Neuroendocrine Carcinoma of the Larynx and Pharynx: A Clinical and Histopathological Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histological and Molecular Analyses
2.2.1. Immunohistochemistry
2.2.2. In Situ Hybridization
2.3. Statistics
3. Results
3.1. Patients
3.2. Treatment
3.3. Immunohistochemical and Molecular Analyses
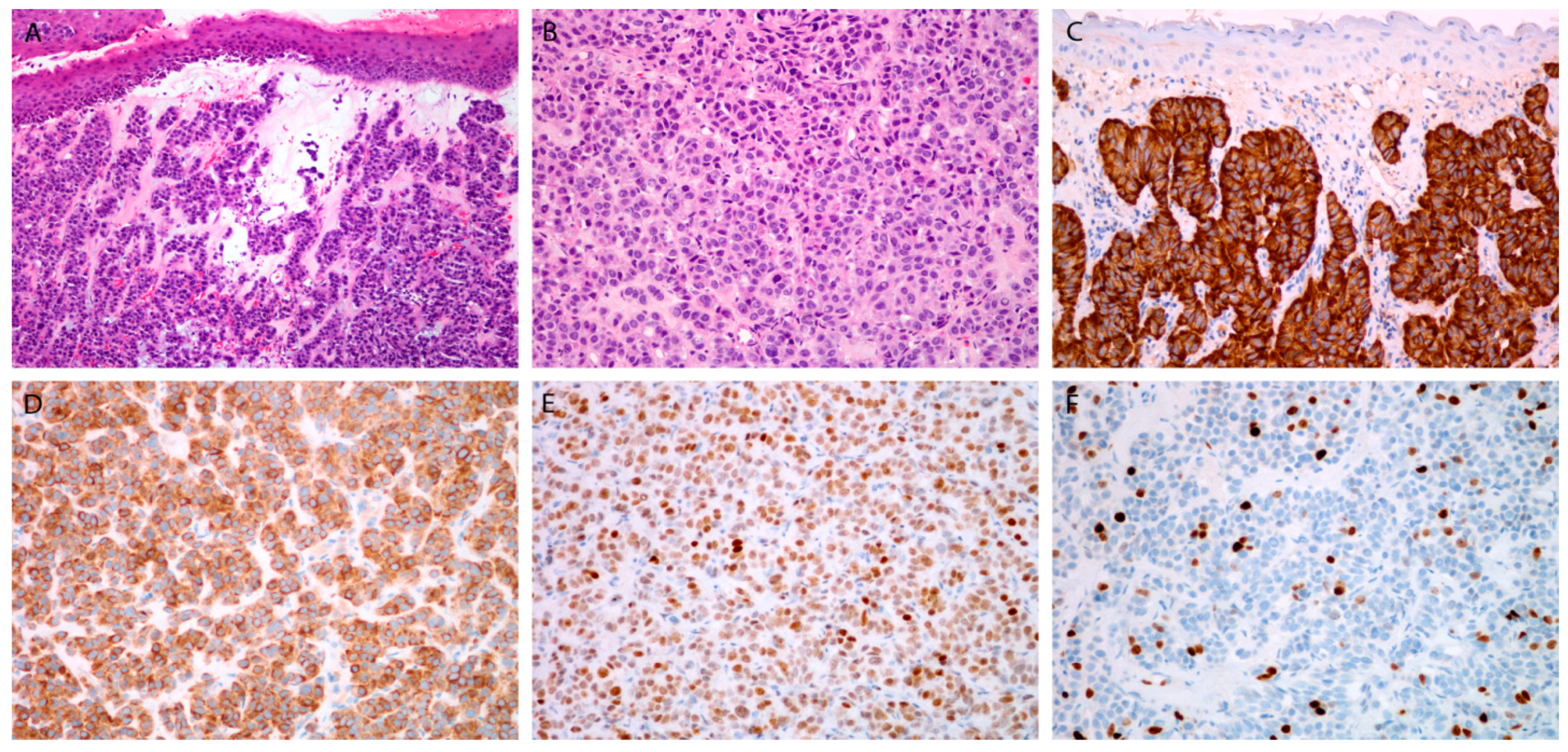
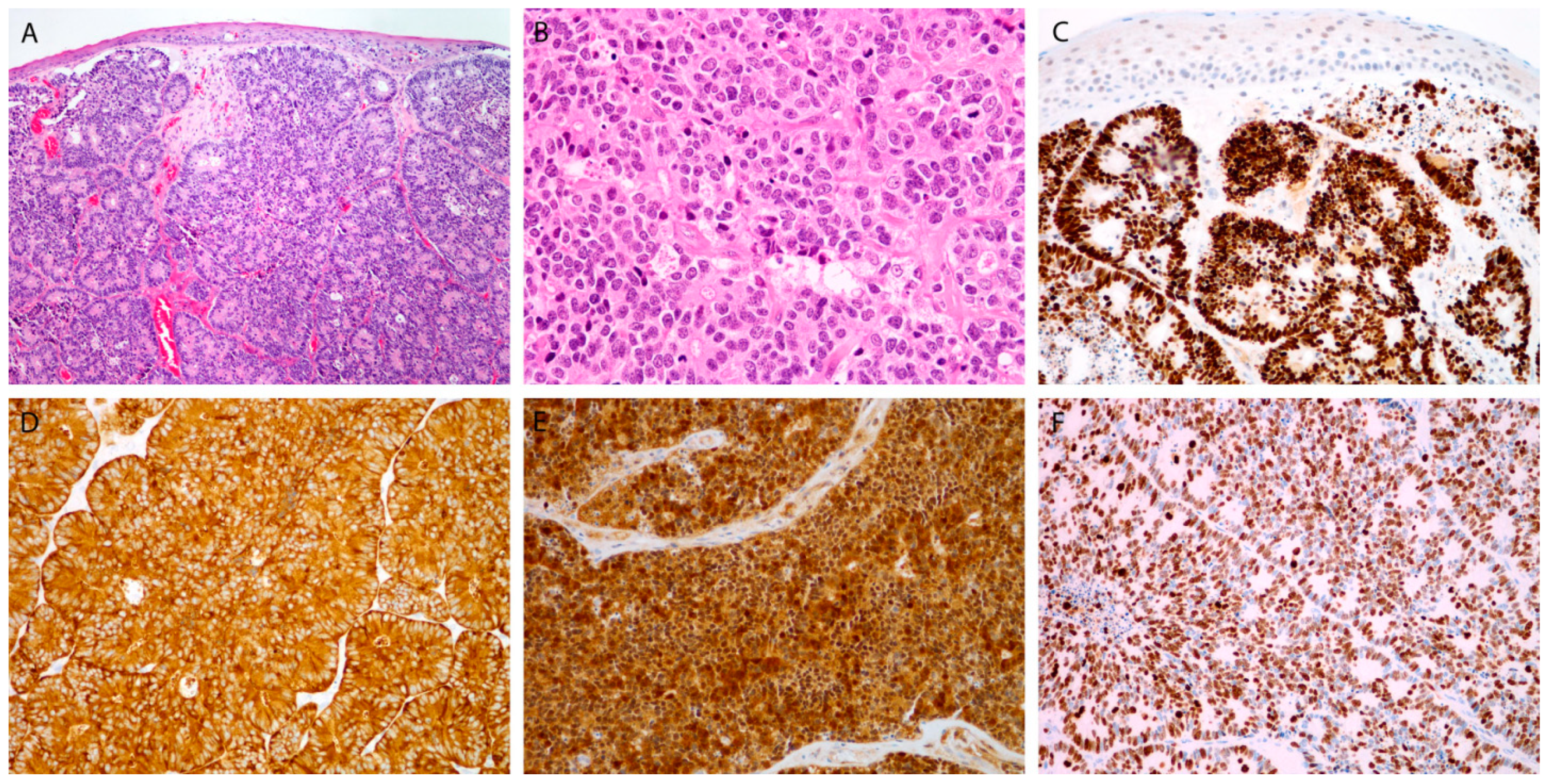
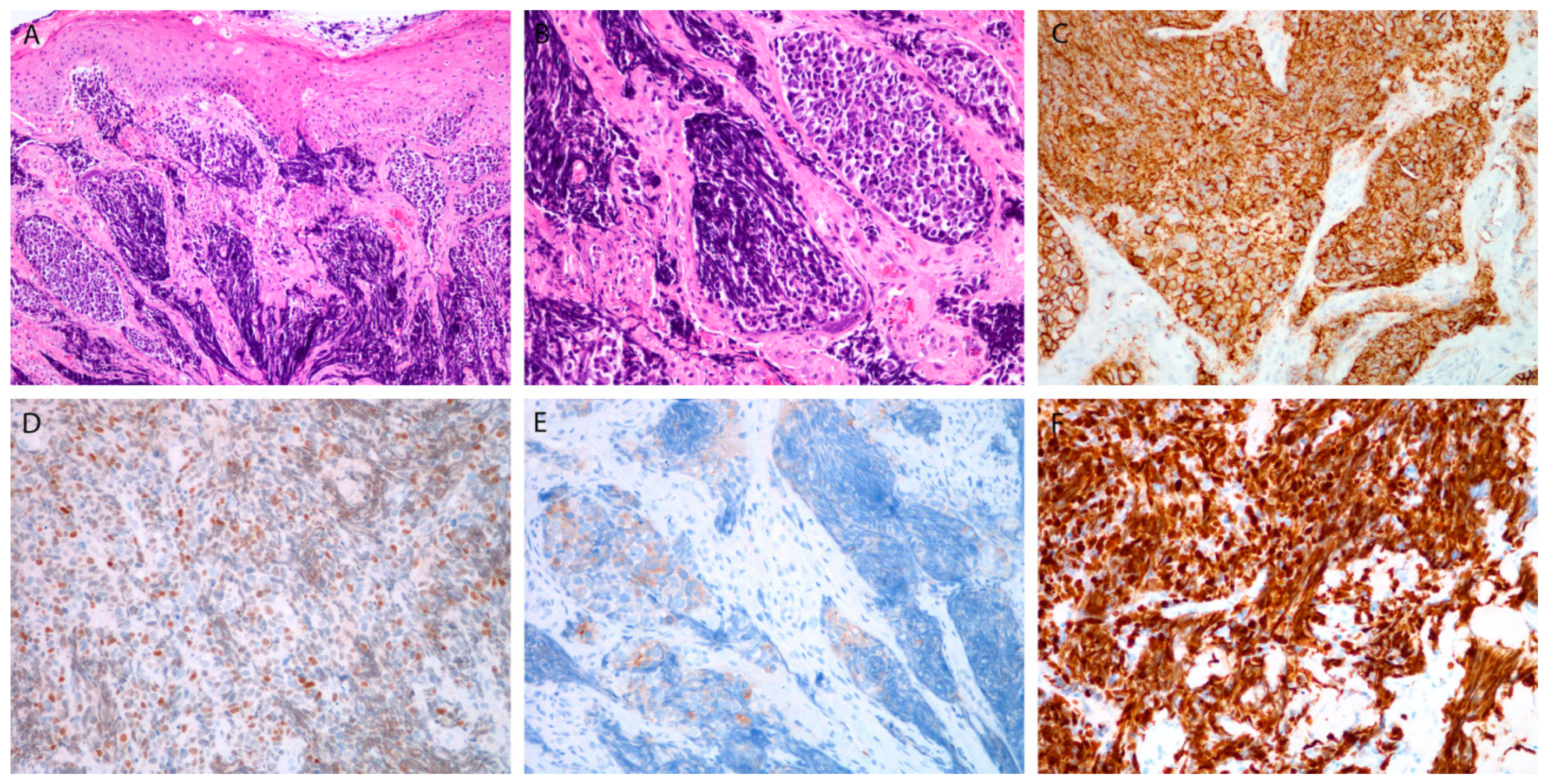
3.3.1. Markers for Diagnosis
3.3.2. Proliferative Markers
3.3.3. Viral Markers
3.3.4. PD-L1
3.4. Treatment Outcome and Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bean, M.B.; Liu, Y.; Jiang, R.; Steuer, C.E.; Patel, M.; McDonald, M.W.; Higgins, K.A.; Beitler, J.J.; Shin, D.M.; Saba, N.F. Small cell and squamous cell carcinomas of the head and neck: Comparing incidence and survival trends based on Surveillance, Epidemiology, and End Results (SEER) data. Oncologist 2019, 24, 1562–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torabi, S.J.; Cheraghlou, S.; Kasle, D.A.; Savoca, E.L.; Judson, B.L. Nonsquamous cell laryngeal cancers: Incidence, demographics, care patterns, and effect of surgery. Laryngoscope 2019, 129, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ordoñez, B. Neuroendocrine Carcinomas of the Larynx and Head and Neck: Challenges in Classification and Grading. Head Neck Pathol. 