DNA Methylation Age Drift Is Associated with Poor Outcomes and De-Differentiation in Papillary and Follicular Thyroid Carcinomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects/Specimens, DNA Methylation and Expression Data
2.2. Calculation for DNAm Age, DNAm Age Acceleration (DNAmaa) and Deceleration (DNAmad)
2.3. Differentially Expressed Genes (DEGs) and Gene Ontology (GO) Term/Pathway Enrichment Analyses
2.4. Thyroid Differentiation Score (TDS)
2.5. Statistical Analyses
3. Results
3.1. Clinic-Pathological Characteristics of the TCGA Cohort of Patients with TC
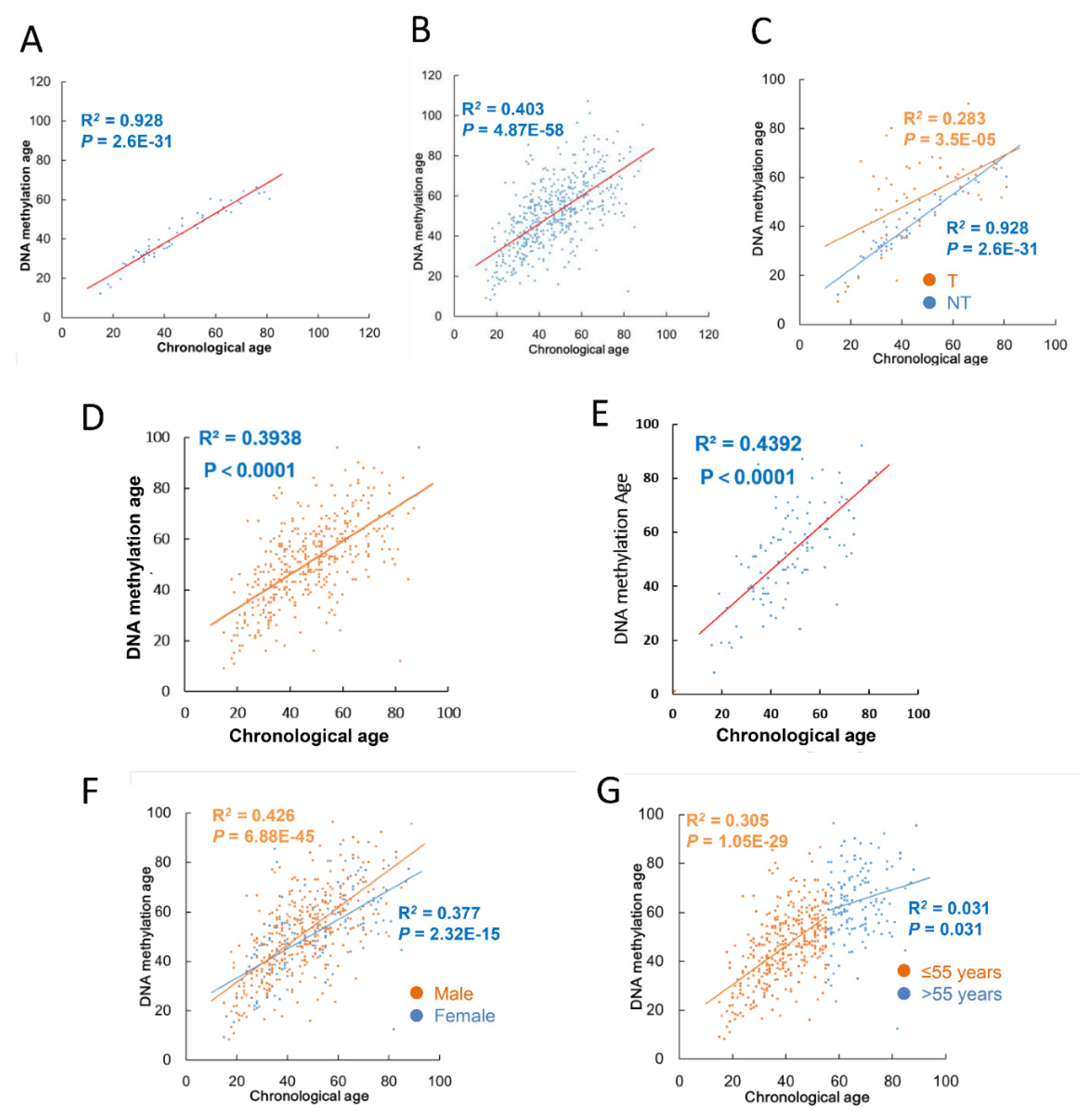
3.2. Correlation between Chronological Age and DNAm Age in NT Specimens and TC Tumors
3.3. DNAmaa and DNAmmad in TC Tumors
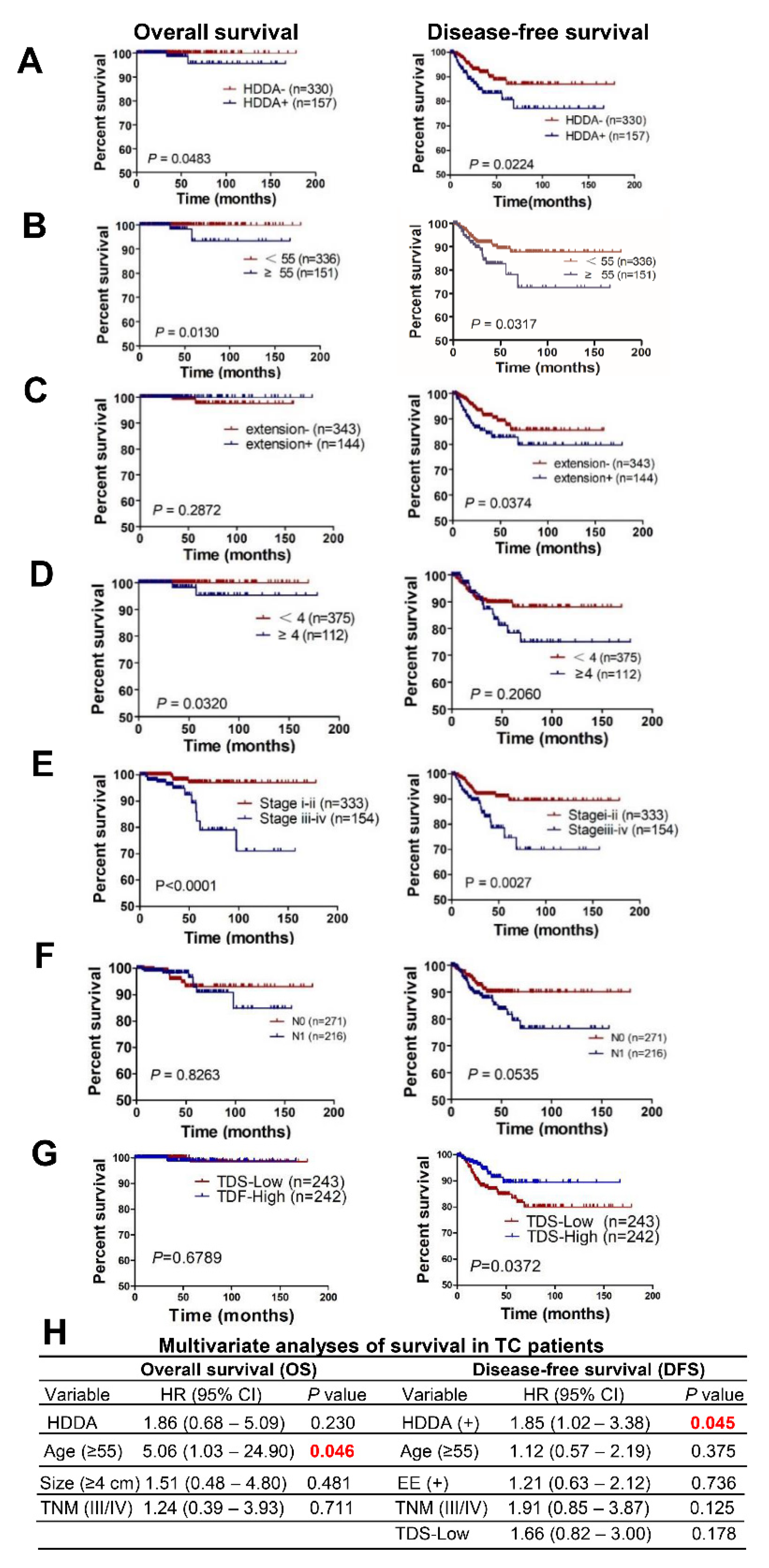
3.4. DNAm Age Drift as a Prognostic Factor for TC Patients
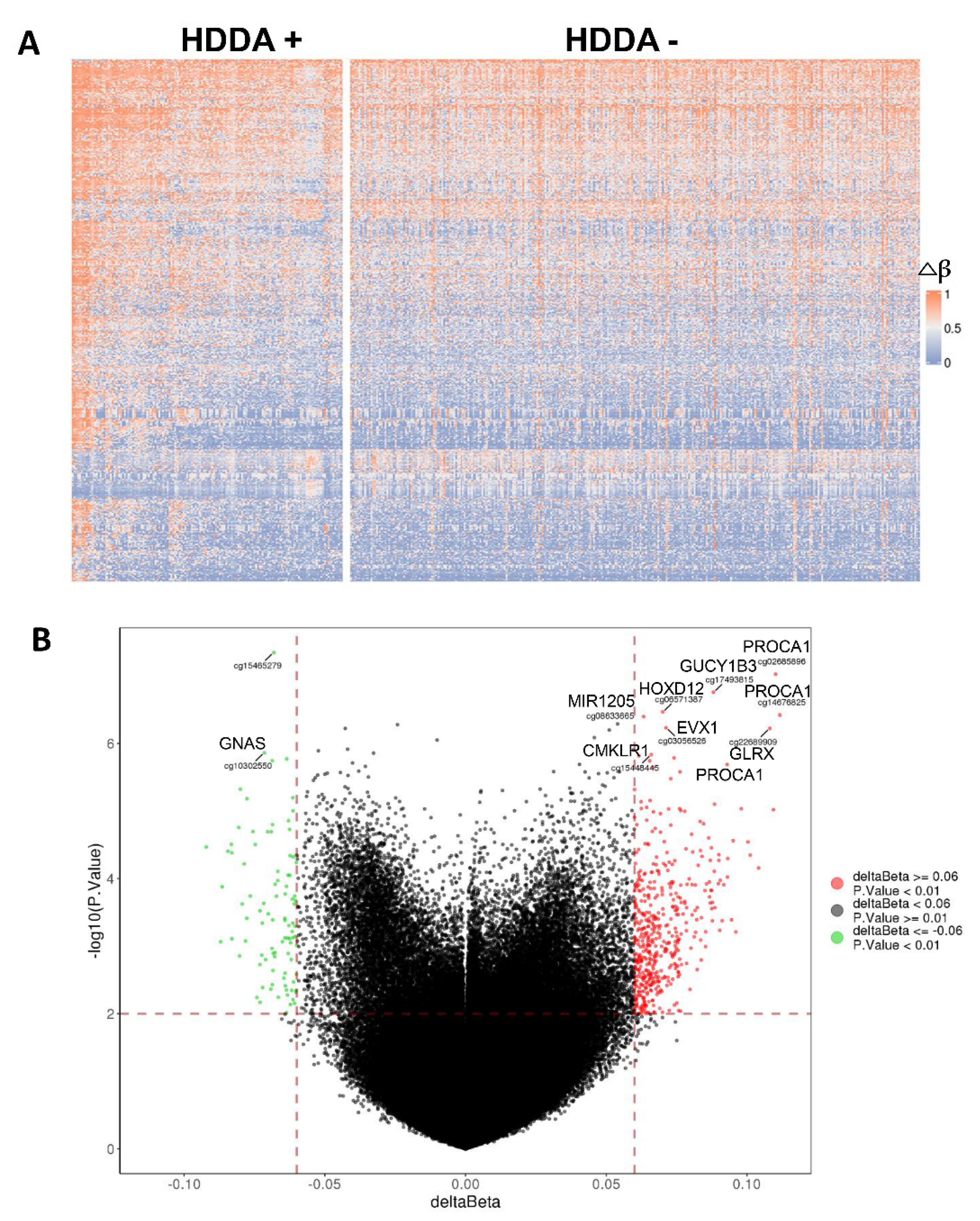
3.5. Alterations in Global DNA Methylation in TC Tumors with HDDA
3.6. DEGs and GO Term/Pathway Enrichments in TC Tumors with HDDA
3.7. Lower TDS in Tumors with HDDA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [Green Version]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tollefsbol, T.O. Age-related epigenetic drift and phenotypic plasticity loss: Implications in prevention of age-related human diseases. Epigenomics 2016, 8, 1637–1651. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S.; Erhart, W.; Brosch, M.; Ammerpohl, O.; von Schonfels, W.; Ahrens, M.; Heits, N.; Bell, J.T.; Tsai, P.C.; Spector, T.D.; et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl. Acad. Sci. USA 2014, 111, 15538–15543. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Huang, Q.; Javed, R.; Zhong, J.; Gao, H.; Liang, H. Effect of tobacco smoking on the epigenetic age of human respiratory organs. Clin. Epigenet. 2019, 11, 183. [Google Scholar] [CrossRef]
