The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Translocation of exHSP70
2.1. Membrane HSP70 (mHSP70)
2.2. EV-Associated HSP70 (evHSP70)
2.3. Soluble Extracellular HSP70 (sHSP70)
3. Role of exHSP70: Regulation of Immune Responses
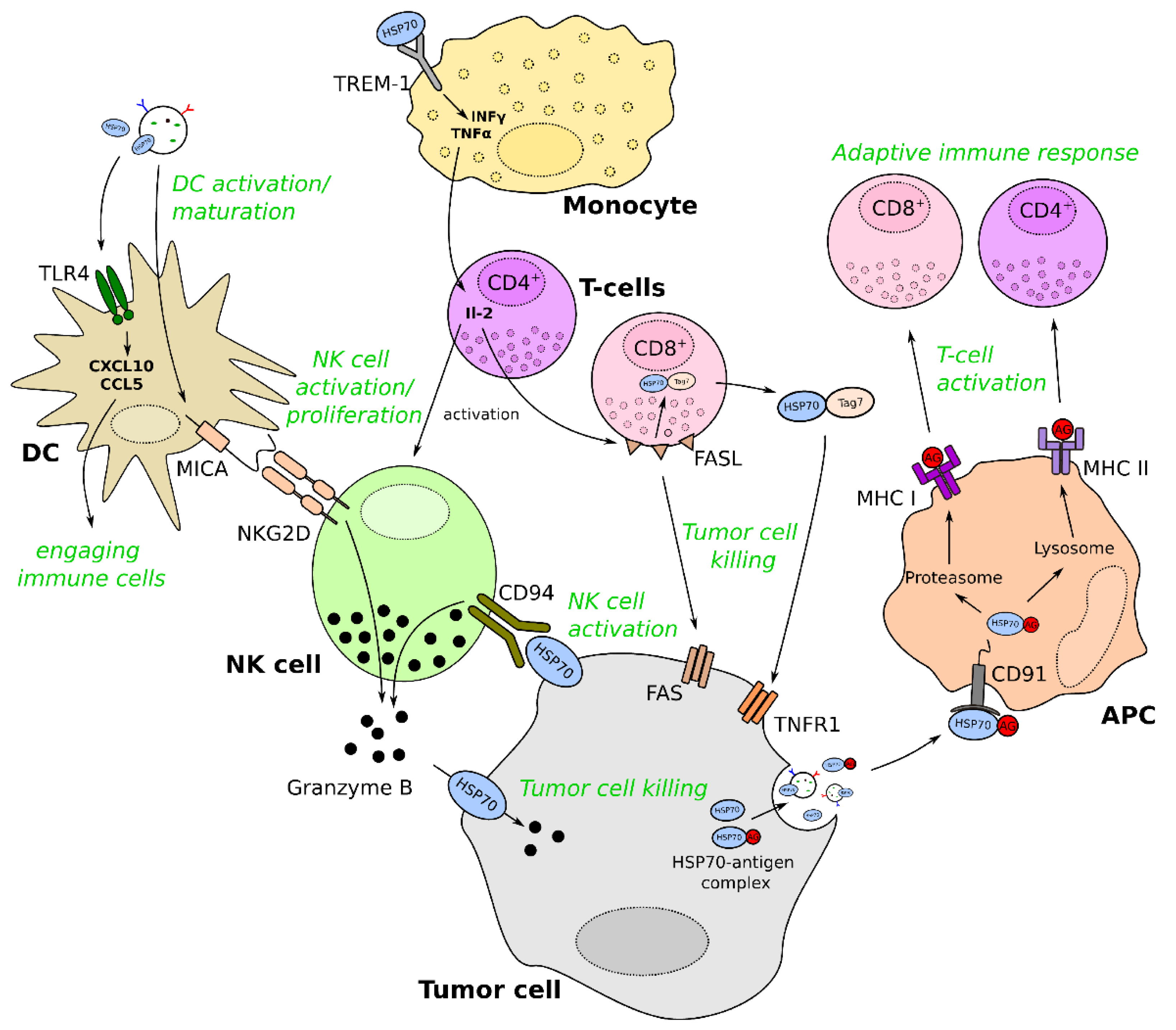
3.1. General Mechanisms of Immunomodulation by exHSP70
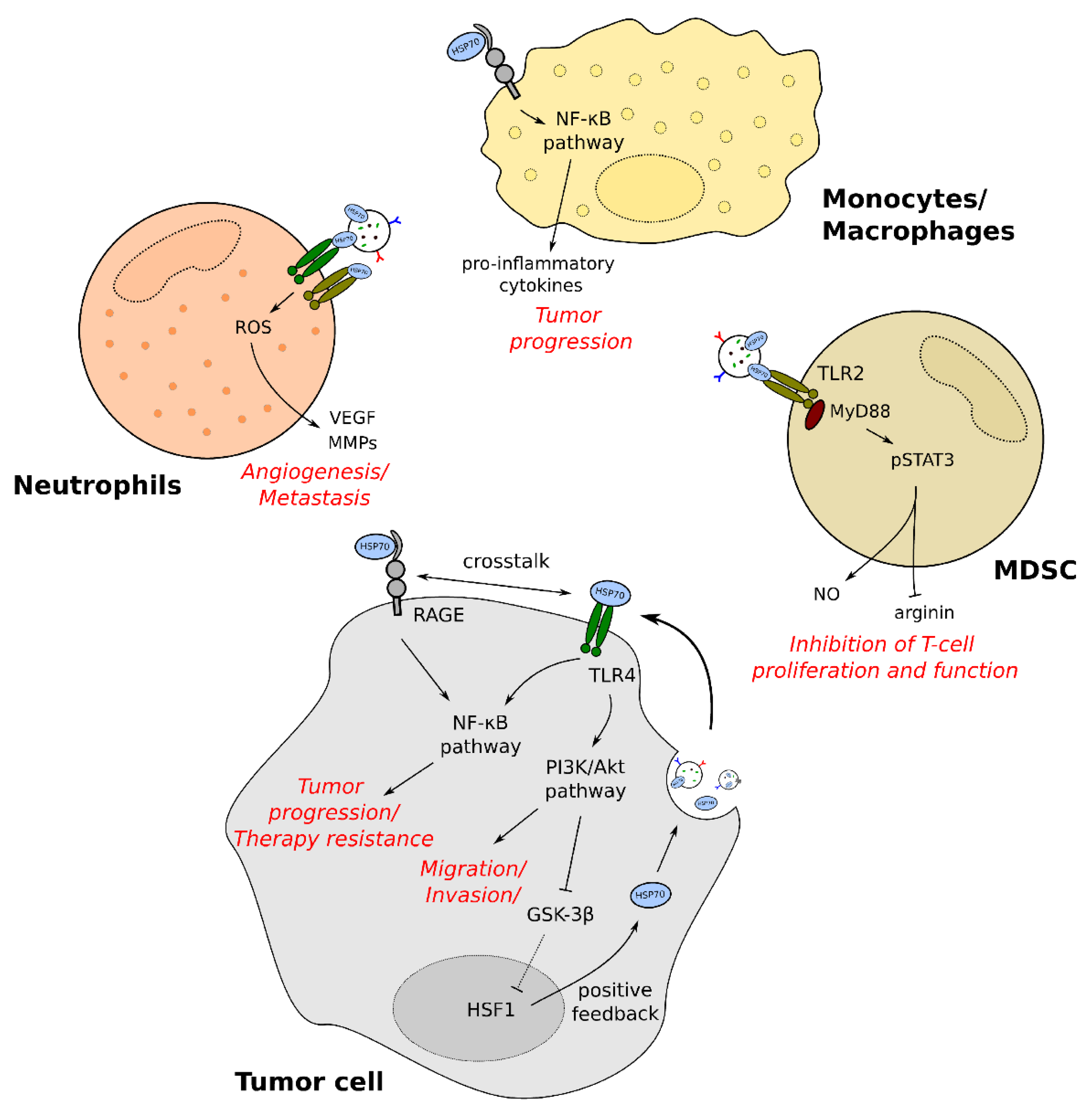
3.2. The Role of exHSP70 in Immunomodulation of Cancer
4. Therapeutic Potential of exHSP70
4.1. HSP70 Antibody
4.2. HSP70 Peptides
4.3. HSP70 Vaccines
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 Chaperone Network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Lv, Q.; Li, X. Exosomes: From Garbage Bins to Translational Medicine. Int. J. Pharm. 2020, 583, 119333. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. Exosome-Dependent Trafficking of HSP70: A Novel Secretory Pathway for Cellular Stress Proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef] [PubMed]
- Vega, V.L.; Rodríguez-Silva, M.; Frey, T.; Gehrmann, M.; Diaz, J.C.; Steinem, C.; Multhoff, G.; Arispe, N.; Maio, A.D. Hsp70 Translocates into the Plasma Membrane after Stress and Is Released into the Extracellular Environment in a Membrane-Associated Form That Activates Macrophages. J. Immunol. 2008, 180, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Müller, E.; Meier, T.; Wilmanns, W.; Issels, R.D. A Stress-Inducible 72-KDa Heat-Shock Protein (HSP72) Is Expressed on the Surface of Human Tumor Cells, but Not on Normal Cells. Int. J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef]
- Pockley, A.G.; Shepherd, J.; Corton, J.M. Detection of Heat Shock Protein 70 (Hsp70) and Anti-Hsp70 Antibodies in the Serum of Normal Individuals. Immunol. Investig. 1998, 27, 367–377. [Google Scholar] [CrossRef]
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A Comprehensive Review of Signal Peptides: Structure, Roles, and Applications. Eur. J. Cell Biol. 2018, 97, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Mambula, S.S.; Stevenson, M.A.; Ogawa, K.; Calderwood, S.K. Mechanisms for Hsp70 Secretion: Crossing Membranes without a Leader. Methods 2007, 43, 168–175. [Google Scholar] [CrossRef]
- Broquet, A.H.; Thomas, G.; Masliah, J.; Trugnan, G.; Bachelet, M. Expression of the Molecular Chaperone Hsp70 in Detergent-Resistant Microdomains Correlates with Its Membrane Delivery and Release. J. Biol. Chem. 2003, 278, 21601–21606. [Google Scholar] [CrossRef]
- Nylander, S.; Kalies, I. Brefeldin A, but Not Monensin, Completely Blocks CD69 Expression on Mouse Lymphocytes:: Efficacy of Inhibitors of Protein Secretion in Protocols for Intracellular Cytokine Staining by Flow Cytometry. J. Immunol. Methods 1999, 224, 69–76. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Hunter-Lavin, C.; Davies, E.L.; Bacelar, M.M.F.V.G.; Marshall, M.J.; Andrew, S.M.; Williams, J.H.H. Hsp70 Release from Peripheral Blood Mononuclear Cells. Biochem. Biophys. Res. Commun. 2004, 324, 511–517. [Google Scholar] [CrossRef]
- Gehrmann, M.; Liebisch, G.; Schmitz, G.; Anderson, R.; Steinem, C.; De Maio, A.; Pockley, G.; Multhoff, G. Tumor-Specific Hsp70 Plasma Membrane Localization Is Enabled by the Glycosphingolipid Gb3. PLoS ONE 2008, 3, e1925. [Google Scholar] [CrossRef]
- Smulders, L.; Daniels, A.J.; Plescia, C.B.; Berger, D.; Stahelin, R.V.; Nikolaidis, N. Characterization of the Relationship between the Chaperone and Lipid-Binding Functions of the 70-KDa Heat-Shock Protein, HspA1A. Int. J. Mol. Sci. 2020, 21, 5995. [Google Scholar] [CrossRef] [PubMed]
- McCallister, C.; Kdeiss, B.; Nikolaidis, N. Biochemical Characterization of the Interaction between HspA1A and Phospholipids. Cell Stress Chaperones 2016, 21, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Schilling, D.; Gehrmann, M.; Steinem, C.; De Maio, A.; Pockley, A.G.; Abend, M.; Molls, M.; Multhoff, G. Binding of Heat Shock Protein 70 to Extracellular Phosphatidylserine Promotes Killing of Normoxic and Hypoxic Tumor Cells. FASEB J. 2009, 23, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Alder, G.M.; Austen, B.M.; Bashford, C.L.; Mehlert, A.; Pasternak, C.A. Heat Shock Proteins Induce Pores in Membranes. Biosci. Rep. 1990, 10, 509–518. [Google Scholar] [CrossRef]
- Bilog, A.D.; Smulders, L.; Oliverio, R.; Labanieh, C.; Zapanta, J.; Stahelin, R.V.; Nikolaidis, N. Membrane Localization of HspA1A, a Stress Inducible 70-KDa Heat-Shock Protein, Depends on Its Interaction with Intracellular Phosphatidylserine. Biomolecules 2019, 9, 152. [Google Scholar] [CrossRef]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat Shock Protein 70 Surface-Positive Tumor Exosomes Stimulate Migratory and Cytolytic Activity of Natural Killer Cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef]
