Comparison of the Clinical Features and Outcomes of Gallbladder Neuroendocrine Carcinoma with Those of Adenocarcinoma: A Propensity Score-Matched Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pathological Classification and Staging
2.3. Statistical Analysis
3. Results
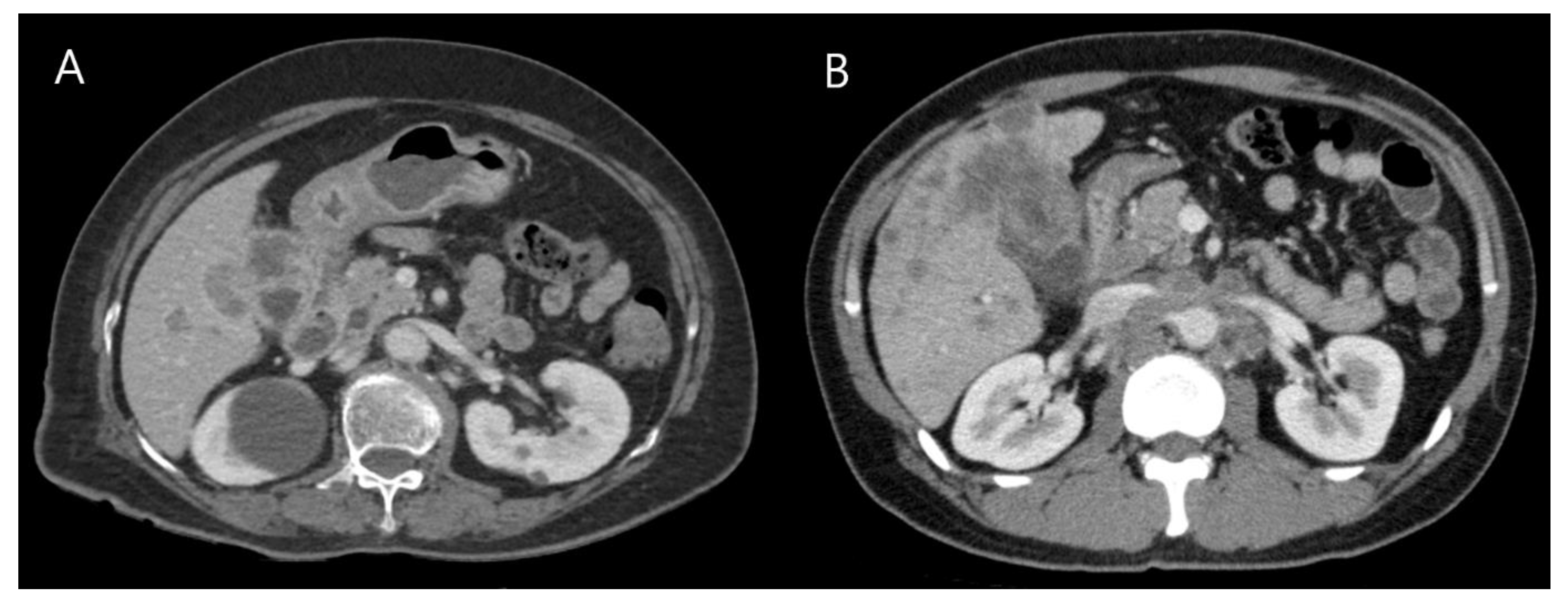
3.1. Clinical Features of GB-NENs
3.2. Histopathological Features and Staging
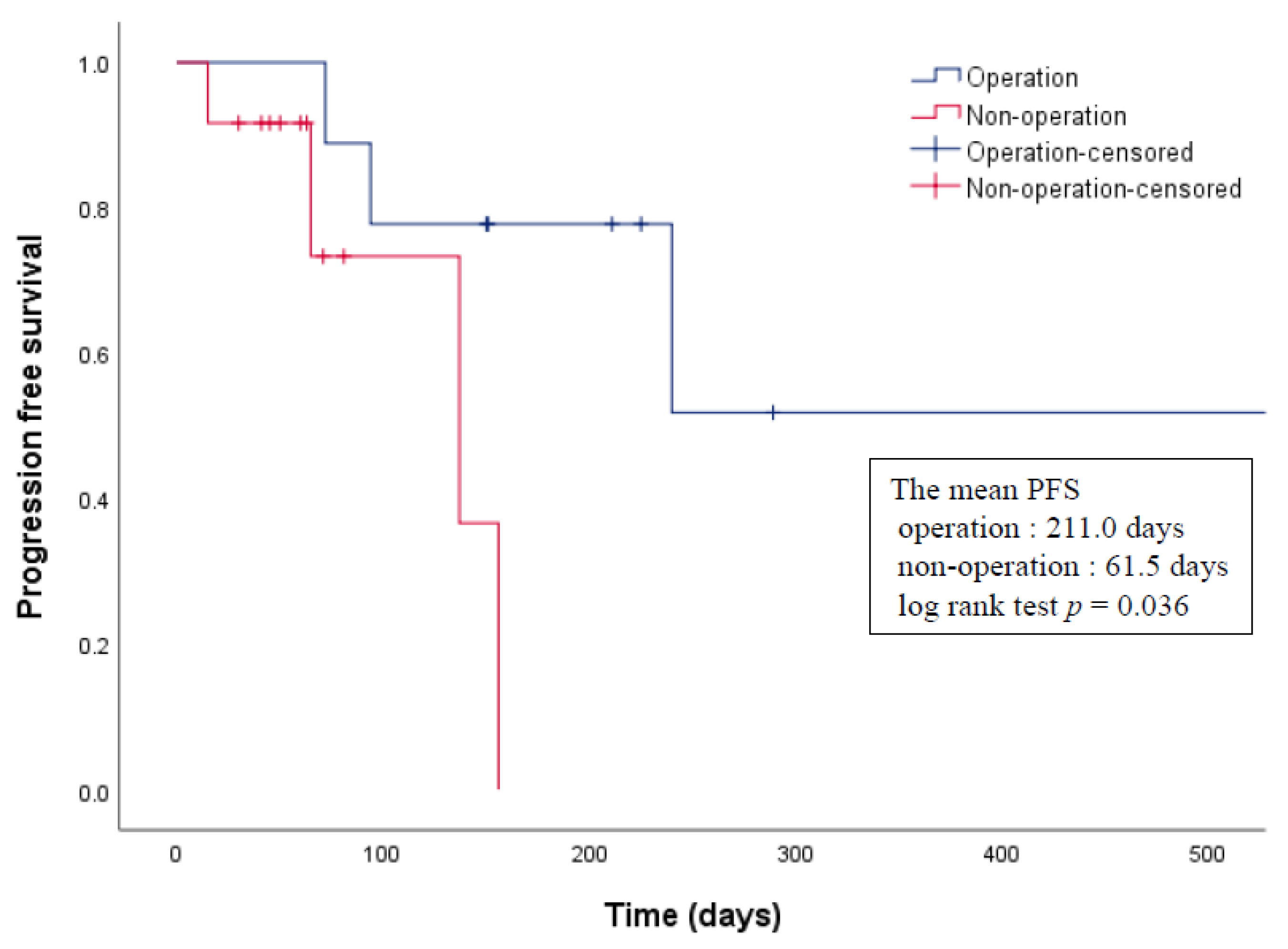
3.3. Treatments and Clinical Outcomes
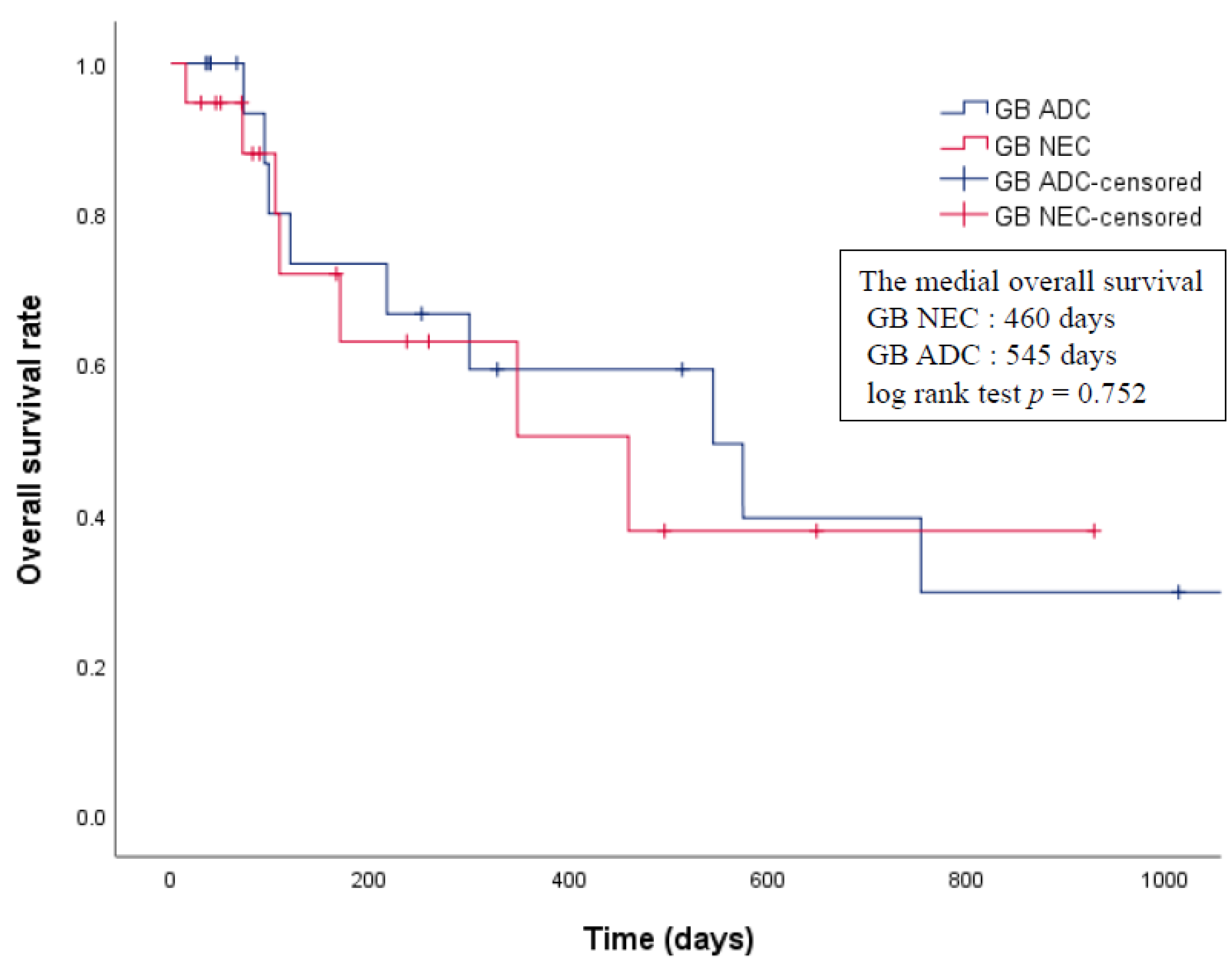
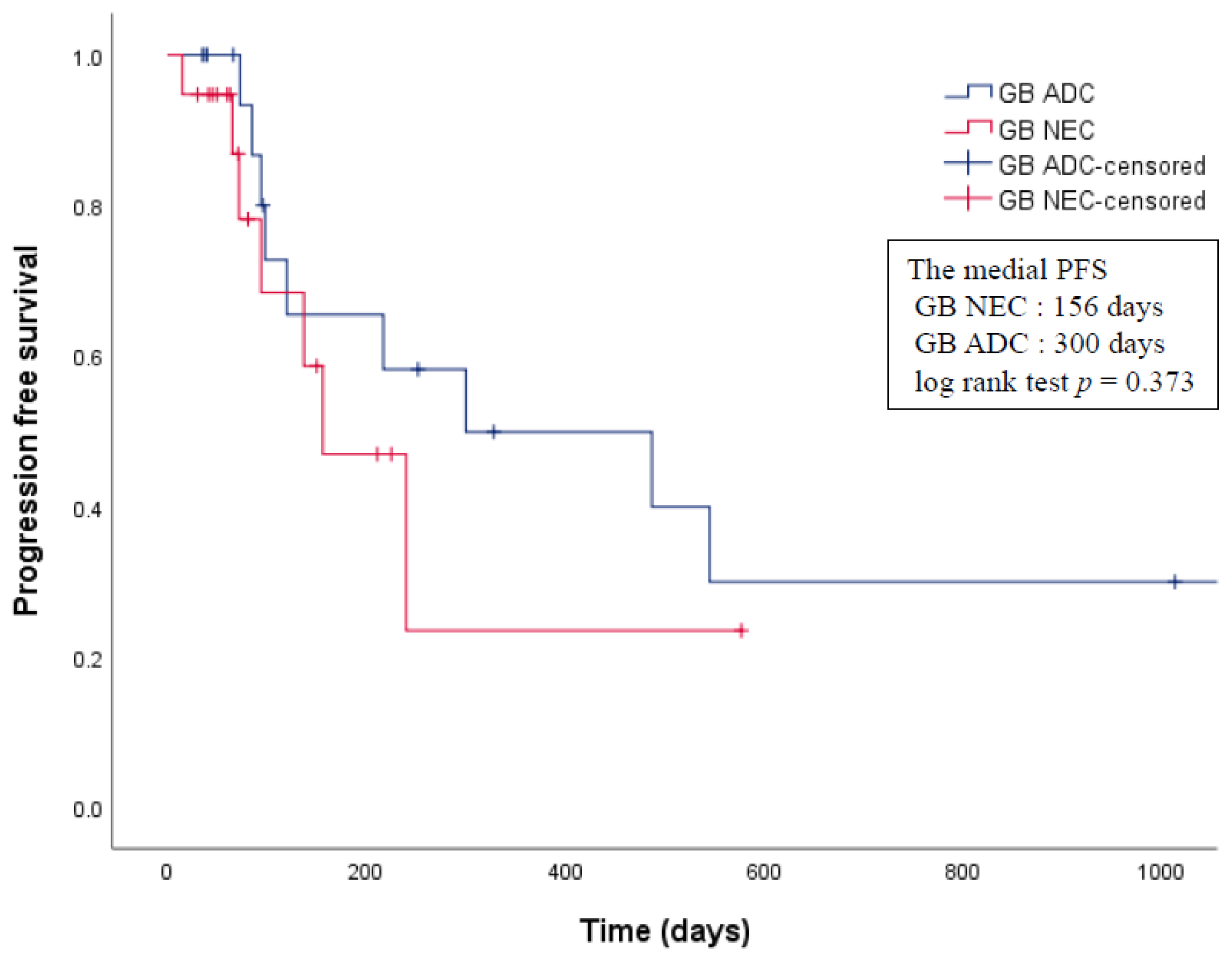
3.4. Propensity Score Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cen, D.; Liu, H.; Wan, Z.; Lin, Z.; Wang, Y.; Xu, J.; Liang, Y. Clinicopathological features and survival for gallbladder NEN: A population-based study. Endocr. Connect. 2019, 8, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Gustafsson, B.I.; Kidd, M.; Modlin, I.M. Neuroendocrine tumors of the diffuse neuroendocrine system. Curr. Opin. Oncol. 2008, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.L.; O’Toole, D. Diagnosis and management of upper gastrointestinal neuroendocrine tumors. Clin. Endosc. 2017, 50, 520–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.H.; Ryu, J.K.; Song, S.Y.; Hwang, J.-H.; Lee, D.K.; Woo, S.M.; Joo, Y.-E.; Jeong, S.; Lee, S.-O.; Park, B.K. Prognostic validity of the American joint committee on cancer and the European neuroendocrine tumors staging classifications for pancreatic neuroendocrine tumors: A retrospective nationwide multicenter study in South Korea. Pancreas 2016, 45, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [Green Version]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Joel, W. Karzinoid der gallenblase. Zent. Allg. Pathl. 1929, 49, 1–4. [Google Scholar]