2018, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, C.; Ferlito, A.; Triantafyllou, A.; Gnepp, D.R.; A Bishop, J.; Hellquist, H.; Strojan, P.; Willems, S.M.; Stenman, G.; Rinaldo, A.; et al. Update on Neuroendocrine Carcinomas of the Larynx. Am. J. Clin. Pathol. 2019, 152, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.-H.; Lee, S.-S.; Shim, Y.-S.; Kim, S.-Y.; Nam, S.-Y.; Kim, D.-H.; Cho, K.-J. A Study of Moderately Differentiated Neuroendocrine Carcinomas of the Larynx and an Examination of Non-Neoplastic Larynx Tissue for Neuroendocrine Cells. Laryngoscope 2004, 114, 1264–1270. [Google Scholar] [CrossRef]
- Nagao, T.; Sugano, I.; Ishida, Y.; Tajima, Y.; Munakata, S.; Asoh, A.; Yamazaki, K.; Muto, H.; Konno, A.; Kondo, Y.; et al. Primary Large-Cell Neuroendocrine Carcinoma of the Parotid Gland: Immunohistochemical and Molecular Analysis of Two Cases. Mod. Pathol. 2000, 13, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Greene, L.; Brundage, W.; Cooper, K. Large cell neuroendocrine carcinoma of the larynx: A case report and a review of the classification of this neoplasm. J. Clin. Pathol. 2005, 58, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.S., Jr.; Spence, D.C.; Chiosea, S.; Barnes, E.L., Jr.; Brandwein-Gensler, M.; El-Mofty, S.K. Large Cell Neuroendocrine Carcinoma of the Larynx: Definition of an Entity. Head Neck Pathol. 