients with mutated-PIK3CA tumors: An additional KRAS mutation was found in 13/24 (54%) of the mutated-PIK3CA tumors and in 74/129 (57%) of the wild-type PIK3CA tumors (Fisher’s exact test, p = 0.82). A total of 34% of the patients with wild-type PIK3CA tumors and 33% of the patients with a tumor mutation in the PIK3CA gene took aspirin regularly after the diagnosis of colorectal cancer.
The subgroups with mutated-PIK3CA tumors and wild-type PIK3CA tumors and the subgroups with mutated-KRAS tumors and wild-type KRAS tumors were similar according to the baseline characteristics (mean age at diagnosis, sex, tumor location, stage of disease, tumor grading, and aspirin use before diagnosis; p > 0.1 for all comparisons).
Moreover, the subgroups with and without aspirin use were similar according to the baseline characteristics (mean age at diagnosis, sex, tumor location, stage of disease, and mutational status; p > 0.1 for all comparisons).
3.2. Aspirin Use and Survival in the Cohort of 153 Patients
With regard to all 153 patients, patients with regular use of aspirin (n = 52) did not show significantly improved 10-year overall survival compared to patients without regular use of aspirin (n = 101) (HR = 0.77; 95% CI = 0.5–1.22; p = 0.29; Figure 2).
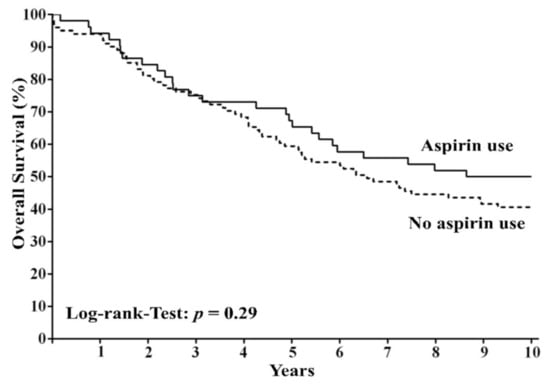
Figure 2.
Overall survival among patients with colorectal cancer according to regular use or nonuse of aspirin after diagnosis. The panel shows overall survival among patients with regular use of aspirin and those with nonuse of aspirin.
3.3. Aspirin Use and Survival According to PIK3CA und KRAS Mutational Status
Among all patients with regular use of aspirin, we found patients with wild-type PIK3CA tumors to harbor a significant overall survival advantage as compared to patients with mutated-PIK3CA tumors (HR = 0.33; 95% CI = 0.05–0.62; p < 0.01). This survival advantage was not evident among the patients without aspirin use (HR = 0.78; 95% CI = 0.38–1.51; p = 0.43). A combined examination of the mutational status in the KRAS and PIK3CA genes revealed clear differences in the absolute 10-year survival rates among patients who regularly used aspirin (Figure 3A) but less differences among patients without regular aspirin use (Figure 3B).
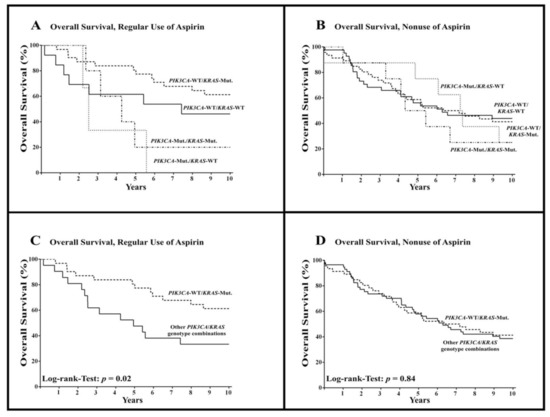
Figure 3.
Overall survival among patients with colorectal cancer according to PIK3CA and KRAS mutational status and regular use or nonuse of aspirin after diagnosis. WT = wild-type, Mut. = mutated. (A,B) show overall survival among all combinations of mutational status in patients with regular use of aspirin (A) and nonuse of aspirin (B), respectively, while (C,D) show overall survival among patients with combined wild-type-PIK3CA and mutated-KRAS tumors compared to other PIK3CA/KRAS genotype combinations (except PIK3CA-WT/KRAS-Mut.).