- Marioni, R.E.; Shah, S.; McRae, A.F.; Chen, B.H.; Colicino, E.; Harris, S.E.; Gibson, J.; Henders, A.K.; Redmond, P.; Cox, S.R.; et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ni, W.; Yao, Y.; Just, A.; Heiss, J.; Wei, Y.; Gao, X.; Coull, B.A.; Kosheleva, A.; Baccarelli, A.A.; et al. DNA methylation-based biomarkers of age acceleration and all-cause death, myocardial infarction, stroke, and cancer in two cohorts: The NAS, and KORA F4. EBioMedicine 2021, 63, 103151. [Google Scholar] [CrossRef]
- Levine, M.E.; Hosgood, H.D.; Chen, B.; Absher, D.; Assimes, T.; Horvath, S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging 2015, 7, 690–700. [Google Scholar] [CrossRef] [Green Version]
- Devall, M.; Sun, X.; Yuan, F.; Cooper, G.S.; Willis, J.; Weisenberger, D.J.; Casey, G.; Li, L. Racial Disparities in Epigenetic Aging of the Right vs Left Colon. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Dugue, P.A.; Bassett, J.K.; Joo, J.E.; Baglietto, L.; Jung, C.H.; Wong, E.M.; Fiorito, G.; Schmidt, D.; Makalic, E.; Li, S.; et al. Association of DNA Methylation-Based Biological Age With Health Risk Factors and Overall and Cause-Specific Mortality. Am. J. Epidemiol. 2018, 187, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aunan, J.R.; Cho, W.C.; Soreide, K. The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging Dis. 2017, 8, 628–642. [Google Scholar] [CrossRef] [Green Version]
- Arora, I.; Sharma, M.; Sun, L.Y.; Tollefsbol, T.O. The Epigenetic Link between Polyphenols, Aging and Age-Related Diseases. Genes 2020, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Tollefsbol, T.O. Epigenetic linkage of aging, cancer and nutrition. J. Exp. Biol. 2015, 218 Pt 1, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Sehl, M.E.; Henry, J.E.; Storniolo, A.M.; Ganz, P.A.; Horvath, S. DNA methylation age is elevated in breast tissue of healthy women. Breast Cancer Res. Treat. 2017, 164, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhou, Y.; Meng, J.; Jiang, L.; Gao, J.; Fan, X.; Chen, Y.; Cheng, Y.; Wang, Y.; Zhang, B.; et al. Epigenetic age acceleration of cervical squamous cell carcinoma converged to human papillomavirus 16/18 expression, immunoactivation, and favourable prognosis. Clin. Epigenet. 