2010, 4, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slootweg, P.J.; Grandis, J.R.; Zidar, N.; Cardesa, A.; Gillison, M.; Helliwell, T.; Hille, J.; Nadal, A. Tumours of the hypopharynx; larynx; trachea and parapharyngeal space. In WHO Classification of Head and Neck Tumours; El-Naggar, A.K., Chan, J.K.C., Grandis, J.R., Takata, T., Slootweg, P.J., Eds.; IARC: Lyon, France, 2017; pp. 77–104. [Google Scholar]
- Van Der Laan, T.P.; Plaat, B.; van der Laan, B.; Halmos, G. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: A meta-analysis of 436 reported cases. Head Neck 2014, 37, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Hernandez-Prera, J.C.; Beitler, J.J.; Eisbruch, A.; Saba, N.F.; Mendenhall, W.M.; Nieto, C.S.; Smee, R.; Rinaldo, A.; Ferlito, A. Small cell and large cell neuroendocrine carcinoma of the larynx: A comparative analysis. Cancer Treat. Rev. 2019, 78, 42–51. [Google Scholar] [CrossRef]
- Rooper, L.; Bishop, J.A.; Westra, W.H. INSM1 is a Sensitive and Specific Marker of Neuroendocrine Differentiation in Head and Neck Tumors. Am. J. Surg. Pathol. 2018, 42, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.L.; Barnes, L.; Triantafyllou, A.; Gnepp, D.R.; Devaney, K.O.; Stenman, G.; Halmos, G.B.; Bishop, J.A.; Skálová, A.; Willems, S.M.; et al. Well-differentiated Neuroendocrine Carcinoma of the Larynx: Confusion of Terminology and Uncertainty of Early Studies. Adv. Anat. Pathol. 2019, 26, 246–250. [Google Scholar] [CrossRef]