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of Heat Shock Proteins in B-Cell Exosomes. J. Cell Sci. 2005, 118, 3631–3638. [Google Scholar] [CrossRef]
- Takeuchi, T.; Suzuki, M.; Fujikake, N.; Popiel, H.A.; Kikuchi, H.; Futaki, S.; Wada, K.; Nagai, Y. Intercellular Chaperone Transmission via Exosomes Contributes to Maintenance of Protein Homeostasis at the Organismal Level. Proc. Natl. Acad. Sci. USA 2015, 112, E2497–E2506. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.L.; Jackson, L.; Schorey, J.S. Ubiquitination as a Mechanism To Transport Soluble Mycobacterial and Eukaryotic Proteins to Exosomes. J. Immunol. 2015, 195, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalo, O.; Villarroya-Beltri, C.; Sánchez-Madrid, F. Post-Translational Modifications of Exosomal Proteins. Front. Immunol. 2014, 5, 383. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, D.J.; Odorizzi, G.; Emr, S.D. Receptor Downregulation and Multivesicular-Body Sorting. Nat. Rev. Mol. Cell Biol. 2002, 3, 893–905. [Google Scholar] [CrossRef]
- Nikita; Porter, C.M.; Truman, A.W.; Truttmann, M.C. Post-translational modifications of Hsp70 family proteins: Expanding the chaperone code. J. Biol. Chem. 2020, 295, 10689–10708. [Google Scholar] [CrossRef]
- Soss, S.E.; Rose, K.L.; Hill, S.; Jouan, S.; Chazin, W.J. Biochemical and Proteomic Analysis of Ubiquitination of Hsc70 and Hsp70 by the E3 Ligase CHIP. PLoS ONE 2015, 10, e0128240. [Google Scholar] [CrossRef]
- Quintana-Gallardo, L.; Martín-Benito, J.; Marcilla, M.; Espadas, G.; Sabidó, E.; Valpuesta, J.M. The Cochaperone CHIP Marks Hsp70- and Hsp90-Bound Substrates for Degradation through a Very Flexible Mechanism. Sci. Rep. 2019, 9, 5102. [Google Scholar] [CrossRef]
- Jiang, J.; Ballinger, C.A.; Wu, Y.; Dai, Q.; Cyr, D.M.; Höhfeld, J.; Patterson, C. CHIP Is a U-Box-Dependent E3 Ubiquitin Ligase: Identification of Hsc70 as a Target for Ubiquitylation. J. Biol. Chem. 2001, 276, 42938–42944. [Google Scholar] [CrossRef]
- Evdonin, A.L.; Guzhova, I.V.; Margulis, B.A.; Medvedeva, N.D. Phospholipse c Inhibitor, U73122, Stimulates Release of Hsp-70 Stress Protein from A431 Human Carcinoma Cells. Cancer Cell Int. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Zhuang, M.; Zhang, L.; Zheng, X.; Yang, P.; Li, Z. Acetylation Modification Regulates GRP78 Secretion in Colon Cancer Cells. Sci. Rep. 2016, 6, 30406. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fiskus, W.; Yong, B.; Atadja, P.; Takahashi, Y.; Pandita, T.K.; Wang, H.-G.; Bhalla, K.N. Acetylated Hsp70 and KAP1-Mediated Vps34 SUMOylation Is Required for Autophagosome Creation in Autophagy. Proc. Natl. Acad. Sci. USA 2013, 110, 6841–6846. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, N.; Gan, X.; Yan, W.; Morrell, J.C.; Gould, S.J. Higher-Order Oligomerization Targets Plasma Membrane Proteins and HIV Gag to Exosomes. PLoS Biol. 2007, 5, e158. [Google Scholar] [CrossRef] [PubMed]
- Nimmervoll, B.; Chtcheglova, L.A.; Juhasz, K.; Cremades, N.; Aprile, F.A.; Sonnleitner, A.; Hinterdorfer, P.; Vigh, L.; Preiner, J.; Balogi, Z. Cell Surface Localised Hsp70 Is a Cancer Specific Regulator of Clathrin-Independent Endocytosis. FEBS Lett. 2015, 589, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Takakuwa, J.E.; Nitika; Knighton, L.E.; Truman, A.W. Oligomerization of Hsp70: Current Perspectives on Regulation and Function. Front. Mol. Biosci. 2019, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but Not Apoptotic Cell Death Releases Heat Shock Proteins, Which Deliver a Partial Maturation Signal to Dendritic Cells and Activate the NF-ΚB Pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef]
- Mambula, S.S.; Calderwood, S.K. Heat Shock Protein 70 Is Secreted from Tumor Cells by a Nonclassical Pathway Involving Lysosomal Endosomes. J. Immunol. 2006, 177, 7849–7857. [Google Scholar] [CrossRef]
- Evdonin, A.L.; Martynova, M.G.; Bystrova, O.A.; Guzhova, I.V.; Margulis, B.A.; Medvedeva, N.D. The Release of Hsp70 from A431 Carcinoma Cells Is Mediated by Secretory-like Granules. Eur. J. Cell Biol. 2006, 85, 443–455. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Joly, A.-L.; Wettstein, G.; Mignot, G.; Ghiringhelli, F.; Garrido, C. Dual Role of Heat Shock Proteins as Regulators of Apoptosis and Innate Immunity. J. Innate Immun. 2010, 2, 238–247. [Google Scholar] [CrossRef]
- Gross, C.; Koelch, W.; DeMaio, A.; Arispe, N.; Multhoff, G. Cell Surface-Bound Heat Shock Protein 70 (Hsp70) Mediates Perforin-Independent Apoptosis by Specific Binding and Uptake of Granzyme B. J. Biol. Chem. 2003, 278, 41173–41181. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P. Roles of Heat-Shock Proteins in Innate and Adaptive Immunity. Nat. Rev. Immunol. 2002, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Wittmann, M.; Wang, D.; Dressel, R.; Seltsam, A.; Blasczyk, R.; Eiz-Vesper, B. Heat Shock Protein 70 (HSP70) Induces Cytotoxicity of T-Helper Cells. Blood 2009, 113, 3008–3016. [Google Scholar] [CrossRef]