2020, 12, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Bewerunge-Hudler, M.; Schick, M.; Burwinkel, B.; Herpel, E.; Hoffmeister, M.; Brenner, H. Blood-derived DNA methylation predictors of mortality discriminate tumor and healthy tissue in multiple organs. Mol. Oncol. 2020, 14, 2111–2123. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Boakye, D.; Li, X.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Whole blood DNA methylation aging markers predict colorectal cancer survival: A prospective cohort study. Clin. Epigenet. 2020, 12, 184. [Google Scholar] [CrossRef]
- Liao, P.; Ostrom, Q.T.; Stetson, L.; Barnholtz-Sloan, J.S. Models of epigenetic age capture patterns of DNA methylation in glioma associated with molecular subtype, survival, and recurrence. Neuro Oncol. 2018, 20, 942–953. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Wagner, W. Epigenetic Aging Signatures Are Coherently Modified in Cancer. PLoS Genet. 2015, 11, e1005334. [Google Scholar] [CrossRef]
- Dugue, P.A.; Bassett, J.K.; Wong, E.M.; Joo, J.E.; Li, S.; Yu, C.; Schmidt, D.F.; Makalic, E.; Doo, N.W.; Buchanan, D.D.; et al. Biological Aging Measures Based on Blood DNA Methylation and Risk of Cancer: A Prospective Study. JNCI Cancer Spectr. 2021, 5, pkaa109. [Google Scholar] [CrossRef]
- Lau, C.E.; Robinson, O. DNA methylation age as a biomarker for cancer. Int. J. Cancer 2021, 148, 2652–2663. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, T.; Xu, D. Telomerase reverse transcriptase promoter mutations in thyroid carcinomas: Implications in precision oncology-a narrative review. Ann. Transl. Med. 2020, 8, 1244. [Google Scholar] [CrossRef]