- Barker, J.L.; Glisson, B.S.; Garden, A.S.; El-Naggar, A.K.; Morrison, W.H.; Ang, K.K.; Chao, K.S.; Clayman, G.; Rosenthal, D. Management of nonsinonasal neuroendocrine carcinomas of the head and neck. Cancer 2003, 98, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T.; et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobin, L.H.; Gospodarowicz, M.; Wittekind, C. International Union Against Cancer (UICC). In TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 25–45. [Google Scholar]
- Lewis, J.S., Jr.; A Khan, R.; Masand, R.P.; Chernock, R.; Zhang, Q.; Al-Naief, N.S.; Muller, S.; McHugh, J.B.; Prasad, M.L.; Brandwein-Gensler, M.; et al. Recognition of nonkeratinizing morphology in oropharyngeal squamous cell carcinoma—A prospective cohort and interobserver variability study. Histopathology 2012, 60, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burtness, B.; Harrington, K.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Bishop, J.A.; Ma, X.-J.; Wang, H.; Luo, Y.; Illei, P.B.; Begum, S.; Taube, J.M.; Koch, W.M.; Westra, W.H. Detection of Transcriptionally Active High-risk HPV in Patients With Head and Neck Squamous Cell Carcinoma as Visualized by a Novel E6/E7 mRNA In Situ Hybridization Method. Am. J. Surg. Pathol. 2012, 36, 1874–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadnik, V.; Primic Žakelj, M. SLORA: Slovenia and Cancer. Epidemiology and Cancer Registry. Istitute of Oncology Ljubljana. Available online: http://www.slora.si (accessed on 25 June 2021).
