Development of Population-Based Cancer Indicators and a Measurement of Cancer Care Continuum Using a Modified Delphi Method
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Gray Literature
2.3. Delphi Rounds
2.3.1. Round 1
2.3.2. Round 2
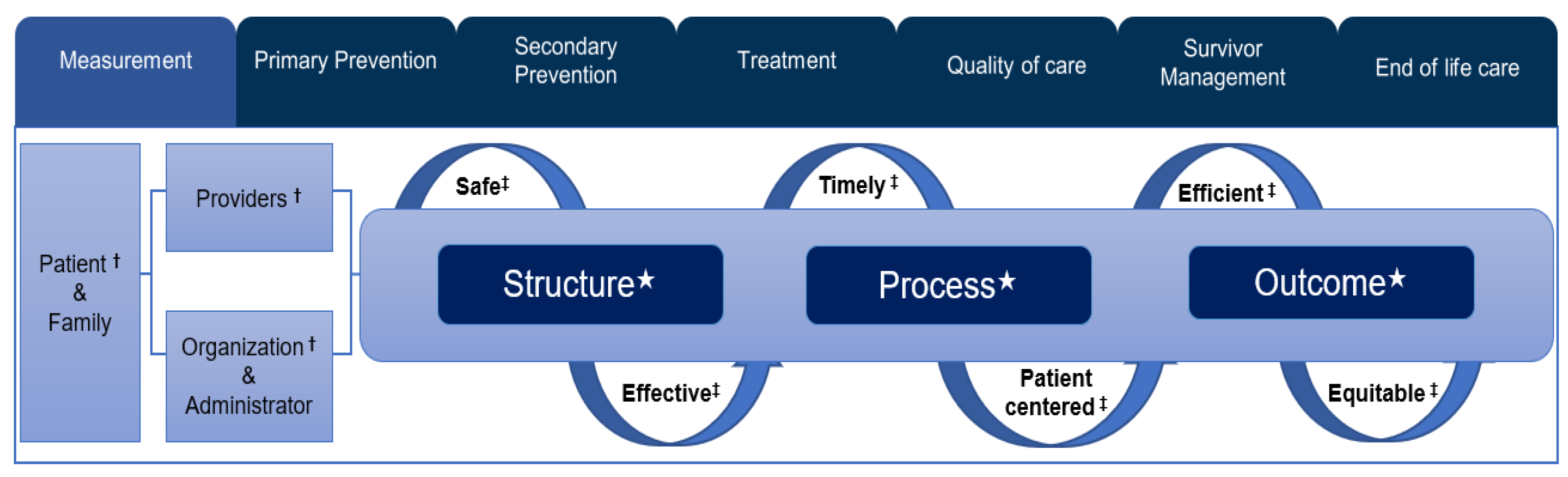
2.4. Quality of the Healthcare Measurement Framework
3. Results
3.1. Literature Search and Gray Literature
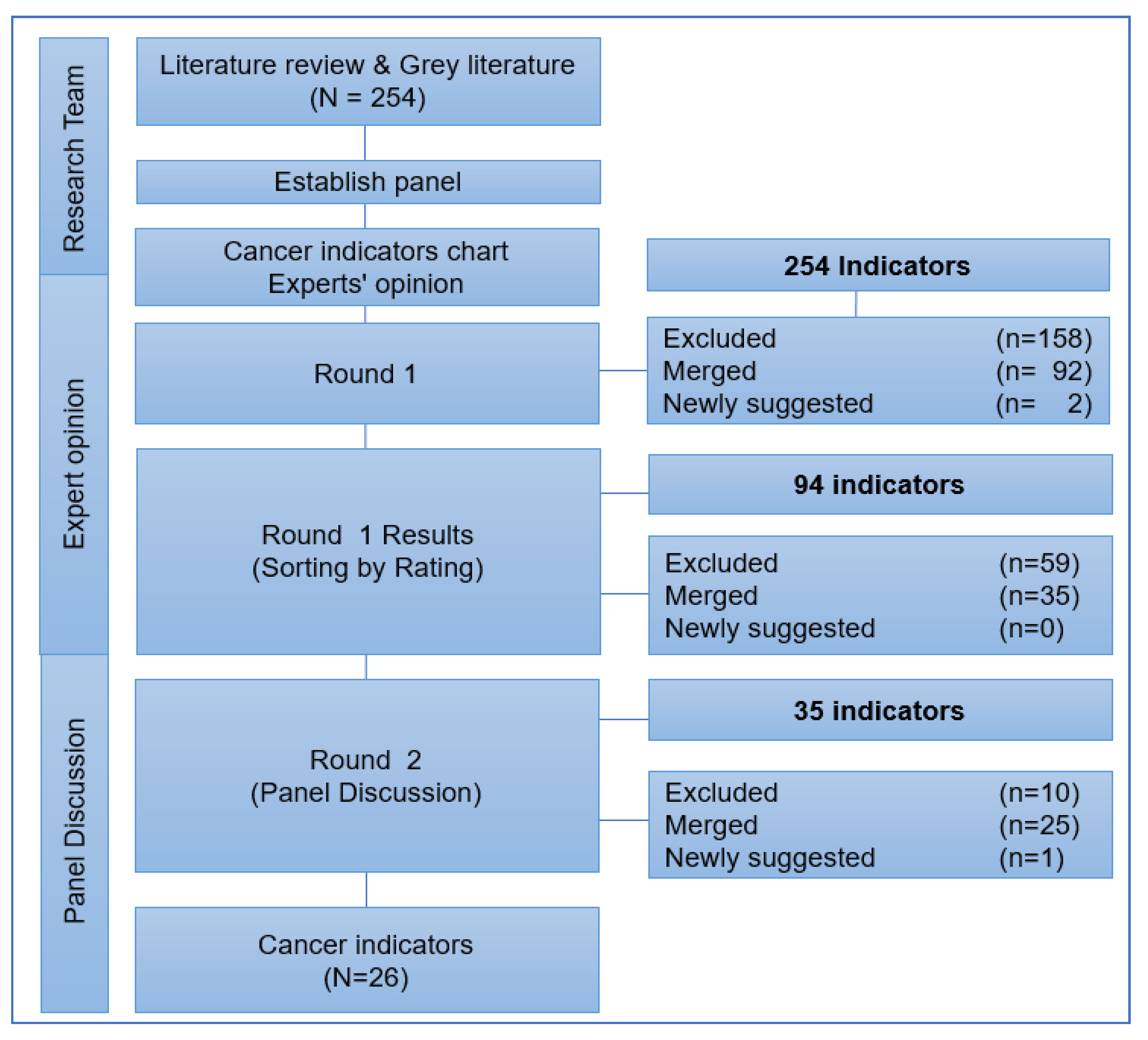
3.2. Selection Process of Cancer Indicators in the Delphi Rounds
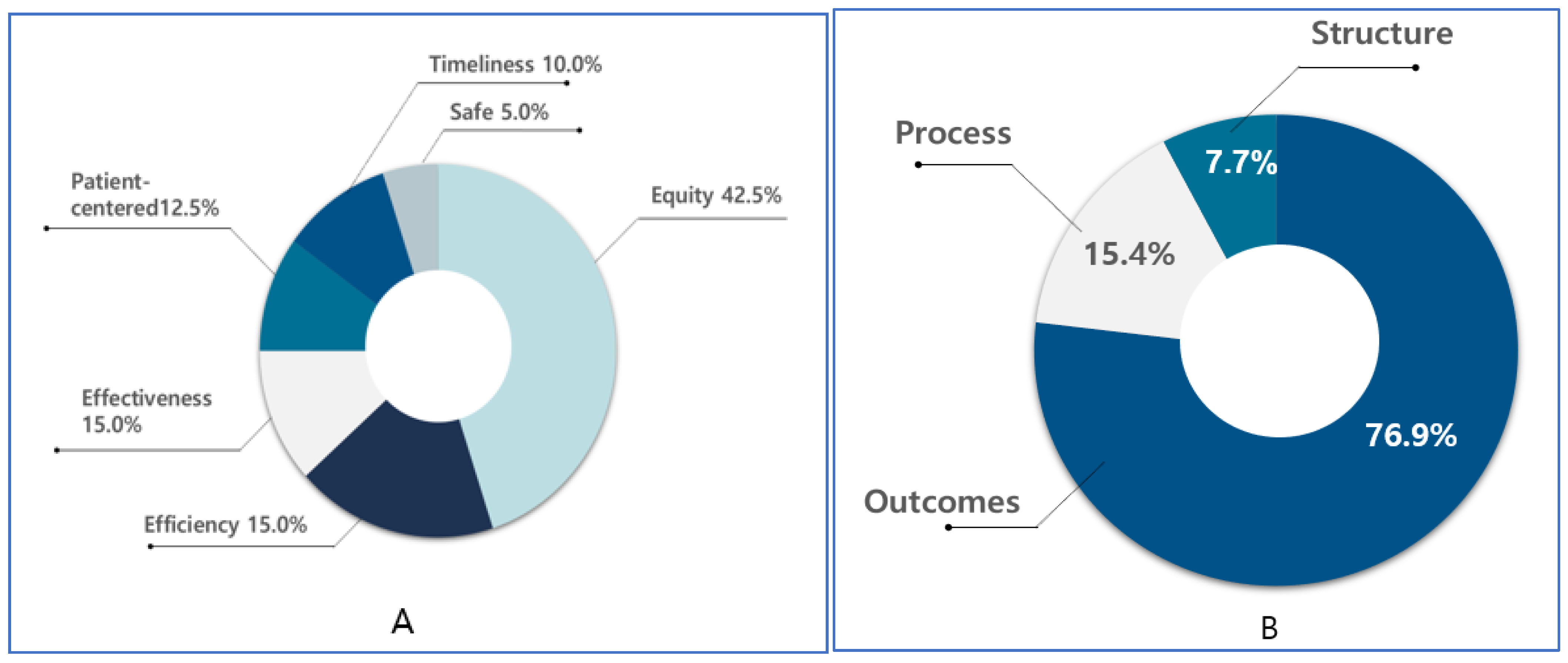
3.3. Distribution of Cancer-Related Monitoring Indicators According to Donabedian’s Quality of Healthcare
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Statistics Korea. Daejeon: Statistics Korea. 2017. Available online: http://kosis.kr (accessed on 15 February 2019).
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Lee, E.S. The Community of Population-based Regional Cancer Registries Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2015. Cancer Res. Treat. 2018, 50, 303–316. [Google Scholar] [CrossRef]
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Lee, E.S. The Community of Population-based Regional Cancer Registries Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res. Treat. 2019, 51, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Global Cancer Observatory. Lyon: International Agency for Research on Cancer. 2018. Available online: https://gco.iarc.fr/ (accessed on 23 January 2019).
- Australian Institute of Health and Welfare. Cancer in Australia 2019. Cancer Series No.119. Cat. No. CAN 122. Canberra: AIHW. Available online: https://www.aihw.gov.au/reports/cancer/cancer-in-australia-2019-in-brief/summary (accessed on 11 September 2020).
- Malin, J.L.; Schneider, E.C.; Epstein, A.M.; Adams, J.; Emanuel, E.J.; Kahn, K.L.; Weekes, C.D.; Vose, J.M.; Lynch, J.C.; Weisenburger, D.D.; et al. Results of the National Initiative for Cancer Care Quality: How Can We Improve the Quality of Cancer Care in the United States? J. Clin. Oncol. 2006, 24, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.E.; Holman, C.D.; Sheiner, H. The influence of socio-economic and locational disadvantage on patterns of surgical care for lung cancer in Western Australia 1982–2001. Aust. Health Rev. 2004, 27, 68–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwamena, F.; Holmes-Rovner, M.; Gaulden, C.M.; Jorgenson, S.; Sadigh, G.; Sikorskii, A.; Lewin, S.; Smith, R.C.; Coffey, J.; Olomu, A. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst. Rev. 2012, 12, CD003267. [Google Scholar] [CrossRef]