- Asea, A.; Kraeft, S.-K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 Stimulates Cytokine Production through a CD14-Dependant Pathway, Demonstrating Its Dual Role as a Chaperone and Cytokine. Nat. Med. 2000, 6, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Asea, A. Stress Proteins and Initiation of Immune Response: Chaperokine Activity of Hsp72. Exerc. Immunol. Rev. 2005, 11, 34. [Google Scholar] [PubMed]
- Hulina, A.; Grdić Rajković, M.; Jakšić Despot, D.; Jelić, D.; Dojder, A.; Čepelak, I.; Rumora, L. Extracellular Hsp70 Induces Inflammation and Modulates LPS/LTA-Stimulated Inflammatory Response in THP-1 Cells. Cell Stress Chaperones 2018, 23, 373–384. [Google Scholar] [CrossRef]
- Campisi, J.; Fleshner, M. Role of Extracellular HSP72 in Acute Stress-Induced Potentiation of Innate Immunity in Active Rats. J. Appl. Physiol. (1985) 2003, 94, 43–52. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel Signal Transduction Pathway Utilized by Extracellular HSP70: Role of Toll-like Receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef]
- Becker, T.; Hartl, F.-U.; Wieland, F. CD40, an Extracellular Receptor for Binding and Uptake of Hsp70-Peptide Complexes. J. Cell Biol. 2002, 158, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tsan, M.-F. Endotoxin Contamination in Recombinant Human Heat Shock Protein 70 (Hsp70) Preparation Is Responsible for the Induction of Tumor Necrosis Factor Alpha Release by Murine Macrophages. J. Biol. Chem. 2003, 278, 174–179. [Google Scholar] [CrossRef]
- Bausinger, H.; Lipsker, D.; Ziylan, U.; Manié, S.; Briand, J.-P.; Cazenave, J.-P.; Muller, S.; Haeuw, J.-F.; Ravanat, C.; de la Salle, H.; et al. Endotoxin-Free Heat-Shock Protein 70 Fails to Induce APC Activation. Eur. J. Immunol. 2002, 32, 3708–3713. [Google Scholar] [CrossRef]
- Stocki, P.; Wang, X.N.; Dickinson, A.M. Inducible Heat Shock Protein 70 Reduces T Cell Responses and Stimulatory Capacity of Monocyte-Derived Dendritic Cells. J. Biol. Chem. 2012, 287, 12387–12394. [Google Scholar] [CrossRef]
- Stocki, P.; Dickinson, A.M. The Immunosuppressive Activity of Heat Shock Protein 70. Autoimmune Dis. 2012, 2012, 617213. [Google Scholar] [CrossRef] [PubMed]
- Tsan, M.-F.; Gao, B. Pathogen-Associated Molecular Pattern Contamination as Putative Endogenous Ligands of Toll-like Receptors. J. Endotoxin Res. 2007, 13, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Gan, Y.-H. Flagellin Contamination of Recombinant Heat Shock Protein 70 Is Responsible for Its Activity on T Cells. J. Biol. Chem. 2007, 282, 4479–4484. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.J.; Sreedhara, K.; Deng, L.; Varki, N.M.; Angata, T.; Liu, Q.; Nizet, V.; Varki, A. Immunomodulatory Activity of Extracellular Hsp70 Mediated via Paired Receptors Siglec-5 and Siglec-14. EMBO J. 2015, 34, 2775–2788. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.S.; Ligabue-Braun, R.; Souza, C.S.; Heimfarth, L.; Verli, H.; Gelain, D.P.; Moreira, J.C.F. Putative Model for Heat Shock Protein 70 Complexation with Receptor of Advanced Glycation End Products through Fluorescence Proximity Assays and Normal Mode Analyses. Cell Stress Chaperones 2017, 22, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Somensi, N.; Brum, P.O.; de Miranda Ramos, V.; Gasparotto, J.; Zanotto-Filho, A.; Rostirolla, D.C.; da Silva Morrone, M.; Moreira, J.C.F.; Pens Gelain, D. Extracellular HSP70 Activates ERK1/2, NF-KB and Pro-Inflammatory Gene Transcription Through Binding with RAGE in A549 Human Lung Cancer Cells. Cell Physiol. Biochem. 2017, 42, 2507–2522. [Google Scholar] [CrossRef]
- Broere, F.; van der Zee, R.; van Eden, W. Heat Shock Proteins Are No DAMPs, Rather “DAMPERs”. Nat. Rev. Immunol. 2011, 11, 565. [Google Scholar] [CrossRef]
- Ferat-Osorio, E.; Sánchez-Anaya, A.; Gutiérrez-Mendoza, M.; Boscó-Gárate, I.; Wong-Baeza, I.; Pastelin-Palacios, R.; Pedraza-Alva, G.; Bonifaz, L.C.; Cortés-Reynosa, P.; Pérez-Salazar, E.; et al. Heat Shock Protein 70 Down-Regulates the Production of Toll-like Receptor-Induced pro-Inflammatory Cytokines by a Heat Shock Factor-1/Constitutive Heat Shock Element-Binding Factor-Dependent Mechanism. J. Inflamm. 2014, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Pinsky, M.R.; Kellum, J.A. Heat Shock Factor 1 Inhibits Nuclear Factor-KappaB Nuclear Binding Activity during Endotoxin Tolerance and Heat Shock. J. Crit. Care 2008, 23, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Lobb, R.; Wharton, W.; Ma, T.Y.; Moseley, P.L. LPS-Induced Cytokine Levels Are Repressed by Elevated Expression of HSP70 in Rats: Possible Role of NF-KappaB. Cell Stress Chaperones 2010, 15, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Paladino, L.; Vitale, A.M.; Caruso Bavisotto, C.; Conway de Macario, E.; Campanella, C.; Macario, A.J.L.; Marino Gammazza, A. Functions and Therapeutic Potential of Extracellular Hsp60, Hsp70, and Hsp90 in Neuroinflammatory Disorders. Appl. Sci. 2021, 11, 736. [Google Scholar] [CrossRef]