- Ferlito, A. Diagnosis and treatment of small cell carcinoma of the larynx: A critical review. Ann. Otol. Rhinol. Laryngol. 1986, 95, 590–600. [Google Scholar] [CrossRef]
- Feola, T.; Puliani, G.; Sesti, F.; Modica, R.; Biffoni, M.; Di Gioia, C.; Carletti, R.; Anastasi, E.; Di Vito, V.; Centello, R.; et al. Laryngeal Neuroendocrine Tumor With Elevated Serum Calcitonin: A Diagnostic and Therapeutic Challenge. Case Report and Review of Literature. Front. Endocrinol. 2020, 11, 397. [Google Scholar] [CrossRef]
- Ghosh, R.; Dutta, R.; Dubal, P.M.; Park, R.C.; Baredes, S.; Eloy, J.A. Laryngeal neuroendocrine carcinoma: A population-based analysis of incidence and survival. Otolaryngol. Head Neck Surg. 2015, 153, 966–972. [Google Scholar] [CrossRef]
- Yu, C.-X.; Yibulayin, F.; Feng, L.; Wang, M.; Lu, M.-M.; Luo, Y.; Liu, H.; Yang, Z.-C.; Wushou, A. Clinicopathological characteristics, treatment and prognosis of head & neck small cell carcinoma: A SEER population-based study. BMC Cancer 2020, 20, 1208. [Google Scholar] [CrossRef]
- Pointer, K.B.; Ko, H.C.; Brower, J.V.; Witek, M.E.; Kimple, R.J.; Lloyd, R.V.; Harari, P.M.; Baschnagel, A.M. Small cell carcinoma of the head and neck: An analysis of the National Cancer Database. Oral Oncol. 2017, 69, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sone, M.; Uchida, I.; Tominaga, M.; Sugiura, S.; Nagasaka, T.; Nakashima, T. Small cell carcinoma of the larynx treated with irinotecan and cisplatin. Auris Nasus Larynx 2006, 33, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Paleri, V.; Moor, J.; Dobrowsky, W.; Kelly, C.; Kovarik, J. Small cell neuroendocrine carcinoma of larynx: Case series and literature review. J. Laryngol. Otol. 2015, 129, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Mody, M.D.; Saba, N.F. Systemic therapy in non-conventional cancers of the larynx. Oral Oncol. 2018, 82, 61–68. [Google Scholar] [CrossRef]
- Lambrescu, I.; Fica, S.; Martins, D.; Spada, F.; Cella, C.; Bertani, E.; Rubino, M.; Gibelli, B.; Grana, C.; Bonomo, G.; et al. Metronomic and metronomic-like therapies in neuroendocrine tumors–Rationale and clinical perspectives. Cancer Treat. Rev. 2017, 55, 46–56. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Ho, W.J.; Rooper, L.; Sagorsky, S.; Kang, H. A robust response to combination immune checkpoint inhibitor therapy in HPV-related small cell cancer: A case report. J. Immunother. Cancer 2018, 6, 33. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [Green Version]
- Özdirik, B.; Jann, H.; Bischoff, P.; Fehrenbach, U.; Tacke, F.; Roderburg, C.; Wiedenmann, B. PD-L1-inhibitors in neuroendocrine neoplasia: Results from a real-life study. Medicine 2021, 100, e23835. [Google Scholar] [CrossRef]
- Bahr, K.; Zimmer, S.; Springer, E.; Fottner, C.; Becker, S.; Ernst, B.P.; Matthias, C.; Künzel, J. High-Grade Neuroendocrine Carcinoma of the Head and Neck: Human Papillomavirus Status and PD-L1 Expression. Otorhinolaryngol. Relat. Spec. 2019, 81, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickman, D.S.; Beltran, H.; Demichelis, F.; Rubin, M.A. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat. Med. 2017, 23, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Halmos, G.B.; Van Der Laan, T.P.; Van Hemel, B.M.; Dikkers, F.G.; Slagter-Menkema, L.; van der Laan, B.; Schuuring, E. Is human papillomavirus involved in laryngeal neuroendocrine carcinoma? Eur. Arch. Oto Rhino Laryngol. 2012, 270, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Westra, W.H. Human Papillomavirus-Related Neuroendocrine Carcinomas of the Head and Neck. Head Neck Pathol. 2018, 12, 9–12. [Google Scholar] [CrossRef]