When reviewing the combined PIK3CA and KRAS mutational status of patients with regular aspirin use, a significant overall survival benefit for patients with combined wild-type PIK3CA and mutated-KRAS tumors was observed (HR = 0.38; 95% CI = 0.17–0.87; p = 0.02; Figure 3C, Table 2). This survival advantage was not observed in patients without aspirin use (HR = 0.95; 95% CI = 0.58–1.57; p = 0.84; Figure 3D, Table 2).

Table 2.
The 10-year overall survival among patients with regular use or nonuse of aspirin after diagnosis of colorectal cancer according to PIK3CA and KRAS mutational status.
4. Discussion
We found that patients with regular aspirin use after the diagnosis of colorectal cancer and combined wild-type PIK3CA and mutated-KRAS tumors showed a significant survival benefit. Several studies have investigated a potential correlation between PIK3CA mutational status and benefit from postdiagnosis aspirin use [13,14,15,16,17]. Some studies have indicated a better survival or longer recurrence-free survival of aspirin users with mutated-PIK3CA tumors [13,14], while others found a favorable survival of aspirin users with wild-type PIK3CA tumors [17]. Two other studies did not point to survival differences with regard to PIK3CA mutational status and aspirin use [15,16]. To the best of our knowledge, our study is the first report that describes a positive effect of aspirin usage in a cohort of colon cancer patients in the context of both PIK3CA- and KRAS-mutational status.
Colorectal cancer is a heterogeneous group of diseases, and it can be assumed that combinations of prognostic factors and not individual biomarkers alone influence the response to therapy, such as an “adjuvant” therapy with aspirin [11,12]. A possible explanation for the divergent results of the studies [13,14,15,16,17] concerning the PIK3CA mutational status, aspirin use, and overall survival could be that the patient cohorts of the studies differed with regard to further molecular tumor markers. When comparing the patient collective of this work with the patient collectives of similar works, differences in tumor location and KRAS mutational status were found [13,14,15,16,17]. This could indicate that patient collectives differed in terms of molecular tumor properties. In this study, 25% of the patients had a right-sided colorectal cancer and 57% showed KRAS mutations, whereas the proportion of patients with a right-sided colorectal cancer in the study of Liao et al. [13] was significantly higher (45%), and the number of KRAS mutations was significantly lower (35%) [13]. Left-sided colorectal cancers as compared to right-sided colorectal cancers show a different distribution of mutations and harbor a divergent prognosis [26]. For example, the incidence of microsatellite instability and mutations in the BRAF gene is increasing from the rectum to the proximal colon [26,27]. The patient cohorts in the different papers could therefore differ with regard to the molecular background. Unfortunately, based on the available data, it was not possible to compare this.
A possible alternative explanation for our findings could be that there are other biomarkers that predict response to aspirin and correlate with the KRAS or PIK3CA mutational status. Large prospective randomized studies are required to evaluate the response to “adjuvant” therapy with aspirin in colorectal cancer by considering various combinations of possible biomarkers.
Furthermore, it is important to understand the underlying molecular mechanism of how aspirin could improve the prognosis of colorectal cancer to detect relevant biomarkers. It has recently been shown that the interaction between KRAS and the PIK3CA catalytic subunit p110α decreases significantly after the addition of aspirin, and leads to an inhibition of cell proliferation and a cell-cycle arrest in the G0/G1 phase [28]. Thus, aspirin may inhibit cell growth of leiomyoma cells by regulating the K-Ras-p110α interaction. In particular, cells with overexpressed KRAS displayed an increased K-Ras-p110α interaction [28].
It is not clear whether these molecular mechanisms also play a role in colorectal cancer cells. Due to the inhibition of the K-Ras-p110α interaction by aspirin, it would be plausible that especially patients with simultaneous occurrence of mutated-KRAS and wild-type PIK3CA tumors could benefit from aspirin use. Further studies are required to determine if these signaling pathways play a role in patients with colorectal cancer in the context of aspirin use.