- Noessner, E.; Gastpar, R.; Milani, V.; Brandl, A.; Hutzler, P.J.S.; Kuppner, M.C.; Roos, M.; Kremmer, E.; Asea, A.; Calderwood, S.K.; et al. Tumor-Derived Heat Shock Protein 70 Peptide Complexes Are Cross-Presented by Human Dendritic Cells. J. Immunol. 2002, 169, 5424–5432. [Google Scholar] [CrossRef] [PubMed]
- Thériault, J.R.; Mambula, S.S.; Sawamura, T.; Stevenson, M.A.; Calderwood, S.K. Extracellular HSP70 Binding to Surface Receptors Present on Antigen Presenting Cells and Endothelial/Epithelial Cells. FEBS Lett. 2005, 579, 1951–1960. [Google Scholar] [CrossRef]
- Li, Z.; Menoret, A.; Srivastava, P. Roles of Heat-Shock Proteins in Antigen Presentation and Cross-Presentation. Curr. Opin. Immunol. 2002, 14, 45–51. [Google Scholar] [CrossRef]
- Bendz, H.; Ruhland, S.C.; Pandya, M.J.; Hainzl, O.; Riegelsberger, S.; Braüchle, C.; Mayer, M.P.; Buchner, J.; Issels, R.D.; Noessner, E. Human Heat Shock Protein 70 Enhances Tumor Antigen Presentation through Complex Formation and Intracellular Antigen Delivery without Innate Immune Signaling. J. Biol. Chem. 2007, 282, 31688–31702. [Google Scholar] [CrossRef]
- Haug, M.; Dannecker, L.; Schepp, C.P.; Kwok, W.W.; Wernet, D.; Buckner, J.H.; Kalbacher, H.; Dannecker, G.E.; Holzer, U. The Heat Shock Protein Hsp70 Enhances Antigen-Specific Proliferation of Human CD4+ Memory T Cells. Eur. J. Immunol. 2005, 35, 3163–3172. [Google Scholar] [CrossRef]
- Fischer, N.; Haug, M.; Kwok, W.W.; Kalbacher, H.; Wernet, D.; Dannecker, G.E.; Holzer, U. Involvement of CD91 and Scavenger Receptors in Hsp70-Facilitated Activation of Human Antigen-Specific CD4+ Memory T Cells. Eur. J. Immunol. 2010, 40, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Seidl, T.; Whittall, T.; Babaahmady, K.; Lehner, T. Stress-Activated Dendritic Cells Interact with CD4+ T Cells to Elicit Homeostatic Memory. Eur. J. Immunol. 2010, 40, 1628–1638. [Google Scholar] [CrossRef]
- Wang, Y.; Rahman, D.; Lehner, T. A Comparative Study of Stress-Mediated Immunological Functions with the Adjuvanticity of Alum. J. Biol. Chem. 2012, 287, 17152–17160. [Google Scholar] [CrossRef] [PubMed]
- Kane, B.A.; Bryant, K.J.; McNeil, H.P.; Tedla, N.T. Termination of Immune Activation: An Essential Component of Healthy Host Immune Responses. J. Innate Immun. 2014, 6, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, M.I.; Hashiguchi, N.; Chen, Y.; Yip, L.; Junger, W.G. Surface Expression of HSP72 by LPS-Stimulated Neutrophils Facilitates GammadeltaT Cell-Mediated Killing. Eur. J. Immunol. 2006, 36, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, H.; Jiang, X.; He, H.; Cui, L.; He, W. Membrane HSP70: The Molecule Triggering Gammadelta T Cells in the Early Stage of Tumorigenesis. Immunol. Investig. 2005, 34, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Patil, R.S.; Chiplunkar, S.V. Insights into the Relationship between Toll Like Receptors and Gamma Delta T Cell Responses. Front. Immunol. 2014, 5, 366. [Google Scholar] [CrossRef]
- Kang, H.-J.; Moon, H.-S.; Chung, H.-W. The Expression of FAS-Associated Factor 1 and Heat Shock Protein 70 in Ovarian Cancer. Obstet. Gynecol. Sci. 2014, 57, 281–290. [Google Scholar] [CrossRef]
- Gao, G.; Liu, S.; Yao, Z.; Zhan, Y.; Chen, W.; Liu, Y. The Prognostic Significance of Hsp70 in Patients with Colorectal Cancer Patients: A PRISMA-Compliant Meta-Analysis. Biomed. Res. Int. 2021, 2021, 5526327. [Google Scholar] [CrossRef]
- Thorsteinsdottir, J.; Stangl, S.; Fu, P.; Guo, K.; Albrecht, V.; Eigenbrod, S.; Erl, J.; Gehrmann, M.; Tonn, J.-C.; Multhoff, G.; et al. Overexpression of Cytosolic, Plasma Membrane Bound and Extracellular Heat Shock Protein 70 (Hsp70) in Primary Glioblastomas. J. Neurooncol. 2017, 135, 443–452. [Google Scholar] [CrossRef]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring HSP70 Exosomes in Cancer Patients’ Follow up: A Clinical Prospective Pilot Study. J. Extracell Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef]