- Eltawil, K.M.; Gustafsson, B.I.; Kidd, M.; Modlin, I.M. Neuroendocrine tumors of the gallbladder: An evaluation and reassessment of management strategy. J. Clin. Gastroenterol. 2010, 44, 687–695. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Wang, Y.; Chen, X.; Zhang, Y.; Huang, Z.; Zhao, J.; Zhou, J.; Li, Z.; Bi, X.; Luo, Z. Clinical analysis of 15 cases of gallbladder neuroendocrine carcinoma and comparison with gallbladder adenocarcinoma using a propensity score matching. Cancer Manag. Res. 2020, 12, 1437–1446. [Google Scholar] [CrossRef] [Green Version]
- You, Y.H.; Choi, D.W.; Heo, J.S.; Han, I.W.; Choi, S.H.; Jang, K.-T.; Han, S. Can surgical treatment be justified for neuroendocrine carcinoma of the gallbladder? Medicine 2019, 98, e14886. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Shin, N.; Seo, H.I. Clinical outcomes of small cell neuroendocrine carcinoma and adenocarcinoma of the gallbladder. World J. Gastroenterol. 2015, 21, 269–275. [Google Scholar] [CrossRef]
- Kamboj, M.; Gandhi, J.S.; Gupta, G.; Sharma, A.; Pasricha, S.; Mehta, A.; Chandragouda, D.; Sinha, R. Neuroendocrine carcinoma of gall bladder: A series of 19 cases with review of literature. J. Gastrointest. Cancer 2015, 46, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.; Byrd, D. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Duffy, A.; Capanu, M.; Abou-Alfa, G.; Huitzil, D.; Jarnagin, W.; Fong, Y.; D’Angelica, M.; Dematteo, R.; Blumgart, L.; O’Reilly, E. Gallbladder cancer (GBC): 10-year experience at memorial Sloan-Kettering cancer centre (MSKCC). J. Surg. Oncol. 