- Partanen, V.-M.; Anttila, A.; Heinävaara, S.; Pankakoski, M.; Sarkeala, T.; Bzhalava, Z.; Elfström, K.M.; Tropé, A.; Skare, G.B.; Thorsteinsdottir, S.; et al. NordScreen—An interactive tool for presenting cervical cancer screening indicators in the Nordic countries. Acta Oncol. 2019, 58, 1199–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahabi, M.; Lofters, A.; Kumar, M.; Glazier, R.H. Breast cancer screening disparities among urban immigrants: A population-based study in Ontario, Canada. BMC Public Health 2015, 15, 679. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Jin, A.; Xie, G.; Wang, L.; Liu, K.; Jia, G.; Liang, X.; Xu, J. The 20 Most Important and Most Preventable Health Problems of China: A Delphi Consultation of Chinese Experts. Am. J. Public Health 2018, 108, 1592–1598. [Google Scholar] [CrossRef]
- Jimmy, B.; Jose, J. Patient Medication Adherence: Measures in Daily Practice. Oman Med. J. 2011, 26, 155–159. [Google Scholar] [CrossRef]
- Hartmann-Johnsen, O.J.; Kåresen, R.; Schlichting, E.; Naume, B.; Nygård, J.F. Using clinical cancer registry data for estimation of quality indicators: Results from the Norwegian breast cancer registry. Int. J. Med. Inf. 2019, 125, 102–109. [Google Scholar] [CrossRef]
- Schneider, P.M.; Vallbohmer, D.; Ploenes, Y.; Lurje, G.; Metzger, R.; Ling, F.C.; Brabender, J.; Drebber, U.; Hoelscher, A.H. Evaluation of quality indicators following implementation of total mesorectal excision in primarily resected rectal cancer changed future management. Int. J. Color. Dis. 2011, 26, 903–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nag, N.; Millar, J.; Davis, I.D.; Costello, S.; Duthie, J.B.; Mark, S.; Delprado, W.; Smith, D.; Pryor, D.; Galvin, D.; et al. Development of Indicators to Assess Quality of Care for Prostate Cancer. Eur. Urol. Focus 2018, 4, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Biganzoli, L.; Marotti, L.; Hart, C.D.; Cataliotti, L.; Cutuli, B.; Kühn, T.; Mansel, R.E.; Ponti, A.; Poortmans, P.; Regitnig, P.; et