- Nowak, M.; Glowacka, E.; Kielbik, M.; Kulig, A.; Sulowska, Z.; Klink, M. Secretion of Cytokines and Heat Shock Protein (HspA1A) by Ovarian Cancer Cells Depending on the Tumor Type and Stage of Disease. Cytokine 2017, 89, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, C.M.; Kleinberg, L.; Davidson, B.; Berner, A.; Gius, D.; Tchabo, N.; Steinberg, S.M.; Kohn, E.C. BAG-4/SODD and Associated Antiapoptotic Proteins Are Linked to Aggressiveness of Epithelial Ovarian Cancer. Clin. Cancer Res. 2007, 13, 6585–6592. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Hua, T.; Li, Y.; Tian, Y.-J.; Huo, Y.; Kang, S. The HSP70 Gene Predicts Prognosis and Response to Chemotherapy in Epithelial Ovarian Cancer. Ann. Transl. Med. 2021, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Finkernagel, F.; Reinartz, S.; Schuldner, M.; Malz, A.; Jansen, J.M.; Wagner, U.; Worzfeld, T.; Graumann, J.; von Strandmann, E.P.; Müller, R. Dual-Platform Affinity Proteomics Identifies Links between the Recurrence of Ovarian Carcinoma and Proteins Released into the Tumor Microenvironment. Theranostics 2019, 9, 6601–6617. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Bu, N.; Yu, Y.-C.; Hua, W.; Xin, X.-Y. Exvivo Experiments of Human Ovarian Cancer Ascites-Derived Exosomes Presented by Dendritic Cells Derived from Umbilical Cord Blood for Immunotherapy Treatment. Clin. Med. Oncol. 2008, 2, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Klink, M.; Nowak, M.; Kielbik, M.; Bednarska, K.; Blus, E.; Szpakowski, M.; Szyllo, K.; Sulowska, Z. The Interaction of HspA1A with TLR2 and TLR4 in the Response of Neutrophils Induced by Ovarian Cancer Cells in Vitro. Cell Stress Chaperones 2012, 17, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Adkins, I.; Sadilkova, L.; Hradilova, N.; Tomala, J.; Kovar, M.; Spisek, R. Severe, but Not Mild Heat-Shock Treatment Induces Immunogenic Cell Death in Cancer Cells. Oncoimmunology 2017, 6, e1311433. [Google Scholar] [CrossRef] [PubMed]
- Park, G.B.; Chung, Y.H.; Kim, D. Induction of Galectin-1 by TLR-Dependent PI3K Activation Enhances Epithelial-Mesenchymal Transition of Metastatic Ovarian Cancer Cells. Oncol. Rep. 2017, 37, 3137–3145. [Google Scholar] [CrossRef]
- Barreca, M.M.; Spinello, W.; Cavalieri, V.; Turturici, G.; Sconzo, G.; Kaur, P.; Tinnirello, R.; Asea, A.A.A.; Geraci, F. Extracellular Hsp70 Enhances Mesoangioblast Migration via an Autocrine Signaling Pathway. J. Cell Physiol. 2017, 232, 1845–1861. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Kim, Y.M.; Kim, D.Y.; Jeoung, D.; Han, K.; Lee, S.-T.; Lee, Y.-S.; Park, K.H.; Park, J.H.; Kim, D.J.; et al. Release of Heat Shock Protein 70 (Hsp70) and the Effects of Extracellular Hsp70 on Matric Metalloproteinase-9 Expression in Human Monocytic U937 Cells. Exp. Mol. Med. 2006, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Jeong, J.; Yoo, C.-G. Positive Feedback Regulation of Heat Shock Protein 70 (Hsp70) Is Mediated through Toll-like Receptor 4-PI3K/Akt-Glycogen Synthase Kinase-3β Pathway. Exp. Cell Res. 2013, 319, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, J.; Marcion, G.; Cordonnier, M.; Dias, A.M.M.; Pernet, N.; Hammann, A.; Richaud, S.; Mjahed, H.; Isambert, N.; Clausse, V.; et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes With a HSP70 Peptide Aptamer. J. Natl. Cancer Inst. 2016, 108, djv330. [Google Scholar] [CrossRef]
- Diao, J.; Yang, X.; Song, X.; Chen, S.; He, Y.; Wang, Q.; Chen, G.; Luo, C.; Wu, X.; Zhang, Y. Exosomal Hsp70 Mediates Immunosuppressive Activity of the Myeloid-Derived Suppressor Cells via Phosphorylation of Stat3. Med. Oncol. 2015, 32, 35. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.-P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-Associated Hsp72 from Tumor-Derived Exosomes Mediates STAT3-Dependent Immunosuppressive Function of Mouse and Human Myeloid-Derived Suppressor Cells. J. Clin. Investig. 2010, 120, 457–471. [Google Scholar] [CrossRef]
- Rébé, C.; Végran, F.; Berger, H.; Ghiringhelli, F. STAT3 Activation: A Key Factor in Tumor Immunoescape. JAKSTAT 2013, 2, e23010. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Bingisser, R.M.; Tilbrook, P.A.; Holt, P.G.; Kees, U.R. Macrophage-Derived Nitric Oxide Regulates T Cell Activation via Reversible Disruption of the Jak3/STAT5 Signaling Pathway. J. Immunol. 1998, 160, 5729–5734. [Google Scholar] [PubMed]
- Wu, F.-H.; Yuan, Y.; Li, D.; Liao, S.-J.; Yan, B.; Wei, J.-J.; Zhou, Y.-H.; Zhu, J.-H.; Zhang, G.-M.; Feng, Z.-H. Extracellular HSPA1A Promotes the Growth of Hepatocarcinoma by Augmenting Tumor Cell Proliferation and Apoptosis-Resistance. Cancer Lett. 2012, 317, 157–164. [Google Scholar] [CrossRef] [PubMed]
- De Larco, J.E.; Wuertz, B.R.K.; Furcht, L.T. The Potential Role of Neutrophils in Promoting the Metastatic Phenotype of Tumors Releasing Interleukin-8. Clin. Cancer Res. 2004, 10, 4895–4900. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Chase, M.A.; Senft, A.P.; Poynter, S.E.; Wong, H.R.; Page, K. Extracellular Hsp72, an Endogenous DAMP, Is Released by Virally Infected Airway Epithelial Cells and Activates Neutrophils via Toll-like Receptor (TLR)-4. Respir. Res. 2009, 10, 31. [Google Scholar] [CrossRef]