2008, 98, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.; Liu, X.; Zhang, G.; Zhao, Y.; Geng, Z. Gallbladder neuroendocrine carcinoma: Report of 10 cases and comparision of clinicopathologic features with gallbladder adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 8218–8226. [Google Scholar]
- Lee, K.J.; Cho, J.H.; Lee, S.H.; Lee, K.H.; Park, B.K.; Lee, J.K.; Woo, S.M.; Ryu, J.K.; Lee, J.K.; Kim, Y.S. Clinicopathological characteristics of biliary neuroendocrine neoplasms: A multicenter study. Scand. J. Gastroenterol. 2017, 52, 437–441. [Google Scholar] [CrossRef]
- Kim, J.; Lee, W.J.; Lee, S.H.; Lee, K.B.; Ryu, J.K.; Kim, Y.-T.; Kim, S.-W.; Yoon, Y.B.; Hwang, J.H.; Han, H.-S. Clinical features of 20 patients with curatively resected biliary neuroendocrine tumours. Dig. Liver Dis. 2011, 43, 965–970. [Google Scholar] [CrossRef]
- Iida, Y.; Tsutsumi, Y. Small cell (endocrine cell) carcinoma of the gallbladder with squamous and adenocarcinomatous components. Pathol. Int. 1992, 42, 119–125. [Google Scholar] [CrossRef]
- Shimizu, T.; Tajiri, T.; Akimaru, K.; Arima, Y.; Yoshida, H.; Yokomuro, S.; Mamada, Y.; Taniai, N.; Mizuguchi, Y.; Kawahigashi, Y. Combined neuroendocrine cell carcinoma and adenocarcinoma of the gallbladder: Report of a case. J. Nippon. Med. Sch. 2006, 73, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Shimono, C.; Suwa, K.; Sato, M.; Shirai, S.; Yamada, K.; Nakamura, Y.; Makuuchi, M. Large cell neuroendocrine carcinoma of the gallbladder: Long survival achieved by multimodal treatment. Int. J. Clin. Oncol. 2009, 14, 351–355. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, S.H.; Lee, K.B.; Han, J.K. Outcome and CT differentiation of gallbladder neuroendocrine tumours from adenocarcinomas. Eur. Radiol. 