al. Quality indicators in breast cancer care: An update from the EUSOMA working group. Eur. J. Cancer 2017, 86, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Maso, L.D.; Laversanne, M.; Bray, F.; Plummer, M.; Franceschi, S. The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Elnaggar, A.C.; Hade, E.M.; O’Malley, D.M.; Liang, M.I.; Copeland, L.J.; Fowler, J.M.; Salani, R.; Backes, F.J.; Cohn, D.E. Time to chemotherapy in ovarian cancer: Compliance with ovarian cancer quality indicators at a National Cancer Institute-designated Comprehensive Cancer Center. Gynecol. Oncol. 2018, 151, 501–505. [Google Scholar] [CrossRef]
- Johnston, E.E.; Martinez, I.; Wolfe, J.; Asch, S.M. Quality measures for end-of-life care for children with cancer: A modified Delphi approach. Cancer 2021, 127, 2571–2578. [Google Scholar] [CrossRef]
- De Meyrick, J. The Delphi method and health research. Health Educ. 2003, 103, 7–16. [Google Scholar] [CrossRef]
- Niederberger, M.; Spranger, J. Delphi Technique in Health Sciences: A Map. Front. Public Health 2020, 8, 457. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Mahood, Q.; Van Eerd, D.; Irvin, E. Searching for grey literature for systematic reviews: Challenges and benefits. Res. Synth. Methods 2014, 5, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Uphoff, E.P.; Wennekes, L.; Punt, C.J.; Grol, R.P.; Wollersheim, H.C.; Hermens, R.P.; Ottevanger, P.B. Development of generic quality indicators for patient-centered cancer care by using a RAND modified Delphi method. Cancer Nurs. 2012, 35, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Donabedian, A. Evaluating the Quality of Medical Care. Milbank Q. 2005, 83, 691–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine. IOM Definition of Quality. Available online: http://www.iom.edu/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx (accessed on 12 December 2020).
| No. | Domains | Participants | Sub Domains | Indicators | Measurement/Total Population | Results |
|---|---|---|---|---|---|---|
| 1 | Primary prevention | Patient | Obesity | Obesity rate |
| Importance |
| 2 | Physical activity | Physical activity/Exercise rate |
| Importance | ||
| 3 | Patient/Administrator | Alcohol consumption | High risk of Alcohol consumption rate |
| Importance | |