- Network CGAR. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Beltrami, C.M.; Dos Reis, M.B.; Barros-Filho, M.C.; Marchi, F.A.; Kuasne, H.; Pinto, C.A.L.; Ambatipudi, S.; Herceg, Z.; Kowalski, L.P.; Rogatto, S.R. Integrated data analysis reveals potential drivers and pathways disrupted by DNA methylation in papillary thyroid carcinomas. Clin. Epigenet. 2017, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Bisarro Dos Reis, M.; Barros-Filho, M.C.; Marchi, F.A.; Beltrami, C.M.; Kuasne, H.; Pinto, C.A.L.; Ambatipudi, S.; Herceg, Z.; Kowalski, L.P.; Rogatto, S.R. Prognostic Classifier Based on Genome-Wide DNA Methylation Profiling in Well-Differentiated Thyroid Tumors. J. Clin. Endocrinol. Metab. 2017, 102, 4089–4099. [Google Scholar] [CrossRef] [Green Version]
- Ambatipudi, S.; Horvath, S.; Perrier, F.; Cuenin, C.; Hernandez-Vargas, H.; Le Calvez-Kelm, F.; Durand, G.; Byrnes, G.; Ferrari, P.; Bouaoun, L.; et al. DNA methylome analysis identifies accelerated epigenetic ageing associated with postmenopausal breast cancer susceptibility. Eur. J. Cancer 2017, 75, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Kresovich, J.K.; Xu, Z.; O’Brien, K.M.; Weinberg, C.R.; Sandler, D.P.; Taylor, J.A. Methylation-Based Biological Age and Breast Cancer Risk. J. Natl. Cancer Inst. 2019, 111, 1051–1058. [Google Scholar] [CrossRef]
- Yang, Z.; Wong, A.; Kuh, D.; Paul, D.S.; Rakyan, V.K.; Leslie, R.D.; Zheng, S.C.; Widschwendter, M.; Beck, S.; Teschendorff, A.E. Correlation of an epigenetic mitotic clock with cancer risk. Genome Biol. 2016, 17, 205. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.; Ruan, M.; Zhao, N.; Koestler, D.C.; De Vivo, I.; Kelsey, K.T.; Michaud, D.S. DNA methylation ageing clocks and pancreatic cancer risk: Pooled analysis of three prospective nested case-control studies. Epigenetics 2021, 1–11. [Google Scholar] [CrossRef]
- Hofstatter, E.W.; Horvath, S.; Dalela, D.; Gupta, P.; Chagpar, A.B.; Wali, V.B.; Bossuyt, V.; Storniolo, A.M.; Hatzis, C.; Patwardhan, G.; et al. Increased epigenetic age in normal breast tissue from luminal breast cancer patients. Clin. Epigenet. 2018, 10, 112. [Google Scholar] [CrossRef]