2017, 27, 507–517. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Kim, M.-A.; Shin, C.-i.; Han, J.K.; Choi, B.I. CT differentiation of poorly-differentiated gastric neuroendocrine tumours from well-differentiated neuroendocrine tumours and gastric adenocarcinomas. Eur. Radiol. 2015, 25, 1946–1957. [Google Scholar] [CrossRef]
- Chang, S.; Choi, D.; Lee, S.J.; Lee, W.J.; Park, M.-h.; Kim, S.W.; Lee, D.K.; Jang, K.-T. Neuroendocrine neoplasms of the gastrointestinal tract: Classification, pathologic basis, and imaging features. Radiographics 2007, 27, 1667–1679. [Google Scholar] [CrossRef]
- Klimstra, D.S.; Modlin, I.R.; Coppola, D.; Lloyd, R.V.; Suster, S. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas 2010, 39, 707–712. [Google Scholar] [CrossRef]
- Liu, W.; Chen, W.; Chen, J.; Hong, T.; Li, B.; Qu, Q.; He, X. Neuroendocrine carcinoma of gallbladder: A case series and literature review. Eur. J. Med. Res. 2019, 24, 8. [Google Scholar] [CrossRef] [Green Version]
- Janson, E.T.; Sorbye, H.; Welin, S.; Federspiel, B.; Grønbæk, H.; Hellman, P.; Ladekarl, M.; Langer, S.W.; Mortensen, J.; Schalin-Jäntti, C. Nordic guidelines 2014 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. 2014, 53, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-h.; Yang, Q.-c.; Lin, Y.; Xue, L.; Chen, M.-h.; Chen, J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine 2014, 93, e247. [Google Scholar] [CrossRef] [PubMed]