- Sinno, S.; Assaad, A.M.; Salem Shabb, N. Human papillomavirus-associated oropharyngeal high-grade neuroendocrine car-cinoma in an adolescent: Case report and review of literature. Clin. Med. Insights Pediatr. 2019, 13, 1179556519870520. [Google Scholar] [CrossRef]
- Alos, L.; Hakim, S.; Larque, A.-B.; De La Oliva, J.; Rodriguez-Carunchio, L.; Caballero, M.; Nadal, A.; Marti, C.; Guimera, N.; Fernandez-Figueras, M.-T.; et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: Potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016, 469, 277–284. [Google Scholar] [CrossRef]
- Benzerdjeb, N.; Traverse-Glehen, A.; Philouze, P.; Bishop, J.; Devouassoux-Shisheboran, M. Poorly differentiated neuroendocrine carcinoma of the head and neck: Human papillomavirus tumour status/p16 status and impact on overall survival. Histopathology 2019, 76, 581–591. [Google Scholar] [CrossRef]
- Cai, Z.; Lin, M.; Blanco, A.I.; Liu, J.; Zhu, H. Epstein–Barr Virus-Positive Large Cell Neuroendocrine Carcinoma of the Nasopharynx: Report of One Case and Review of the Literature. Head Neck Pathol. 2018, 13, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Mesolella, M.; Allosso, S.; Varricchio, S.; Russo, D.; Pignatiello, S.; Buono, S.; Motta, G. Small-cell carcinoma of nasopharynx: A case report of unusual localization. Ear Nose Throat J. 2020, 145561320973780. [Google Scholar] [CrossRef]
- Grillo, F.; Bruzzone, M.; Pigozzi, S.; Prosapio, S.; Migliora, P.; Fiocca, R.; Mastracci, L. Immunohistochemistry on old archival paraffin blocks: Is there an expiry date? J. Clin. Pathol. 2017, 70, 988–993. [Google Scholar] [CrossRef]
- Rinaldo, A.; Coca-Pelaz, A.; Silver, C.E.; Ferlito, A. Paraneoplastic Syndromes Associated with Laryngeal Cancer. Adv. Ther. 2019, 37, 140–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlito, A.; Rinaldo, A.; Bishop, J.A.; Hunt, J.L.; Poorten, V.V.; Williams, M.D.; Triantafyllou, A.; Devaney, K.O.; Gnepp, D.R.; Kusafuka, K.; et al. Paraneoplastic syndromes in patients with laryngeal neuroendocrine carcinomas: Clinical manifestations and prognostic significance. Eur. Arch. Oto Rhino Laryngol. 2016, 273, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Jackson, L.; Sharma, S. Primary combined small cell carcinoma of larynx with lateralized histologic components and corresponding side-specific neck nodal metastasis: Report of a unique case and review of literature. Int. J. Clin. Exp. Pathol. 2010, 4, 111–117. [Google Scholar]
- Robinson, L.; Schouwstra, C.-M.; van Heerden, W.F.P. Oropharyngeal Mixed Neuroendocrine-Nonneuroendocrine Neoplasm (MiNEN): A Case Report and Literature Review. Head Neck Pathol. 2021, 1–6. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Devaney, K.O.; Rodrigo, J.P.; Halmos, G.B.; Strojan, P.; Mendenhall, W.M.; Eisbruch, A.; Smee, R.; Kusafuka, K.; Rinaldo, A.; et al. Should patients with laryngeal small cell neuroendocrine carcinoma receive prophylactic cranial irradiation? Eur. Arch. Oto Rhino Laryngol. 2015, 273, 2925–2930. [Google Scholar] [CrossRef]