- Oltra, S.S.; Pena-Chilet, M.; Flower, K.; Martinez, M.T.; Alonso, E.; Burgues, O.; Lluch, A.; Flanagan, J.M.; Ribas, G. Acceleration in the DNA methylation age in breast cancer tumours from very young women. Sci. Rep. 2019, 9, 14991. [Google Scholar] [CrossRef]
- Panjarian, S.; Madzo, J.; Keith, K.; Slater, C.M.; Sapienza, C.; Jelinek, J.; Issa, J.J. Accelerated aging in normal breast tissue of women with breast cancer. Breast Cancer Res. 2021, 23, 58. [Google Scholar] [CrossRef]
- Joo, J.E.; Clendenning, M.; Wong, E.M.; Rosty, C.; Mahmood, K.; Georgeson, P.; Winship, I.M.; Preston, S.G.; Win, A.K.; Dugue, P.A.; et al. DNA Methylation Signatures and the Contribution of Age-Associated Methylomic Drift to Carcinogenesis in Early-Onset Colorectal Cancer. Cancers 2021, 13, 2589. [Google Scholar] [CrossRef]
- Cui, Y.; Mubarik, S.; Li, R.; Nawsherwan; Yu, C. Trend dynamics of thyroid cancer incidence among China and the U.S. adult population from 1990 to 2017: A joinpoint and age-period-cohort analysis. BMC Public Health 2021, 21, 624. [Google Scholar] [CrossRef]
- Vaccarella, S.; Lortet-Tieulent, J.; Colombet, M.; Davies, L.; Stiller, C.A.; Schuz, J.; Togawa, K.; Bray, F.; Franceschi, S.; Dal Maso, L.; et al. Global patterns and trends in incidence and mortality of thyroid cancer in children and adolescents: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 144–152. [Google Scholar] [CrossRef]
- Zheng, C.; Berger, N.A.; Li, L.; Xu, R. Epigenetic age acceleration and clinical outcomes in gliomas. PLoS ONE 2020, 15, e0236045. [Google Scholar] [CrossRef]
- Dugue, P.A.; Bassett, J.K.; Joo, J.E.; Jung, C.H.; Ming Wong, E.; Moreno-Betancur, M.; Schmidt, D.; Makalic, E.; Li, S.; Severi, G.; et al. DNA methylation-based biological aging and cancer risk and survival: Pooled analysis of seven prospective studies. Int. J. Cancer 2018, 142, 1611–1619. [Google Scholar] [CrossRef]
- Zheng, C.; Li, L.; Xu, R. Association of Epigenetic Clock with Consensus Molecular Subtypes and Overall Survival of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1720–1724. [Google Scholar]