- Nikou, G.; Marinou, K.; Thomakos, P.; Papageorgiou, D.; Sanzanidis, V.; Nikolaou, P.; Kosmidis, C.; Moulakakis, A.; Mallas, E. Chromogranin a levels in diagnosis, treatment and follow-up of 42 patients with non-functioning pancreatic endocrine tumours. Pancreatology 2008, 8, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Hung, Y.-S.; Hsu, J.-T.; Chen, J.-S.; Lu, C.-H.; Hwang, T.-L.; Rau, K.-M.; Yeh, K.-Y.; Chen, T.-C.; Sun, C.-F. Chromogranin A is a reliable biomarker for gastroenteropancreatic neuroendocrine tumors in an Asian population of patients. Neuroendocrinology 2012, 95, 344–350. [Google Scholar] [CrossRef]
- Nehar, D.; Lombard-Bohas, C.; Olivieri, S.; Claustrat, B.; Chayvialle, J.A.; Penes, M.C.; Sassolas, G.; Borson-Chazot, F. Interest of Chromogranin A for diagnosis and follow-up of endocrine tumours. Clin. Endocrinol. 2004, 60, 644–652. [Google Scholar] [CrossRef]
- Campana, D.; Nori, F.; Piscitelli, L.; Morselli-Labate, A.M.; Pezzilli, R.; Corinaldesi, R.; Tomassetti, P. Chromogranin A: Is it a useful marker of neuroendocrine tumors? J. Clin. Oncol. 2007, 25, 1967–1973. [Google Scholar] [CrossRef] [Green Version]
- Pobłocki, J.; Jasińska, A.; Syrenicz, A.; Andrysiak-Mamos, E.; Szczuko, M. The neuroendocrine neoplasms of the digestive tract: Diagnosis, treatment and nutrition. Nutrients 2020, 12, 1437. [Google Scholar] [CrossRef]
- Porter, J.; Kalloo, A.; Abernathy, E.; Yeo, C.J. Carcinoid tumor of the gallbladder: Laparoscopic resection and review of the literature. Surgery 1992, 112, 100–105. [Google Scholar] [PubMed]
- Deehan, D.; Heys, S.; Kernohan, N.; Eremin, O. Carcinoid tumour of the gall bladder: Two case reports and a review of published works. Gut 1993, 34, 1274–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskal, T.L.; Zhang, P.J.; Nava, H.R. Small cell carcinoma of the gallbladder. J. Surg. Oncol. 1999, 70, 54–59. [Google Scholar] [CrossRef]