| Antigen | Clone | Manufacturer | Pretreatment | Dilution |
|---|---|---|---|---|
| CD56 | MRQ-42 | Cell Marque, Rocklin, CA, USA | CC1 60 min | Ready to use |
| Chromogranin | polyclonal | Dako, Glostrup, Denmark | CC1 60 min | 1:1000 |
| Cytokeratin AE1/AE3 | AE1AE3 | Novocastra, Wetzlar, Germany | CC1 60 min | 1:50 |
| INSM1 | A-8 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | CC1 56 min | 1:50 |
| Ki-67 | MIB-1 | Dako, Glostrup, Denmark | CC1 60 min | 1:50 |
| p16 | E6H4 | Ventana, Tuscon, AR, USA | CC1 32 min | Ready to use |
| PD-L1 | SP263 | Ventana, Tuscon, AR, USA | CC1 56 min | Ready to use |
| Synaptophysin | MRQ-40 | Cell Marque, Rocklin, CA, USA | CC1 64 min | Ready to use |
| Patients | Tumors | PET-CT | Therapy | Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Sex/Age (Years) | Smoking Status | Site | TNM | Grade/ Type | (Yes/No) | Modality | Surgery | ChT (Drug/Cycles) | RT (Gy/fx) | DFI (mos) | Recurrence | Salvage Therapy | Status (Months) |
| Larynx | ||||||||||||||
| 1 | M/64 | Former/ 15 py | SG | pT1cN0, M0 | WD | No | S | TLM | 46+ | No | NED | |||
| 2 | M/60 | Former/ 15 py | SG | pT2pN2B, M0 ECE+ | MD | No | S→RT | SGL ND(l) | L:60/30 R:64/32 | 12 | Skin | S, ChT, RT | DOD (26) | |
| 3 | F/59 | No | SG | pT1pN0, M0 | MD | No | S | SGL ND(b) | 79 | Neck node | S | NED (109) | ||
| 4 | M/60 | No | SG | cT2cN0, M0 | MD | No | cCRT | CP/5 | L:70/35 R:56/35 | 7 | Gallblader, skin | S ChT | DOD (38) | |
| 5 | F/87 | No | SG | pT2cN0, M0 | MD | Yes | S | T | 6+ | No | NED | |||
| 6 | F/64 | Active/ 26 py | SG | pT2pN1, M0 | PD/LC | No | S→RT | SGL ND(b) | L:52/26 R:52/26 | 0 | No | DOC (3) | ||
| 7 | M/72 | Former/ 20 py | SG | cT3cN0, M0 | PD/LC | No | RT | L:70/35 R:50/25 | 114 | No | DOC | |||
| 8 | M/74 | No | SG | pT1pN2B, M0 ECE+ | PD/LC | No | S→RT | SGL ND(r) | L:60/30 R:64/32 | 8 | Liver, lung | ChT | DOD (13) | |
| 9 | F/26 | Active/ 10 py | SG | cT2cN0, M0 | PD/SC | Yes | cCRT | CP + E/3 | L:70/35 R:56/35 | 80+ | No | NED | ||
| 10 | F/62 | Active/ 40 py | SG | pT1pN0, M0 | PD/LC +SCC | Yes | S | SGL ND(b) | 60+ | No | NED | |||
| 11 | M/74 | Former/ 10 py | SG | pT2pN2C, M0 ECE+ | PD/LC | No | S→cCRT | TLM ND(b) | CaP/5 | L:60/30 R:63/30 | 5,5 | No | DOC | |
| 12 | M/71 | Former/ 60 py | SG | cT3cN0, M0 | PD/SC | Yes | cCRT | CP/7 | L:70/35 R:56/35 | 27+ | No | NED | ||
| Nasopharynx | ||||||||||||||
| 13 | M/67 | No | NP | cT4cN0, M0 | MD | No | iC→cCRT | CP + E/3 CP/5 | L:70/35 R:50/25 | 47 | Brain | RT | DOD (57) | |
| 14 | M/80 | Active/n.s. | NP | cT3cN0, M0 | PD/LC | Yes | RT | L:70/35 R:56/35 | 12 | No | DOC | |||
| 15 | M/54 | Active/ 45 py | NP | cN2cN3, M0 | PD/SC | Yes | iC→cCRT | CP + E/3 CP/6 | L:70/35 R:70/35 | 7,5 | Bone marrow | No | DOD (8,5) | |
| Oropharynx | ||||||||||||||
| 16 | M/67 | Active/n.s. | OP | pT1pN1, M0 | PD/LC | No | S | T(l) ND(l) | 32 | No | DOC (32) | |||