| Variable (Number) | n (%) |
|---|---|
| Age at diagnosis (n = 508) | |
| Median (Min–Max) (years) | 46 (15–89) |
| <55 | 342 (67.3) |
| ≥55 | 166 (32.7) |
| Gender (n = 507) | |
| Female | 371 (73.2) |
| Male | 136 (26.8) |
| Histology (n = 508) | |
| PTC | 399 (78.5) |
| FTC | 107 (21.1) |
| Other/unknown | 2 (0.4) |
| Tumor size (n = 502) | |
| <4 cm | 390 (77.7) |
| ≥4 cm | 112 (22.3) |
| Nstage (n = 458) | |
| N0 | 233 (50.9) |
| N1 | 225 (49.1) |
| TNM stage (n = 506) | |
| I & II | 338 (66.8) |
| III & IV | 168 (33.2) |
| Extrathyroidal extension (n = 489) | |
| No | 336 (68.7) |
| Yes | 153 (31.3) |
| Specimen | Number | Chronological Age (Year)/Range (Median) | DNAm Age (Year)/Range (Median) |
|---|---|---|---|
| NT 1 | 54 | 15–81 (42) | 12–66 (38) |
| TC Tumor | 502 | ||
| All | 502 | 15–89 (46) | 8–121 (51) |
| PTC 2 | 396 | 15–89 (46) | 15–107 (51) |
| FTC 3 | 105 | 16–83 (46) | 8–121 (52) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wang, J.; Xiu, Y.; Wu, Y.; Xu, D. DNA Methylation Age Drift Is Associated with Poor Outcomes and De-Differentiation in Papillary and Follicular Thyroid Carcinomas. Cancers 2021, 13, 4827. https://doi.org/10.3390/cancers13194827
Liu T, Wang J, Xiu Y, Wu Y, Xu D. DNA Methylation Age Drift Is Associated with Poor Outcomes and De-Differentiation in Papillary and Follicular Thyroid Carcinomas. Cancers. 2021; 13(19):4827. https://doi.org/10.3390/cancers13194827
Chicago/Turabian StyleLiu, Tiantian, Jiansheng Wang, Yuchen Xiu, Yujiao Wu, and Dawei Xu. 2021. "DNA Methylation Age Drift Is Associated with Poor Outcomes and De-Differentiation in Papillary and Follicular Thyroid Carcinomas" Cancers 13, no. 19: 4827. https://doi.org/10.3390/cancers13194827
APA StyleLiu, T., Wang, J., Xiu, Y., Wu, Y., & Xu, D. (2021). DNA Methylation Age Drift Is Associated with Poor Outcomes and De-Differentiation in Papillary and Follicular Thyroid Carcinomas. Cancers, 13(19), 4827. https://doi.org/10.3390/cancers13194827