- Iype, S.; Mirza, T.; Propper, D.; Bhattacharya, S.; Feakins, R.; Kocher, H. Neuroendocrine tumours of the gallbladder: Three cases and a review of the literature. Postgrad. Med. J. 2009, 85, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K.; Åkerström, G.; Rindi, G.; Jelic, S. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v223–v227. [Google Scholar] [CrossRef]
- Adachi, T.; Haraguchi, M.; Irie, J.; Yoshimoto, T.; Uehara, R.; Ito, S.; Tokai, H.; Noda, K.; Tada, N.; Hirabaru, M. Gallbladder small cell carcinoma: A case report and literature review. Surg. Case Rep. 2016, 2, 71. [Google Scholar] [CrossRef] [Green Version]
- Kanetkar, A.V.; Patkar, S.; Khobragade, K.H.; Ostwal, V.; Ramaswamy, A.; Goel, M. Neuroendocrine carcinoma of gallbladder: A step beyond palliative therapy, experience of 25 cases. J. Gastrointest. Cancer 2019, 50, 298–303. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.J.; Lee, H.I.; Jeong, S.-H.; Nam, H.J.; Cho, J.H. Upregulation of Peroxiredoxin-2 in Well-Differentiated Pancreatic Neuroendocrine Tumors and Its Utility as a Biomarker for Predicting the Response to Everolimus. Antioxidants 2020, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
| Variables | Median (Range) or n (%) |
|---|---|
| Age, years, median (range) | 62.0 (31–84) |
| Sex, n (%) | |
| Male | 11 (52.4) |
| Female | 10 (47.6) |
| Clinical symptom, n (%) | |
| Abdominal discomfort | 18 (85.8) |
| Jaundice | 6 (28.6) |
| Weight loss | 4 (19.0) |
| Pruritus | 1 (4.8) |
| Tumor marker, median (range) | |
| CA19-9 (U/mL) | 22.6 (0.6–696.7) |
| CEA (ng/mL) | 2.2 (0.5–1265.3) |
| NEN classification, n (%) | |