- Kelly, M.G.; Alvero, A.B.; Chen, R.; Silasi, D.-A.; Abrahams, V.M.; Chan, S.; Visintin, I.; Rutherford, T.; Mor, G. TLR-4 Signaling Promotes Tumor Growth and Paclitaxel Chemoresistance in Ovarian Cancer. Cancer Res. 2006, 66, 3859–3868. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Alvero, A.B.; Chen, W.; O’Malley, D.; Hao, X.-Y.; Dwipoyono, B.; Garg, M.; Kamsteeg, M.; Rutherford, T.; Mor, G. Resistance of Ovarian Carcinoma Cells to Docetaxel Is XIAP Dependent and Reversible by Phenoxodiol. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2004, 14, 567–578. [Google Scholar] [CrossRef]
- Hu, W.; Xu, Z.; Zhu, S.; Sun, W.; Wang, X.; Tan, C.; Zhang, Y.; Zhang, G.; Xu, Y.; Tang, J. Small Extracellular Vesicle-Mediated Hsp70 Intercellular Delivery Enhances Breast Cancer Adriamycin Resistance. Free Radic. Biol. Med. 2021, 164, 85–95. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Liu, D.; Sun, H.; Su, D.; Yang, F.; Liu, J. Extracellular HSP70/HSP70-PCs Promote Epithelial-Mesenchymal Transition of Hepatocarcinoma Cells. PLoS ONE 2013, 8, e84759. [Google Scholar] [CrossRef]
- Zhe, Y.; Li, Y.; Liu, D.; Su, D.-M.; Liu, J.-G.; Li, H.-Y. Extracellular HSP70-Peptide Complexes Promote the Proliferation of Hepatocellular Carcinoma Cells via TLR2/4/JNK1/2MAPK Pathway. Tumour Biol. 2016, 37, 13951–13959. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Karimi, J.; Goodarzi, M.T.; Saidijam, M.; Khodadadi, I.; Razavi, A.N.E.; Nankali, M. Overexpression of Receptor for Advanced Glycation End Products (RAGE) in Ovarian Cancer. Cancer Biomark. 2017, 18, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, T.; Ye, G.; Shen, Z.; Hu, Y.; Mou, T.; Yu, J.; Li, S.; Liu, H.; Li, G. Overexpression of the Receptor for Advanced Glycation Endproducts (RAGE) Is Associated with Poor Prognosis in Gastric Cancer. PLoS ONE 2015, 10, e0122697. [Google Scholar] [CrossRef]
- Prantner, D.; Nallar, S.; Vogel, S.N. The Role of RAGE in Host Pathology and Crosstalk between RAGE and TLR4 in Innate Immune Signal Transduction Pathways. FASEB J. 2020, 34, 15659–15674. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Murata, H.; Yamamoto, K.; Ono, T.; Sakaguchi, Y.; Motoyama, A.; Hibino, T.; Kataoka, K.; Huh, N. TIRAP, an Adaptor Protein for TLR2/4, Transduces a Signal from RAGE Phosphorylated upon Ligand Binding. PLoS ONE 2011, 6, e23132. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Jennen, L.; Schmidt, J.; Ellwart, J.; Issels, R. Heat Shock Protein 72 on Tumor Cells: A Recognition Structure for Natural Killer Cells. J. Immunol. 1997, 158, 4341–4350. [Google Scholar]
- Multhoff, G.; Pfister, K.; Gehrmann, M.; Hantschel, M.; Gross, C.; Hafner, M.; Hiddemann, W. A 14-Mer Hsp70 Peptide Stimulates Natural Killer (NK) Cell Activity. Cell Stress Chaperones 2001, 6, 337–344. [Google Scholar] [CrossRef]
- Specht, H.M.; Ahrens, N.; Blankenstein, C.; Duell, T.; Fietkau, R.; Gaipl, U.S.; Günther, C.; Gunther, S.; Habl, G.; Hautmann, H.; et al. Heat Shock Protein 70 (Hsp70) Peptide Activated Natural Killer (NK) Cells for the Treatment of Patients with Non-Small Cell Lung Cancer (NSCLC) after Radiochemotherapy (RCTx)—From Preclinical Studies to a Clinical Phase II Trial. Front. Immunol. 2015, 6, 162. [Google Scholar] [CrossRef]
- Elsner, L.; Flügge, P.F.; Lozano, J.; Muppala, V.; Eiz-Vesper, B.; Demiroglu, S.Y.; Malzahn, D.; Herrmann, T.; Brunner, E.; Bickeböller, H.; et al. The Endogenous Danger Signals HSP70 and MICA Cooperate in the Activation of Cytotoxic Effector Functions of NK Cells. J. Cell. Mol. Med. 2010, 14, 992–1002. [Google Scholar] [CrossRef]
- Sharapova, T.N.; Romanova, E.A.; Ivanova, O.K.; Yashin, D.V.; Sashchenko, L.P. Hsp70 Interacts with the TREM-1 Receptor Expressed on Monocytes and Thereby Stimulates Generation of Cytotoxic Lymphocytes Active against MHC-Negative Tumor Cells. Int. J. Mol. Sci. 2021, 22, 6889. [Google Scholar] [CrossRef]
- Yashin, D.V.; Ivanova, O.K.; Soshnikova, N.V.; Sheludchenkov, A.A.; Romanova, E.A.; Dukhanina, E.A.; Tonevitsky, A.G.; Gnuchev, N.V.; Gabibov, A.G.; Georgiev, G.P.; et al. Tag7 (PGLYRP1) in Complex with Hsp70 Induces Alternative Cytotoxic Processes in Tumor Cells via TNFR1 Receptor. J. Biol. Chem. 2015, 290, 21724–21731. [Google Scholar] [CrossRef] [PubMed]
- Sashchenko, L.P.; Romanova, E.A.; Ivanova, O.K.; Sharapova, T.N.; Yashin, D.V. FasL and the NKG2D Receptor Are Required for the Secretion of the Tag7/PGRP-S-Hsp70 Complex by the Cytotoxic CD8+ Lymphocytes. IUBMB Life 2017, 69, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, J.; Han, C.; Yang, M.; Cao, X. Heat Shock Protein 70, Released from Heat-Stressed Tumor Cells, Initiates Antitumor Immunity by Inducing Tumor Cell Chemokine Production and Activating Dendritic Cells via TLR4 Pathway. J. Immunol. 2009, 182, 1449–1459. [Google Scholar] [CrossRef]