| 4 | Risk of Chronic disease | Current status of Cancer-related chronic diseases |
| Importance | ||
| 5 | Smoking | Average daily smoking amount per person |
| Importance | ||
| 6 | Secondary prevention | Patient/Administrator | Smoking | Smoking rate |
| Importance |
| 7 | Administrator | Infection | Helicobacter infection rate |
| Selective | |
| 8 | Patient | Incidence | Probability of Developing Cancer (Cumulative incidence rate) |
| Importance | |
| 9 | Administrator | Incidence | Cancer incidence rate (Crude Rate) |
| Importance | |
| 10 | Screening | Cancer Incidence (Age-Standardized Rate) |
| Importance | ||
| 11 | Screening | Cancer Screening Rate |
| Selective | ||
| 12 | Screening | Positive Predictive Value of Eligible Persons in National Cancer Screening Examination |
| Selective | ||
| 13 | Secondary prevention | Administrator | Health professional | Providers’ Excellence in National Cancer Screening Program |
| Selective |
| 14 | Health professional | Regional relevance index of national cancer screening candidates |
| Selective | ||
| 15 | Appropriate procedures | Distribution of invasive extent (stage) at diagnosis |
| Selective | ||
| 16 | Patient/Administrator | Screening | Colonoscopy screening rate of national cancer screening |
| Selective | |
| 17 | Patient/Administrator | Screening | Cancer detection rate among national cancer screening candidates |
| Selective | |
| 18 | Patient/Administrator | Prevalence | Cancer prevalence rate (Crude rate) |
| Selective | |
| 19 | Treatment | Health care provider | Health system | Mean Sojourn Time between National Cancer Screening and Confirmative Diagnosis |
| Selective |
| 20 | Health care provider | Appropriate treatment | Immuno-histopathologic Test for Breast Cancer Patients |
| Importance | |
| 21 | Quality of care | Administrator | Survival/Mortality | Cancer mortality rate |
| Importance |
| 22 | Administrator | Survival/Mortality | 5-year relative survival rate |
| Importance | |
| 23 | Quality of care | Administrator | Survival/Mortality | 5-year absolute survival rate |
| Selective |
| 24 | Survival/Mortality | Disability-adjusted Life Year (DALY) |
| Selective | ||
| 25 | Survivor management | Administrator | Health screening | National Health Screening Rate of Cancer Survivors |