| NEC, small-cell type | 20 (95.2) |
| NEC, large-cell type | 1 (4.8) |
| Differentiated degree, n (%) | |
| Grade 1/2 | 0/0 |
| Grade 3 | 21 (100) |
| Ki-67 index, n (%) | |
| >20 | 17 (81.0) |
| Unknown | 4 (19.0) |
| Immunohistochemical stain, n (%) | |
| CgA | 17 (81.0) |
| Syn | 18 (85.7) |
| NSE (test in 1 case) | 1 (100) |
| CD56 (test in 15 cases) | 11 (73.3) |
| TNM stage, n (%) | |
| IIIB | 6 (28.6) |
| IVA | 1 (4.8) |
| IVB | 14 (66.7) |
| Distant metastasis, n (%) | 13 (61.9) |
| Liver | 11 |
| Bone | 1 |
| Peritoneum | 1 |
| Ovary | 1 |
| Lt. subclavian lymph node | 1 |
| Operation, n (%) | 9 (42.9) |
| Curative | 7 |
| Palliative | 2 |
| Chemotherapy, n (%) | 15 (71.4) |
| Variables | before PS Matching | after PS Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| GB-NEC (n = 21) | GB-ADC (n = 206) | Standardized Difference | p-Value | GB-NEC (n = 19) | GB-ADC (n = 19) | Standardized Difference | p-Value | |
| Age, years, mean (SD) | 64.2 ± 14.3 | 65.0 ± 11.1 | 0.059 | 0.774 | 64.6 ± 15.1 | 63.1 ± 13.8 | −0.102 | 0.755 |
| Sex, n (%) | 0.471 | 0.746 | ||||||
| Male | 11 (52.4) | 91(44.2) | −0.165 | 9 (47.4) | 10 (52.6) | 0.105 | ||
| Female | 10 (47.6) | 115 (55.8) | 0.165 | 10 (52.6) | 9 (47.4) | −0.105 | ||
| TNM stage, n (%) | 0.011 | >0.999 | ||||||
| I | 0 (0.0) | 17 (8.3) | 0.424 | 0 (0.0) | 0 (0.0) | 0.000 | ||
| II | 0 (0.0) | 32 (15.5) | 0.606 | 0 (0.0) | 0 (0.0) | 0.000 | ||
| IIIa | 0 (0.0) | 10 (4.9) | 0.319 | 0 (0.0) | 0 (0.0) | 0.000 | ||
| IIIb | 6 (28.6) | 15 (7.3) | −0.578 | 4 (21.1) | 4 (21.1) | 0.000 | ||
| IVa | 1 (4.8) | 6 (2.9) | −0.096 | 1 (5.3) | 2 (10.5) | 0.196 | ||
| IVb | 14 (66.7) | 126 (61.2) | −0.115 | 14 (73.7) | 13 (68.4) | −0.116 | ||