| 17 | M/64 | Former/ 20 py | OP | cT4AcN2A M0 | PD/LC | No | iC→cCRT | CP + E/4 CP/6 | L:70/35 R:70/35 | 139+ | N0 | NED | ||
| Hypopharynx | ||||||||||||||
| 18 | M/55 | Active/ 35 py | HP | pT3pN2B, M0 ECE+ | PD/SC | No | S→RT | pPH ND(l) | L:60/30 R:64/32 | 10 | Liver, spleen, retroperitoneal | N0 | DOD (10) | |
| 19 | M/53 | Active/ 40 py | HP | cT3cN3, M1/lung | PD/SC | No | iC→RT | CP + E/4 | L:70/35 R:70/35 | 6 | Lung | ChT | DOD (12) | |
| 20 | M/59 | Active/n.s. | HP | cT3cN3, M0 | PD/SC | No | cCRT | CP/6 | L:70/35 R:70/35 | 0 | Residual tumor | No | DOD (5) | |
| Patient’s No. | Sex/Age (Years) | Grade/Type | Chromogranin | Synaptophysin | CD56 | INSM1 | Ki-67 | p16 | HPV | PD-L1 (CPS) | Mitoses (per 10 hpf) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Larynx | |||||||||||
| 1 | M/64 | WD | + | + | + | + | <2% | - | - | Not done 1 | 1 |
| 2 | M/60 | MD | + | + | Not done 1 | + | 5% | + | - | 0 | 3 |
| 3 | F/59 | MD | + | + | + | + | 5% | + | - | 0 | 9 |
| 4 | M/60 | MD | + | + | Not done 1 | + | 15% | + | - | 0 | 6 |
| 5 | F/87 | MD | + | + | + | + | 5% | - | - | 3 | 3 |
| 6 | F/64 | PD/LC | + | + | Not done 1 | + | 20% | + | - | 0 | 95 |
| 7 | M/72 | PD/LC | - | - | + | + | 20% | - | - | 0 | Not possible 2 |
| 8 | M/74 | PD/LC | + | + | + | + | 70% | + | + | 0 | 33 |
| 9 | F/26 | SC/SC | - | + | + | + | 100% | + | + | 0 | Not possible 2 |
| 10 | F/62 | PD/LC + SCC | + | + | + | + | 80% | + | - | 5 | 40 |
| 11 | M/74 | PD/LC | + | + | + | + | 90% | + | - | 0 | 30 |
| 12 | M/71 | PD/SC | - | + | + | + | 90% | - | - | 0 | Not possible 2 |
| Nasopharynx | |||||||||||
| 13 | M/67 | MD | + | + | + | + | >2% | - | - | 0 | 1 |
| 14 | M/80 | PD/LC | + | + | + | + | 30% | + | - | 0 | 20 |
| 15 | M/54 | PD/SC | + | + | + | + | 70% | + | - | 0 | 28 |
| Oropharynx | |||||||||||
| 16 | M/67 | PD/LC | + | + | + | + | 70% | - | - | 0 | 120 |
| 17 | M/64 | PD/LC | - | - | + | + | 80% | + | - | 1 | 25 |
| Hypophyrynx | |||||||||||
| 18 | M/55 | PD/SC | + | + | + | + | 80% | + | - | 0 | 29 |
| 19 | M/53 | PD/SC | - | - | + | + | 80% | + | - | 0 | 30 |
| 20 | M/59 | PD/SC | - | + | + | + | 40% | - | - | 0 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strojan, P.; Šifrer, R.; Ferlito, A.; Grašič-Kuhar, C.; Lanišnik, B.; Plavc, G.; Zidar, N. Neuroendocrine Carcinoma of the Larynx and Pharynx: A Clinical and Histopathological Study. Cancers 2021, 13, 4813. https://doi.org/10.3390/cancers13194813
Strojan P, Šifrer R, Ferlito A, Grašič-Kuhar C, Lanišnik B, Plavc G, Zidar N. Neuroendocrine Carcinoma of the Larynx and Pharynx: A Clinical and Histopathological Study. Cancers. 2021; 13(19):4813. https://doi.org/10.3390/cancers13194813
Chicago/Turabian StyleStrojan, Primož, Robert Šifrer, Alfio Ferlito, Cvetka Grašič-Kuhar, Boštjan Lanišnik, Gaber Plavc, and Nina Zidar. 2021. "Neuroendocrine Carcinoma of the Larynx and Pharynx: A Clinical and Histopathological Study" Cancers 13, no. 19: 4813. https://doi.org/10.3390/cancers13194813
APA StyleStrojan, P., Šifrer, R., Ferlito, A., Grašič-Kuhar, C., Lanišnik, B., Plavc, G., & Zidar, N. (2021). Neuroendocrine Carcinoma of the Larynx and Pharynx: A Clinical and Histopathological Study. Cancers, 13(19), 4813. https://doi.org/10.3390/cancers13194813