| Selective |
| 26 | End-of-life care | Administrator | Health care professional | The availibility of beds at Palliative Care Institutions |
| Selective |
| Measurement | Subjects/Participants | Primary Prevention | Secondary Prevention | Treatment | Quality of Care | Survivor Management | End-of-Life Care | Total N (%) |
|---|---|---|---|---|---|---|---|---|
| A | Patient | 2 | 1 | 3 (11.5) | ||||
| Providers | 2 | 2 (7.7) | ||||||
| Administrator | 1 | 7 | 4 | 1 | 1 | 14 (53.8) | ||
| Patient/ administrator | 4 | 3 | 7 (26.9) | |||||
| Total N (%) | 7 (26.9) | 11 (42.3) | 2 (7.7) | 4 (15.4) | 1 (3.8) | 1 (3.8) | 26 (100) | |
| B | Safety | 2 | 2 (5) | |||||
| Effective | 1 | 4 | 1 | 6 (15) | ||||
| Patient-centered | 2 | 2 | 1 | 5 (12.5) | ||||
| Timely | 3 | 1 | 4 (10) | |||||
| Efficiency | 4 | 1 | 1 | 6 (15) | ||||
| Equity | 7 | 7 | 1 | 1 | 1 | 17 (42.5) | ||
| Total N (%) | 7 (17.5) | 19 (47.5) | 4 (10) | 6 (15) | 1 (2.5) | 3 (7.5) | 40 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaguru, V.; Jang, J.; Kim, J.; Kwon, J.; Cho, O.; Chung, S.; Ahn, M.; Park, J.; Won, Y.; Jung, K.; et al. Development of Population-Based Cancer Indicators and a Measurement of Cancer Care Continuum Using a Modified Delphi Method. Cancers 2021, 13, 4826. https://doi.org/10.3390/cancers13194826
Rajaguru V, Jang J, Kim J, Kwon J, Cho O, Chung S, Ahn M, Park J, Won Y, Jung K, et al. Development of Population-Based Cancer Indicators and a Measurement of Cancer Care Continuum Using a Modified Delphi Method. Cancers. 2021; 13(19):4826. https://doi.org/10.3390/cancers13194826
Chicago/Turabian StyleRajaguru, Vasuki, Jieun Jang, JaeHyun Kim, JeoungA Kwon, Oyeon Cho, SeungYeun Chung, MiSun Ahn, JinHee Park, YoungJoo Won, KyuWon Jung, and et al. 2021. "Development of Population-Based Cancer Indicators and a Measurement of Cancer Care Continuum Using a Modified Delphi Method" Cancers 13, no. 19: 4826. https://doi.org/10.3390/cancers13194826
APA StyleRajaguru, V., Jang, J., Kim, J., Kwon, J., Cho, O., Chung, S., Ahn, M., Park, J., Won, Y., Jung, K., Shin, J., & Chun, M. (2021). Development of Population-Based Cancer Indicators and a Measurement of Cancer Care Continuum Using a Modified Delphi Method. Cancers, 13(19), 4826. https://doi.org/10.3390/cancers13194826