| Operation, n (%) | 9 (42.9) | 115 (55.8) | 0.262 | 0.255 | 7 (36.8) | 9 (47.4) | 0.214 | 0.511 |
| Chemotherapy, n (%) | 15 (71.4) | 141 (68.4) | −0.065 | 0.779 | 13 (68.4) | 14 (73.7) | 0.116 | 0.721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, M.-Y.; Jang, S.-I.; Kang, H.-P.; Kim, E.-J.; Lee, K.-J.; Park, G.-E.; Lee, S.-J.; Lee, D.-K.; Woo, S.-M.; Cho, J.-H. Comparison of the Clinical Features and Outcomes of Gallbladder Neuroendocrine Carcinoma with Those of Adenocarcinoma: A Propensity Score-Matched Analysis. Cancers 2021, 13, 4713. https://doi.org/10.3390/cancers13184713
Do M-Y, Jang S-I, Kang H-P, Kim E-J, Lee K-J, Park G-E, Lee S-J, Lee D-K, Woo S-M, Cho J-H. Comparison of the Clinical Features and Outcomes of Gallbladder Neuroendocrine Carcinoma with Those of Adenocarcinoma: A Propensity Score-Matched Analysis. Cancers. 2021; 13(18):4713. https://doi.org/10.3390/cancers13184713
Chicago/Turabian StyleDo, Min-Young, Sung-Ill Jang, Hua-Pyong Kang, Eui-Joo Kim, Kyong-Joo Lee, Go-Eun Park, Su-Jee Lee, Dong-Ki Lee, Sang-Myung Woo, and Jae-Hee Cho. 2021. "Comparison of the Clinical Features and Outcomes of Gallbladder Neuroendocrine Carcinoma with Those of Adenocarcinoma: A Propensity Score-Matched Analysis" Cancers 13, no. 18: 4713. https://doi.org/10.3390/cancers13184713
APA StyleDo, M.-Y., Jang, S.-I., Kang, H.-P., Kim, E.-J., Lee, K.-J., Park, G.-E., Lee, S.-J., Lee, D.-K., Woo, S.-M., & Cho, J.-H. (2021). Comparison of the Clinical Features and Outcomes of Gallbladder Neuroendocrine Carcinoma with Those of Adenocarcinoma: A Propensity Score-Matched Analysis. Cancers, 13(18), 4713. https://doi.org/10.3390/cancers13184713