- Komarova, E.Y.; Marchenko, L.V.; Zhakhov, A.V.; Nikotina, A.D.; Aksenov, N.D.; Suezov, R.V.; Ischenko, A.M.; Margulis, B.A.; Guzhova, I.V. Extracellular Hsp70 Reduces the Pro-Tumor Capacity of Monocytes/Macrophages Co-Cultivated with Cancer Cells. Int. J. Mol. Sci. 2019, 21, 59. [Google Scholar] [CrossRef]
- Kumar, S.; Stokes, J.; Singh, U.P.; Scissum Gunn, K.; Acharya, A.; Manne, U.; Mishra, M. Targeting Hsp70: A Possible Therapy for Cancer. Cancer Lett. 2016, 374, 156–166. [Google Scholar] [CrossRef]
- Jacobson, B.A.; Chen, E.Z.; Tang, S.; Belgum, H.S.; McCauley, J.A.; Evenson, K.A.; Etchison, R.G.; Jay-Dixon, J.; Patel, M.R.; Raza, A.; et al. Triptolide and Its Prodrug Minnelide Suppress Hsp70 and Inhibit in Vivo Growth in a Xenograft Model of Mesothelioma. Genes Cancer 2015, 6, 144–152. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, T.N.; Mujumdar, N.; Banerjee, S.; Sangwan, V.; Sarver, A.; Vickers, S.; Subramanian, S.; Saluja, A.K. Triptolide Induces the Expression of MiR-142-3p: A Negative Regulator of Heat Shock Protein 70 and Pancreatic Cancer Cell Proliferation. Mol. Cancer Ther. 2013, 12, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Kleinjung, T.; Arndt, O.; Feldmann, H.J.; Bockmühl, U.; Gehrmann, M.; Zilch, T.; Pfister, K.; Schönberger, J.; Marienhagen, J.; Eilles, C.; et al. Heat Shock Protein 70 (Hsp70) Membrane Expression on Head-and-Neck Cancer Biopsy-a Target for Natural Killer (NK) Cells. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 820–826. [Google Scholar] [CrossRef]
- Hantschel, M.; Pfister, K.; Jordan, A.; Scholz, R.; Andreesen, R.; Schmitz, G.; Schmetzer, H.; Hiddemann, W.; Multhoff, G. Hsp70 Plasma Membrane Expression on Primary Tumor Biopsy Material and Bone Marrow of Leukemic Patients. Cell Stress Chaperones 2000, 5, 438–442. [Google Scholar] [CrossRef]
- Stangl, S.; Gehrmann, M.; Riegger, J.; Kuhs, K.; Riederer, I.; Sievert, W.; Hube, K.; Mocikat, R.; Dressel, R.; Kremmer, E.; et al. Targeting Membrane Heat-Shock Protein 70 (Hsp70) on Tumors by CmHsp70.1 Antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 733–738. [Google Scholar] [CrossRef]
- Stangl, S.; Gehrmann, M.; Dressel, R.; Alves, F.; Dullin, C.; Themelis, G.; Ntziachristos, V.; Staeblein, E.; Walch, A.; Winkelmann, I.; et al. In Vivo Imaging of CT26 Mouse Tumours by Using CmHsp70.1 Monoclonal Antibody. J. Cell. Mol. Med. 2011, 15, 874–887. [Google Scholar] [CrossRef]
- Dechant, M.; Beyer, T.; Schneider-Merck, T.; Weisner, W.; Peipp, M.; van de Winkel, J.G.J.; Valerius, T. Effector Mechanisms of Recombinant IgA Antibodies against Epidermal Growth Factor Receptor. J. Immunol. 2007, 179, 2936–2943. [Google Scholar] [CrossRef]
- Stockmeyer, B.; Dechant, M.; van Egmond, M.; Tutt, A.L.; Sundarapandiyan, K.; Graziano, R.F.; Repp, R.; Kalden, J.R.; Gramatzki, M.; Glennie, M.J.; et al. Triggering Fc Alpha-Receptor I (CD89) Recruits Neutrophils as Effector Cells for CD20-Directed Antibody Therapy. J. Immunol. 2000, 165, 5954–5961. [Google Scholar] [CrossRef]
- Russell, M.W.; Reinholdt, J.; Kilian, M. Anti-Inflammatory Activity of Human IgA Antibodies and Their Fab Alpha Fragments: Inhibition of IgG-Mediated Complement Activation. Eur. J. Immunol. 1989, 19, 2243–2249. [Google Scholar] [CrossRef]
- Van der Steen, L.; Tuk, C.W.; Bakema, J.E.; Kooij, G.; Reijerkerk, A.; Vidarsson, G.; Bouma, G.; Kraal, G.; de Vries, H.E.; Beelen, R.H.J.; et al. Immunoglobulin A: Fc(Alpha)RI Interactions Induce Neutrophil Migration through Release of Leukotriene B4. Gastroenterology 2009, 137, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.; Meyer, S.; Meulenbroek, L.A.P.M.; Jansen, J.H.M.; Nederend, M.; Kretschmer, A.; Klausz, K.; Möginger, U.; Derer, S.; Rösner, T.; et al. An Anti-EGFR IgA That Displays Improved Pharmacokinetics and Myeloid Effector Cell Engagement In Vivo. Cancer Res. 2016, 76, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.A.; Yakovleva, L.Y.; Nikolaev, B.P.; Marchenko, Y.Y.; Dobrodumov, A.V.; Onokhin, K.V.; Onokhina, Y.S.; Selkov, S.A.; Mikhrina, A.L.; Guzhova, I.V.; et al. Tumor Targeting Using Magnetic Nanoparticle Hsp70 Conjugate in a Model of C6 Glioma. Neuro-Oncology 2014, 16, 38–49. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Ryzhov, V.A.; Yakovleva, L.Y.; Marchenko, Y.Y.; Parr, M.A.; Rolich, V.I.; Mikhrina, A.L.; Dobrodumov, A.V.; Pitkin, E.; et al. Ionizing Radiation Improves Glioma-Specific Targeting of Superparamagnetic Iron Oxide Nanoparticles Conjugated with CmHsp70.1 Monoclonal Antibodies (SPION–CmHsp70.1). Nanoscale 2015, 7, 20652–20664. [Google Scholar] [CrossRef]