Besides signal transduction changes induced by aspirin, autophagy may also be modulated [29,30,31,32,33]. Whether changes in autophagy or its signal modulation via the classical inhibition of COX (cyclooxygenase) or even ERK (extracellular signal-regulated kinase) was causative for the described effect needs to be addressed in prospective clinical trials.
Of note, although the total number of analyzed patients was rather small, the benefit of aspirin use in patients with combined wild-type PIK3CA and mutated-KRAS tumors was clearly denoted. The frequency of PIK3CA mutations in our study fit well with the data presented by others, and the clinical and pathological data matched very well with the published data [13,14,15,16,17,34,35]. Nevertheless, because of the small numbers of patients in some subgroups, we must be cautious in interpreting our data. However, our data call for larger randomized trials, especially since some of some of the trials included only patients with mutated-PIK3CA tumors.
This was a unicentric German study, and thus may be biased by single-center experience. Recent data indicated a distinct treatment response to chemotherapy particular in German versus non-German patients with rectal adenocarcinoma [36]. Our data from an unicenter German patient cohort may therefore not be representative for patients from other countries, and warrant further exploration in a prospective study.
Taken together, these data suggest that the shared consideration of both the PIK3CA and KRAS mutational status could play an important role. These data must be confirmed in larger prospective clinical trials. This is especially critical, as some clinical trials recruit colorectal cancer patients into aspirin trials only in case the tumors harbor PIK3CA mutations (see: clinicaltrials.gov, NCT02467582).
5. Conclusions
In summary, the results of this work suggest that patients with a combination of wild-type PIK3CA and mutated-KRAS tumors in particular benefit from aspirin use after diagnosis of colorectal cancer. The results of this study conclusively underline that the roles of the PIK3CA and KRAS mutational status as potential predictive markers for adjuvant therapy with aspirin in patients with colorectal cancer require prospective studies.
Author Contributions
Conceptualization, A.N. (Andreas Neubauer) and C.B.; methodology, C.B., R.M., T.S., and A.N. (Andrea Nist), K.S.; software, K.S.; validation, C.B., A.N. (Andrea Nist), and L.G.; formal analysis, U.K., M.M., L.G., and C.B.; investigation, L.G.; resources, C.B.; data curation, L.G.; writing—original draft preparation, L.G.; writing—review and editing, E.M., C.B., and A.N. (Andreas Neubauer); visualization, L.G.; supervision, A.N. (Andreas Neubauer) and C.B.; project administration, A.N. (Andreas Neubauer), E.M., and C.B.; funding acquisition, T.S., A.N. (Andreas Neubauer), and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the German José Carreras Foundation (AH 06-01, AN).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and the Ethics Committee of Philipps University of Marburg (protocol code 98/20; 24 June 2020).
Informed Consent Statement
According to the regulations of the local ethics committee, individual consent of the patients examined was not required until the end of 2004, since only routine samples embedded in paraffin were examined for the present study.
Data Availability Statement
The data presented in this study can be made available upon request from the corresponding author.
Acknowledgments
We thank Anette Fiebeler for her excellent support in data evaluation. We thank Lisa-Marie Thomas, Ute Niebergall, and Viktoria Wischmann for their excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; International Agency for Research on Cancer: Lyon, France; Geneva, Switzerland, 2014. [Google Scholar]
- Chan, A.T.; Ogino, S.; Fuchs, C.S. Aspirin use and survival after diagnosis of colorectal cancer. Jama 2009, 302, 649–658. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Elwin, C.-E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.; Meade, T.W.; Mehta, Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef]
- Bains, S.J.; Mahic, M.; Myklebust, T.A.; Smastuen, M.C.; Yaqub, S.; Dorum, L.M.; Bjornbeth, B.A.; Moller, B.; Brudvik, K.W.; Tasken, K. Aspirin As Secondary Prevention in Patients with Colorectal Cancer: An Unselected Population-Based Study. J. Clin. Oncol. 2016, 34, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Goh, H.H.; Leong, W.Q.; Chew, M.H.; Pan, Y.S.; Tony, L.K.H.; Chew, L.; Tan, I.B.H.; Toh, H.C.; Tang, C.L.; et al. Post-operative aspirin use and colorectal cancer-specific survival in patients with stage I-III colorectal cancer. Anticancer Res. 2014, 34, 7407–7414. [Google Scholar] [PubMed]
- McCowan, C.; Munro, A.J.; Donnan, P.T.; Steele, R.J.C. Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur. J. Cancer 2013, 49, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Schneider, W.R. The need for wider and appropriate utilization of aspirin and statins in the treatment and prevention of cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2008, 6, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ittaman, S.V.; VanWormer, J.J.; Rezkalla, S.H. The role of aspirin in the prevention of cardiovascular disease. Clin. Med. Res. 2014, 12, 147–154. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef]
- Ogino, S.; Fuchs, C.S.; Giovannucci, E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev. Mol. Diagn. 2012, 12, 621–628. [Google Scholar] [CrossRef]
- Coyle, C.; Cafferty, F.H.; Langley, R.E. Aspirin and Colorectal Cancer Prevention and Treatment: Is It for Everyone? Curr. Colorectal Cancer Rep. 2016, 12, 27–34. [Google Scholar] [CrossRef][Green Version]
- Liao, X.; Lochhead, P.; Nishihara, R.; Morikawa, T.; Kuchiba, A.; Yamauchi, M.; Imamura, Y.; Qian, Z.R.; Baba, Y.; Shima, K.; et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N. Engl. J. Med. 2012, 367, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Church, D.N.; Sieber, O.; Ramamoorthy, R.; Yanagisawa, Y.; Johnstone, E.; Davidson, B.; Kerr, D.J.; Tomlinson, I.P.M.; Midgley, R. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J. Clin. Oncol. 2013, 31, 4297–4305. [Google Scholar] [CrossRef] [PubMed]
- Kothari, N.; Kim, R.; Jorissen, R.N.; Desai, J.; Tie, J.; Wong, H.-L.; Faragher, I.; Jones, I.; Day, F.L.; Li, S.; et al. Impact of regular aspirin use on overall and cancer-specific survival in patients with colorectal cancer harboring a PIK3CA mutation. Acta Oncol. 2015, 54, 487–492. [Google Scholar] [CrossRef]
- Murphy, C.; Turner, N.; Wong, H.-L.; Sinnathamby, M.; Tie, J.; Lee, B.; Desai, J.; Skinner, I.; Christie, M.; Hutchinson, R.; et al. Examining the impact of regular aspirin use and PIK3CA mutations on survival in stage 2 colon cancer. Intern. Med. J. 2017, 47, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Reimers, M.S.; Bastiaannet, E.; Langley, R.E.; van Eijk, R.; van Vlierberghe, R.L.P.; Lemmens, V.E.P.; van Herk-Sukel, M.P.P.; van Wezel, T.; Fodde, R.; Kuppen, P.J.K.; et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern. Med. 2014, 174, 732–739. [Google Scholar] [CrossRef]
- Wu, W.K.K.; Sung, J.J.Y.; Lee, C.W.; Yu, J.; Cho, C.H. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: An update on the molecular mechanisms. Cancer Lett. 2010, 295, 7–16. [Google Scholar] [CrossRef]