- Huang, H.S.; Hainfeld, J.F. Intravenous Magnetic Nanoparticle Cancer Hyperthermia. Int. J. Nanomed. 2013, 8, 2521–2532. [Google Scholar] [CrossRef]
- Shevtsov, M.; Huile, G.; Multhoff, G. Membrane Heat Shock Protein 70: A Theranostic Target for Cancer Therapy. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160526. [Google Scholar] [CrossRef]
- Kimm, M.A.; Shevtsov, M.; Werner, C.; Sievert, W.; Zhiyuan, W.; Schoppe, O.; Menze, B.H.; Rummeny, E.J.; Proksa, R.; Bystrova, O.; et al. Gold Nanoparticle Mediated Multi-Modal CT Imaging of Hsp70 Membrane-Positive Tumors. Cancers 2020, 12, 1331. [Google Scholar] [CrossRef]
- Xie, Y.; Bai, O.; Zhang, H.; Yuan, J.; Zong, S.; Chibbar, R.; Slattery, K.; Qureshi, M.; Wei, Y.; Deng, Y.; et al. Membrane-Bound HSP70-Engineered Myeloma Cell-Derived Exosomes Stimulate More Efficient CD8+ CTL- and NK-Mediated Antitumour Immunity than Exosomes Released from Heat-Shocked Tumour Cells Expressing Cytoplasmic HSP70. J. Cell. Mol. Med. 2010, 14, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel Is Incorporated by Mesenchymal Stromal Cells and Released in Exosomes That Inhibit in Vitro Tumor Growth: A New Approach for Drug Delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.W.; Gastpar, R.; Andreesen, R.; Gross, C.; Ullrich, H.; Thonigs, G.; Pfister, K.; Multhoff, G. Treatment of Colon and Lung Cancer Patients with Ex Vivo Heat Shock Protein 70-Peptide-Activated, Autologous Natural Killer Cells: A Clinical Phase I Trial. Clin. Cancer Res. 2004, 10, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Seier, S.; Stangl, S.; Sievert, W.; Shevtsov, M.; Werner, C.; Pockley, A.G.; Blankenstein, C.; Hildebrandt, M.; Offner, R.; et al. Targeted Natural Killer Cell–Based Adoptive Immunotherapy for the Treatment of Patients with NSCLC after Radiochemotherapy: A Randomized Phase II Clinical Trial. Clin. Cancer Res. 2020, 26, 5368–5379. [Google Scholar] [CrossRef]
- Kokowski, K.; Stangl, S.; Seier, S.; Hildebrandt, M.; Vaupel, P.; Multhoff, G. Radiochemotherapy Combined with NK Cell Transfer Followed by Second-Line PD-1 Inhibition in a Patient with NSCLC Stage IIIb Inducing Long-Term Tumor Control: A Case Study. Strahlenther. Onkol. 2019, 195, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Pitkin, E.; Ischenko, A.; Stangl, S.; Khachatryan, W.; Galibin, O.; Edmond, S.; Lobinger, D.; Multhoff, G. Ex Vivo Hsp70-Activated NK Cells in Combination With PD-1 Inhibition Significantly Increase Overall Survival in Preclinical Models of Glioblastoma and Lung Cancer. Front. Immunol. 2019, 10, 454. [Google Scholar] [CrossRef]
- Lin, C.-N.; Tsai, Y.-C.; Hsu, C.-C.; Liang, Y.-L.; Wu, Y.-Y.; Kang, C.-Y.; Lin, C.-H.; Hsu, P.-H.; Lee, G.-B.; Hsu, K.-F. An Aptamer Interacting with Heat Shock Protein 70 Shows Therapeutic Effects and Prognostic Ability in Serous Ovarian Cancer. Mol. Ther. Nucleic Acids 2021, 23, 757–768. [Google Scholar] [CrossRef]
- Shevtsov, M.; Stangl, S.; Nikolaev, B.; Yakovleva, L.; Marchenko, Y.; Tagaeva, R.; Sievert, W.; Pitkin, E.; Mazur, A.; Tolstoy, P.; et al. Granzyme B Functionalized Nanoparticles Targeting Membrane Hsp70-Positive Tumors for Multimodal Cancer Theranostics. Small 2019, 15, 1900205. [Google Scholar] [CrossRef]
- Vanaja, D.K.; Grossmann, M.E.; Celis, E.; Young, C.Y. Tumor Prevention and Antitumor Immunity with Heat Shock Protein 70 Induced by 15-Deoxy-Delta12,14-Prostaglandin J2 in Transgenic Adenocarcinoma of Mouse Prostate Cells. Cancer Res. 2000, 60, 4714–4718. [Google Scholar]
- Gong, J.; Zhang, Y.; Durfee, J.; Weng, D.; Liu, C.; Koido, S.; Song, B.; Apostolopoulos, V.; Calderwood, S.K. A Heat Shock Protein 70-Based Vaccine with Enhanced Immunogenicity for Clinical Use. J. Immunol. 2010, 184, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L.; Peng, S.; Kos, F.; Gravitt, P.; Viscidi, R.; Sugar, E.; Pardoll, D.; Wu, T.C. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin. Cancer Res. 2009, 15, 361–367. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linder, M.; Pogge von Strandmann, E. The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells. Cancers 2021, 13, 4721. https://doi.org/10.3390/cancers13184721
Linder M, Pogge von Strandmann E. The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells. Cancers. 2021; 13(18):4721. https://doi.org/10.3390/cancers13184721
Chicago/Turabian StyleLinder, Manuel, and Elke Pogge von Strandmann. 2021. "The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells" Cancers 13, no. 18: 4721. https://doi.org/10.3390/cancers13184721
APA StyleLinder, M., & Pogge von Strandmann, E. (2021). The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells. Cancers, 13(18), 4721. https://doi.org/10.3390/cancers13184721