- Burn, J.; Gerdes, A.-M.; Macrae, F.; Mecklin, J.-P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet 2011, 378, 2081–2087. [Google Scholar] [CrossRef]
- Tomozawa, S.; Nagawa, H.; Tsuno, N.; Hatano, K.; Osada, T.; Kitayama, J.; Sunami, E.; Nita, M.E.; Ishihara, S.; Yano, H.; et al. Inhibition of haematogenous metastasis of colon cancer in mice by a selective COX-2 inhibitor, JTE-522. Br. J. Cancer 1999, 81, 1274–1279. [Google Scholar] [CrossRef]
- Yao, M.; Lam, E.C.; Kelly, C.R.; Zhou, W.; Wolfe, M.M. Cyclooxygenase-2 selective inhibition with NS-398 suppresses proliferation and invasiveness and delays liver metastasis in colorectal cancer. Br. J. Cancer 2004, 90, 712–719. [Google Scholar] [CrossRef]
- Alfonso, L.; Ai, G.; Spitale, R.C.; Bhat, G.J. Molecular targets of aspirin and cancer prevention. Br. J. Cancer 2014, 111, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Ogino, S.; Fuchs, C.S. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 2007, 356, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Ferrández, A.; Piazuelo, E.; Castells, A. Aspirin and the prevention of colorectal cancer. Best practice & research. Clin. Gastroenterol 2012, 26, S185–S195. [Google Scholar]
- Nosho, K.; Kawasaki, T.; Ohnishi, M.; Suemoto, Y.; Kirkner, G.J.; Zepf, D.; Yan, L.; Longtine, J.A.; Fuchs, C.S.; Ogino, S. PIK3CA mutation in colorectal cancer: Relationship with genetic and epigenetic alterations. Neoplasia 2008, 10, 534–541. [Google Scholar] [CrossRef]
- Lee, M.S.; Menter, D.G.; Kopetz, S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J. Natl. Compr. Cancer Netw. 2017, 15, 411–419. [Google Scholar] [CrossRef]
- Yamauchi, M.; Morikawa, T.; Kuchiba, A.; Imamura, Y.; Qian, Z.R.; Nishihara, R.; Liao, X.; Waldron, L.; Hoshida, Y.; Huttenhower, C.; et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012, 61, 847–854. [Google Scholar] [CrossRef]
- Gao, M.; Guo, K.-M.; Wei, Y.-M.; Ma, M.-M.; Cai, J.-R.; Xia, T.-T.; Ye, Q.-J. Aspirin inhibits the proliferation of human uterine leiomyoma cells by downregulation of KRasp110α interaction. Oncol. Rep. 2017, 38, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, W.B.; Liu, J.B.; Lu, J.W.; Feng, J.F. Autophagy: Novel applications of nonsteroidal anti-inflammatory drugs for primary cancer. Cancer Med. 2018, 7, 471–484. [Google Scholar] [CrossRef]
- Castoldi, F.; Humeau, J.; Martins, I.; Lachkar, S.; Loew, D.; Dingli, F.; Durand, S.; Enot, D.; Bossut, N.; Chery, A.; et al. Autophagy-mediated metabolic effects of aspirin. Cell Death Discov. 2020, 6, 129. [Google Scholar] [CrossRef]
- Huang, Z.; Fang, W.; Liu, W.; Wang, L.; Liu, B.; Liu, S. Aspirin induces Beclin-1-dependent autophagy of human hepatocellular carcinoma cell. Eur. J. Pharmacol. 2018, 823, 58–64. [Google Scholar] [CrossRef]
- Zeng, X.; Overmeyer, J.H.; Maltese, W.A. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J. Cell Sci. 2006, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Zhang, C.; Yi, H.; Wu, C.; Wang, J.; Liu, Y.; Tan, J.; Wen, J. Downregulation of Beclin 1 and impairment of autophagy in a small population of colorectal cancer. Dig. Dis. Sci. 2013, 58, 2887–2894. [Google Scholar] [CrossRef]
- Robert Koch Institut (Ed.) Bericht zum Krebsgeschehen in Deutschland 2016; Zentrum für Krebsregisterdaten: Berlin, Germany, 2016. [Google Scholar]
- Barault, L.; Veyrie, N.; Jooste, V.; Lecorre, D.; Chapusot, C.; Ferraz, J.-M.; Lièvre, A.; Cortet, M.; Bouvier, A.-M.; Rat, P.; et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int. J. Cancer 2008, 122, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Stein, A.; Van Cutsem, E.; Price, T.; Hofheinz, R.D.; Nordlinger, B.; Daisne, J.F.; Janssens, J.; Brenner, B.; Reinel, H.; et al. Pre- and Postoperative Capecitabine Without or With Oxaliplatin in Locally Advanced Rectal Cancer: PETACC 6 Trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J. Clin. Oncol. 2021, 39, 17–29. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).